Introduction
Esophageal cancer (EC) ranks as the eighth most
common cancer type, with the sixth highest mortality rate worldwide
(1). Esophageal squamous cell
carcinoma (ESCC) contributed to 80% of all EC. Chemoprevention is
the chronic administration of a synthetic, natural or biological
agent to reduce the concurrency, or delay the occurrence of
malignancy, and is a fast evolving field (2). Great efforts have being made to
identify effective chemoprotective agents. However, toxicity is
always present alongside efficacy in the common preventive agents,
including non-steroidal anti-inflammatory drugs. Therefore, there
is a requirement to develop highly efficient chemopreventive agents
with fewer side-effects, and natural compounds, including
polyphenols and antioxidants, are regarded as important sources for
this objective.
Camellia nitidissima Chi (CN), is distributed
in a narrow region of Southern China and North Vietnam (3,4). The
leaves, flowers and seed oils of CN are used in foodstuffs and
Chinese traditional medicines (5).
CN has several similar constituents as other Camellia
sinensis species, including green tea, however, it also has
some unique phytochemicals as well (5,6).
Ethanol extracts of the seeds of CN exhibit cytotoxicity against
human lymphoma cells, and cervical and prostate cancer cells
(7). The amount of bioactive
components in CN flowers is reported to be higher compared with
that in the leaves (6). A previous
study reported that flavonoid glycoside extracted from the flowers
of CN slowed down the proliferation of human lymphoma U937 cells
(8). The toxicity of CN is quite
low. A previous study by Peng et al recently showed that
fresh CN leaf water extracts caused no acute, subacute or genetic
hazards in the mouse acute oral toxicity test, 90-day feeding in
male Wistar rat, Ames test, mouse teratospermia test and mice sperm
abnormality test (9). Another
previous study showed that the lethal dose, 50% (LD50)
on mice oral toxicity test was up to 106.7 g crude drug/kg
(10).
However, the effect of CN flower extracts on the
prevention of ESCC remains to be studied. Drinking is the most
common way for humans to consume teas. Therefore, water extracts of
the CN flower represents the components that would be consumed by
humans through daily tea drinking.
In the present study, the CN flower water extract
(CNFE) was used to investigate possible chemopreventative effects
of CN on an ESCC cell line, Eca109. The cell viability change and
apoptosis induced by CNFE were first evaluated, and the effect of
CNFE on the cell cycle was further analyzed using flow
cytometry.
Materials and methods
Preparation of CNFE
Dry CN flowers were provided by Guangxi Nongyi
Organic Agriculture Company (Guangxi, China). A total of 10 g dry
CN flowers were steeped in 100 ml near boiling double distilled
H2O for 1 h. The infusion was filtered twice through a
0.45 µm polyvinylidene difluoride (PVDF) filter disk (EMD
Millipore, Billerica, MA, USA) and vacuum freeze dried at −80°C to
produce a powdered crude extract, which was stored at −20°C until
use. Prior to the experiments, the powdered crude extract was
dissolved in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at a concentration of
100 mg/ml and filtered twice through a 0.22 µm PVDF filter
disk (EMD Millipore).
Cell culture
The ESCC cell line, Eca109, was purchased from
Shanghai Institute for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). The Eca109 cells were cultured in DMEM,
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) and incubated at 37°C in an atmosphere of 5%
CO2.
Trypan blue exclusion assay
Eca109 cells were cultured in 6-well plates
(8×105 cells/well) for 24 h. When the cells reached ~70%
confluence, they were treated with CNFE at five different
concentrations (100, 200, 300, 400 and 500 µg/ml) for 24, 48
or 72 h. Then the cells were trypsinized and mixed 1:1 with 0.4%
trypan blue solution for 2–3 min. The cells were counted on a
hemocytometer using an inverted phase contrast microscope. Viable
cells exclude trypan blue, while dead cells stain blue due to
trypan blue uptake. The ratio of the numbers of dead cells divided
by the total number of cells is calculated as the percentage of
cell death. At each time point, the effect of CNFE was compared
with the non-treated control.
