Introduction
Hepatitis B virus (HBV) is a persistent infection
that has become a global threat to human health. Despite having an
effective preventive vaccine, there are ~1 million deaths each year
from complications resulting from chronic HBV infection. The
present study suggests that functional depletion of T cell is an
important feature of chronic HBV infection. The presence of
increased quantities of antigen stimulation is thought to be an
important reason for the T cell exhaustion in patients with chronic
hepatitis B (CHB) (1,2). In the progression of chronic viral
infection, abnormal expression of co-stimulatory and inhibitory
receptors are often identified on the surfaces of exhaustive T
cells, and vary in diversity and quantity depending on the course
of the disease (3).
The cluster of differentiation (CD)28 family is the
primary co-stimulatory molecule expressed on T cells. The CD28
family consists of the co-stimulatory receptors, CD28 and inducible
T-cell co-stimulator (ICOS), and three co-inhibitory receptors,
programmed cell death protein 1 (PD-1), cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4), and B- and T-lymphocyte
attenuator (BTLA) (4). The members
of the CD28 family possess a similar gene structure and they are
important in the regulation of T cell function. Co-stimulatory
receptors provide positive signals for T cells to promote
proliferation. Co-inhibitory receptors produce inhibitory signals
for T cells to limit, terminate and attenuate the T cell response
(5). Upon ligand combination,
these receptors regulate the function of various stages of T
cell-mediated immune responses, including numerous positive or
negative regulations of cellular and humoral immunity (6–8). T
cells regulate immune responses by maintaining the balance between
stimulation and inhibition. Understanding these pathways may
provide novel insight into the diagnosis and treatment of viral
diseases.
The association of the CD28 family of receptors with
chronic human viral infections has been investigated in the past;
however, previous research has been limited to a single study of
co-stimulatory or co-inhibitory receptors in the human
immunodeficiency virus (HIV) infection (9). Only a small number of studies have
investigated HBV (10,11), whereas PD-1 has been researched to
a greater extent and is well understood. The function of exhausted
T cells in CHB has been demonstrated to be reversed or improved by
PD-1 blockade in ex vivo studies (12-14).
However, the underlying mechanism of this effect is not fully
understood. Whether or not this blockade may tip the balance among
the co-stimulatory or co-inhibitory receptors of the CD28 family or
molecules requires further investigation, as the PD-1 blockade may
trigger immune injury while effectively removing the virus.
The current study demonstrated the overall
expression characteristics of the CD28 family of receptors and
effects on T cells in persistent HBV infection, and correlated the
expression with clinical parameters. In addition, in order to
demonstrate the effect of PD-1 block on receptors of the CD28
family, cell function and differentiation, the expression levels of
receptors in the CD28 family, interferon (IFN)γ and the ratio of
T-box 21 (T-bet)/GATA binding protein 3 (GATA-3) mRNA in peripheral
blood mononuclear cells (PBMCs) was tested. It was determined that
blockage of PD-1 may lead to the increased expression of receptors
of the CD28 family, cell function and differentiation may change
accordingly. Thus, the therapeutic potential of PD-1 blockade
demonstrates a correlation with the expression levels of the
co-stimulatory and co-inhibitory receptors of the CD28 family in
chronic HBV infection.
Materials and methods
Subjects
A total of 52 patients with CHB from the Department
of Infectious Disease, Union Hospital (Wuhan, China) served as the
experimental group and 26 healthy volunteers served as controls.
Patients were diagnosed with CHB according to the guidelines for
the prevention and treatment of the disease (15). Patients with other chronic liver
diseases (i.e. caused by other viruses, autoimmunity, alcohol
consumption, non-alcoholic fatty liver, medicines and toxins) were
excluded. None of the patients had been treated with antiviral
drugs or by immunological methods for a minimum of 1 year prior to
recruitment. There were no statistically significant differences in
age between the experimental and control groups. All patients were
hepatitis B virus surface antigen (HBsAg) seropositive; 19 of which
were hepatitis E antigen (HBeAg) seropositive. Patient alanine
transaminase (ALT) levels ranged between 87 and 954 U/l (median,
205 U/l), and HBV DNA ranged between 5.67×102 and
4.98×108 IU/ml (median, 7.52×105 IU/ml). The
polymerase chain reaction (PCR) method was used to screen for human
leukocyte antigen (HLA)-A2+ and 22 HLA-A2+
patients were identified. The study was approved by the Medical
Ethical Committee of Huazhong University of Science and Technology
and written informed consent was obtained from all subjects.
Virologic determination
The presence or absence of HBsAg, HBeAg, antibody to
hepatitis B surface antigen (anti-HBs), anti-HBe, anti-HBc,
antibody to hepatitis C virus (anti-HCV), anti-hepatitis D virus
(HDV) and anti-HIV (1/2) were determined using commercial enzyme
immunoassay kits (Kehua Bio-Engineering Co., Ltd., Shanghai,
China). HBV DNA quantification was performed at the laboratory of
Hepatology and Infectious Disease, Union Hospital (Hubei, China)
using a commercial PCR diagnostic kit (DaAn Gene Co., Ltd.,
Guangzhou, China). The cut-off value of HBV DNA was
5.0×102 IU/ml and the lower limit of HBV DNA detection
was 1.0×102 IU/ml.
Synthetic peptides, antibodies and
primers
A synthetic peptide with HLA-A2-restricted epitope
HBcAg18-27 (FLPSDFFPSI) was synthesized by the Chinese Peptide
Company (Hangzhou, China). Antibodies against CD8 allophycocyanin
(APC; cat. no.17-0088; 1:20), CD8 fluorescein isothiocyanate (FITC;
cat. no. 11-0088; 1:20), CD4 phycoerythrin (PE; cat. no. 12-0049;
1:20), CD4 FITC (cat. no. 11-0049; 1:20), CD3 peridinin chlorophyll
protein (cat. no. 45-0037; 1:20), ICOS APC (cat. no. 17-9948;
1:20), CD28 PE (cat. no. 12-0289; 1:20), IFN-γ FITC (cat. no.
11-7319; 1:20), PD-L1 (cat. no. 16-5983; 1:200) and their
corresponding isotype control antibodies (ICOS; cat. no. 17-4714;
1:20, CD28; cat. no. 12-4714; 1:20, IFN-γ; cat. no. 11-4714; 1:20,
PD-L1; cat. no. 16-4714; 1:200) were purchased from eBioscience,
Inc. (San Diego, CA, USA). Antibodies against PD-1 FITC (cat. no.
