Introduction
Acute necrotizing pancreatitis (ANP) is a sudden and
serious inflammatory disease of the human digestive system
(1). Clinical data has indicated
that ANP is a serious inflammatory disease with a systemic
inflammatory response and multiple organ dysfunction (2). Although the pathogenesis of ANP is
not completely clear, the activation of inflammatory cytokines is
key in the etiology (3–5). During ANP, a number of
pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α
and interleukin (IL)-6 are released into the blood, which can
result in sepsis (6), and
mortality (7). Thus, controlling
the initial inflammatory response is important for controlling ANP
and renal injury induced by ANP. ANP is not only localized to the
pancreas but can also activate inflammatory responses throughout
the body, it is a systemic disease and can result in systemic
inflammatory response syndrome (SIRS) and multiple organ
dysfunction (8). ANP-induced renal
injury is a frequent and early complication of ANP, and the
mortality rate of patients with ANP-induced renal failure is ~71.2%
(9). Thus, it is essential to
protect renal function in patients with ANP.
Paeoniflorin (PF) is the active ingredient of Radix
Paeoniae (10), a traditional
herbal medicine and the dried root of Paeonia lactiflora
Pallas. PF exhibits numerous pharmacological effects, such as
anti-inflammatory, anticancer, analgesic (11) and immunomodulatory effects
(12–14). PF can inhibit the systemic
inflammatory response and improve the survival of rats with
lipopolysaccharide (LPS)-induced sepsis (15). However, the pharmacological effect
of PF on ANP and renal injury induced by ANP remains unclear.
Evidence demonstrates that the p38 mitogen-activated protein kinase
(MAPK) signaling pathway is closely associated with the nuclear
factor (NF)-κB signaling pathway, which is a critical cell
signaling pathway in the inflammatory response. Thus the p38 MAPKs
signaling pathway is a focus of research into the inflammatory
response. p38MAPKs are members of the MAPKs signaling pathway,
which includes p38MAPKs, c-Jun NH2-terminal kinase (JNK) and
extracellular signal-regulated kinase kinase. Its function is
associated with the growth and apoptosis of cells (16). The p38 and JNK signaling molecules
are key in the inflammatory signaling pathway. p38MAPKs can be
triggered by proinflammatory cytokines, such as TNF-α and IL-6
(17,18), and can mediate the inflammatory
response induced by LPS (19). The
p38MAPK signaling pathway has been demonstrated to be important in
the progress of ANP and the organ dysfunction following ANP
(20–22).
Thus, the present study used sodium taurocholate to
generate an ANP model in rats and investigate the pharmacological
effect of PF in renal injury induced by ANP, and whether the p38
MAPK pathway is important in this process. The aim of the present
study was to obtain the optimal dose of PF for preventing acute
renal injury induced by ANP, and to investigate the effects and
underlying mechanisms of PF on acute renal injury following
ANP.
Materials and methods
Reagents
PF (C23O28H11; FW,
480.45; purity ≥95%) was purchased from Nanjing Tcm Institute of
Chinese Materia Medica (Nanjing, China). Rat TNF-α, IL-1β and IL-6
enzyme-linked immunosorbent assay (ELISA) kits were purchased from
the Huamei Biology Technology Company (Wuhan, China). Nitric oxide
(NO) detection kits were purchased from the Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Rabbit anti-NF-κBp65
(1:200; ab16502), anti-glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; 1:2,500; ab8245) and anti-caspase3 (1:100; ab2302)
polyclonal antibodies were purchased from Abcam (Cambridge, UK).
Rabbit anti-p38 (1:1,000; 8690) and anti-p-p38 (1:1,000; 4511)
polyclonal antibodies were purchased from Cell Signaling
Technologies, Inc. (Hercules, CA, USA). The terminal
deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling
(TUNEL) assay kits was purchased from Roche Diagnostics
(Indianapolis, IN, USA).
Animals
A total of 128 Sprague Dawley (SD) rats (weight,
180–200 g), were purchased from the Center of Experimental Animals
of Wuhan University (Wuhan, China). The rats were maintained in
specific pathogen-free conditions at room temperature under a 12 h
light-dark cycle with ad libitum access to water. The rats
underwent fasting prior to operation. The rats were maintained in
accordance with the principles of the 1983 Declaration of Helsinki.
The present study was approved by the Ethics Committee of Wuhan
University (Wuhan, China).
Experimental design
In the first part of the present study, 48 SD rats
were randomly divided into six groups (8 rats/group), as follows:
i) The sham-operated group; ii) the ANP group; iii) the ANP+P1
group (50 mg/kg PF); iv) the ANP+P2 group (100 mg/kg PF); v) the
ANP+P3 group (150 mg/kg PF); and vi) the vehicle group (normal
saline at the same volume). In order to induce severe ANP, the rats
were fasted overnight and given fresh tap water ad libitum.
Anesthesia was administered by intraperitoneal injection with 10%
chloraldurat (3 ml/kg; Wuhan Goodbio Technology Co., Ltd., Wuhan,
China). The pancreatic bile duct was cannulated through the
duodenum, and ANP was induced by a standardized retrograde infusion
of a freshly prepared 5% sodium taurocholate solution (1 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) into the biliary-pancreatic
duct. Isotonic saline solution (20 ml/kg) was injected into the
back of the rats to compensate for fluid loss. The sham-operated
group received isotonic saline solution infusion instead of sodium
taurochlorate. For the PF-treated groups, PF was dissolved in
vehicle (normal saline) and intravenously injected into the femoral
vein 30 min following the generation of the ANP model. The rats
were sacrificed 12 h following the induction of ANP by
exsanguination following anesthesia, as the pancreatic damage
reached a climax at that time point (23). The effect of PF was evaluated by
determining the levels of serum amylase (AMY), serum lipase (LIPA),
aspartate-transaminase (AST), alanine-aminotransferase (ALT), and
the pathological changes of pancreas shown by hematoxylin &
eosin (H&E) staining.
Following the initial experiment, the 100 mg/kg dose
of PF was determined to be the optimum dose. Subsequently, 72 SD
rats were divided into three groups: i) The 3 h group; ii) the 6 h
group; and iii) the 12 h group. These groups were further divided
into three subgroups (8 rats/subgroup), including the sham-operated
group, the ANP group and the ANP+PF group (PF group in the
figures). For each part of study the rats were sacrificed by
exsanguination under anesthesia. Blood samples were collected from
the postcava and plasma was collected following centrifugation at
12,000 × g for 10 min at 4°C. The pancreas and kidneys were
harvested and immediately frozen in liquid nitrogen, after which
they were stored at −80°C for subsequent experiments. Sections of
the pancreas and kidneys were fixed in 4% paraformaldehyde (Wuhan
Goodbio Technology Co., Ltd.).
