Introduction
The incidence of acute myocardial infarction (MI)
has exhibited an increasing trend. The most effective therapeutic
measure against myocardial ischemia is the restoration of
myocardial perfusion; however, myocardial ischemia/reperfusion
(I/R) injury is a serious consequence. Myocardial I/R may induce
excessive reactive oxygen species to attack cells in the reperfused
area. Ultrastructural damage to the myocardium is irreversible, and
can lead to disordered metabolism and function.
Dexmedetomidine (DEX) is a novel α-2 agonist with
sedative properties. DEX has been used as a sedative in numerous
settings, increasing the cooperation of patients without depressing
their respiration (1,2). DEX has high potential for use in
clinical anesthesia, and due to its wide effect on inflammatory
responses, may have a protective role in myocardial I/R (3). It has previously been reported that
DEX pre-treatment improves myocardial I/R injury in isolated hearts
(4); however, whether DEX
post-treatment can protect the heart from I/R injury in vivo
remains unknown (5).
Phosphatidylinositol-3 kinase/protein kinase B
(PI3K/Akt) is an intracellular signaling pathway, which has diverse
biological actions, and is involved in cell survival, apoptosis,
growth, energy metabolism and migration (6). Akt, also known as PKB, is a
downstream target of PI3K; when Akt is activated by upstream
signaling factors (PI3K-phosphoinositide-dependent kinase-1), it
can activate glycogen synthase kinase (GSK)-3β to phosphorylated
(p)-GSK-3β, which is believed to be the integration point of
several pathways and has an important role in cardioprotection
(7). In addition, it has been
hypothesized that GSK-3β is a functional downstream target of Akt
(7). Cysteine aspartate protease-3
(caspase-3) is one of the most important enzymes in the caspase
family, and is involved in the apoptotic process following
activation by other members of the caspase family. Caspase-3 is a
significant protease in the cascade reaction, and is therefore
known as the 'hallmark enzyme' of cell apoptosis (8). Cleaved caspase-3 is the activated
form of caspase-3. Therefore, when Akt is activated, I/R-induced
myocardial injury may be reduced, via the involvement of GSK-3β,
the B-cell lymphoma 2 (Bcl-2) family and caspase-3.
The present study used an in vivo I/R rat
model to determine whether post-ischemic treatment with DEX exerted
protective effects against I/R-induced MI. Lactate dehydrogenase
(LDH), cardiac troponin I (cTnI), creatine kinase isoenzymes
(CK-MB), superoxide dismutase (SOD) and malondialdehyde (MDA) serum
levels were measured, as well as the infarct size of the heart. The
most adverse effect of myocardial I/R injury is irreversible
necrosis. Myocardial enzyme serum levels are one of the most
important indexes used to determine the degree of myocardial
necrosis (9). LDH, CK-MB and cTnI
are markers of myocardial injury (10–12).
CTnI is only expressed in the myocardium, and is therefore a
specific marker of myocardial injury and is considered the 'gold
standard (13)' for the
identification of myocardial injury. MDA is commonly used as a
biomarker of lipid peroxidation, whereas SOD is an antioxidant
enzyme that protects tissues against oxidative stress. All of these
parameters indicate the severity of myocardial ischemia.
In order to investigate the underlying mechanisms,
the present study measured the expression levels of
apoptosis-associated genes and proteins, and evaluated alterations
to the PI3K/Akt signal transduction pathway. Bcl-2 was the first
gene to be identified as an inhibitor of apoptosis, which has been
reported to work as a downstream target of GSK-3β (7). When the expression levels of Bax are
increased, Bax can form homodimers with Bcl-2, and proapoptotic
factors, such as cytochrome c can be released, eventually
activating the downstream factor caspase-3 and promoting apoptosis.
Conversely, Bcl-2 exerts an anti-apoptotic role via its effects on
Bax; therefore, the ratio of Bcl-2/Bax (13,14)
regulates apoptosis to some degree.
The results of the present study indicated that
post-ischemic treatment with DEX exerted protective effects against
I/R-induced MI via the PI3K/Akt signal transduction pathway. These
effects were very likely induced via p-GSK-3β. Further studies are
required to delineate the effects of DEX with α-2 adrenergic
receptor stimulation.
Materials and methods
Reagents
DEX was purchased from Jiangsu Nhwa Pharmaceutical
Corporation Ltd. (Jiangsu, China). Wortmannin (Wort) was obtained
from Sigma-Aldrich (St. Louis, MO, USA). Rat CK-MB isoenzyme enzyme
linked-immunosorbent assay (ELISA) kit (cat. no. E006), LDH assay
kit cat. no. A020-2), cTnI ELISA kit (cat. no. H149-2), SOD assay
kit (cat. no. A001-3) and MDA assay kit (cat. no. A003-1) were
obtained from Jiancheng Institute of Biotechnology (Nanjing,
China). TRIzol® reagent was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Bcl-2, Bax and
β-actin primers were acquired from Sangon Biotech Co., Ltd.
(Shanghai, China). The following primary antibodies were obtained
from Cell Signaling Technology, Inc. (Danvers MA, USA): Mouse
anti-GSK-3β (1:2,000 dilution; cat. no. BF0695), rabbit polyclonal
anti-p-GSK-3β (1:2,000 dilution; cat. no. AF2016), rabbit
polyclonal anti-Akt (1:1,000 dilution; cat. no. 9272S), rabbit
polyclonal anti-p-Akt (1:1,000 dilution; cat. no. 4060S) and rabbit
polyclonal anti-cleaved caspase-3 (1:2,000 dilution; cat. no.