329904; 1:20), CTLA-4 APC (cat. no. 349908; 1:20), BTLA PE (cat.
no. 344505; 1:20), CD28 (cat. no. 302902), their isotype control
antibodies (PD-1; cat. no. 400107; 1:20, CTLA-4; cat. no. 400121;
1:20, BTLA; cat. no. 400211; 1:20) and 7-AAD Viability Staining
Solution [7-amino-actinomycin D (7-AAD)] were purchased from
Biolegend, Inc. (San Diego, CA, USA). PCR primers for HLA-A2
(forward, 5′-GTGGATAGAGCAGGAGGGT-3′ and reverse,
5′-CCAAGAGCGCAGGTCCTCT-3′) were purchased from Invitrogen; Thermo
Fisher Scientific, Inc. Real-time quantitative primers specific for
the transcription factors, T-bet (cat. no. QT00042217), GATA-3
(cat. no. QT00095501) and β-actin (cat. no. QT01680476) were
purchased from Qiagen GmbH (Hilden, Germany).
Selection of HLA-A2+
individuals
Selection of HLA-A2+ individuals was
completed by the method of ordinary PCR. A total of 5 ml venous
blood was collected. DNA was extracted from fresh heparinized blood
using a commercial blood DNA kit (Omega Bio-Tek, Inc., Norcross,
GA, USA). HLA-A2+ individuals were selected by PCR as
previously described (16). The
cycling conditions for the PCR reaction were as follows: 94.0°C for
5 min, 30 cycles of 94.0°C for 45 sec, 60.0°C for 45 sec and 72.0°C
for 45 sec. The products of PCR were determined using 2% agarose
gel electrophoresis.
Isolation of PBMCs, in vitro culture and
PD-1:PD-L1 blocking
PBMCs were isolated from fresh heparinized blood
using Ficoll-Hypaque density gradient centrifugation at 800 × g for
20 min (HaoYang Biological Manufacture Co., Ltd., Tianjin, China)
and were resuspended in RPMI-1640 medium supplemented with 10%
fetal bovine serum and 1% penicillin-streptomycin (Gibco; Thermo
Fisher Scientific, Inc.).
To assess the effects of blocking the PD-1:PD-L1
signaling pathway, PBMCs from HLA-A2+ individuals were
cultured in a flat-bottomed 96-well plate (2–10×105
cells/well) in the presence of 5 µg/ml HBcAg18-27 peptide, 5
µg/ml anti-PD-L1 or control IgG for PD-1:PD-L1 for 10 days,
supplemented with 25 IU/ml interleukin (IL)-2 (PeproTech, Inc.,
Rocky Hill, NJ, USA) and 0.5 µg/ml anti-CD28 at 0 and 5
days.
Flow cytometric analysis of CD28 family
receptors and intracellular IFN-γ
PBMCs were surface-stained with anti-CD28,
anti-ICOS, anti-PD-1, anti-CTLA-4, anti-BTLA, 7-AAD, anti-CD4,
anti-CD8 and their corresponding isotype fluorescent antibodies.
Subsequent to staining, cells were detected by flow cytometry
(FACSCalibur; BD Biosciences, San Jose, CA, USA), and data were
analyzed based on the levels of background of isotype-matched
controls using the Flowjo 7.6.1 software (FlowJo, LLC., Ashland,
OR, USA). In order to evaluate the effect of anti-PD-L1 on the
expression of the CD28 family receptors on CD4+ and
CD8+ T cells, cells cultured into 96-well plates in the
presence of anti-PD-L1 or control antibody for 10 days, were
collected, washed twice with PBS and analyzed as described above.
For intracellular IFN-γ staining, following exposure to anti-PD-L1
for 10 days, cells were transferred from flat-bottomed 96-well
plates to round-bottomed 96-well plates, washed once with RPMI-1640
with 10% FBS medium and incubated with 5 µg/ml HBcAg18-27
peptide, 0.5 µg/ml anti-CD28 and 1 µg/ml brefeldin A
(eBioscience, Inc.) for 5 h at 37°C. The cells were washed twice
with PBS and then stained with anti-CD4, anti-CD8, and 7-AAD and
fixed. Cells were then fixed/permeabilized using
Fixation/Permeabilization kit following manufacturer's protocol (BD
Biosciences) for intracellular staining with anti-IFN-γ and
analyzed as described above.
Expression of T-bet and GATA-3 genes in
PBMCs
To assess the effect of anti-PD-L1 on T-bet and
GATA-3 in PBMCs, total RNA from PBMCs treated with anti-PD-L1 or
control antibody for 10 days was extracted using RNAiso Plus
reagent (Takara Biotechnology Co., Ltd., Dalian, China) according
to the manufacturer's instructions. T-bet, GATA-3 and β-actin mRNA
were amplified by a Real Time One Step RT-PCR method with the One
Step SYBR RT-PCR kit (Takara Biotechnology Co., Ltd.). The
conditions used for qPCR were according to the manufacturer's
protocol. PCR reactions were performed on a real-time fluorescent
quantitative PCR analyzer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The conditions for reverse transcription reaction were
42.0°C for 5 min and 95.0°C for 10 sec. The cycling conditions for
the PCR reaction were: 95.0°C for 5 sec, 57.0°C for 30 sec
(+fluorescence detection), 39 cycles of 95.0°C for 5 sec and 57.0°C
for 30 sec, melt curve 65.0–95.0°C (0.5°C increments for 5 sec,
+fluorescence detection). The relative gene expression levels of
T-bet and GATA-3 normalized to the corresponding β-actin were
calculated automatically by the CFX Manager 2.1 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data were analyzed using the GraphPad Prism 6.01
software (San Diego, USA). The Mann-Whitney U test was used to
analyze the differences among the various groups. The Wilcoxon
paired test was used to compare differences in expression inside
and outside the cell membranes, prior and subsequent to blocking of
PD-1:PD-L1. Correlations among various molecules, and the HBV DNA
values and molecules were evaluated using the Spearman test. Data
are presented as the mean ± standard error. A two-tailed test was
used to analyze all data and P<0.05 was considered to indicate a
statistically significant difference.
Results
T cells in CHB express different levels
of CD28 family receptors compared with those of healthy
individuals
To compare the in vitro expression frequency
of the CD28 family, 5 receptors on total peripheral CD4+
and CD8+ T cells from patients with CHB (n=52) and
healthy controls (n=26) were investigated. Representative flow
cytometric scatter plots of the CD28 family receptors on peripheral
CD4+ and CD8+ T cells from a patient with CHB
are demonstrated in Fig. 1A.
Levels of PD-1 and CTLA-4 on CD4 T cells (Fig. 1B) and ICOS, PD-1, and BTLA on CD8 T
cells (Fig. 1C) were increased in
cells from patients with CHB compared with those from the healthy
controls.