Quantification of apoptosis by flow
cytometry
The Eca109 cells were plated at a density of
4×105 cells in 6-well plates and treated with CNFE at
100, 200, 300, 400 and 500 µg/ml. The cells were harvested
at the indicated time points, washed twice with cold
phosphate-buffered saline, centrifuged for 5 min at 300 × g at room
temperature. The cells were subsequently stained with Annexin V and
propidium iodide (PI) using the fluorescein isothiocyanate
(FITC)-Annexin V Apoptosis Detection Kit I (BD Bioscience, Franklin
Lakes, NJ, USA). Briefly, the cells were incubated at room
temperature with 3 µl FITC-Annexin V staining solution for
10 min and 2 µl PI staining solution for 5 min in sequence.
A total of 100 µl 1X binding buffer was added at room
temperature in the dark and the labeled cells were subsequently
analyzed by flow cytometry.
DNA cell cycle analysis
The Eca109 cells (70% confluence) were serum starved
for 36 h to synchronize in G0 phase. They were treated
with CNFE at 100, 200, 300, 400 and 50 0 µg/ml in complete
culture medium for 24 h. The cells were subsequently trypsinized,
washed twice with buffer solution (BD Biosciences) and centrifuged
for 5 min at 300 × g at room temperature each time. The pellet was
re-suspended in buffer and frozen at −80°C until analysis. The
frozen samples were thawed rapidly in a water-bath at 37°C. The
cells were centrifuged for 5 min at 400 × g at room temperature and
stained with the Cycle TEST™ PLUS DNA Reagent kit (BD Biosciences),
according to the manufacturer's instructions. The labeled cells
were subsequently analyzed by flow cytometry.
Statistical analysis
The probit regression model was applied to estimate
the half-maximal inhibitory concentration (IC50) values
of CNFE on Eca109 cells. χ2 was applied to determine the
event probability of the cytostatic or cytotoxic effect of CNFE,
and to assess the effects of CNFE on the induction of apoptosis and
cell-cycle perturbation. All statistical analyses were performed
using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
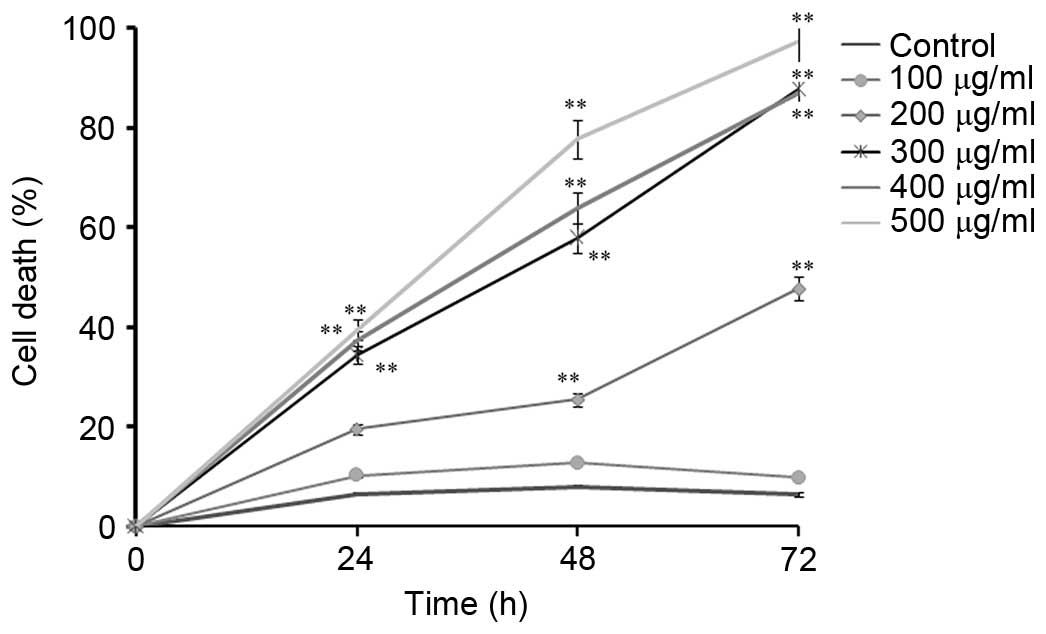
CNFE inhibited the growth of Eca109
cells
Firstly, the present study investigated the effects
of CNFE on the viability of Eca109 cells by trypan blue exclusion
assay (Fig. 1). As shown in
Fig. 1, the percentage of dead
cells increased both in a time- and dose-dependent manner. Compared
with the non-treated control, the percentage of dead cells when
Eca109 cells were treated with 300 µg/ml of CNFE for 24 h
was significantly increased (34.43 vs. 6.43%). The cell death of
Eca109 cells increased gradually when treated with CNFE at 300, 400
and 500 µg/ml for 24 h (P<0.001, compared with the
control; Fig. 1). The percentage
of dead cells when Eca109 cells were treated with CNFE (300, 400 or
500 µg/ml) for 24 h was 34.42, 37.30 and 39.43%,
respectively. Treatment with 200 µg/ml CNFE for 48 or 72 h
significantly reduced the cell viability (P<0.001, compared with
control; Fig. 1). The
antiproliferative effect of CNFE was also time-dependent. The
IC50 of CNFE on Eca109 cells, calculated accordingly at
24, 48 and 72 h, was 513.64, 326.88 and 217.31 µg/ml,
respectively (Fig. 1).