9661S). Horseradish peroxidase (HRP)-linked anti-mouse
immunoglobulin (IgG) (1:10,000 dilution; cat. no. BA1050),
HRP-linked anti-rabbit IgG (1:10,000 dilution; cat. no. BA1054) and
anti-β-actin (1:1,000 dilution; cat. no. BM0627) were acquired from
Wuhan Boster Biological Technology Co., Ltd. (Wuhan, China).
Experimental animals
Male Sprague Dawley (SD) rats (weight, 250–300 g)
were purchased from the Experimental Animal Center of Bengbu
Medical Collage (Bengbu, China). All of the rats had ad
libitum access to food and water, were maintained in a
temperature and humidity-controlled environment (22–26°C; 50%
humidity) with a 12 hours light/dark cycle, and were raised in
plastic cages. All animal procedures were conducted in accordance
with the United States National Institutes of Health Guide and were
approved by the Animal Use and Care Committee of Bengbu Medical
College.
Animal preparation and experimental
design
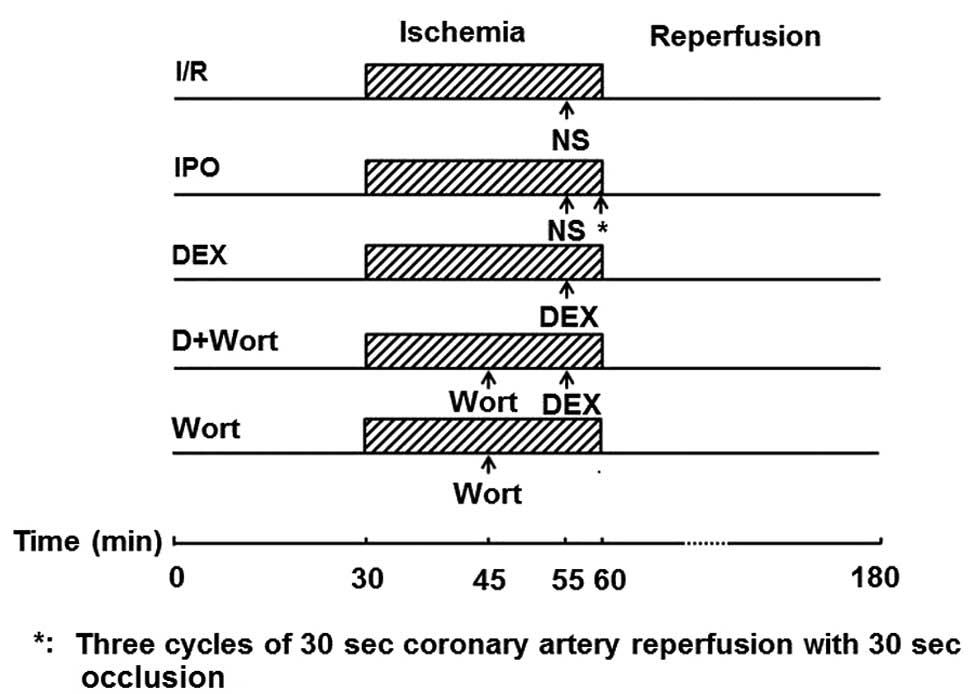
A total of 64 male SD rats were randomly assigned to
the following eight groups: Group 1, sham group (S; n=8), the left
anterior descending artery (LAD) was threaded but not ligated for
150 min; group 2, I/R group (n=8), the rats were injected with 0.5
ml normal saline (NS) after 25 min of LAD ligation, following 30
min of LAD ligation the rats underwent reperfusion for 120 min;
group 3, ischemic post-conditioning group (IPO; n=8), the rats
received 0.5 ml normal saline (NS) after 25 min of LAD ligation,
following 30 min of LAD ligation the hearts were subjected to three
cycles of 30 sec coronary artery reperfusion with 30 sec occlusion,
followed by 120 min of reperfusion; groups 4–6, DEX5, DEX10
(15) and DEX20 groups
(n=8/group), the rats were intravenously injected with 5, 10 or 20
µg/kg DEX after 25 min of LAD ligation, respectively,
following 30 min of LAD ligation the rats underwent reperfusion for
120 min; group 7, DEX20 + Wort group (n=8), the rats were
intravenously injected with 15 µg/kg Wort (16) 15 min after LAD ligation and were
then injected with 20 µg/kg DEX 5 min prior to 120 min
reperfusion; group 8, Wort group (n=8), the rats were intravenously
injected with 15 µg/kg Wort after 15 min of LAD ligation,
following 30 min of LAD ligation the rats underwent reperfusion for
120 min (Fig. 1).