Enzyme assay
The serum levels of AMY, LIPA, AST, ALT, BUN and Cr
were measured using the Automatic Biochemistry Analyzer (Olympus
Corporation, Tokyo, Japan), according to a standard technique
(24).
Histopathological examination
The paraffin-embedded tissues were cut into
3-µm sections for pathological examination by H&E
staining. The H&E results were observed under a light
microscope (Olympus BX53; Olympus Corporation) by two experienced
pathologists who were blinded to the groups. The pancreatic
pathological scores were measured according to the degree of edema,
inflammation, vacuolization and necrosis as described by Schmidt
et al (25). The kidney
pathology score was used for evaluating the severity of the renal
injury. For each kidney, 100 cortical tubules from at least 10
different views were analyzed. Higher scores represented more
severe damage (maximum score per tubule was 10), with points given
for the following: The presence and extent of tubular epithelial
cell flattening (1 point), brush border loss (1 point), cell
membrane bleb formation (1 or 2 points), cytoplasmic vacuolization
(1 point), cell necrosis (1 or 2 points), interstitial edema (1
point) and tubular lumen obstruction (1 or 2 points) (26).
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of TNF-α, IL-1β and IL-6 were
measured using ELISA kits, according to the manufacturer's
instructions.
Immunohistochemistry assay
The paraffin-embedded kidney tissues were cut into
3-µm sections, and the levels of NF-κB and caspase-3 were
determined by immunohistochemical analyses. Briefly, sections were
deparaffinized and dehydrated using a graded series of ethanol
solutions. The slides were immersed in 10 mM citrate buffer (pH
6.0) and boiled for 15 min in a microwave for antigen retrieval.
Then the slides were allowed to cool at room temperature. Hydrogen
peroxidase (0.3%) was used to halt the endogenous peroxidase
activity for 15 min at room temperature. Non-specific binding was
blocked by incubation with goat serum (5%) for 10 min, after which
the sections were incubated overnight at 4°C with. rabbit
anti-NF-κBp65 and anti-caspase3 primary antibodies, followed by
incubation with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (1:5,000; 10811; Pierce
Biotechnology, Inc., Rockford, IL, USA). The staining results were
visualized using 3,5-diaminobenzidine (DAB; Wuhan Goodbio
Technology Co., Ltd.) and images of the slides were captured under
the BX53 microscope. In each immunohistochemistry staining
analysis, phosphate-buffered saline was used instead of the primary
antibody as a negative control.
TUNEL
The TUNEL assay was performed for evaluating renal
cell apoptosis. The renal paraffin-embedded tissues were cut into
3-µm sections as reported in the H&E and
immunohistochemistry assay. The sections were deparaffinized,
hydrated and incubated with proteinase-K (Wuhan Goodbio Technology
Co., Ltd.) for 15 min at room temperature. The residual steps were
conducted according to the protocol provided by the recommendations
in situ cell death detection kit, POD (Roche Diagnostics).
Hematoxylin was used for staining the nucleus after DAB staining.
The results of TUNEL were observed with the Olympus BX53
microscope. The positive results of TUNEL were brown staining in
the nucleus.
NO assay
Kidney tissues were thawed and homogenized in
phosphate buffer containing 0.5% hexadeyltrimethylammmonium bromide
(Nanjing Jiancheng Bioengineering Institute). NO production was
measured using an NO detection kit, according to the manufacturer's
instructions.
Western blot analysis
The fresh kidney tissues were homogenized in
ice-cold lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) in the presence of a protease inhibitor cocktail (Wuhan
Goodbio Technology Co., Ltd.). Proteins were measured using the
Bradford Protein Assay with bovine serum albumin (Beyotime
Institute of Biotechnology) as a standard. In brief, equal
quantities of protein samples were separated on 10% sodium dodecyl
sulfate-polyacrylamide gels and then transferred to a
poly-vinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 5% skimmed milk in TBST buffer
(Tris-buffered saline containing 0.1% Tween-20) at room temperature
for 2 h and then incubated with a rabbit polyclonal anti-GAPDH
antibody (1:2,500, Abcam), rabbit polyclonal antibody anti-P38
(1:1,000, Cell Signaling Technologies, Inc.) and rabbit polyclonal
anti-p-P38 antibody (1:1,000, Cell Signaling Technologies, Inc.) at
4°C overnight. After extensive rinsing with TBST, the blots were
incubated with secondary antibody HRP-conjugated goat anti-rabbit
(1:5,000, Pierce Biotechnology, Inc.) at room temperature for 1.5
h, and then detected the expression of GAPDH, P38 and p-P38 by
enhanced chemiluminescent reagent (Immobilon Western HRP Substrate;
EMD Millipore). The intensities of the bands on the western blots
were quantified using Quantity One software, version 4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation. Data analyses were conducted using SPSS software,
version 19.0 (IBM SPSS, Armonk, NY, USA). Data between all groups
was compared by one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Optimum dose of PF
The present study analyzed the serum AMY, LIPA, ALT
and AST levels in ANP rats treated with various doses of PF (50,
100 and 150 mg/kg) in order to determine the optimum dose of PF.
All doses of PF were able to significantly reduce the levels of AMY
and LIPA, as compared with the ANP group (P<0.05). However, the
100 mg/kg PF group was the only PF-treated group in which both the
levels of AST and ALT were significantly decreased, as compared
with the ANP group. Therefore, 100 mg/kg PF was selected as the
optimum dose for treating ANP (Table
I).
 | Table IEffect of paeoniflorin on enzyme
levels. |
Table I
Effect of paeoniflorin on enzyme
levels.