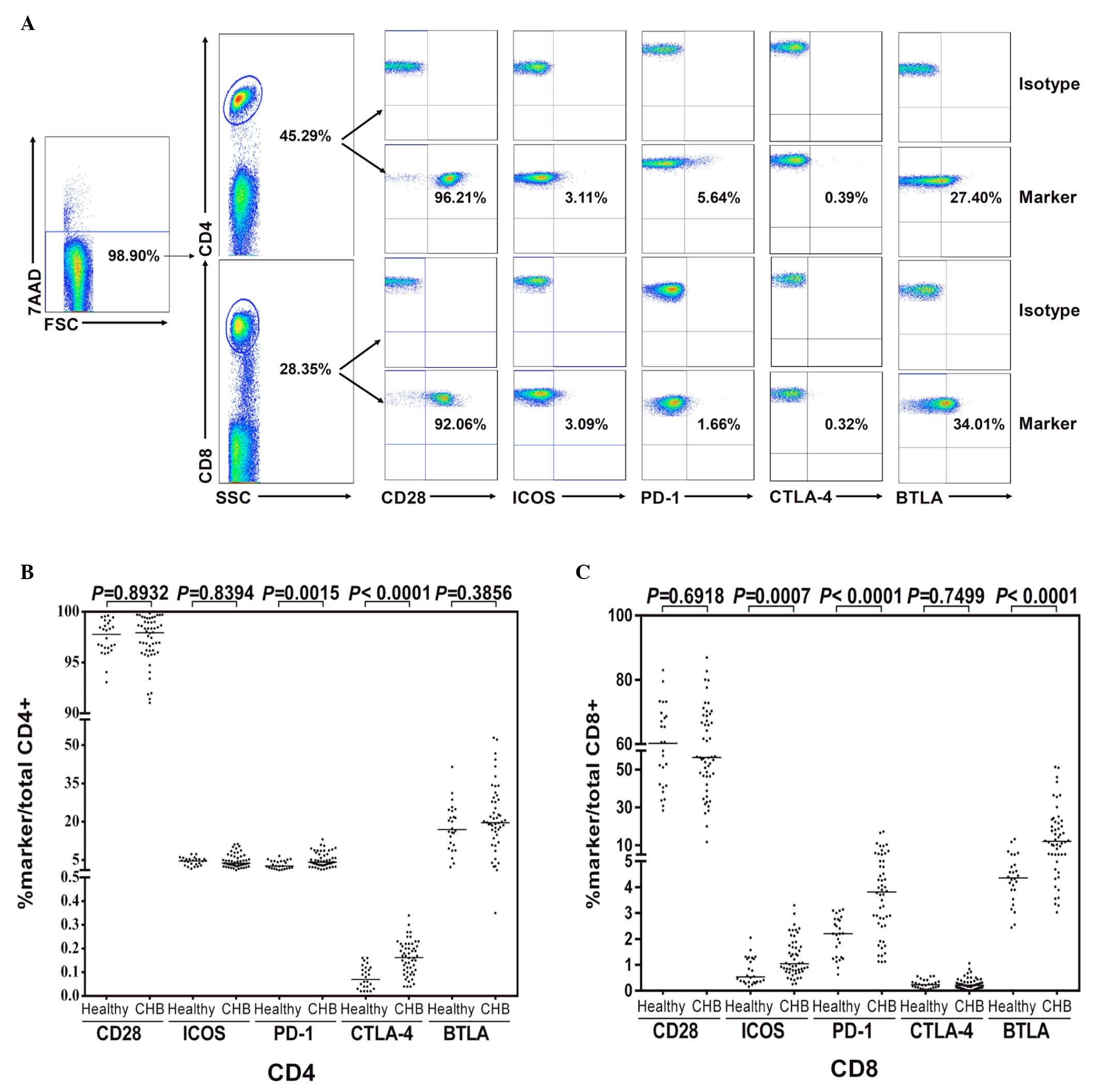 | Figure 1Expression of CD28 family receptors
on CD4+ and CD8+ T cells of patients with
CHB. Peripheral blood mononuclear cells were isolated from healthy
donors (n=26) or patients with CHB (n=52), and stained with
anti-CD4, CD8, CD28, CTLA-4, PD-1, ICOS, BTLA or isotype control
antibodies. Dead cells were excluded by 7-AAD staining. (A)
Representative flow cytometric scatter plots of CD28 family
receptors on peripheral CD4+ and CD8+ T cells
from a patient with CHB. (B and C) Expression of CD28, CTLA-4,
PD-1, ICOS and BTLA receptors. CD, cluster of differentiation; CHB,
chronic hepatitis B; CTLA-4, cytotoxic T-lymphocyte-associated
protein 4; PD-1, programmed cell death protein 1; ICOS, inducible
T-cell co-stimulator; BTLA, B- and T-lymphocyte attenuator; 7-AAD,
7-aminoactinomycin D. |
Correlations among the CD28 family
receptors and clinical parameters in CHB
A significant positive correlation was demonstrated
among the serum HBV DNA titers and the levels of PD-1 on
CD8+ T cells with the highest expression of PD-1
corresponding to viremia levels >106 IU/ml
(P<0.0001; Fig. 2A). A
significant positive correlation was observed among the serum HBV
DNA titers and the expression levels of BTLA on CD8+T
cells with the highest expression of BTLA corresponding to viremia
levels >106 IU/ml (P<0.0001; Fig. 2B). However, a significant inverse
correlation was indicated among the serum HBV DNA titers and
expression levels of ICOS on CD8+ T cells with the
lowest expression of ICOS corresponding to viremia levels
>106 IU/ml (P<0.001; Fig. 2C). No significant difference was
demonstrated compared with the healthy controls (P>0.05;
Fig. 2C). No correlation was
observed among the serum HBV DNA titers and expression levels of
CD28 (Fig. 2D) or CTLA-4 (Fig. 2E) on CD8+ T cells. In
addition, no correlations were observed among the virological
parameters and the abnormal expression of the CD28 family receptors
on CD4+ T cells in patients with CHB (data not shown).
Furthermore, expression of PD-1 on CD8+ T cells was
significantly higher in the HBeAg+ group compared with
the HBeAg− group (P<0.0001; Fig. 2F).