Establishment of a myocardial I/R
model
The rats were anesthetized with 4% chloral hydrate
(1 ml/100 g body weight; i.p.), and in order to maintain
anesthesia, 0.5 ml 4% chloral hydrate was intraperitoneally
injected periodically (0.5 ml/100 g body weight). Following
tracheal intubation, ventilation was provided via respiratory
equipment, at a respiratory rate of 70–80 times/min and a tidal
volume of 2–3 ml/100 g. Standard electrocardiograms (ECG) were
recorded. Using the Medlab biological signal collecting and
processing system (Nanjing Mei Yi Technology Co., Ltd., Nanjing,
China), hemodynamic parameters were continuously measured by
catheterization of the left common carotid artery. A left
parasternal incision was made through the third and fourth ribs,
and the pericardium was gently opened to expose the heart. The LAD
was ligated with a 5-0 silk suture (2 mm) below the left atrial
appendage and the left edge of the pulmonary cone. Its circumflex
branch and the suture ends were threaded through a polyethylene
tube to form snares for reversible coronary artery occlusion.
Myocardial ischemia was induced via compression of the LAD by
tightening the silk suture around the polyethylene tube following
20 min stabilization. An elevated ST-segment in the ECG indicated
the success of ischemia. After 30 min of ischemia, the polyethylene
tubes were loosened for 120 min to mimic reperfusion (17).
Measurement of serum LDH, cTnI, CK-MB,
SOD and MDA levels
At the end of reperfusion, arterial blood samples
were collected, placed in test tubes with heparin, and centrifuged
at 3,000 × g for 15 min at 4°C. The perfusion fluid was collected
and stored at −80°C, and was thawed once prior to analysis. LDH,
cTnI, CK-MB and MDA contents, and SOD activity were measured using
commercially available kits according to the manufacturer's
protocols.
Assessment of myocardial infarct
size
At the end of reperfusion, heart tissues were
collected from rats that had been anesthetized 4% chloral hydrate(1
ml/100 g body weight; i.p.) and the excess blood was removed using
Krebs-Henseleit saline. Following re-occlusion of the left coronary
artery in isolated Langendorff-perfused equipment, the hearts were
perfused with 1% Evans blue dye, in order to delineate the
non-ischemic area, since the risk and infarct areas remained
undyed. The tissues were then frozen at −20°C for several hours,
prior to being sliced transversely into 2–3 mm sections. The slices
were subsequently incubated in 1% tetrazolium chloride buffer
solution (pH 7.4) for 10–15 min at 37°C, and were fixed in 10%
buffered formalin. Infarct (pale) and risk (red) areas were
calculated by planimetry using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA). Infarct size was
expressed as the percentage of risk area.
Western blot analysis of Akt, p-Akt,
cleaved caspase-3, GSK-3β and p-GSK-3β
Western bot analysis was performed using proteins
extracted from heart tissue samples. The DEX20 group was chosen as
the treatment group used to investigate the underlying mechanisms
of DEX post-conditioning, since the synthetic protective effects
were more obvious in the DEX20 group compared with the DEX5 and
DEX10 groups, according to serum enzyme level alterations. Western
blot analyses were performed as previously described (17).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of Bax and Bcl-2 mRNA expression
Gene expression analysis was performed via RT-PCR
using RNA from the heart tissue samples, as previously described
(16). The primer sequences are
presented in Table I.
 | Table IReverse transcription-polymerase chain
reaction primers for Bax, Bcl-2 and β-actin. |
Table I
Reverse transcription-polymerase chain
reaction primers for Bax, Bcl-2 and β-actin.
| Gene | Primer | Sequence | Product (bp) |
|---|
| Bax | Forward |
5′-GGATCGAGCAGAGAGGATGG-3′ | 464 |
| Reverse |
5′-TGGTGAGTGAGGCAGTGAGG-3′ | |
| Bcl-2 | Forward |
5′-CTGGTGGACAACATCGCTCTG-3′ | 227 |
| Reverse |
5′-GGTCTGCTGACCTCACTTGTG-3′ | |
| β-actin | Forward |
5′-GATGGTGGGTATGGGTCAGAAGGAC-3′ | 630 |
| Reverse |
5′-GCTCATTGCCGATAGTGATGACT-3′ | |
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences between treatment groups were assessed by
one-way analysis of variance and Student-Newman-Keuls multiple
comparison test using SPSS 16.0 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Alterations to the serum LDH, CK-MB,
cTnI, SOD and MDA levels in each group
Compared with the S group, LDH, cTnI, CK-MB and MDA
levels in all of the other groups were increased, whereas SOD
activity was decreased. Compared with the I/R group, the activities
of LDH and CK-MB, and cTnI and MDA levels were reduced in the IPO,
DEX10 and DEX20 groups; however, SOD activity was elevated. In
addition, LDH, cTnI, CK-MB and MDA levels were significantly
increased in the DEX20 + Wort group compared with the DEX20 group,
whereas SOD activity was decreased (Fig. 2).