| Group | AMY (U/l) | LIPA (U/l) | ALT (U/l) | AST (U/l) |
|---|
| Sham-operated | 1755.7±214.0 | 4417.9±553.1 | 40.1±4.8 | 127.9±37.2 |
| ANP |
8287.5±1394.5a |
14599.5±1741.5a | 183.5±54.6a | 618.8±165.7a |
| ANP+Paeoniflorin
(mg/kg) |
| 50 |
7244.1±793.2a,b |
14190.3±803.1a | 158.6±24.8a | 480.3±70.4a |
| 100 |
6333.1±683.9a–c |
12675.0±735.2a–c | 114.1±9.4a–c | 357.6±51.7a–c |
| 150 |
5244.3±445.8a–c |
11362.8±905.0a–c | 275.2±84.5a–c | 514.0±65.6a |
| NS | 1865.3±205.9 | 4489.7±649.3 | 50±17.9 | 142.6±29.3 |
Histopathological assay
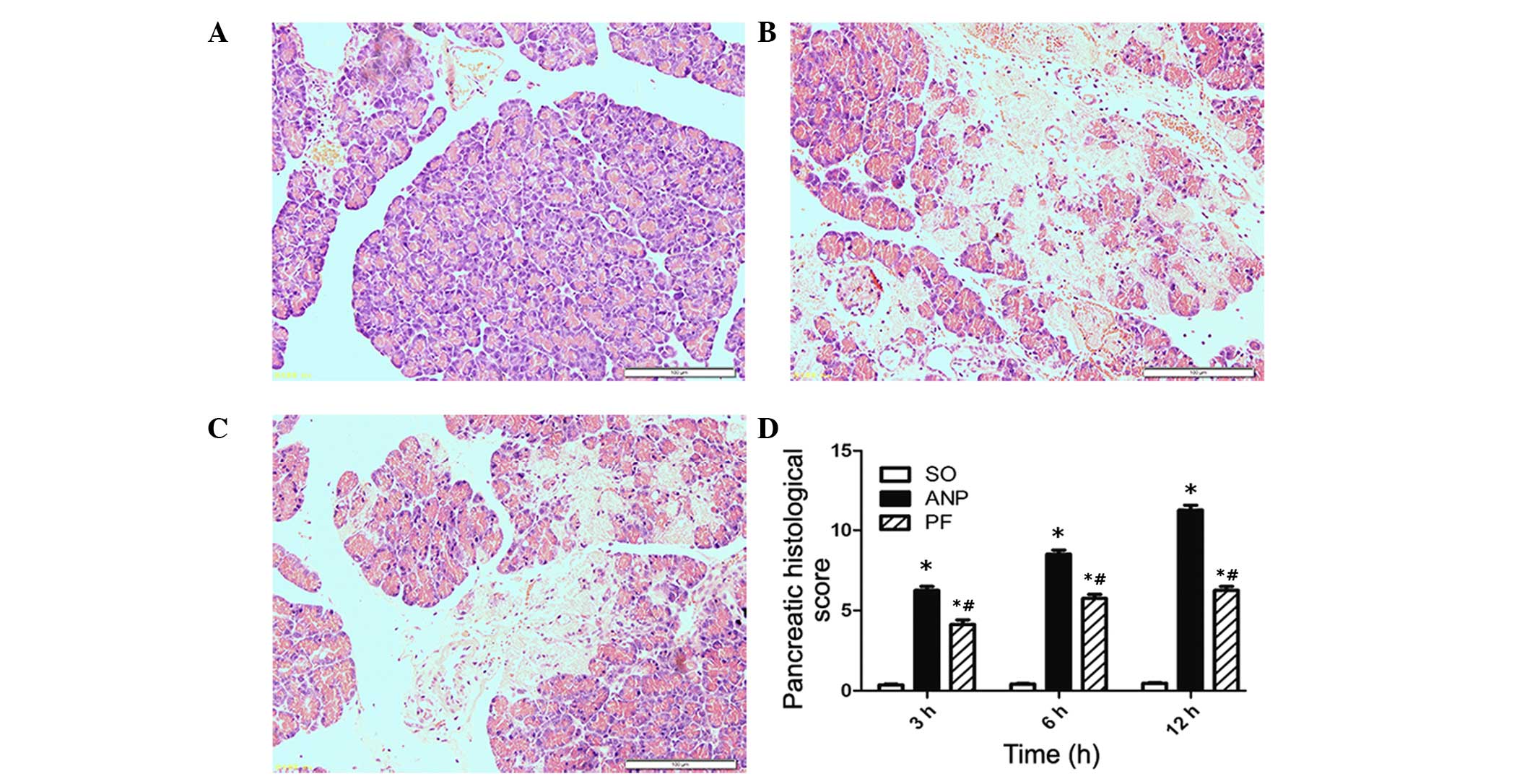
Representative pathological changes in pancreatic
tissue are shown in the Fig. 1.
Normal histological features of the pancreas were observed in the
sham-operated group (Fig. 1A). In
the ANP group, pancreatic edema, interstitial leukocyte
infiltration, hemorrhage and necrosis were observed (Fig. 1B). In the ANP+100 mg/kg PF group,
the severity of pancreatic histological injuries was significantly
reduced compared with the ANP group (Fig. 1C). As shown in Fig. 1D the histopathological score in the
pancreas was lower in the PF group compared with that in the ANP
group at the corresponding time points (P<0.05).
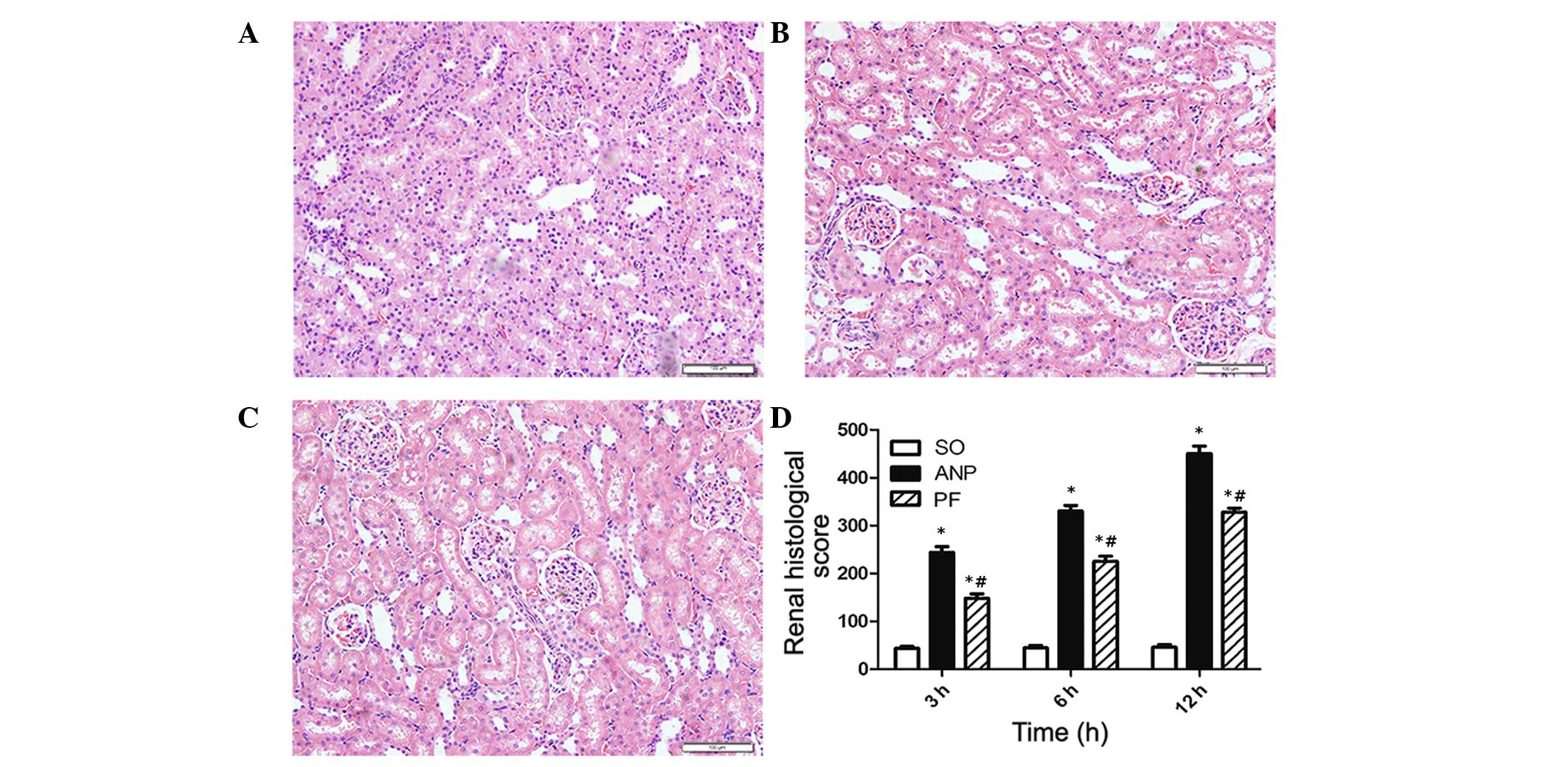
To examine the effect of PF on acute renal injury
induced by ANP, the pathological changes in the kidney were
analyzed 3, 6 and 12 h after rat ANP models were successfully
generated. The renal damage was shown to be the most severe at the
12 h time point. The loss of brush border, cell necrosis, sloughing
of tubular epithelial cell, cast formation and dilation of tubules
in the cortex were observed clearly in the ANP group. The ANP+PF
