Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
broad term for cases of SCC arising in the paranasal sinuses, nasal
cavity, oral cavity, pharynx and larynx. The majority of these are
epithelial malignancies (1), and
tobacco and alcohol consumption are the most important risk factors
(2). Regional lymph node
metastasis is a prominent characteristic of HNSCC and is an
important prognostic factor for patients with HNSCC. Patients with
regional metastasis have markedly reduced survival rates, compared
with those without. Neck dissection is a standard technique used
for the treatment of regional metastasis, however, predicting
metastasis at early stages of HNSCC remains a challenge clinically,
as the technology using to detect regional metastasis is not
totally reliable. In patients with an N0 tumor status, 20–30% have
been found to show occult metastasis during elective neck
dissection, but remained pathologically N0 (3). In addition, cervical lymph node
metastasis cannot be accurately judged or predicted.
Currently, biomarkers, including A15-3 and
carcinoembryonic antigen, can be used as indices for the detection
of tumor progression and metastasis. However, novel biomarkers are
urgently required, particularly for detecting metastasis in early
stage cancer. Neuromedin U (Nmu) is a secreted neuropeptide, which
is isolated from the porcine spinal cord (4). It is named due to its potent uterine
contraction-inducing activity. Following its identification,
multiple physiological roles have been suggested. The first
biological function of Nmu to be identified was smooth muscle
contraction of the uterus (5).
Current evidence suggests that Nmu is involved in pain, regulation
of feeding and energy homeostasis, stress and immune-mediated
inflammatory diseases, including asthma (6–11).
Nmu has been shown to exist in several peripheral and central
activities. Two types of Nmu receptor have been identified in
humans; Nmu-R1 is expressed predominantly in the periphery, whereas
Nmu-R2 is predominantly expressed in the central nervous system
(12).
Until now, few studies have examined the role of Nmu
in cancer. Pharmacological unmasking of HNSCC and esophageal
squamous cell carcinoma revealed that the expression of Nmu was
silenced in these tumors (13,14).
The downregulation of genes encoding Nmu indicates that Nmu is
potentially involved in preventing the action of tumor suppressors
(15). Several studies have
reported that Nmu is overexpressed in several types of cancer
(16–19). Furthermore, Nmu has a close
correlation with metastasis. It is a Rho GDP Dissociation Inhibitor
2-regulated gene, which appears to be important in lung metastasis
(18). It may be involved in the
hepatocyte growth factor-c-Met paracrine loop regulating cell
migration and the invasiveness of pancreatic cancer (16). In addition, Rani et al
(20) defined Nmu as a candidate
drug response biomarker for HER2-overexpressing cancer, and as a
candidate therapeutic target to limit metastatic progression.
In the present study, the potential role for Nmu as
a novel biomarker of metastasis was investigated to determine
whether it may offer value as a novel therapeutic target to inhibit
the tumor growth and metastasis of HNSCC. The results demonstrated
overexpression of the Nmu protein in the metastatic tissues of
HNSCC, and this was correlated with the Tumor Node Metastasis (TNM)
stage of HNSCC.
Materials and methods
Patient selection and tissue
microassay
The study was approved by the ethics committee of
Renmin Hospital of Wuhan University (Wuhan, China). A total of 240
patients were recruited between 2012 to 2014, who were
histologically diagnosed with HNSCC and were analyzed
retrospectively at the Department of Otolaryngology Head and Neck
Surgery of Renmin Hospital of Wuhan University (Table I). The group contained 236 men and
four women. The average age of the patients was 60 years old
(range, 31–80 years old). Tumor localization included the larynx,
pharynx and nasopharynx. None of the patients received preoperative
radiotherapy, and neck dissection had been performed on all
patients during surgery. The patients were divided into two groups,
consisting of those who had regional metastasis and those who did
not, which was confirmed histologically. Detailed information,
including tumor type, age, gender, differentiation grade and
regional metastasis were obtained (Table I). The present study was approved
by the appropriate ethical committees of the institutions in which
the study was performed. Formal consent was not required
 | Table ICharacteristics of patients
selected. |
Table I
Characteristics of patients
selected.
| Characteristic | Metastasis group
(n=123) | No metastasis group
(n=117) | P-value |
|---|
| Gender | | | 0.123 |
| Male | 123 | 113 | |
| Female | 0 | 4 | |
| Age at diagnosis
(years) | | | 0.085 |
| Median (range) | 59.6 (31–73) | 60.5 (41–80) | |
| Tumor type | | | 0.023 |
| Glottis | 105 | 99 | |
| Hypopharynx | 10 | 10 | |
| Supraglottis | 3 | 1 | |
| Nasopharynx | 5 | 2 | |
| Tonsil | 0 | 1 | |
| Differentiation
grade | | | 0.065 |
| Well
differentiated | 118 | 106 | |
| Moderate | 5 | 7 | |
| Poor | 0 | 0 | |
| Total | 123 | 117 | |
A total of 180 paraffin-embedded tissue blocks from
the primary tumors of the patients were obtained, which was
consistent with the retrospective samples selected from the
Pathology Department of Renmin Hospital of Wuhan University. The
paraffin-embedded tissue blocks were divided into two groups:
Primary tumor with neck lymph node metastasis; and primary tumor
without neck lymph node metastasis. All paraffin-embedded tissue
blocks were cut and dried on 4 µm-thick paraffin slides for
immunohistochemistry.