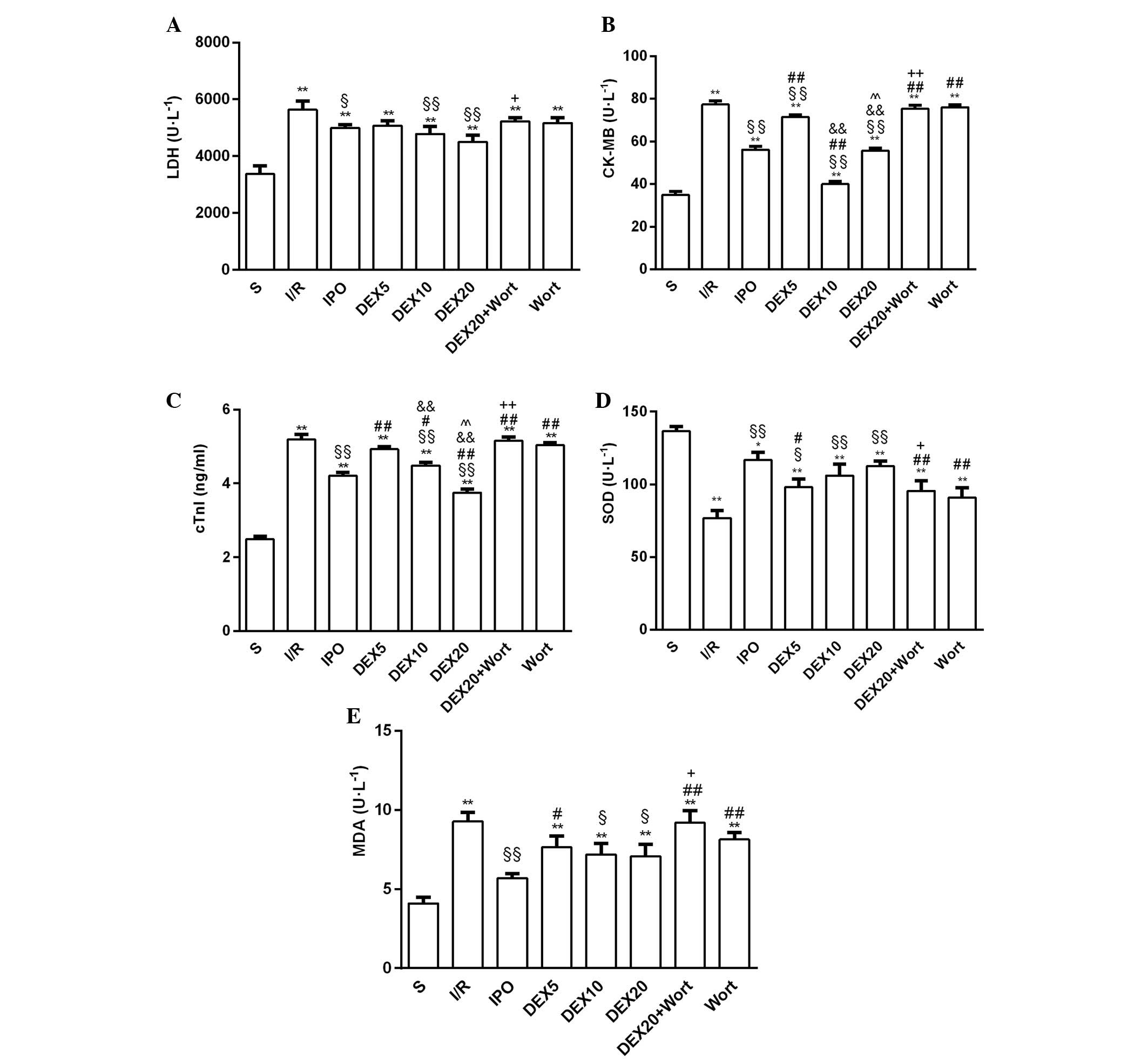 | Figure 2(A) LDH, (B) CK-MB, (C) cTnI, (D) SOD
and (E) MDA levels in the serum of rats from each group. Data are
presented as the mean ± standard error of the mean from at least
six independent experiments. *P<0.05,
**P<0.01 vs. S group, §P<0.05,
§§P<0.01 vs. I/R group; #P<0.05,
##P<0.01 vs. IPO group,
&&P<0.01 vs. DEX5 group;
^^P<0.01 vs. DEX10 group; +P<0.05,
++P<0.01 vs. DEX20 group. LDH, lactate dehydrogenase;
cTnI, cardiac troponin I; CK-MB, creatine kinase isoenzymes; MDA,
malondialdehyde; SOD, superoxide dismutase; S, sham; I/R,
ischemia/reperfusion; IPO, ischemic post-conditioning; DEX,
dexmedetomidine; Wort, wortmannin. |
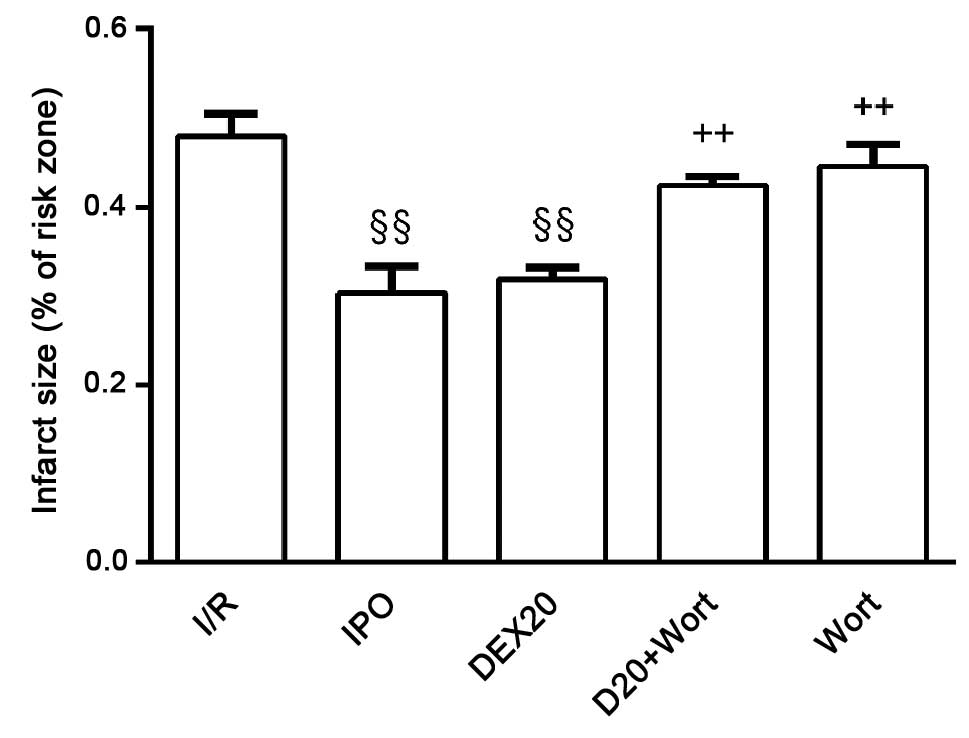
Effects on myocardial infarct size in
each group
Compared with the I/R group, infarct size in the IPO
and DEX20 groups was decreased. Compared with the DEX20 group,
infarct size in the DEX20 + Wort group was markedly increased
(Fig. 3).