group had less severe features of renal injury and the renal
histological scores were significantly decreased compared with the
ANP group at the corresponding time points as shown in Fig. 2 (P<0.05).
Analysis of serum AMY, LIPA, BUN and
Cr
Compared with the levels of AMY, LIPA, BUN and Cr in
the shame-operated group, the levels in the ANP group were
significantly increased at each time point (Fig. 3). Peak serum AMY level was observed
at the 6 h time point in the ANP group and the peak serum LIPA, BUN
and Cr levels were observed at the 12 h time point. In the ANP+PF
groups the serum enzyme levels were significantly reduced at the
corresponding time points compared with the ANP group
(P<0.05).
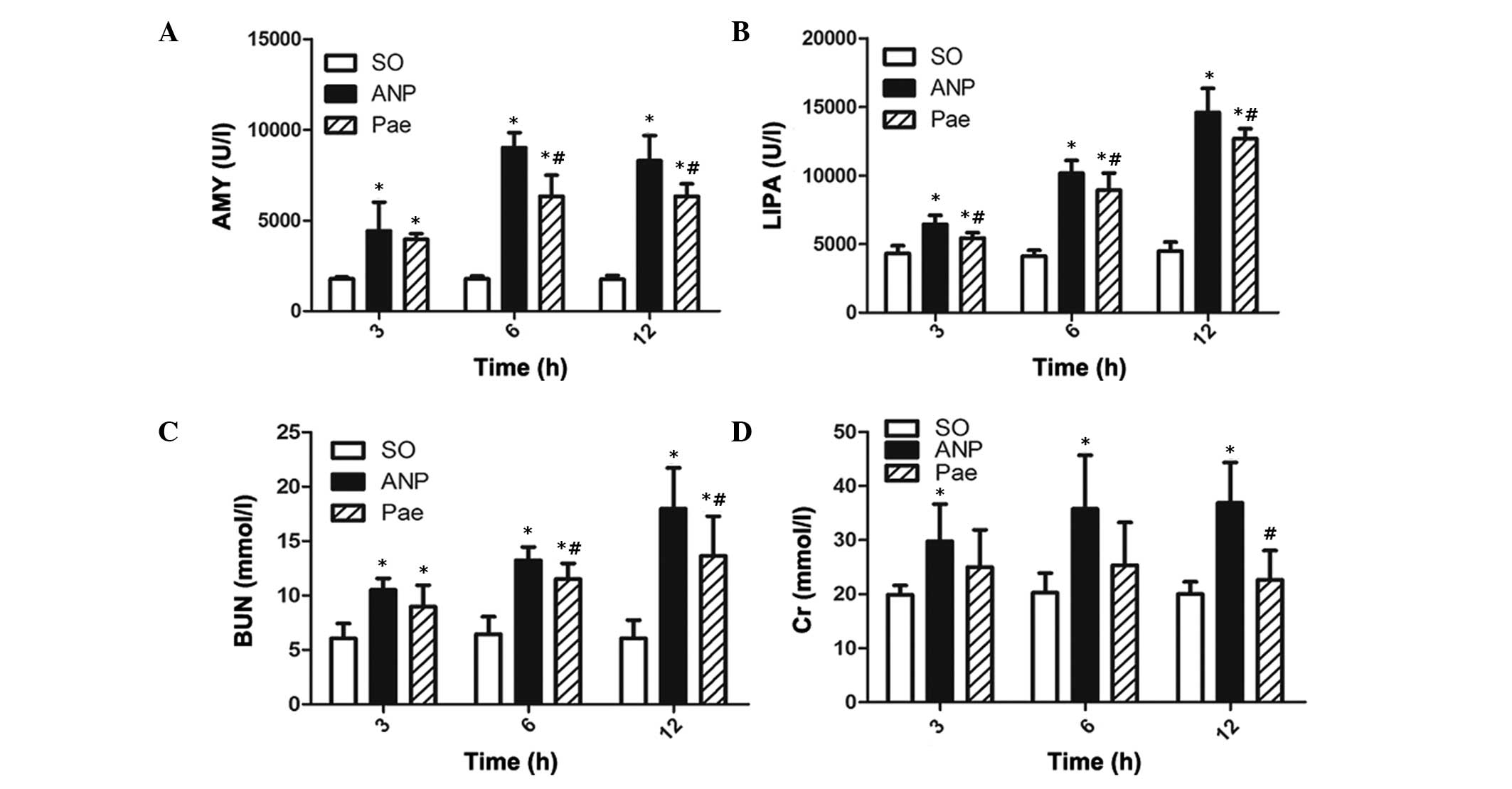 | Figure 3Serum levels of AMY, LIPA, BUN and Cr
in all groups. Serum levels of (A) AMY, (B) LIPA, (C) BUN and (D)
Cr. Data are expressed as the mean ± standard deviation; n=8 in
each group. *P<0.05 vs. the sham-operated group;
#P<0.05 vs. the ANP group. SO, sham-operated; ANP,
acute necrotizing pancreatitis; PF, paeoniflorin; AMY, amylase;
LIPA, lipase; BUN, blood urea nitrogen; Cr, creatine. |
ELISA assay
PF reduced the serum levels of TNF-α, IL-1β and IL-6
in the ANP rats. The serum levels of TNF-α, IL-1β and IL-6 were
determined using ELISA. It was found that the serum levels of
TNF-α, IL-1β and IL-6 were progressively upregulated from 3 to 12
h. The ANP group showed significantly higher levels of TNF-α, IL-1β
and IL-6 compared with the sham-operated group. The PF treatment
resulted in a marked decrease of serums of TNF-α, IL-1β and IL-6,
however, the levels of the enzymes in the PF group were
significantly higher than the sham-operated group at each time
point (Table II).