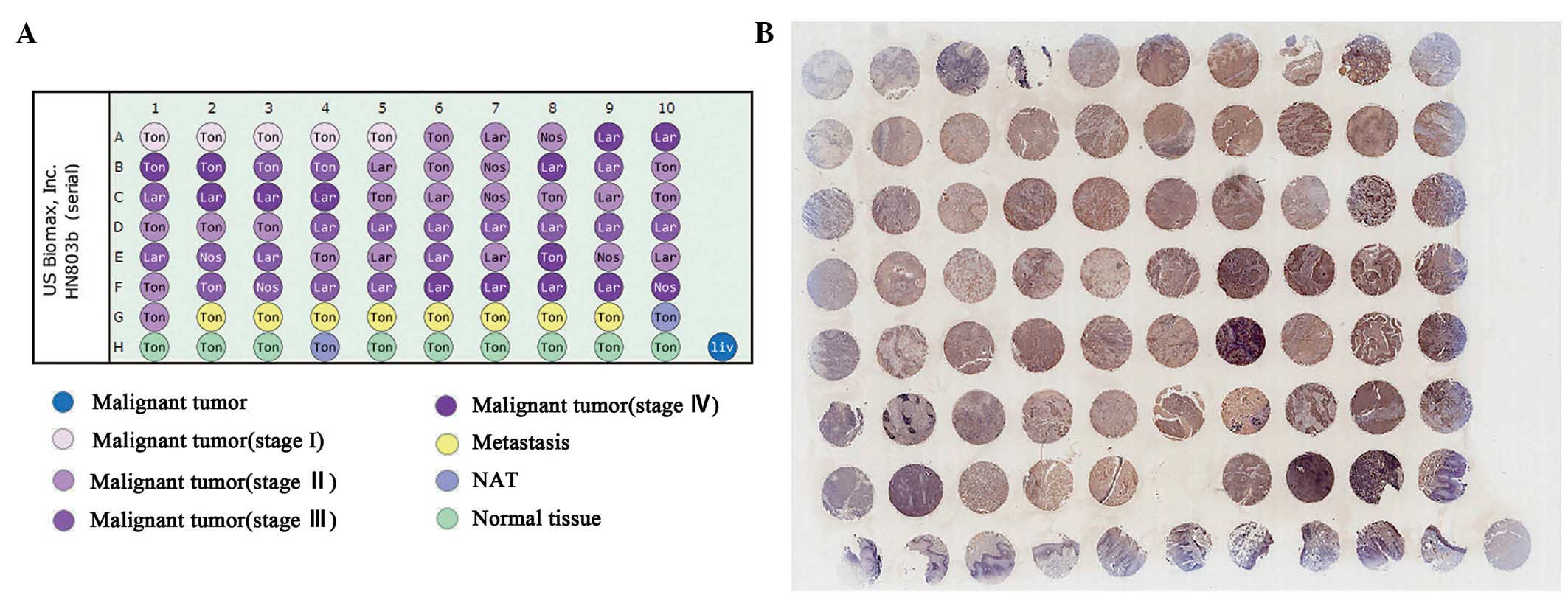
An independent tissue microassay (TMA) was purchased
from US Biomax, Inc. (Rockville, MD, USA; cat. no. HN803b). The TMA
consisted of three types of tumors, including tongue carcinoma,
laryngeal carcinoma and nasal carcinoma. Normal tissues were also
included. In total, 62 men and 18 women formed the group, and the
mean age was 53.4 years old (range, 18–90 years old). Detailed
information of the TMA is shown in Table II.
 | Table IICharacteristics of the tissue
microassay comprising 80 samples. |
Table II
Characteristics of the tissue
microassay comprising 80 samples.
| Characteristic | n (%) |
|---|
| Age (years) | 53.4±10.5 |
| Localization |
| Tongue | 42 (52.5) |
| Larynx | 31 (38.6) |
| Nose | 7 (8.9) |
| T classification |
| T1 | 5 (8.3) |
| T2 | 30 (50.0) |
| T3 | 17 (28.3) |
| T4 | 8 (13.3) |
| N classification |
| N0 | 42 (68.9) |
| N1 | 16 (26.2) |
| N2 | 3 (5.0) |
|
Differentiation |
| 1 | 12 (16.7) |
| 2 | 36 (50.0) |
| 3 | 24 (33.3) |
| Staging |
| I | 5 (0.81) |
| II | 33 (54.1) |
| III | 14 (23.0) |
| IV | 9 (14.8) |
| Metastasis | 8 (10.0) |
| Normal adjacent
tongue tissue | 2 (2.5) |
| Normal | 9 (11.2) |
Immunohistochemical staining
The tissue sections and the TMA tissues were
deparaffinized with standard pure xylene for 15 min three times at
room temperature, and hydrated in graded alcohols.