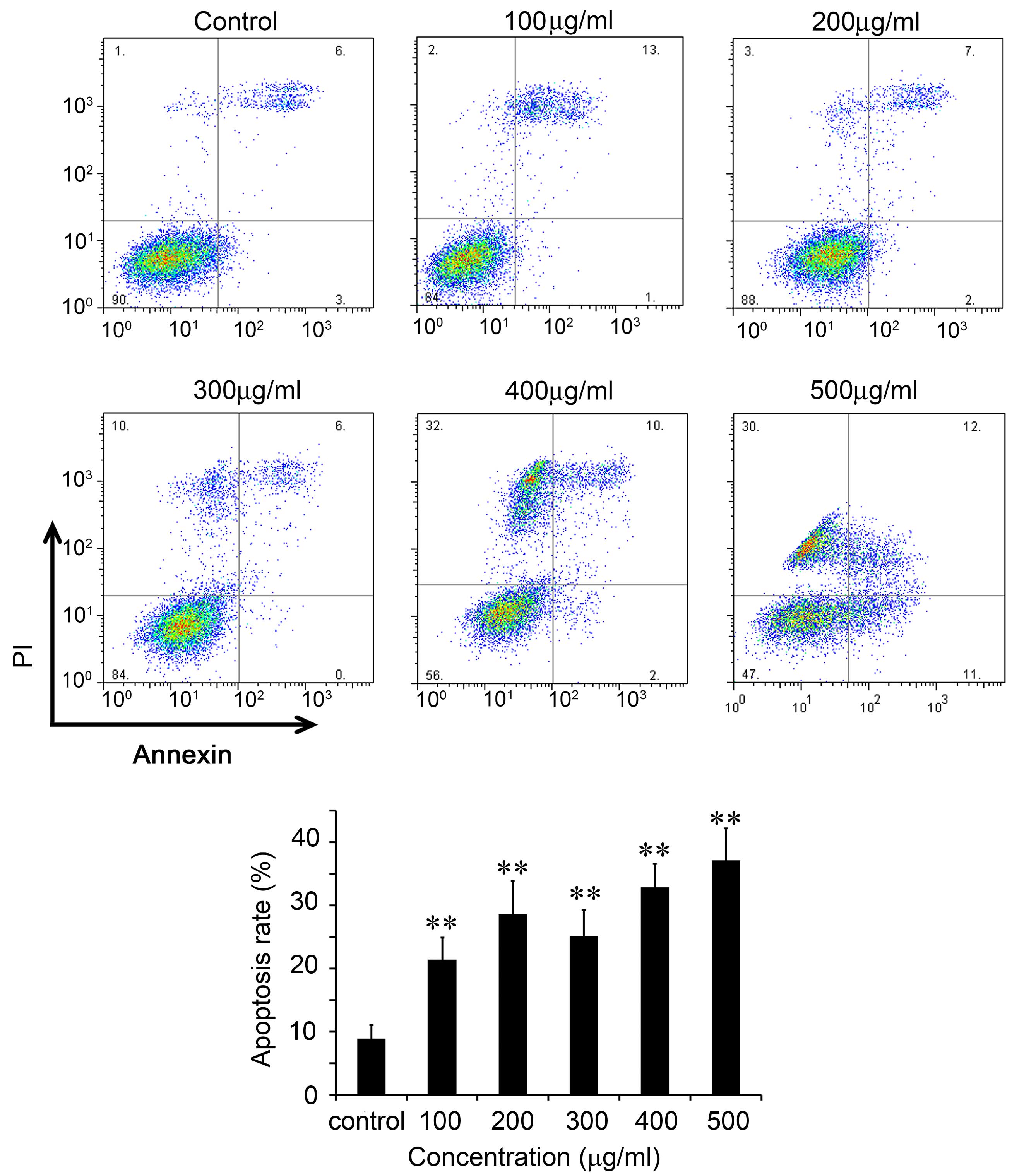
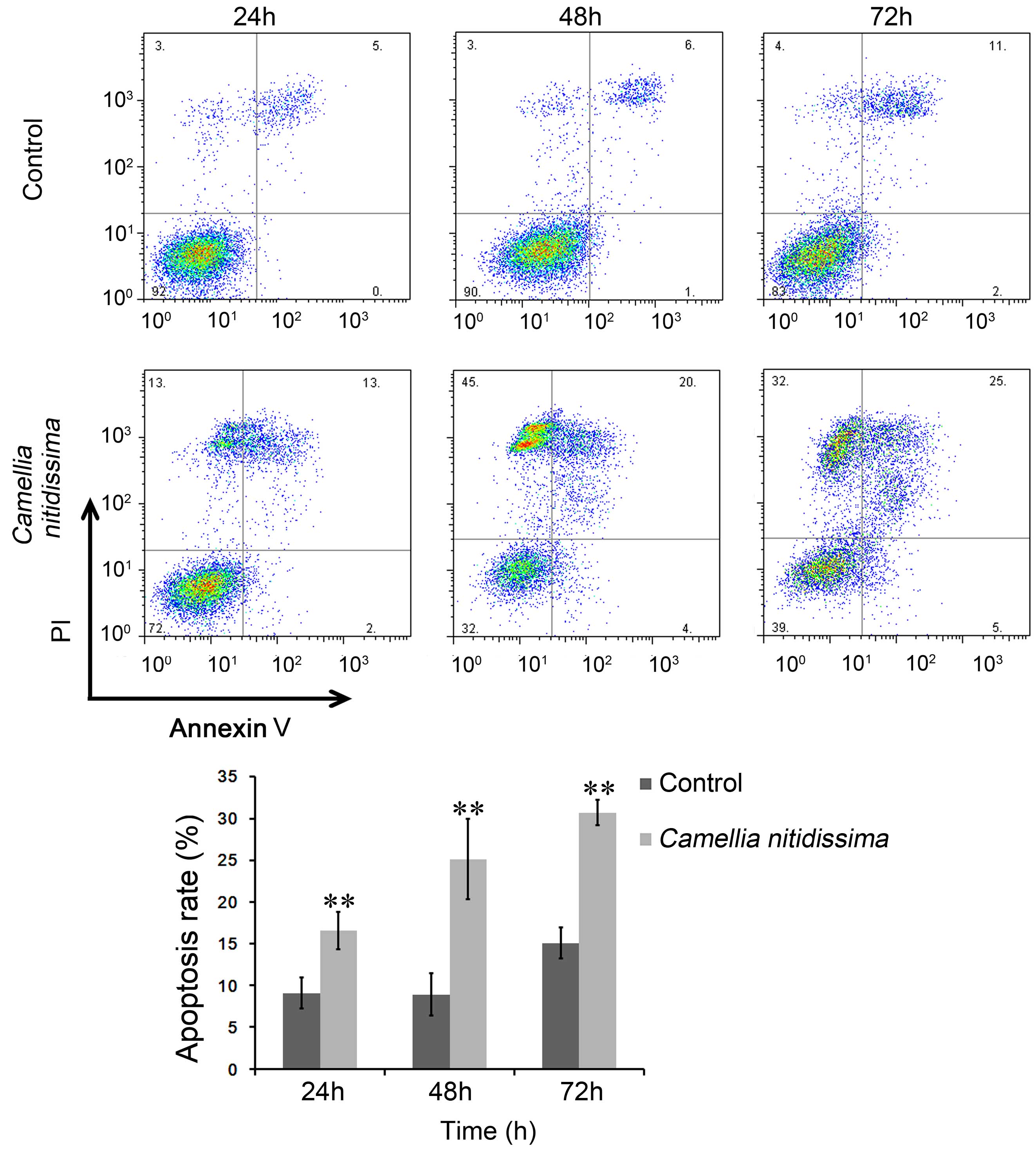
CNFE induced apoptosis of Eca109
cells
The apoptosis of Eca109 cells was further analyzed
by flow cytometry using FITC-Annexin V and PI (Fig. 2). In control cells, the percentage
of early apoptotic cells was 3.01% and the percentage of late
apoptotic/dead cells was 5.91% (Fig.