 | Table IISerum levels of TNF-α, IL-1β and
IL-6. |
Table II
Serum levels of TNF-α, IL-1β and
IL-6.
| Group | TNF-α (pg/ml) | TNF-α (pg/ml) | IL-6 (pg/ml) |
|---|
| Sham-operated |
| 3 h | 154.78±11.94 | 106.21±6.52 | 113.36±9.77 |
| 6 h | 155.84±14.59 | 103.98±6.28 | 116.56±11.17 |
| 12 h | 152.45±12.83 | 105.89±9.02 | 118.78±11.43 |
| ANP |
| 3 h |
251.39±18.06a |
257.50±16.31a |
271.44±17.00a |
| 6 h |
317.35±20.76a |
351.41±14.94a |
355.00±27.80a |
| 12 h |
366.90±13.13a |
430.39±26.26a |
441.64±31.19a |
|
ANP+Paeoniflorin |
| 3 h |
189.44±11.84a,b |
178.54±13.23a,b |
173.33±15.03a,b |
| 6 h |
261.88±16.47a,b |
275.76±20.28a,b |
244.51±15.37a,b |
| 12 h |
286.23±11.23a,b |
315.60±11.04a,b |
288.29±12.71a,b |
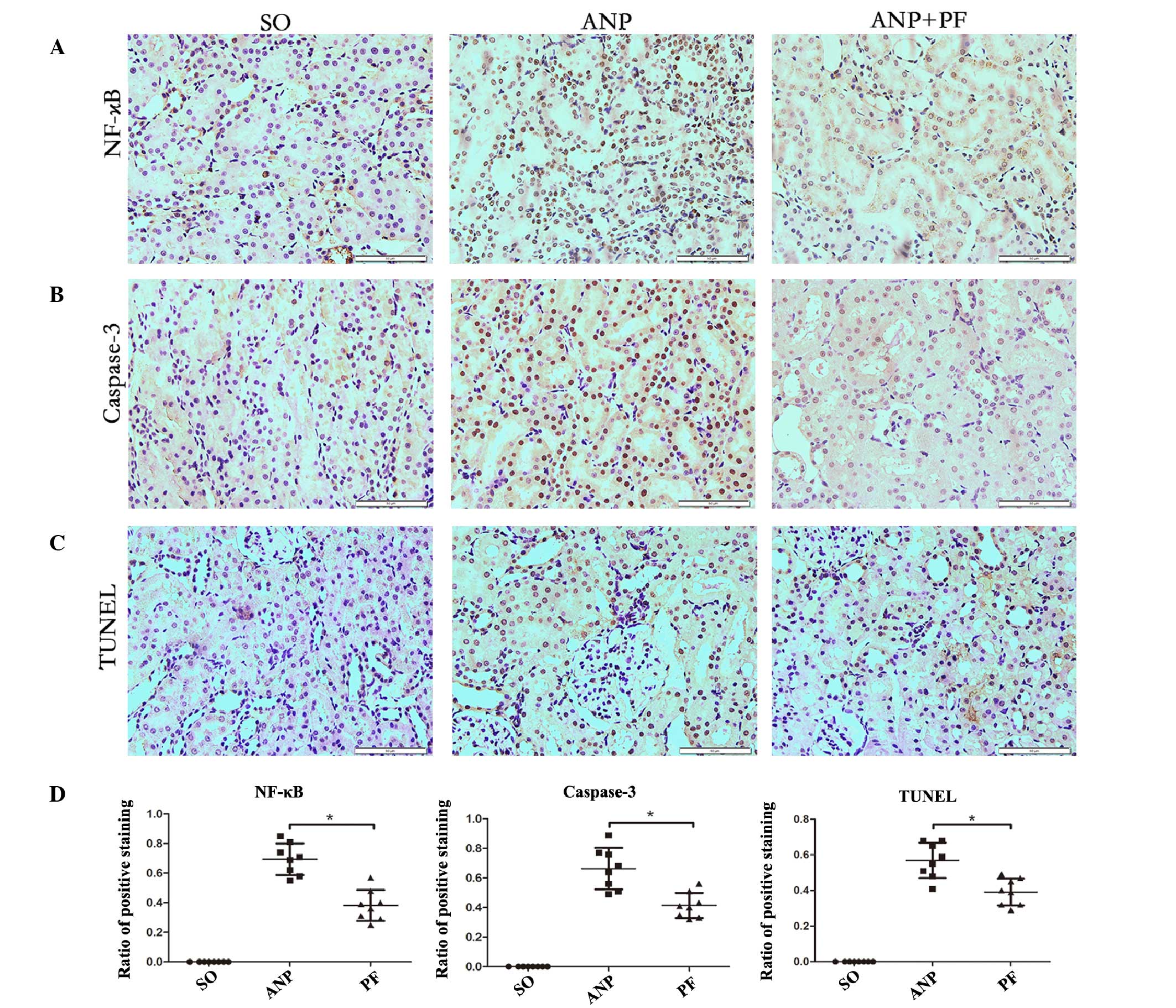
Immunohistochemistry analysis of NF-κB
and caspase-3 levels, and the ratio of apoptotic cells in the renal
tissue by TUNEL
In order to detect the degree of inflammation in
renal tissues, immunostaining of NF-κB was measured in the kidney
samples at the 12 h time point in each group. In the ANP+PF group,
the expression of NF-κB was lower than in the ANP group (Fig. 4A). To identify the effects of PF on
apoptotic changes in renal tissues, caspase-3 protein expression
was detected with immunohistochemistry. A marked increase in
caspase-3 staining was found in the nucleus in the ANP group. In
the ANP+PF group the expression of caspase-3 was markedly decreased
(Fig. 4B). To further confirm the
apoptotic changes, numbers of TUNEL-positive cells were measured.