Phosphate-buffered saline (PBS) was used to wash the sections.
Antigen retrieval was performed in boiling citrate buffer (pH 6.0)
for 15 min. The sections were then cooled down to room temperature
in the buffers. Following washing the sections in PBS for 5 min
three times, 0.3% hydrogen peroxide phosphate-citrate buffer was
used to block endogenous peroxidase activity for 10 min. The
sections were then rinsed with PBS for 5 min, following which they
were incubated with primary Nmu antibody (cat. no. HPA025926;
Sigma-Aldrich; St. Louis, MO, USA; dilution 1:100) for 12 h at 4°C.
The sections were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit antibody (cat. no. KIT-9710;
Maixin-bio, Fuzhou, China) for 30 min at room temperature. The
slides were then stained with diaminobenzidine for 5 min.
Hematoxylin was used to counterstain the nuclei, followed by
dehydration and mounting. Images of the sections were captured
using an Olympus BX40 microscope and CC-12 Soft-Imaging system
(Olympus Corporation, Tokyo, Japan).
Evaluation of immunohistochemical
staining
The staining revealed that Nmu was expressed in the
cytoplasm and membrane. To further analyze the results, all the
immunostained sections and TMAs were quantified and scored for
intensity (0–3) and frequency (0–4). The intensity was scored as
follows: Grade 0, negative; grade 1, weak intensity; grade 2,
moderate intensity; grade 3, strong intensity. The frequency scores
were respectively assigned as 1, 2, 3 and 4 when 0–25, 26–50, 51–75
and 76–100% of the tumor cells were positive. To perform
statistical analysis, the Nmu protein intensity and frequency were
transformed into a composite expression score (CES) utilizing the
following formula: CES = intensity x frequency. The CES range was
between 0 to 12, with scores of 0 considered negetive, 1–4
considered weakly positive, 5–8 considered positive and 9–12
considered strongly positive.
Statistic analysis
All values are expressed as the mean ± standard
deviation. All statistical analysis was performed using SPSS
software (version 19; IBM SPSS, Armonk, NY, USA). The expression
levels of Nmu in primary tumor tissues were analyzed using a
χ2 test. The expression levels of Nmu measured in the
TMA was analyzed by one-way of analysis of variance and
Bonferroni's multiple comparison tests among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the retrospectively
selected patients and the TMA
The characteristics of all patients recruited in the