2). Following incubation with 100 µg/ml CNFE for 48 h,
the percentages of early apoptotic cells and late apoptotic cells
increased to 14.16 and 7.27%, respectively (P<0.01; Fig. 2). The apoptosis of Eca109 cells
after treatment with 100–500 µg/ml CNFE for 48 h was
significantly increased in a dose-dependent manner (Fig. 2). The percentage of apoptotic cells
(early and late apoptosis in total) gradually increased as the
incubating time was extended. The percentage of apoptotic Eca109
cells following treatment with 300 µg/ml CNFE for 24, 48 and
72 h were 16.55, 25.14 and 30.72% respectively, each significantly
increased compared with that of the control cells (9.09, 8.92 and
15.07%, respectively; P<0.001; Fig.
3).
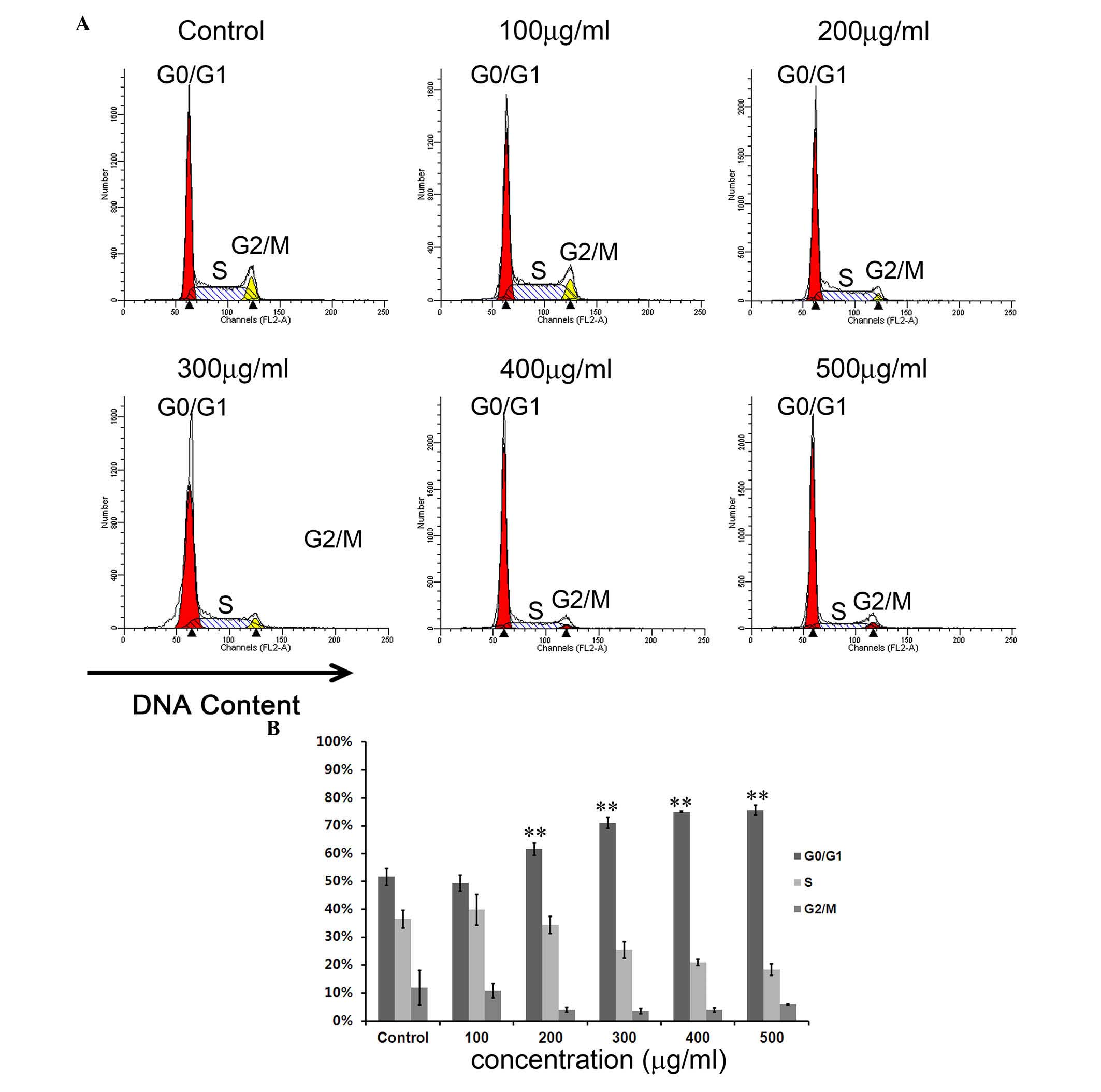
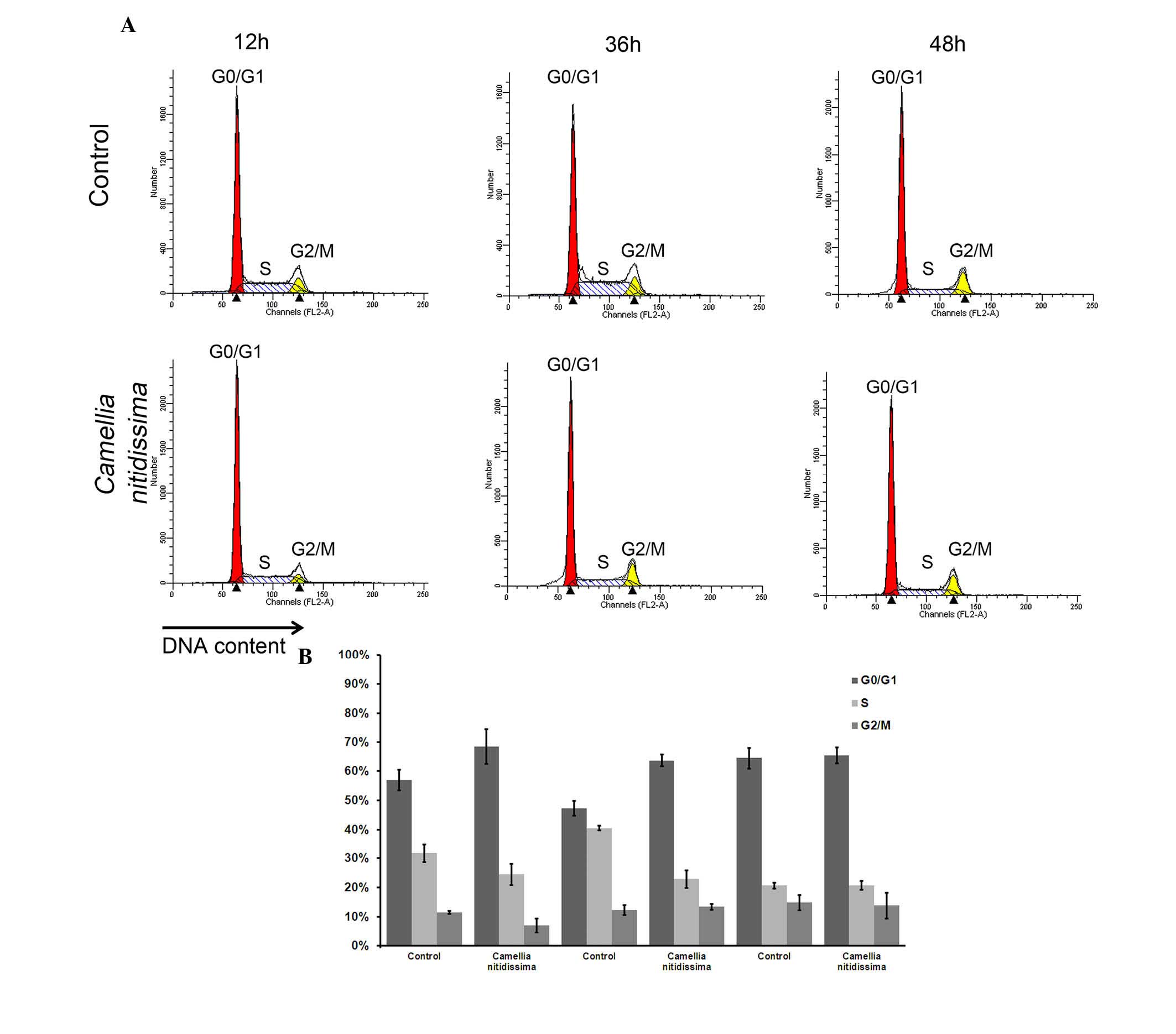
CNFE resulted in
G0/G1 arrest in Eca109 cells
To study whether CNFE can interrupt the cell cycle,
flow cytometric analysis with PI staining was used to analyze the
distribution of the cell cycle. After treatment with CNFE at 200
µg/ml for 24 h, the number of cells in
G0/G1 phase was 61.67%, which was
significantly more compared with the 51.66% observed in control
cells (P<0.001; Fig. 4). The S
phase cell population and G2/M phase cell population
were significantly reduced to 34.30 and 4.04%, compared with the
36.44 and 11.91% observed in control cells (P<0.001; Fig. 4), respectively. The
G0/G1 arrest effect of CNFE on Eca109 cells
in G0/G1 phase was dose-dependent