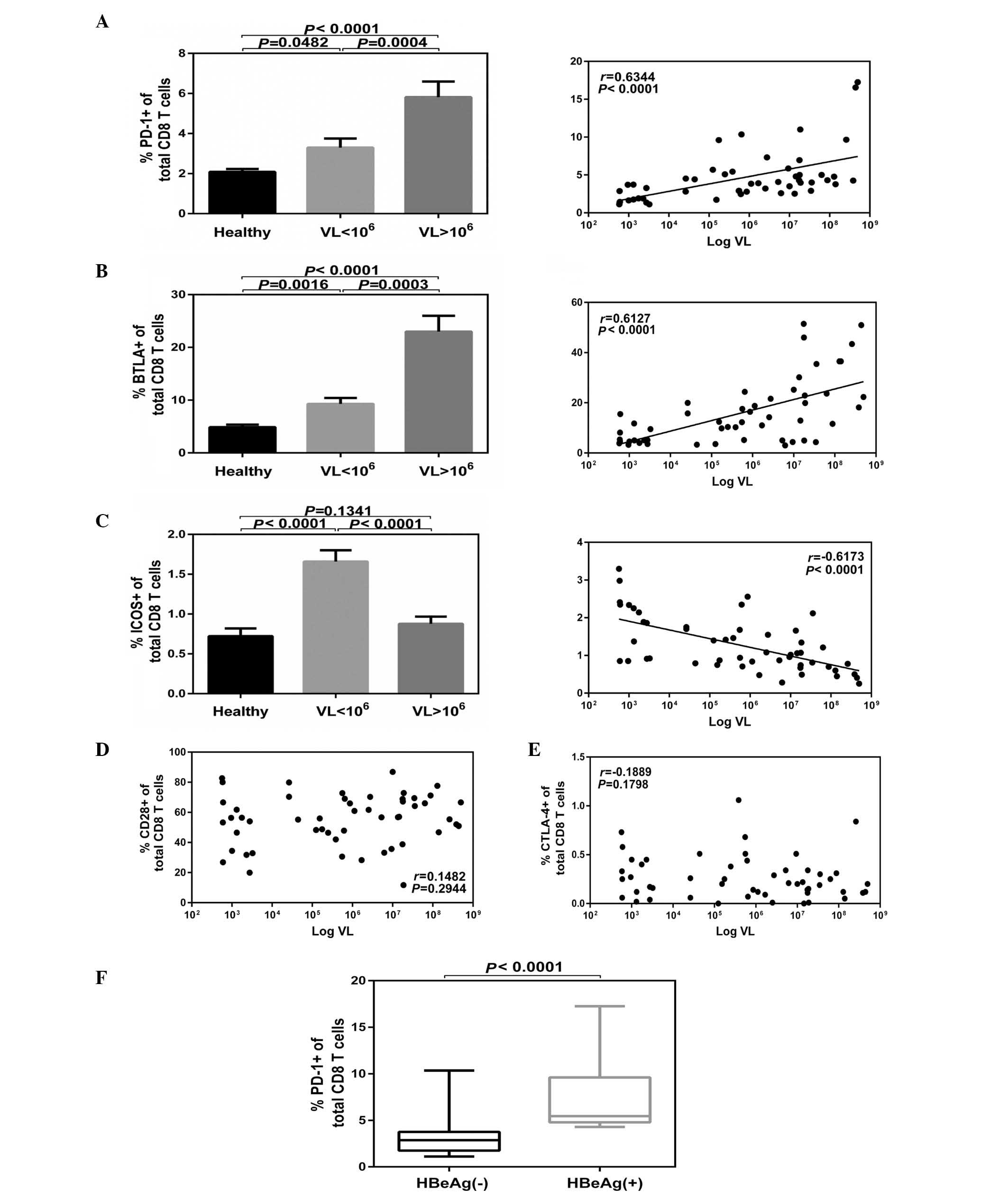 | Figure 2Expression of CD28 family receptors
associated with the HBV DNA levels. Patients were classified as low
(n=27) and high (n=25) VL, and CD4 or CD8 cells expressing CD28
family receptors were analyzed. (A) PD-1 and (B) BTLA on CD8 T
cells were positively correlated with VL, (C) ICOS were inversely
correlated, while (D) CD28 and (E) CTLA-4 demonstrated no
correlation with HBV DNA levels. (F) Cumulative PD-1 expression
data on CD8+ T cells in HBeAg+ (n=19) and
HBeAg− groups (n=33). Data are presented as the mean ±
standard error. CD, cluster of differentiation; HBV, hepatitis B
virus; PD-1, programmed cell death protein 1; BTLA, B- and
T-lymphocyte attenuator; ICOS, inducible T-cell co-stimulator;
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; HBeAg,
hepatitis E antigen; VL, viral levels. |
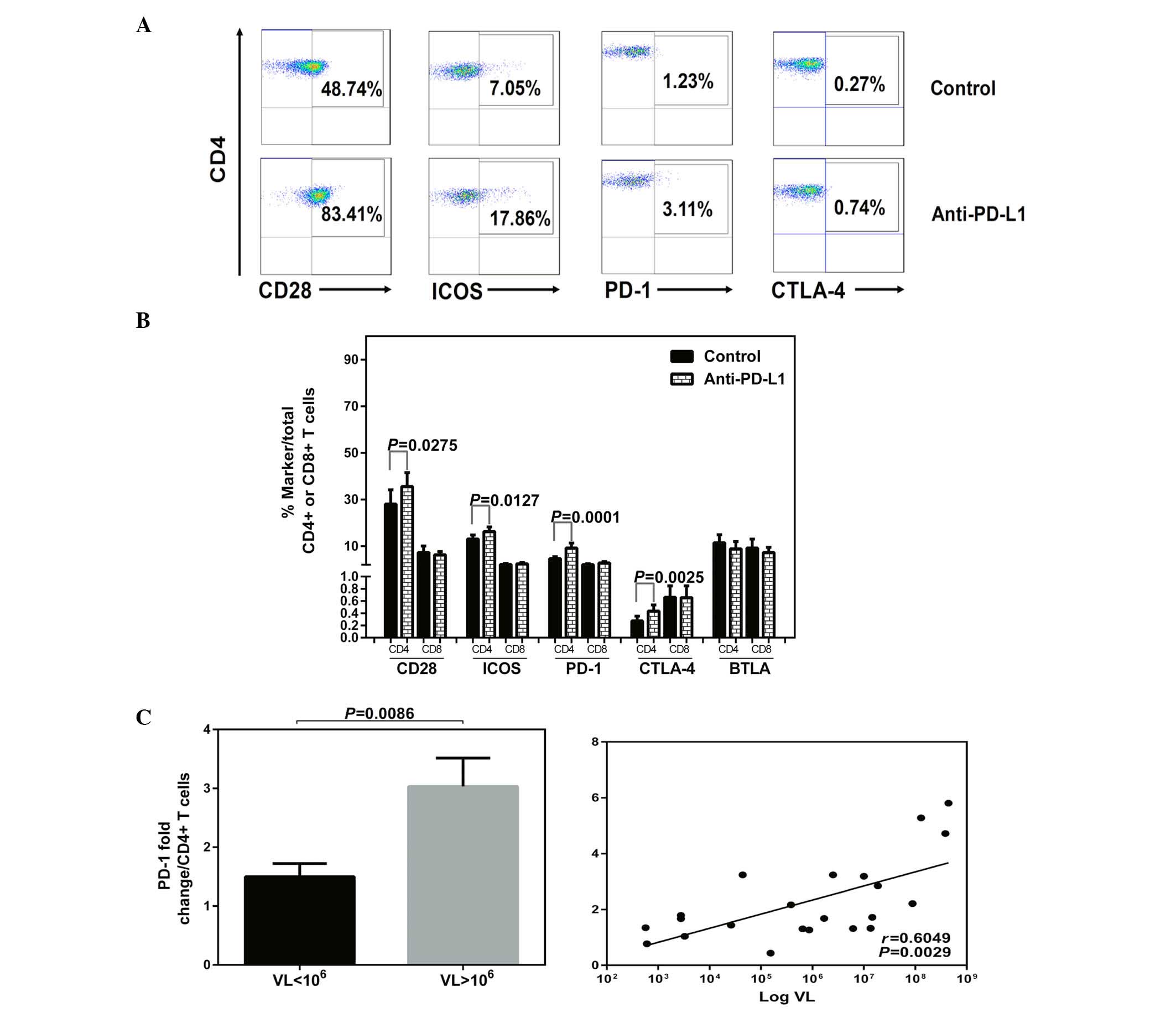
Effects of blocking PD-1 on the
expression profile of the CD28 family receptors and function of CD4
T cells in CHB
Besides assessing function, the effects of blocking
PD-1 on the levels of co-stimulatory and co-inhibitory receptors
were investigated. The expression of the CD28 family receptors and
intracellular IFN-γ were measured simultaneously in T cells in
HLA-A2+ patients with CHB following exposure to
anti-PD-L1 or a control antibody. Increased expression of the
receptors with PD-1 blockade or isotype antibody treatment are
demonstrated by flow cytometric dot plots of peripheral
CD4+ T cells from a patient with CHB (Fig. 3A). Following anti-PD-L1 exposure,
the expression levels of CD28, ICOS, PD-1 and CTLA-4 were increased
in the CD4+ T cells (Fig.