clinic and the TMA are presented in Tables I and II, respectively. Of the patients
recruited from the Department of Otolaryngology Head and Neck
Surgery at Renmin Hospital of Wuhan University, men formed the
majority of the group and the average age was 60 years old. No
statistically significant differences between gender or age and
metastasis were observed. The glottis was the most common site of
tumor formation (85.0%), and 93.3% patients exhibited
well-differentiated tumors.
In the present study, all patients underwent neck
dissection. A total of 123 patients were confirmed to have regional
metastasis using histological examination following surgery. The
positive rate of neck dissection was only 51.3%, with 48.7% of the
patients confirmed as having negative lymph nodes histologically.
These data suggested that certain patients may avoid unnecessary
neck dissection.
Analysis of the expression of Nmu in
primary tumors
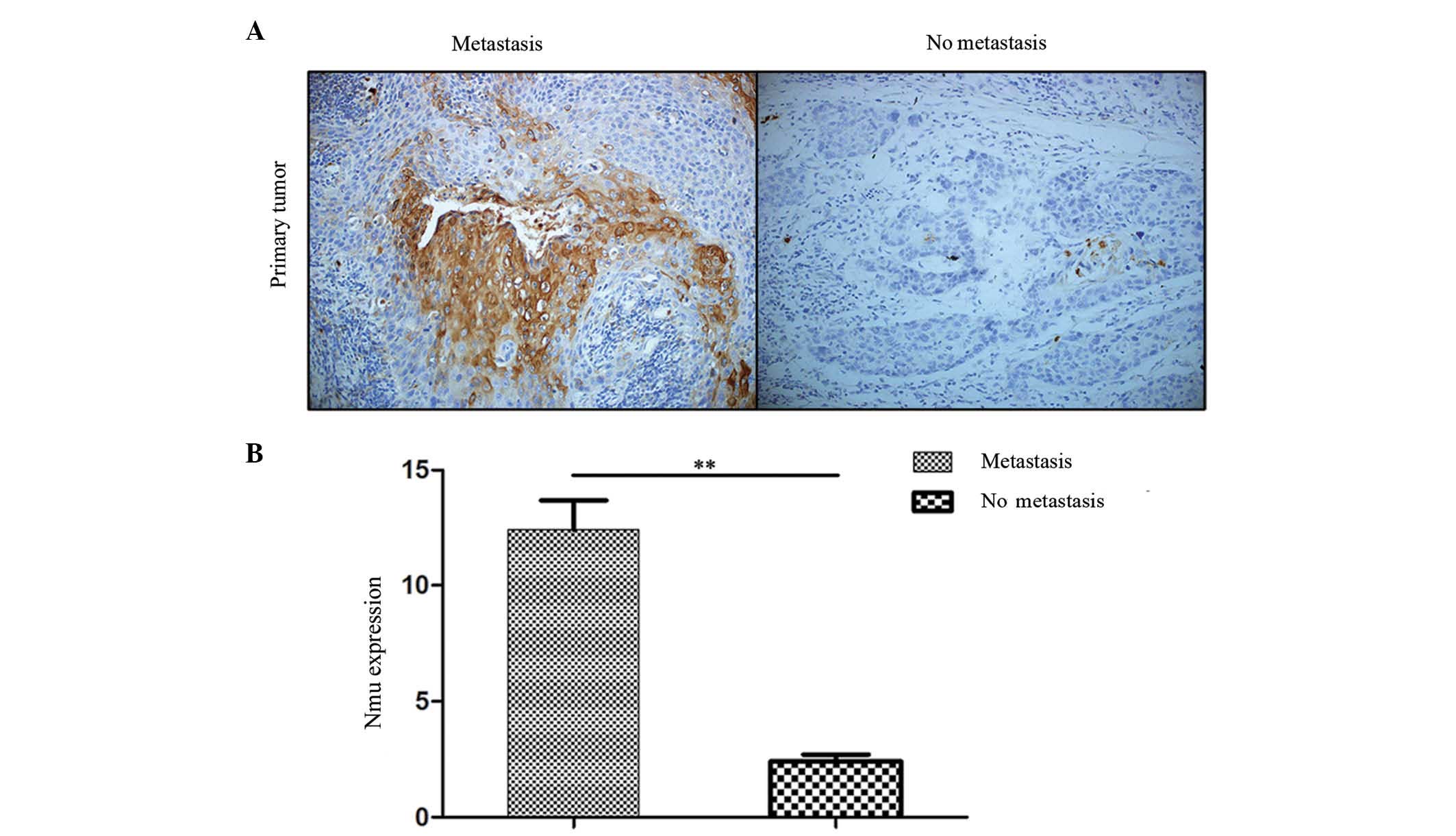
The present study first identified the expression of
Nmu in the clinical tissue sections of HNSCC. The frequency and
intensity of the immunohistochemical staining in all tissue
sections were analyzed. Representative images of the expression of
Nmu in the tissues are shown in Fig.
1A. Nmu was expressed in the cytoplasmic membrane. The
expression level of Nmu was transformed into a single numerical
measurement, CES. A significant increase in CES was noted in the
primary tumor with metastasis group, compared with the group
without metastasis (P<0.01; Fig.
1B). From the images, it appeared that the Nmu protein was
expressed predominantly among the carcinoma nests in primary tumors
with metastasis. These data suggested that Nmu may be involved in
the process of metastasis in HNSCC.
Analysis of the expression of Nmu in a
TMA of HNSCC
Following completion of the analysis of Nmu using
the clinical samples of HNSCC, A TMA, which contained three types
of head and neck cancer (tongue, larynx and nose) was performed to
provide a subset analysis, which also included normal tissues. As
shown in Fig. 2, integral images
of immunohistochemical staining of the TMA with an anti-Nmuantibody
were captured. The immunohistochemical (IHC) score of every sample
in the TMA was determined. The expression of Nmu among the T-stage,
N-stage and grades of differentiation were compared,
respectively.
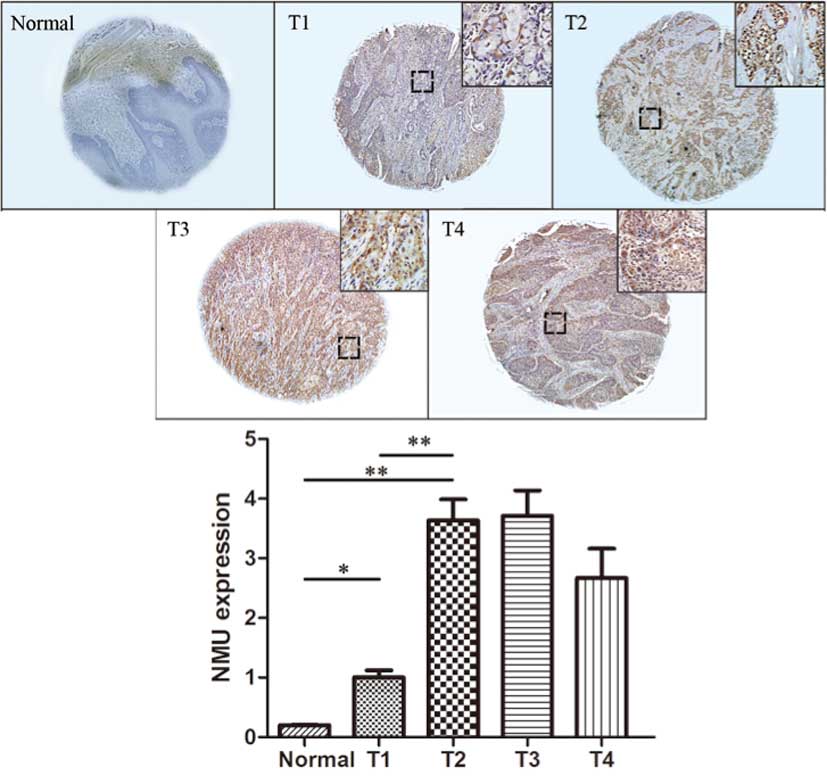
For the T-stage, representative images of the
expression of Nmu are shown in Fig.