The number of TUNEL-positive cells increased in the ANP group
compared with those in sham-operated group. PF attenuated the
increase of TUNEL-positive cells number in renal tissue (Fig. 4C). The ratios of positively stained
cells were determined and it was shown that the number of cells
expression NF-κB and caspase-3 were significantly decreased in the
ANP+PF group, as compared with the ANP group (P<0.05; Fig. 4D). Furthermore, the numbers of
TUNEL-positive cells were significantly decreased in the ANP+PF
group, as compared with the ANP group (P<0.05; Fig. 4D).
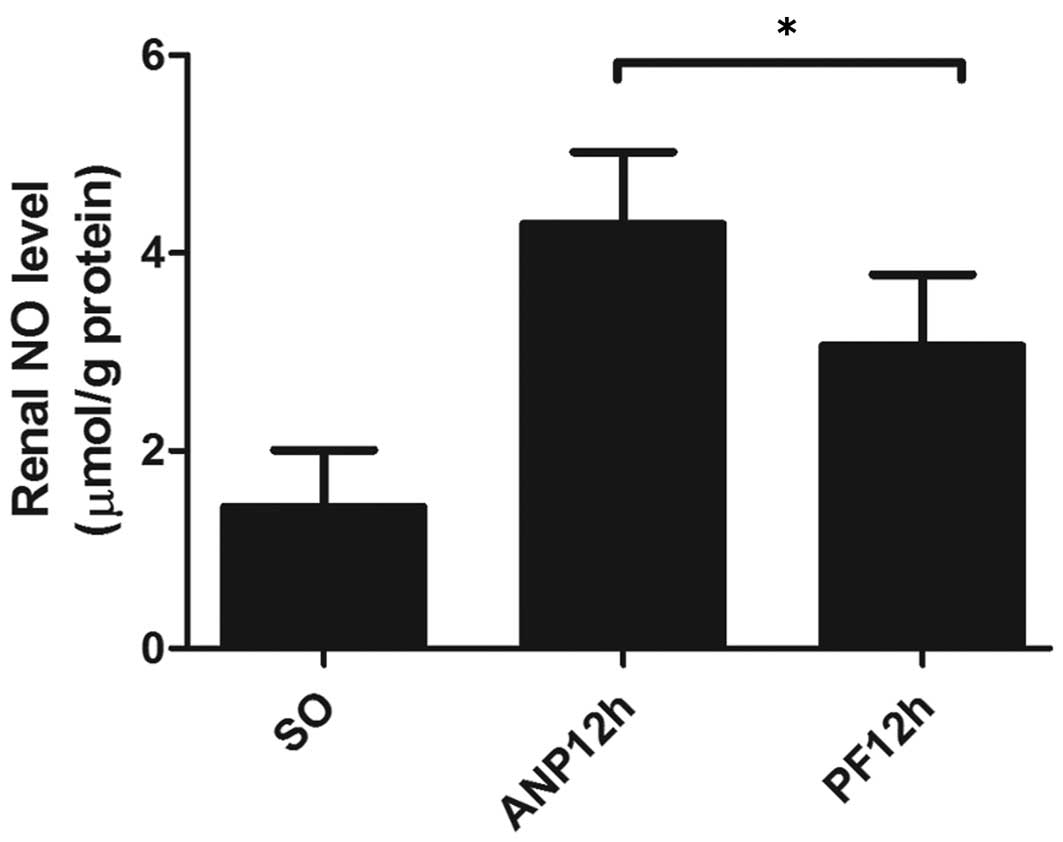
PF decreases renal NO production
The levels of NO in renal tissues from the ANP group
were markedly increased compared with those in the sham-operated
group. However, treatment with PF significantly reduced the NO
production (Fig. 5).
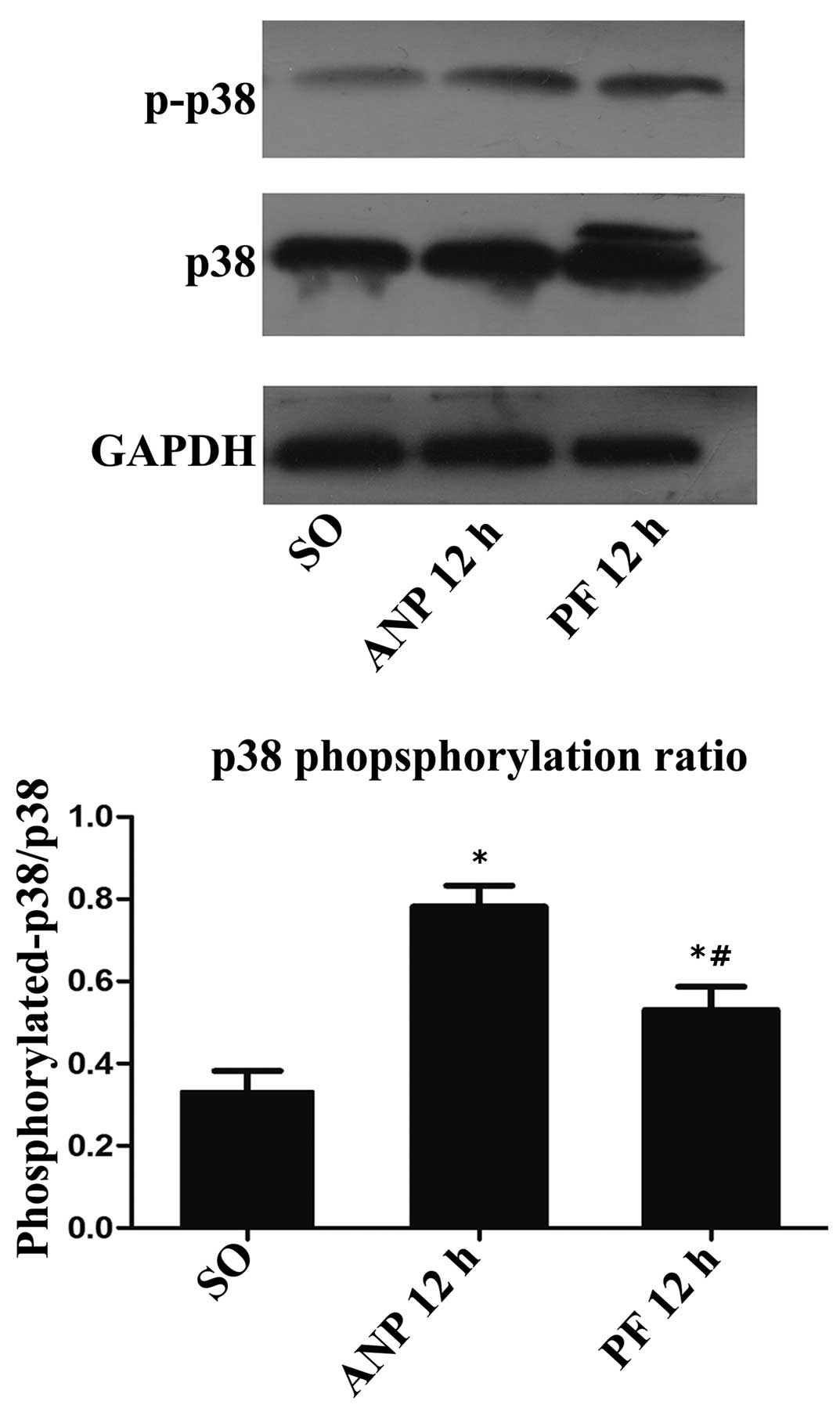
Role of the p38 MAPK pathway in the
effect of PF in renal injury induced by ANP
The p38 MAPK signaling pathway can regulate the
expression of NF-κB in numerous inflammatory diseases. Therefore,
this study detected the effect of PF on the activation of p38 MAPKs
in renal tissues using western blot analysis. As shown in the
Fig. 6, the ANP group exhibited a
higher ratio of p-p38/p38 compared with the sham-operated group.