3B). No significant impact on the expression of the CD28 family
receptors was observed on the CD8+ T cells following
anti-PD-L1 exposure (Fig. 3B;
P>0.05).
In addition, following anti-PD-L1 exposure, a
significant positive correlation was detected among the HBV DNA
titers and the fold changes of PD-1, with the highest fold-change
of PD-1 in CD4+ T cells corresponding to viremia levels
>106 IU/ml (VL<106 vs.
VL>106, P=0.0086; Log VL vs. PD-1 fold change,
P=0.0029; Fig. 3C).
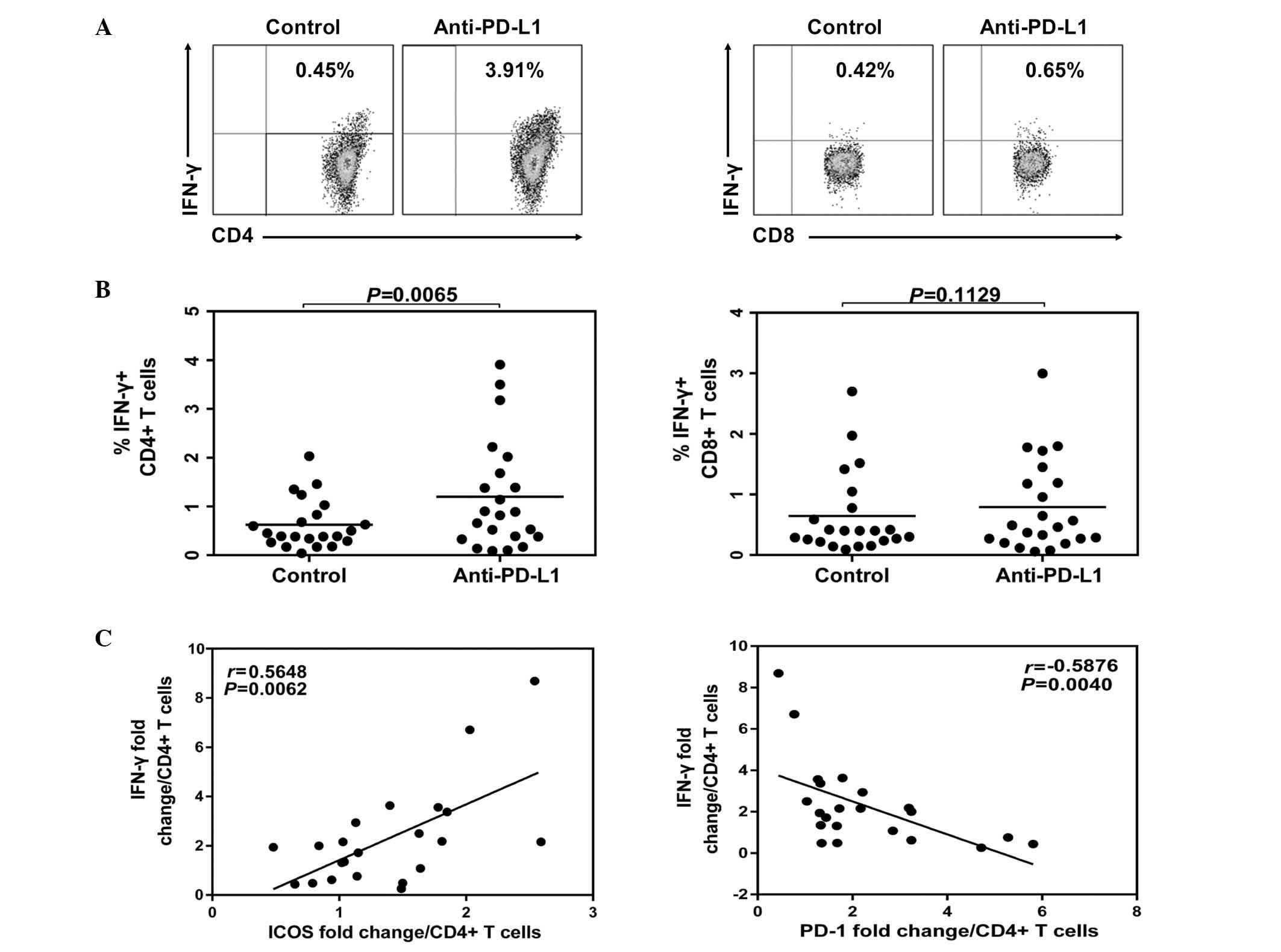
The representative flow cytometric dot plots of
intracellular IFN-γ expression in CD4+ and
CD8+ T cells with PD-1 blockade or isotype antibody
treatment are demonstrated in Fig.
4. Following anti-PD-L1 treatment, the levels of IFN-γ were
increased in CD4+ T cells (Fig. 4B) and IFN-γ fold changes in
CD4+ T cells had a positive correlation with ICOS
fold-change (P=0.0062; Fig. 4C).
However, there was an inverse correlation with PD-1 fold-change
(P=0.004; Fig. 4C). In the current
study, no significant impact was demonstrated on the expression of
IFN-γ in the CD8+ T cells following anti-PD-L1 treatment
(Fig. 4B; P>0.05).