3. The protein expression of Nmu had a significantly increased
CES in T1 tissues, compared with the normal tissues (P<0.05),
and the CES in the T2, T3 and T4 tissues was markedly increased,
compared with T1 tissues (P<0.01). There was a statistically
significant difference between T3 and T2 tissues, however, no
statistically significant differences were observed between T2 and
T1 tissues or between T3 and T4 tissues, These data suggested that
the expression of Nmu was correlated with T-stage, and an increase
in the expression of Nmu was found in HNSCC at a high T-stage.
The N-stage represents the lymph node metastasis.
The present study analyzed the correlation between the N-stage and
the expression levels of Nmu, for which representative images are
shown in Fig. 4A. The CES of the
Nmu protein showed that there was a statistically significant
difference in the expression of Nmu in the N0 tissues, compared
with normal tissues (P<0.05). There was also a statistically
significant difference between the N1 and N0 tissues (P<0.01).
In addition, a statistically significant difference in the
expression of Nmu was observed between the N2 and N1 tissues
(P<0.01; Fig. 4B).
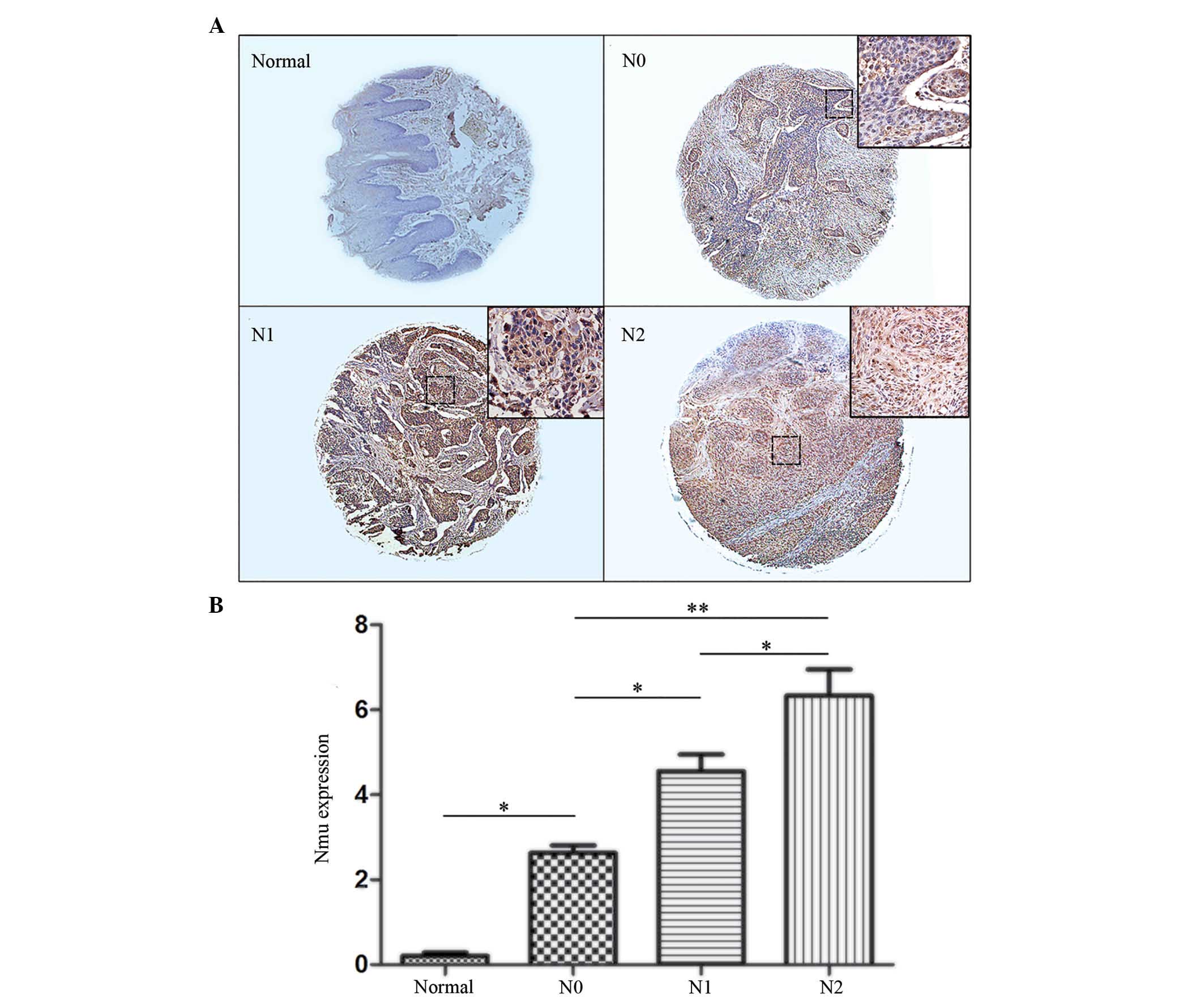 | Figure 4(A) Representative images of IHC
staining for Nmu protien in a tissue microassay of tumor tissues of
different N-stages. Normal tissue stained negative for Nmu protein.