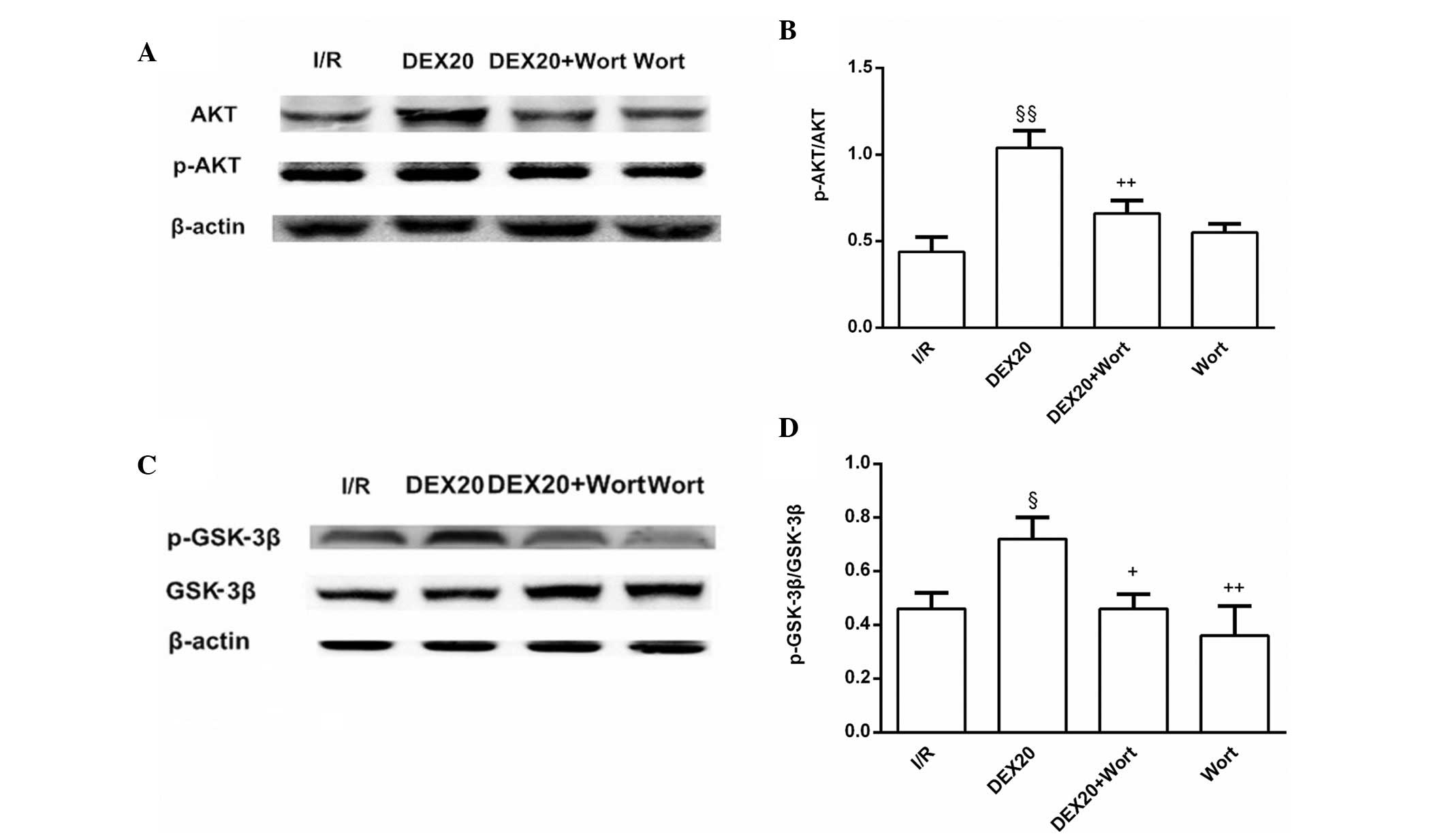
Alterations to the protein expression
levels of p-Akt and p-GSK-3β
The protein expression levels of p-Akt and p-GSK-3β
in the DEX20 group were increased compared with those in the I/R
group. Conversely, p-Akt and p-GSK-3β levels in the DEX20 + Wort
group were lower than in the DEX20 group (Fig. 4).