(P<0.001; Fig. 4). However,
this effect appeared not to be time-dependent. Even when the
incubation time was extended to 48 h, the cell cycle distribution
revealed no significant change when compared with that at 12 or 36
h (Fig. 5).
Discussion
As a part of the ongoing program of investigating
the CN flower in relation to cancer chemoprevention, the present
study evaluated the cancer preventive effect of CNFE on the human
ESCC cell line, Eca109. It was demonstrated that CNFE was able to
reduce Eca109 viability both in a time- and dose-dependent manner.
CNFE induced the apoptosis of Eca109 cells and caused
G0/G1 arrest.
Similar to other C. sinensis species (e.g.
green tea), CN is rich in antioxidants, including numerous
polyphenols such as gallocatechin, epicatechin,
epigallocatechin-3-gallate and gallocatechin gallate (4,6).
Only a few previous studies have investigated the possibility of CN
extracts in preventing cancer (4,6,7).
Water extracts of CN leaf reduce the viability of breast cancer
cells (7), and ethanol extracts
from the flowers of CN inhibit proliferation and induce apoptosis
in human lymphoma cells (8). CN
flowers contain more phytochemicals compared with those in the
leaves and these include flavonoids, tea polyphenols and saponin
(6). Peng et al (8) and his colleagues reported that a
unique acylated flavonoid glycoside in the ethanol extract of CN
flowers induced the apoptosis of human lymphoma cells (8). These bioactive components may serve a