N0, N1 and N2 stages showed weak positive, positive and strongly
positive staining, respectively. (Images, magnification, ×100;
enlarged area of image in top right corner, magnification, ×200).
(B) IHC index (intensity x percentage of tumor cells). Expression
levels of Nmu in the N0 tissues were significantly higher, compared
with those in normal tissues. There was a statistically significant
difference between N1 and N0, and the N2 index was significantly
higher, compared with the N1 index Data are expressed as the mean ±
standard deviation (*P<0.05 and
**P<0.01). Nmu, neuromedin U; IHC,
immunohistochemistry. |
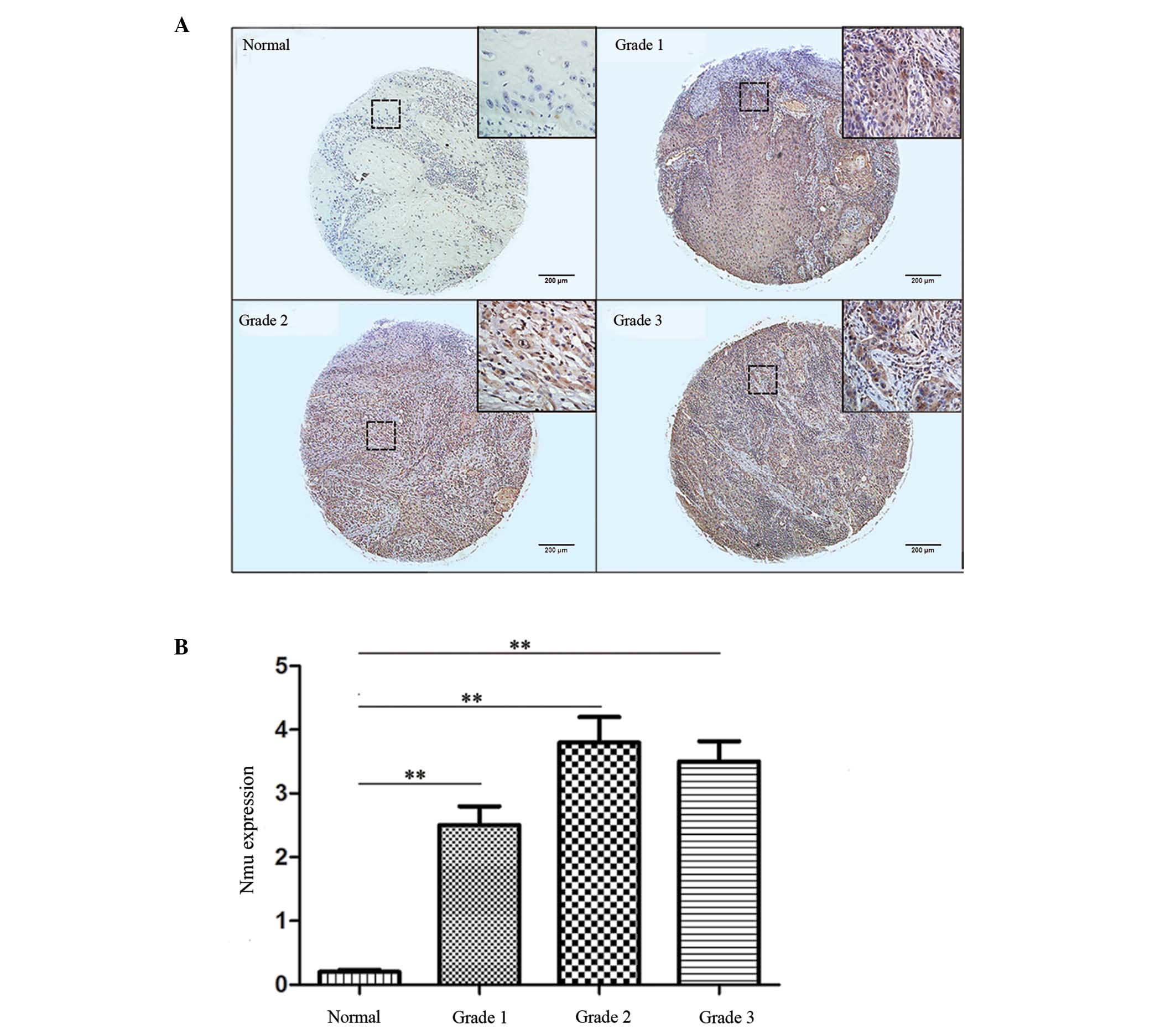
Finally, the expression levels of Nmu between tumor
grades were compared. Grades 1, 2 and 3 represented well, moderate
and poorly differentiated tumor tissues, respectively. The CES was
significantly increased in all three grades, compared with the
normal tissues (P<0.05), however, no statistically significant
difference was found among the grades. Representative images and
the IHC indices are shown in Fig. 5A
and B. The results suggested that the expression of Nmu was not
correlated with tumor grade.