Furthermore, the ANP+PF group exhibited a significant decrease in
the ratio of p-p38/p38 compared with the ANP group at the 12 h time
point (P<0.05).
Discussion
This study is the first to observe the therapeutic
effect of PF on ANP and the acute renal injury induced by ANP in
rat models. PF is one of the active ingredients of Radix Paeoniae,
and it is an important traditional herbal medicine with a number of
pharmacological effects, including antihyperlipidemic and
anti-inflammatory effects. The results of this study showed that PF
can ameliorate ANP and protect the kidneys in ANP. This effect was
demonstrated by the serum levels of enzymes and the histological
changes in the pancreas and kidneys. Acute renal injury is a common
complication of ANP and it is associated with increased risk of
mortality (27). The mechanism of
the acute renal injury following ANP is complicated. The
inflammatory cascades that are initiated by endothelial dysfunction
can be augmented by the generation of pro-inflammatory cytokines,
such as TNF-α, IL-1 and IL-6. Using ELISA, the serum levels of the
TNF-α, IL-1β and IL-6 were shown to be lower in the PF+ANP group
compared with the ANP group at each time point. In a previous study
by Jiang et al (15), PF
was shown to inhibit systemic inflammation and improve survival in
the experimental rat models of sepsis.
Another important mechanism underlying the
development of acute renal injury following ANP is acute ischemia
(28). ANP results in a decreased
circulating blood volume, which causes renal ischemia due to
decreased blood flow to the kidneys. Ischemia results in apoptosis
of renal cells which reduces kidney function. Therefore, in order
to investigate the effect of PF on kidney cell apoptosis, a TUNEL
assay was conducted and the expression of caspase-3 was analyzed.
The ratio of the TUNEL-positive cells was shown to increase in the
ANP groups and the majority of TUNEL-positive cells were located at
the cortex of the kidney. The mechanisms underlying the apoptosis
of renal tissues are associated with cellular cytotoxicity and DNA
damage. In addition, NO was shown to have an important role in the
initiation of apoptosis in experimental renal IR (29). A previous study demonstrated that
NO production resulted in cytotoxicity and DNA damage (29). In the present study, PF was shown
to inhibit NO-mediated apoptosis in acute renal injury induced by
ANP. The results showed that PF was able to ameliorate NO
production and inhibit tubular cell apoptosis. PF is known to
promote the dilatation of blood vessels, which may increase blood
perfusion to the renal cortex and inhibit cell apoptosis.
Therefore, these results suggested that PF may attenuate renal
damage by reducing NO-induced apoptosis of renal cells.
Caspase-3, which is a member of the caspase family
that includes caspase-1 to caspase-12, has a key function in
apoptosis (30). In a previous
study, the role of caspase-3 in apoptosis was closely associated
with the Bax/Bcl-2 signaling pathway. Activation of the initiator
caspase-8 is triggered by the extrinsic death signaling pathway
(31). The other initiator
caspase, caspase-9, is predominantly activated by the release of
pro-apoptotic proteins in the intrinsic pathway (32). The initiator caspases culminate and
trigger downstream caspase-3, which is the core executioner caspase
during the extrinsic and intrinsic apoptosis pathways. In the
present study, PF treatment significantly suppressed the expression
of caspase-3 in renal tissues in the ANP rat model. These results
suggested that PF may inhibit apoptosis.
The alterations in renal apoptosis and inflammation
in the ANP rat model were closely associated, since inflammation
has been shown to lead to apoptosis. Inflammatory cytokines, such
as IL-1, TNF-α and IL-6, promote cell death by inducing the
expression of connective molecules on the surface of the blood
vessel endothelium, and these molecules can promote the
inflammatory cells to form multinucleated cells that have been
associated with apoptosis (33).
NF-κB is essential in the inflammatory response to acute
pancreatitis as well as in apoptosis (34,35).
The most common forms of NF-κB are heterodimers composed of p50 and
p65 or p52 and p65 (36). In the
cytoplasm, NF-κB exists as an inactive form associated with its
regulatory protein, IκB. During inflammation, inflammatory
cytokines such as IL-1, TNF-α and IL-6 activate NF-κB by
phosphorylating IκB; phosphorylated IκB releases NF-κB dimers from
the cytoplasmic NF-κB-IκB complex, such that they are able to
translocate into the nucleus (37). The p38MAPK signaling pathway, a
classic MAPK signal transduction pathway, has been shown to be
important in cell proliferation, differentiation, cytokine
synthesis and T-cell activation (38). When stimulated by specific
inflammatory cytokines, pathogens or cell stress, p38MAPK is
activated, phosphorylated and transferred into the nucleus. The
phosphorylated p38MAPKs can activate IκB and induce the NF-κB
signal transduction pathway. In the present study,
immunohistochemistry results demonstrated an increase in the level
of NF-κB in the ANP group, as compared with the sham-operated
group, and a significant decrease in the level of NF-κB in the
PF+ANP group, as compared with the ANP group. Western blotting
demonstrated that PF treatment decreased the level of
phosphorylated p38MAPKs. Thus, the anti-inflammatory action of PF
may be associated with the p38MAPKs/NF-κB signaling pathway. A
limitation of this study is that no experiment was conducted using
a p38MAPKs signal pathway inhibitor. It remains to be determined
whether the p38MAPK/NF-κB signaling pathway is the only signal
transduction pathway mediating the effects of PF. Although further
investigation is required to determine the precise mechanism
underlying the effects of PF, the present results demonstrate that
PF does exhibit a protective effect against ANP-induced renal
injury.