role in the anticancer ability of CNFE. Previous studies have
confirmed that the water extract of CN leaves and flowers can
inhibit the proliferation of hepatoma cells and the
diethylnitrosamine-induced precancerous lesions in rat liver
(11,12). In the present study, CNFE resulted
in loss of Eca109 cell viability in a dose- and time-dependent
manner, and also induced apoptosis in a dose- and time-dependent
manner.
Cells can be arrested in G1, S and G2/M phases of
the cell cycle to prevent replication of damaged DNA or to prevent
aberrant mitosis. The present study also confirmed that CNFE was
able to arrest Eca109 cells in G0/G1 phase,
dose-dependently. It was similar to that of green tea and tea
polyphenols, which most likely arrest cells in
G0/G1 phase (13).
However, the cycle arrest and the apoptosis were
affected differently in a time-dependent manner. When the Eca109
cells were treated with CNFE at 200 µg/ml, the apoptosis
rate increased sharply from 24 to 72 h, however, the percentage of
cells arrested in G0/G1 phase appeared to
remain the same. It is possible that the cell cycle arrest is not
the predominant cause of the CNFE-induced the Eca109 cell death. A
previous study demonstrated that compounds in CN flower
EtOAc-soluble fraction can activate caspase 3 (8). Therefore, CNFE may possibly cause
cell death by directly activating the caspase pathway; however,
this requires further investigation.