Discussion
Clinically, the importance of regional metastasis is
well recognized. However, the positive rate of selective neck
dissection requires further improvement, particularly for early
stage cancer. Ferlito at al showed that only three of 211
patients diagnosed with laryngeal cancer with clinical N0 in the
neck were confirmed to have positive lymph node metastasis
(21), suggesting that certain
aspects of undesirable selective neck dissection in the clinic may
be avoided. In the present study, the positive rate of neck
dissection was 51.4%, and >40% of patients were confirmed to
have negative lymph nodes histologically. This suggested that areas
in these patients may have been overtreated. This increases the
potential for the incidence rate of surgical complications, and
reduces quality of life in patients with HNSCC. Therefore,
techniques to detect regional metastasis more accurately requires
further investigation.
Biomarkers offer potential for predicting tumor
progression and metastasis during the process of cancer therapy in
the future. At present, no clinic biomarkers are used for the
precise prediction of regional metastasis in HNSCC. The role of Nmu
in cancer remains to be fully elucidated. Several studies have
reported that Nmu acts as a tumor suppressor gene (15), and Nmu and its receptors are
reported to be correlated with cancer (22). Euer et al (23) investigated the transcriptional
profile of 11 ovarian tumor cell lines and two immortalized ovarian
surface epithelial cell lines using GeneChip technology, and
identified Nmu as an ovarian cancer-associated antigen. Using the
same method, Nmu was revealed as an oncogene, which had not been
previously implicated, in oral cancer (15). Although Nmu has been shown to have
a potential role in preventing the action of tumor suppressor genes
(15), its functions in cancer
require further investigation.
In the present study, the overexpression of Nmu was
identified in clinical tissue sections of HNSCC. The expression of
Nmu was increased in primary tumors with metastasis, compared with
those without metastasis. A TMA was then performed to confirm the
expression of Nmu in HNSCC. Analysis of the pathological specimens
demonstrated for the first time, to the best of our knowledge, that
Nmu was correlated with tumor progression and regional metastasis.
As further conformation, the TMA found that Nmu was overexpressed
in advanced tumor tissues. These results indicated that Nmu may be
a potential biomarker of tumor and metastasis in HNSCC. Currently,
computed tomography and magnetic resonance imaging are important
technologies for the detection of metastases clinically. However,
statistics have shown that the results of all the pretreatment
examinations are significantly different from histopathological
results (24). Thus, combining
these two forms of examination are likely to improve accuracy when
predicting regional metastasis, and may increase the positive rate
of neck dissection.
Generally, poorly differentiated tumors show a
higher rate of metastasis, however, in the present study, no
statistically significant difference was observed in the expression
of Nmu among tumor grades. It was concluded that Nmu was not
correlated with tumor grade, possibly due to the fact that the
poorly differetiated tumor may be in the early stage, whereas well
differentiated tumors may be of a later stage. In addition, the
present study found that the protein expression of Nmu in the T4
tissues was decreased, compared with the T3 tissues, although there
was no statistical significance between them.
It is important to note that, despite increased data
confirming the function of Nmu in metastasis, the mechanism
underlying the contribution of Nmu to metastasis remains to be
fully elucidated. Further investigations on this mechanism are
required in the future to provide a strategy in targeted therapy
for metastasis. It may be that the sensitivity of detecting
metastasis using the single biomarker of Nmu is low, and the
identification of additional biomarkers is required to improve the
positive rate of detecting regional metastasis.
In conclusion, the results of the present study
suggested that Nmu may be used as a biomarker of regional
metastasis. It may be also used as a therapeutic target in the
treatment of regional metastasis.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (grant nos. 81372880 and 81172569), the
doctoral program of Higher Education Research Fund (grant nos.
20130141120093 and 20110141110062) and the Natural Science
Foundation of Hubei Province (grant no. 2012FFA045).
References
|
1
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Argiris A and Eng C: Epidemiology, staging
and screening of head and neck cancer. Cancer Treat Res. 114:15–60.
2003. View Article : Google Scholar
|
|
3
|
Pimenta Amaral TM, Da Silva Freire AR,
Carvalho AL, Pinto CA and Kowalski LP: Predictive factors of occult
metastasis and prognosis of clinical stages I and II squamous cell
carcinoma of the tongue and floor of the mouth. Oral Oncol.
40:780–786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Minamino N, Kangawa K and Matsuo H:
Neuromedin U-8 and U-25: Novel uterus stimulating and hypertensive
peptides identified in porcine spinal cord. Biochem Biophys Res
Commun. 130:1078–1085. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minamino N, Kangawa K and Matsuo H:
Neuromedin U-8 and U-25: Novel uterus stimulating and hypertensive
peptides in porcine spinal cord. Biochem Biophys Res Commun.