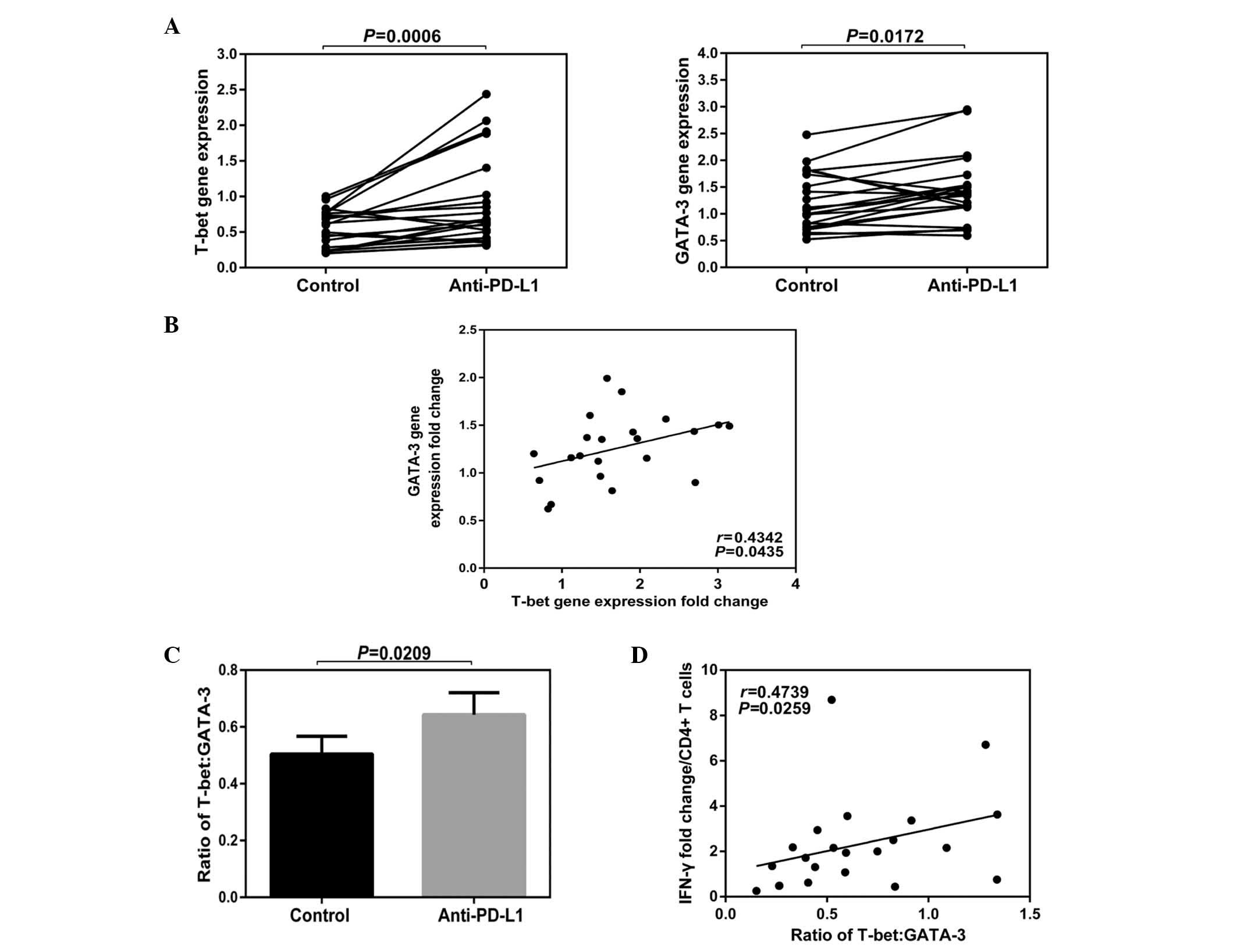
Effects of blocking PD-1 on the ratio of
T-bet to GATA-3 in PBMCs in CHB
Blocking the PD-1:PD-L1 signaling pathway
demonstrated a significant impact on the function of
CD4+ T cells. The blocking step enhanced their
expression levels in IFN-γ, but had no significant effect on the
function of the CD8+ T cells. Changes in cell function
tend to alter the expression levels of a variety of molecules in a
cell; therefore, it is relatively limited to investigate the cell
function by measuring certain protein levels. Due to this fact, the
effect of blocking the PD-1:PD-L1 signaling pathway on total T-bet
and GATA-3 mRNA levels from peripheral PBMCs was investigated.
The results of the current study demonstrated that
the total gene expression levels of T-bet and GATA-3 in PBMCs were
increased following anti-PD-L1 exposure (T-bet Control vs.
Anti-PD-L1; P=0.0006; GATA-3 Contol vs. Anti-PD-L1; P=0.0172;
Fig. 5A). A positive correlation
was demonstrated among the fold-changes of T-bet and GATA-3
(P=0.0435; Fig. 5B) and following
anti-PD-L1 exposure, the rate of T-bet/GATA-3 was increased
(P=0.0209; Fig. 5C). In addition,
a positive correlation was identified between the IFN-γ fold-change
and the rate of T-bet/GATA-3 (P=0.0259; Fig. 5D).
Discussion
The functional exhaustion of virus-specific T cells
is an important feature of chronic HBV. Although the mechanism of T
cell functional exhaustion in patients with CHB remains unclear,
the co-stimulatory or co-inhibitory receptors on T cell surfaces
vary in type and quantity. These receptors regulate and control T
cell function, positively or negatively, in the progression of
persistent human viral infections (3). The present study demonstrated that
co-signaling molecules of the CD28 family are crucial in positive
and negative regulation of T lymphocyte responses (17).
The results of the current study demonstrated that
the overall expression status of the CD28 family receptors on
peripheral T cells in patients with CHB had distinctive
characteristics that were different from those of healthy control
subjects. Furthermore, the results demonstrated that the CTLA-4
expression levels of CD4+ and CD8+ T cells,
from patients with CHB and healthy controls, were weak, but were
increased on global CD4+ T cells from patients with CHB
compared with the healthy control subjects. CTLA-4 is expressed at
low basal levels on naive T cells with increased expression
following T cell activation (5).
The present study hypothesized that the increased expression of
CTLA-4 may be associated with increased frequencies of
CD4+CD25+Foxp3+ regulatory T
cells. CTLA-4 was demonstrated to be constitutively expressed on
CD4+CD25+Foxp3+ regulatory T
cells, which were increased in the peripheral blood of patients
with CHB (18,19). The current study demonstrated that
in chronic HBV infection, the expression of PD-1 on CD4+
and CD8+ T cells was increased. The level of PD-1
expression on CD8+ T cells in HBeAg-positive patients
was significantly higher than that of HBeAg-negative patients, and
PD-1 expression on the CD8+ T cells was positively
correlated with HBV DNA titers. These data demonstrated that the
expression of PD-1 was associated with the chronic HBV infection,
and that HBeAg and HBV DNA promoted the expression of PD-1,
resulting in injury to T cells. Highly expressed PD-1 is the
primary indicator of exhausted T cells. In patients with chronic
HBV infection, up-regulation of PD-1 on T cells has frequently been
observed (20,21). The reason ICOS expression on
CD8+ T cells in patients with CHB was increased compared
with that in healthy controls remains unclear, and was negatively
correlated with the HBV DNA levels. Previous studies have
demonstrated that ICOS is a co-stimulatory molecule, and in HIV
infection, ICOS expression on CD4+ and CD8+ T
cells were up-regulated and associated with the disease process
(22–24). However, previous studies have
indicated that ICOS is similar to CD28 in structure and function,
thus ICOS provided stimulatory signals to T cells affecting
expansion, survival and differentiation (25,26).