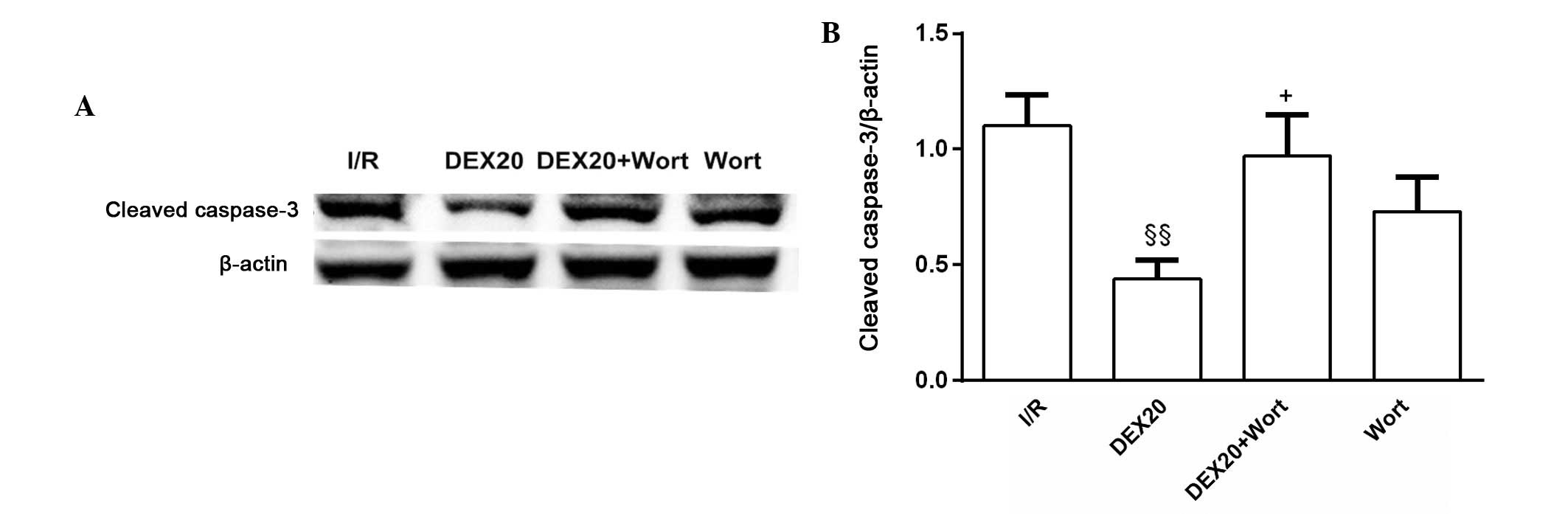
Alterations to the protein expression
levels of cleaved caspase-3
A significant decrease in the expression levels of
cleaved caspase-3 was detected in the DEX20 group compared with in
the I/R group. However, in the DEX20 + Wort group, a significant
increase in cleaved caspase-3 was detected, as compared with in the
DEX20 group. The protein expression levels for each sample were
determined as a percentage of the corresponding β-actin levels
(Fig. 5).
Alterations to the mRNA expression levels
of Bcl-2 and Bax
A marked increase in the expression levels of Bcl-2
and the ratio of Bcl-2/Bax were detected in the DEX20 group
compared with the I/R group, whereas Bax expression was decreased.
In the DEX20 + Wort group, Bcl-2 levels and the ratio of Bcl-2/Bax
were significantly decreased compared with the DEX20 group, whereas
Bax expression was increased (Fig.
6).