130:1078–1085. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Budhiraja S and Chugh A: Neuromedin U:
Physiology, pharmacology and therapeutic potential. Fundam Clin
Pharmacol. 23:149–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu XH, Cao CQ, Mennicken F, Puma C, Dray
A, O'Donnell D, Ahmad S and Perkins M: Pro-nociceptive effects of
neuromedin U in rat. Neuroscience. 120:467–474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kojima M, Haruno R, Nakazato M, Date Y,
Murakami N, Hanada R, Matsuo H and Kangawa K: Purification and
identification of neuromedin U as an endogenous ligand for an
orphan receptor GPR66 (FM3). Biochem Biophys Res Commun.
276:435–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakazato M, Hanada R, Murakami N, Date Y,
Mondal MS, Kojima M, Yoshimatsu H, Kangawa K and Matsukura S:
Central effects of neuromedin U in the regulation of energy
homeostasis. Biochem Biophys Res Commun. 277:191–194. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niimi M, Murao K and Taminato T: Central
administration of neuromedin U activates neurons in ventrobasal
hypothalamus and brainstem. Endocrine. 16:201–206. 2001. View Article : Google Scholar
|
|
11
|
Funes S, Hedrick JA, Yang SJ, Shan LX,
Bayne M, Monsma FJ and Gustafson EL: Cloning and characterization
of murine neuromedin U receptors. Peptides. 23:1607–1615. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domin J, Ghatei MA, Chohan P and Bloom SR:
Neuromedin U - a study of its distribution in the rat. Peptides.
8:779–784. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita K, Upadhyay S, Osada M, Hoque
MO, Xiao Y, Mori M, Sato F, Meltzer SJ and Sidransky D:
Pharmacologic unmasking of epigenetically silenced tumor suppressor
genes in esophageal squamous cell carcinoma. Cancer Cell.
2:485–495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokumaru Y, Yamashita K, Osada M, Nomoto
S, Sun DI, Xiao Y, Hoque MO, Westra WH, Califano JA and Sidransky
D: Inverse correlation between cyclin A1 hypermethylation and p53
mutation in head and neck cancer identified by reversal of
epigenetic silencing. Cancer Res. 64:5982–5987. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alevizos I, Mahadevappa M, Zhang X, Ohyama
H, Kohno Y, Posner M, Gallagher GT, Varvares M, Cohen D, Kim D, et
al: Oral cancer in vivo gene expression profiling assisted by laser
capture microdissection and microarray analysis. Oncogene.
20:6196–6204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ketterer K, Kong B, Frank D, Giese NA,
Bauer A, Hoheisel J, Korc M, Kleeff J, Michalski CW and Friess H:
Neuromedin U is overexpressed in pancreatic cancer and increases
invasiveness via the hepatocyte growth factor c-Met pathway. Cancer
Lett. 277:72–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shetzline SE, Rallapalli R, Dowd KJ, Zou
S, Nakata Y, Swider CR, Kalota A, Choi JK and Gewirtz AM:
Neuromedin U: A Myb-regulated autocrine growth factor for human
myeloid leukemias. Blood. 104:1833–1840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, McRoberts K, Berr SS, Frierson HF
Jr, Conaway M and Theodorescu D: Neuromedin U is regulated by the
metastasis suppressor RhoGDI2 and is a novel promoter of tumor
formation, lung metastasis and cancer cachexia. Oncogene.
26:765–773. 2007. View Article : Google Scholar
|
|
19
|
Harten SK, Esteban MA, Shukla D, Ashcroft
M and Maxwell PH: Inactivation of the von Hippel-Lindau tumour
suppressor gene induces Neuromedin U expression in renal cancer
cells. Mol Cancer. 10:892011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rani S, Corcoran C, Shiels L, Germano S,
Breslin S, Madden S, McDermott MS, Browne BC, O'Donovan N, Crown J,
et al: Neuromedin U: A candidate biomarker and therapeutic target
to predict and overcome resistance to HER-tyrosine kinase
inhibitors. Cancer Res. 74:3821–3833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferlito A, Rinaldo A, Silver CE, Shah JP,
Suárez C, Medina JE, Kowalski LP, Johnson JT, Strome M, Rodrigo JP,
et al: Neck dissection: Then and now. Auris Nasus Larynx.
33:365–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brighton PJ, Szekeres PG and Willars GB:
Neuromedin U and its receptors: Structure, function and
physiological roles. Pharmacol Rev. 56:231–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Euer NI, Kaul S, Deissler H, Möbus VJ,
Zeillinger R and Weidle UH: Identification of L1CAM, Jagged2 and
Neuromedin U as ovarian cancer-associated antigens. Oncol Rep.
13:375–387. 2005.PubMed/NCBI
|
|
24
|
Haberal I, Celik H, Göçmen H, Akmansu H,
Yörük M and Ozeri C: Which is important in the evaluation of
metastatic lymph nodes in head and neck cancer: Palpation,
ultrasonography, or computed tomography? Otolaryngol Head Neck
Surg. 130:197–201. 2004. View Article : Google Scholar : PubMed/NCBI
|