The results of the present study demonstrated that the BTLA
expression on CD8+ T cells in patients with CHB was
significantly higher compared with the healthy control subjects,
and was positively correlated with the HBV DNA titers. No
significant difference was demonstrated in the BTLA expression on
CD4+ T cells between patients with CHB and healthy
control subjects, which is consistent with the results of Nan et
al (27). Previous studies
demonstrated that, similar with PD-1 and CTLA-4, BTLA inhibits the
activation of T cells, the initialization of CD4+ T
cells, and the second reaction of CD4+ and
CD8+ T cells (28,29).
T cells express a diverse array of co-stimulatory and co-inhibitory
receptors during chronic infection (3). It is likely that extrinsic regulation
of T cells by a number of suppressive mechanisms may alter the
balance of co-stimulatory versus co-inhibitory signals (30). Collectively, the balance of
expression between the co-stimulatory and co-inhibitory receptors
of the CD28 family may correlate with disease progression,
suggesting that the co-expression profiles of these molecules are
potential clinical indicators for rapid monitoring of changes in T
cell function during CHB treatment.
Following anti-PD-L1 exposure, the expression
profile of the CD28 family receptors in T-cells in patients with
CHB was altered, and the receptor fold-changes were most prominent
in the CD4+ T cells. The results of the present study
demonstrated that blocking the PD-1:PD-L1 signaling pathway may
enhance the peripheral CD4+ T cell function in patients
with CHB. CD4+ T cells are involved in the antiviral
response, which occurs primarily through the secretion of cytokines
and supports CD8+ T cells. Similarly, virus-specific
CD4+ T-cells have been indicated to lose efficacy in
chronic viral infections (31).
Therefore, the current study speculated that functional improvement
of CD4+ T cells in CHB patients may promote functional
recovery or reverse the exhausted CD8+ T cells. A
previous study demonstrated that CD4+ T-cell activity is
important for CD8+ T-cells to establish an effective
response in the liver during viral infections (32). These data indicated that PD-1
blockade has a therapeutic potential, which may be enhanced by
modulating the expression of co-stimulatory and co-inhibitory
receptors of the CD28 family.
Although blocking the PD-1:PD-L1 signaling pathway
did not effectively improve the function of the CD8+ T
cells, it significantly enhanced the IFN-γ expression in the
CD4+ T cells corresponding to the increasing rate of
T-bet/GATA-3. T-bet and GATA-3 are transcription factors and T-bet
promotes T helper (Th)0 cells to differentiate towards
Th1 cells (33). GATA-3
makes Th0 cells differentiate into Th2 cells
and the ratio of T-bet/GATA-3 determines the final direction of
differentiation (33). T-bet is a
member of the T-box transcription factor family, and promotes
CD4+ T cells to secrete IFN-γ and induce the
differentiation of CD4+ T cells (34). It may be speculated that the
PD-1:PD-L1 signaling pathway may result in an increased ratio of
T-bet/GATA-3 in CD4+ T cells, thus contributing to the
secretion of CD4+ T cell IFN-γ and promoting the
differentiation of CD4+ T cells from Th0 to
Th1 cells. In addition, the current results demonstrated
that the CTLA-4 expression on CD4+ T cells was
significantly increased following blocking. Nasta et al
(35) demonstrated that during the
differentiation of helper T cells, CTLA-4 may inhibit the
expression of GATA-3 mRNA, but not inhibit the T-bet mRNA
expression. The present study hypothesized that the increased rate
of T-bet/GATA-3 may have been due to the increase of the CTLA-4
expression on CD4+ T cells following blocking, which in
turn promoted the Th0 cells to differentiate to
Th1 cells, leading to improvement of T cell function.
However, further research is required to confirm this.
In conclusion, the results of the current study
demonstrate that the expression profiles of co-stimulatory and
co-inhibitory receptors of the CD28 family serve an important role
in chronic HBV infection. In particular, the expression profiles of
the CD28 family receptors, and the ratio of T-bet/GATA-3 gene
expression were changed when T cell function was enhanced following
PD-1 blockade. These results suggested that the balance between
these molecules may have an important correlation with the
improvement of T cell function induced by PD-1 blockade. However,
considering the complexity of correlations among various receptors
on CD4+ or CD8+ T cells, additional studies
are required to assess whether PD-1 blockade may lead to the
possibly life-threatening damage, which may result from biological
effects when T cell function is improved by PD-1 blockade. This may
aid to better understand the pathogenic mechanism of HBV infection,
and provide novel means to assess curative effects, evaluate
prognosis, as well as to investigate novel immunological
therapeutic strategies in future.
Acknowledgments
The present study would like to thank Professor
Haiming Wei (University of Science and Technology of China, Hefei,
China) for assisting with the writing of this manuscript. The study
was supported by grants from the National Major Science and
Technology Project for Infectious Diseases of China (grant nos.
2008ZX10002-011, 2012ZX10004503 and 2013ZX10002001), the
International Science & Technology Cooperation Program of China
(grant no. 2011DFA31030), the Natural Science Foundation of China
(grant nos. 81271884 and 81461130019) and the Deutsche
Forschungsgemeinschaft (Transregio TRR60).
References
|
1
|
Yi JS, Cox MA and Zajac AJ: T-cell
exhaustion: Characteristics, causes and conversion. Immunology.
129:474–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crawford A and Wherry EJ: The diversity of
costimulatory and inhibitory receptor pathways and the regulation
of antiviral T cell responses. Curr Opin Immunol. 21:179–186. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carreno BM, Carter LL and Collins M:
Therapeutic opportunities in the B7/CD28 family of ligands and
receptors. Curr Opin Pharmacol. 5:424–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anand S and Chen L: Control of autoimmune
diseases by the B7-CD28 family molecules. Curr Pharm Des.