The CNFE exerted its effect by inhibiting cell
proliferation and inducing apoptosis; however, its role in
chemoprevention on ESCC and its targets were predominantly unknown.
Further studies on the underlying mechanisms may assist with
highlighting the chemopreventive potential of this rare plant.
Furthermore, as CNFE contains a variety of
water-soluble constituents, the individual constituent that is the
most important in preventing cancer remains unknown. Future studies
must focus on the most effective antioxidant fractions and their
functions in vivo, and this may assist in developing
effective chemoprevention agents from CN extracts.
In conclusion, the present study has demonstrated
that CNFE can reduce the viability of Eca109 cells by affecting the
cell cycle and by inducing apoptosis in vitro. The results
of the present study suggested that CNFE or some of its
constituents may be potential chemopreventive agents for ESCC in
the future.
Acknowledgments
The authors would like to thank Dr Dev Sooranna
(Imperial College, London, UK) for his assistance in editing the
manuscript.
The present study was supported by grants from the
Key Research Projects of Guangxi Health Department (no. Z2011083),
the Guangxi Graduate Education Innovation Projects 2011 (no.
2011105981002M179) and the Administration of Traditional Chinese
Medicine in Guangxi Research projects (no. GZZC1144).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2012. View Article : Google Scholar
|
|
2
|
Steward WP and Brown K: Cancer
chemoprevention: A rapidly evolving field. Br J Cancer. 109:1–7.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang S, Bin X, Wang L and Zhong Y: Genetic
diversity and population structure of yellow camellia (Camellia
nitidissima) in China as revealed by RAPD and AFLP markers. Biochem
Genet. 44:449–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin XL, Shi YC, Li CZ, Wei X, Huang RS,
Kong DX and Huang SS: Study on camellia sect. Chrysantha Chang
species identification by FTIR technology. Guang Pu Xue Yu Guang Pu
Fen Xi. 32:2685–2689. 2012.In Chinese.
|
|
5
|
Huang Y, Wen Y, Chen Y, et al: Extraction
technology and dynamic change of flavonoids in Camellia nitidissima
leaves. Shipin Kexue. 30:72–75. 2009.
|
|
6
|
Lin H, Qin X, Zeng Q, Yang J and Zhong J:
Analysis on chemical and bioactive components in flower of Camellia
chrysantha (Hu) Tuyama. Food Science and Technology. 35:88–91.
2010.
|
|
7
|
Han LC, Shi LY, Yu DY, et al: Inhibitive
effect of seeds of camellia chrysantha (Hu) tuyama on gonadal
hormones dependent tumour in vitro. Lishizhen Med Mat Med Res.
20:31462009.
|
|
8
|
Peng X, Yu DY, Feng BM, Wang YQ and Shi
LY: A new acylated flavonoid glycoside from the flowers of Camellia
nitidissima and its effect on the induction of apoptosis in human
lymphoma U937 cells. J Asian Nat Prod Res. 14:799–804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng L, Zhang P, Bing L, Huang CP, Yao SY
and HY Q: Toxicological safety studies of Camellia nitidissima chi
fresh leaf. J Toxicol February. 25:72–74. 2011.
|
|
10
|
Xia X, Huang JJ, Wang ZP, Wang Q and WG P:
The hypoglycemic effects and acute toxicity studies of nitidissima
chi. Arch Iran med lishizhen medicine and materia medica research.
1281–1282. 2013.
|
|
11
|
Duan X, Tang X, Su J, et al: Study on
inhibition of C. chrysantha on DEN induction of murine liver
cancer. Journal of Medical Research. 35:14–19. 2006.
|
|
12
|
Li C, Duan X, Su J, et al: Impact of
leaves and flowers of camellia chrysantha (Hu) tuyama of different
concentrations on Diethylnitrosaminal-induced precancerous lision
to liver of rat and hepatoma cells BEL-7404. Journal of Guangxi
Medical University. 24:660–663. 2007.
|
|
13
|
Ahmad N, Feyes DK, Nieminen AL, Agarwal R
and Mukhtar H: Green tea constituent epigallocatechin-3-gallate and
induction of apoptosis and cell cycle arrest in human carcinoma
cells. J Natl Cancer Inst. 89:1881–1886. 1997. View Article : Google Scholar : PubMed/NCBI
|