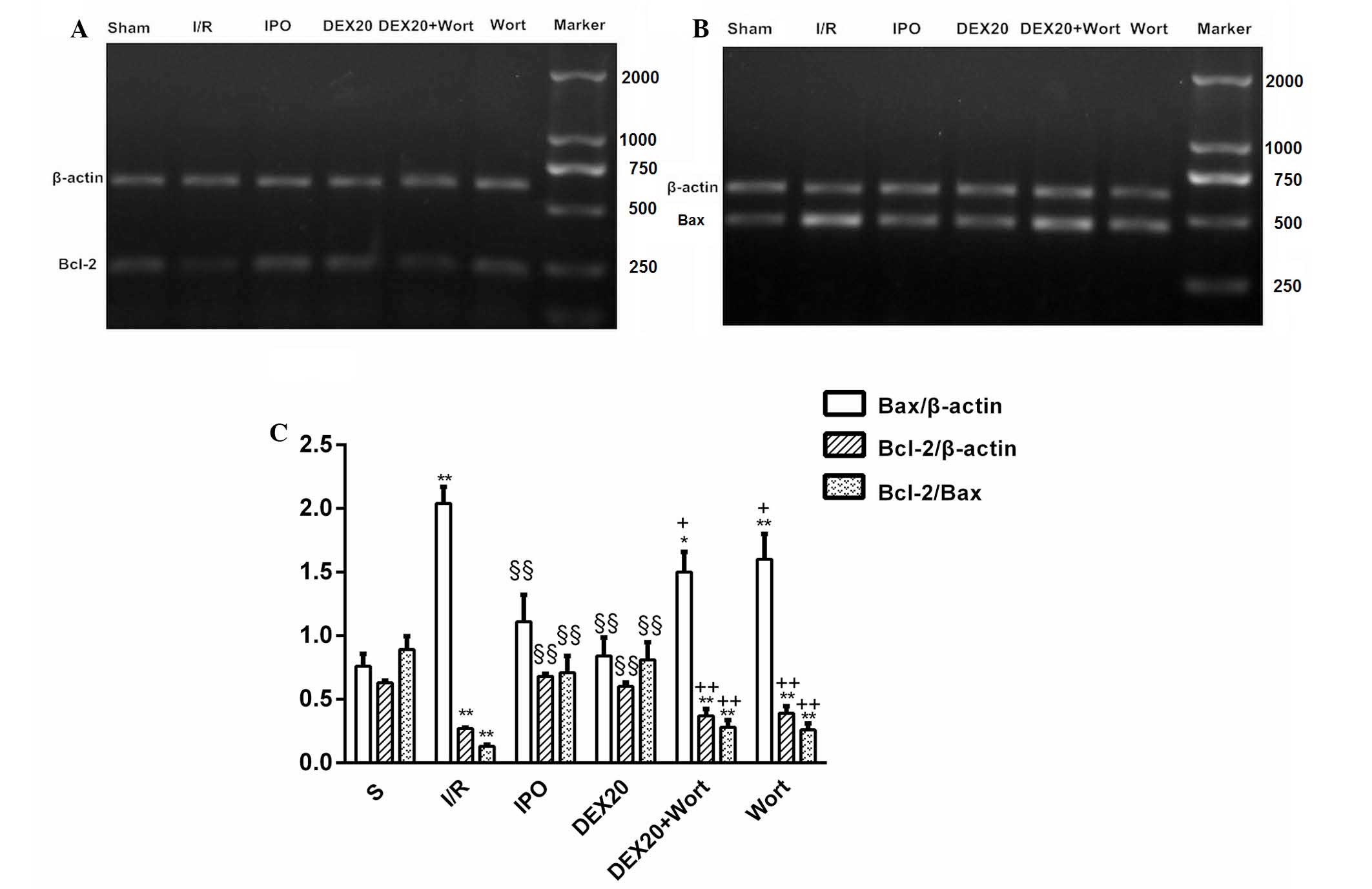 | Figure 6mRNA expression levels of myocardial
Bcl-2 and Bax, and quantification of the Bcl-2/Bax ratio in the
various groups. (A and B) Results of RT-PCR analysis in the heart
tissue. β-actin was used as a loading control. (C) Quantification
of the Bcl-2/Bax ratio obtained by densitometric analysis of
RT-PCR. Data are presented as the mean ± standard error of the mean
from at least six independent experiments. *P<0.05,
**P<0.01 vs. S group; §§P<0.01 vs. I/R
group; +P<0.05, ++P<0.01 vs. DEX20
group. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
RT-PCR, reverse transcription-polymerase chain reaction; S, sham;
I/R, ischemia/reperfusion; IPO, ischemic post-conditioning; DEX,
dexmedetomidine; Wort, wortmannin. |
Discussion
The present study demonstrated that post-ischemic
treatment with DEX, a novel α-2 agonist with sedative properties,
significantly attenuated the increased levels of LDH, cTnI, CK-MB
and MDA, and the reduced activity of SOD induced by I/R, similar to
post-ischemic preconditioning. In addition, DEX reduced infarct
size. These observations indicated that DEX may exert
cardioprotective effects.
Notably, the present study revealed that DEX
increased the expression levels of p-Akt and p-GSK3β in myocardium.
In addition, DEX decreased the expression levels of the downstream
proteins of the PI3K/Akt signaling pathway, Bax and cleaved
caspase-3, and increased the expression levels of Bcl-2 and the
ratio of Bcl-2/Bax. Conversely, the effects of DEX on I/R-induced
myocardial injury and PI3K/Akt signaling were attenuated by Wort, a
noncompetitive inhibitor of PI3Ks. These results indicated that DEX
may have an important role in myocardial protection by activating
the PI3K/Akt signaling pathway possibly via activation of
GSK-3β.
Mimuro et al (5) reported that treatment with DEX after
myocardial ischemia in an isolated perfused heart preparation did
not have a beneficial effect on infarct size, DEX induced an
increase in infarct size compared with the reduction in infarct
size observed in the present study. However, the preparations used
in the two studies differed: In vitro in the previous study
compared with in vivo in the present study. Furthermore, a
previous study reported that the cardioprotective effects of
morphine preconditioning on doxorubicin-induced heart failure were
associated with the extracellular signal-regulated kinase/GSK-3β
pathway, independent of PI3K/Akt (18). In the previous study, morphine
preconditioning reduced infarct size and LDH activity caused by I/R
injury, in accordance with the results of the present study.
However, in the previous study, the protective effect of morphine
preconditioning was abolished by extracellular-regulated kinase
inhibition (PD98059), but not by PI3K inhibition (wortmannin). The
current study also used wortmannin to block PI3K demonstrating that
the protective effects of DEX on heart injury were dependent on
PI3K/Akt signaling pathway.