10:121–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma L, Cai YJ, Yu L, Feng JY, Wang J, Li C,
Niu JQ and Jiang YF: Treatment with telbivudine positively
regulates antiviral immune profiles in Chinese patients with
chronic hepatitis B. Antimicrob Agents Chemother. 57:1304–1311.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zelinskyy G, Myers L, Dietze KK, Gibbert
K, Roggendorf M, Liu J, Lu M, Kraft AR, Teichgräber V, Hasenkrug KJ
and Dittmer U: Virus-specific CD8+ T cells upregulate
programmed death-1 expression during acute friend retrovirus
infection but are highly cytotoxic and control virus replication. J
Immunol. 187:3730–3737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tassiopoulos K, Landay A, Collier AC,
Connick E, Deeks SG, Hunt P, Lewis DE, Wilson C and Bosch R:
CD28-negative CD4+ and CD8+ T cells in
antiretroviral therapy-naive HIV-infected adults enrolled in adult
clinical trials group studies. J Infect Dis. 205:1730–1738. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schurich A, Khanna P, Lopes AR, Han KJ,
Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G and
Maini MK: Role of the coinhibitory receptor cytotoxic T lymphocyte
antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B
virus infection. Hepatology. 53:1494–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lopes AR, Kellam P, Das A, Dunn C, Kwan A,
Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A and Maini MK:
Bim-mediated deletion of antigen-specific CD8 T cells in patients
unable to control HBV infection. J Clin Invest. 118:1835–1845.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisicaro P, Valdatta C, Massari M, Loggi
E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P,
Missale G and Ferrari C: Antiviral intrahepatic T-cell responses
can be restored by blocking programmed death-1 pathway in chronic
hepatitis B. Gastroenterology. 138:682–693. 693.e1–e4. 2010.
View Article : Google Scholar
|
|
13
|
Schurich A, Pallett LJ, Lubowiecki M,
Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W
and Maini MK: The third signal cytokine IL-12 rescues the
anti-viral function of exhausted HBV-specific CD8 T cells. PLoS
Pathog. 9:e10032082013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bengsch B, Martin B and Thimme R:
Restoration of HBV-specific CD8+ T-cell function by PD-1
blockade in inactive carrier patients is linked to T-cell
differentiation. J Hepatol. 61:1212–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinese Society of Hepatology and Chinese
Society of Infectious Diseases Chinese Medical Association: The
guideline of prevention and treatment for chronic hepatitis B (2010
version). Zhonghua Gan Zang Bing Za Zhi. 19:13–24. 2011.In
Chinese.
|
|
16
|
Bunce M: PCR-sequence-specific primer
typing of HLA class I and class II alleles. Methods Mol Biol.
210:143–171. 2003.
|
|
17
|
Mikami N and Sakaguchi S: CD28 signals the
differential control of regulatory T cells and effector T cells.
Eur J Immunol. 44:955–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng M, Li MH, Liu SA, Liu F, Sang Y, Song
SJ, Zang SF, Guan XP, Yao X, Wu XP, et al: Studies about the level
of CD4+ CD25+ regulatory T cells and relation
between expression of Foxp3 and CD127 in peripheral blood of
chronic HBV infection. Zhonghua Shi Yan He Lin Chuang Bing Du Xue
Za Zhi. 24:21–23. 2010.In Chinese. PubMed/NCBI
|
|
19
|
Sojka DK, Hughson A and Fowell DJ: CTLA-4
is required by CD4+CD25+ Treg to control
CD4+ T-cell lymphopenia-induced proliferation. Eur J
Immunol. 39:1544–1551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Sun XH, Zhu XJ, Jin SG, Zeng ZJ,
Zhou ZH, Yu Z and Gao YQ: HBcAg induces PD-1 upregulation on
CD4+T cells through activation of JNK, ERK and PI3K/AKT
pathways in chronic hepatitis-B-infected patients. Lab Invest.
92:295–304. 2012. View Article : Google Scholar
|
|
21
|
Liu XY, Shi F, Zhao H and Wang HF:
Research of PD-1 expression in CD8+ T cell of peripheral
blood with HBV-associated acute-on-chronic liver failure. Zhonghua
Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 24:125–127. 2010.In
Chinese. PubMed/NCBI
|
|
22
|
Prendergast A, Klenerman P and Goulder P:
Expression of inducible costimulator (ICOS) on T cells is
associated with HIV disease progression. Aids Research and Human
Retroviruses. 24:1302008.
|
|
23
|
Jurado JO, Pasquinelli V, Alvarez IB,
Martínez GJ, Laufer N, Sued O, Cahn P, Musella RM, Abbate E,
Salomón H and Quiroga M: ICOS, SLAM and PD-1 expression and
regulation on T lymphocytes reflect the immune dysregulation in
patients with HIV-related illness with pulmonary tuberculosis. J
Int AIDS Soc. 15:174282012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rudd CE, Taylor A and Schneider H: CD28
and CTLA 4 coreceptor expression and signal transduction. Immuno
Rev. 229:12–26. 2009. View Article : Google Scholar
|
|
25
|
van Berkel ME and Oosterwegel MA: CD28 and
ICOS: Similar or separate costimulators of T cells? Immunol Lett.
105:115–122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simpson TR, Quezada SA and Allison JP:
Regulation of CD4 T cell activation and effector function by
inducible costimulator (ICOS). Curr Opin Immunol. 22:326–332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nan XP, Zhang Y, Yu HT, Li Y, Sun RL, Wang
JP and Bai XF: Circulating CD4+CD25 high
regulatory T cells and expression of PD-1 and BTLA on
CD4+ T cells in patients with chronic hepatitis B virus
infection. Viral Immunol. 23:63–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe N, Gavrieli M, Sedy JR, Yang J,
Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et
al: BTLA is a lymphocyte inhibitory receptor with similarities to
CTLA-4 and PD-1. Nat Immunol. 4:670–679. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otsuki N, Kamimura Y, Hashiguchi M and
Azuma M: Expression and function of the B and T lymphocyte
attenuator (BTLA/CD272) on human T cells. Biochem Biophys Res
Commun. 344:1121–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maini MK and Schurich A: The molecular
basis of the failed immune response in chronic HBV: Therapeutic
implications. J Hepatol. 52:616–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brooks DG, Teyton L, Oldstone MB and
McGavern DB: Intrinsic functional dysregulation of CD4 T cells
occurs rapidly following persistent viral infection. J Virol.
79:10514–10527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trautmann T, Kozik JH, Carambia A, Richter
K, Lischke T, Schwinge D, Mittrücker HW, Lohse AW, Oxenius A,
Wiegard C and Herkel J: CD4+ T-cell help is required for
effective CD8+ T cell-mediated resolution of acute viral
hepatitis in mice. PLoS One. 9:e863482014. View Article : Google Scholar
|
|
33
|
Chakir H, Wang H, Lefebvre DE, Webb J and
Scott FW: T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine
profile in mixed cell populations: Predominant role of GATA-3. J
Immunol Methods. 278:157–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szabo SJ, Sullivan BM, Stemmann C,
Satoskar AR, Sleckman BP and Glimcher LH: Distinct effects of T-bet
in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T
cells. Science. 295:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nasta F, Ubaldi V, Pace L, Doria G and
Pioli C: Cytotoxic T-lymphocyte antigen-4 inhibits GATA-3 but not
T-bet mRNA expression during T helper cell differentiation.
Immunology. 117:358–367. 2006. View Article : Google Scholar : PubMed/NCBI
|