Since DEX is a selective α-2 adrenergic agonist, the
cardio-protective effects observed in the present study may be due
to activation of the α-2 adrenergic receptor. Therefore, further
studies are required to delineate the role of the receptor. In
summary, DEX postconditioning exhibited protective effects on
myocardial ischemia reperfusion injury and the effects may be
dependent on the PI3K/Akt signaling pathway. The results of the
current study may potentially provide a basis the use of DEX as a
clinical therapy for cardiac I/R injury.
Acknowledgments
The present study was supported by the Anhui
Educational Committee (grant no. KJ2012Z246), China. The authors
would like to thank Professor Tak-Ming Wong (University of Hong
Kong, Hong Kong, China) for reading and revising the
manuscript.
References
|
1
|
Souter MJ, Rozet I, Ojemann JG, Souter KJ,
Holmes MD, Lee L and Lam AM: Dexmedetomidine sedation during awake
craniotomy for seizure resection: Effects on electrocorgraphy. J
Neurosurg Anesthesiol. 19:38–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ard J, Doyle W and Bekker A: Awake
craniotomy with dexmedetomidine in pediatric patients. J Neurosurg
Anesthesiol. 15:263–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai Y, Xu H, Yan J, Zhang L and Lu Y:
Molecular targets and mechanism of action of dexmedetomidine in
treatment of ischemia/reperfusion injury. Mol Med Rep. 9:1542–1550.
2014.PubMed/NCBI
|
|
4
|
Ibacache M, Sanchez G, Pedrozo Z, Galvez
F, Humeres C, Echevarria G, Duaso J, Hassi M, Garcia L, Díaz-Araya
G and Lavandero S: Dexmedetomidine preconditioning activates
pro-survival kinases and attenuates regional ischemia/reperfusion
injury in rat heart. Biochim Biophys Acta. 1822.537–545. 2012.
|
|
5
|
Mimuro S, Katoh T, Suzuki A, Yu S, Adachi
YU, Uraoka M, Sano H and Sato S: Deterioration of myocardial injury
due to dexmedetomidine administration after myocardial ischaemia.
Resuscitation. 81:1714–1717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wetzker R and Rommel C: Phosphoinositide
3-kinases as targets for therapeutic intervention. Curr Pharm Des.
10:1915–1922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juhaszova M, Zorov DB, Yaniv Y, Nuss HB,
Wang S and Sollott SJ: Role of glycogen synthase kinase-3beta in
cardioprotection. Circ Res. 104:1240–1252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou PY, Zhang Z, Guo YL, Xiao ZZ, Zhu P,
Mai MJ and Zheng SY: Protective effect of antiapoptosis potency of
prolonged preservation by desiccation using high-pressure carbon
monoxide on isolated rabbit hearts. Transplant Proc. 47:2746–2751.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dahlin LG, Kågedal B, Nylander E, Olin C,
Rutberg H and Sved-jeholm R: Early identification of
permanentmyocrdial damage after coronary surgery is aided by
repeated measurements of CK-MB. Stand Cardiovasc J. 36:35–40. 2002.
View Article : Google Scholar
|
|
10
|
Yang C, Wu K, Li SH and You Q: Protective
effect of curcumin against cardiac dysfunction in sepsis rats.
Pharm Biol. 51:482–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdelrahman RS, El-Awady MS, Nader MA and
Ammar EM: Hydrogen sulfide ameliorates cardiovascular dysfunction
induced by cecal ligation and puncture in rats. Hum Exp Toxicol.
34:953–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ran X, Diao JX, Sun XG, Wang M, An H,
Huang GQ, Zhao XS, Ma WX, Zhou FH, Yang YG and Miao CM: Huangzhi
oral liquid prevents arrhythmias by upregulating caspase-3 and
apoptosis network proteins in myocardial ischemia-reperfusion
injury in rats. Evid Based Complement Alternat Med.
2015:5189262015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: The sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brooks C and Dong Z: Regulation of
mitochondrial morphological dynamics during apoptosis by Bcl-2
family proteins: A key in Bak? Cell Cycle. 6:3043–3047. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lempiäinen J, Finckenberg P, Mervaala EE,
Storvik M, Kaivola J, Lindstedt K, Levijoki J and Mervaala EM:
Dexmedetomidine preconditioning ameliorates kidney
ischemia-reperfusion injury. Pharmacol Res Perspect. 2:e000452014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Y, Jia XJ, Zong QF, Zhang GJ, Ye HW, Hu
J, Gao Q and Guan SD: Remote ischemic postconditioning protects the
heart by upregulating ALDH2 expression levels through the PI3K/Akt
signaling pathway. Mol Med Rep. 10:536–542. 2014.PubMed/NCBI
|
|
17
|
Zhou H, Hou SZ, Luo P, Zeng B, Wang JR,
Wong YF, Jiang ZH and Liu L: Ginseng protects rodent hearts from
acute myocardial ischemia reperfusion injury through
GR/ER-activated RISK pathway in an endothelial NOS-dependent
mechanism. J Ethnopharmacol. 135:287–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He SF, Jin SY, Wu H, Wang B, Wu YX, Zhang
SJ, Irwin MG, Wong TM and Zhang Y: Morphine preconditioning confers
cardioprotection in doxorubicin-induced failing rat hearts via
ERK/GSK-3β pathway independent of PI3K/Akt. Toxicol Appl Pharmacol.
288:349–358. 2015. View Article : Google Scholar : PubMed/NCBI
|