In conclusion, PF prevented ANP-induced acute renal
injury by inhibiting inflammation and apoptosis. Thus, PF may be a
potential therapeutic agent for the treatment or prevention of ANP
and acute renal injury induced by ANP.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370562) and the
Projects of Medical and Health Technology Development Program in
Shandong province (grant no. 2013GGB14096). The authors would like
to thank Dr. Yongfei Tang of Department of Pathology, (Renmin
Hospital, Wuhan University).
References
|
1
|
Fritz S, Hartwig W, Lehmann R,
Will-Schweiger K, Kommerell M, Hackert T, Schneider L, Büchler MW
and Werner J: Prophylactic antibiotic treatment is superior to
therapy on-demand in experimental necrotising pancreatitis. Cri
Care. 12:R1412008. View
Article : Google Scholar
|
|
2
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. J Pathol. 190:117–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Büchler MW, Gloor B, Müller CA, Friess H,
Seiler CA and Uhl W: Acute necrotizing pancreatitis: Treatment
strategy according to the status of infection. Ann Surg.
232:619–626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norman JG, Fink G, Franz M, Guffey J,
Carter G, Davison B, Sexton C and Glaccum M: Active interleukin-1
receptor required for maximal progression of acute pancreatitis.
Ann Surg. 223:163–169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felderbauer P, Müller C, Bulut K, Belyaev
O, Schmitz F, Uhl W and Schmidt WE: Pathophysiology and treatment
of acute pancreatitis: New therapeutic targets-a ray of hope? Basic
Clin Pharmacol Toxicol. 97:342–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong W, Dong L, Huang Q, Wu W, Wu J and
Wang Y: Prediction of severe acute pancreatitis using
classification and regression tree analysis. Dig Dis Sci.
56:3664–3671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang
JM, Cai W, Shen HP and Cai J: Influence of dexamethasone on
inflammatory mediators and NF-kappaB expression in multiple organs
of rats with severe acute pancreatitis. World J Gastroenterol.
13:548–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herrera Gutiérrez ME, Seller Pérez G, de
La Rubia De Gracia C, Chaparro Sánchez MJ and Nacle López B: Acute
renal failure profile and prognostic value in severe acute
pancreatitis. Med Clin (Barc). 115:721–725. 2000.In Spanish.
View Article : Google Scholar
|
|
10
|
Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL and
Zhu XZ: Neuroprotective effect of paeoniflorin on cerebral ischemic
rat by activating adenosine A1 receptor in a manner different from
its classical agonists. Br J Pharmacol. 146:604–611. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugishita E, Amagaya S and Ogihara Y:
Studies on the combination of Glycyrrhizae Radix in
Shakuyakukanzo-To. J Pharmacobiodyn. 7:427–435. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu ZY, Xu L, Yan R, Huang Y, Liu G, Zhou
WX and Zhang YX: Advance in studies on effect of paeoniflorin on
nervous system. Zhongguo Zhong Yao Za Zhi. 38:297–301. 2013.In
Chinese. PubMed/NCBI
|
|
13
|
Zhang W and Dai SM: Mechanisms involved in
the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid
arthritis. Int Immunopharmacol. 14:27–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamahara J, Yamada T, Kimura H, Sawada T
and Fujimura H: Biologically active principles of crude drugs. II.
Anti-allergic principles in 'Shoseiryu-To' anti-inflammatory
properties of paeoniflorin and its derivatives. J Pharmacobiodyn.
5:921–929. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang WL, Chen XG, Zhu HB, Gao YB, Tian JW
and Fu FH: Paeoniflorin inhibits systemic inflammation and improves
survival in experimental sepsis. Basic Clin Pharmacol Toxicol.
105:64–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huh JE, Kang KS, Chae C, Kim HM, Ahn KS
and Kim SH: Roles of p38 and JNK mitogen-activated protein kinase
pathways during cantharidin-induced apoptosis in U937 cells.
Biochem Pharmacol. 67:1811–1818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv KY, Yu XY, Bai YS, Zhu SH, Tang HT, Ben
DF, Xiao SC, Wang GY, Ma B and Xia ZF: Role of inhibition of p38
mitogen-activated protein kinase in liver dysfunction after
hemorrhagic shock and resuscitation. J Surg Res. 178:827–832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: Conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
19
|
Schnyder-Candrian S, Quesniaux VF, Di
Padova F, Maillet I, Noulin N, Couillin I, Moser R, Erard F,
Vargaftig BB, Ryffel B and Schnyder B: Dual effects of p38 MAPK on
TNF-dependent bronchoconstriction and TNF-independent neutrophil
recruitment in lipopolysaccharide-induced acute respiratory
distress syndrome. J Immunol. 175:262–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XY, Tang QQ, Zhang JL, Fang MY and Li
YX: Effect of SB203580 on pathologic change of pancreatic tissue
and expression of TNF-α and IL-1β in rats with severe acute
pancreatitis. Eur Rev Med Pharmacol Sci. 18:338–343. 2014.
|
|
21
|
Lv W, Lv C, Yu S, Yang Y, Kong H, Xie J,
Sun H, Andersson R, Xu D, Chen B and Zhou M: Lipoxin A4 attenuation
of endothelial inflammation response mimicking pancreatitis-induced
lung injury. Exp Biol Med (Maywood). 238:1388–1395. 2013.
View Article : Google Scholar
|
|
22
|
Pereda J, Sabater L, Cassinello N,
Gómez-Cambronero L, Closa D, Folch-Puy E, Aparisi L, Calvete J,
Cerdá M, Lledó S, et al: Effect of simultaneous inhibition of
TNF-alpha production and xanthine oxidase in experimental acute
pancreatitis: The role of mitogen activated protein kinases. Ann
Surg. 240:108–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paszkowski AS, Rau B, Mayer JM, Möller P
and Beger HG: Therapeutic application of caspase
1/interleukin-1beta-converting enzyme inhibitor decreases the death
rate in severe acute experimental pancreatitis. Ann Surg.
235:68–76. 2002. View Article : Google Scholar
|
|
24
|
Saitoh M, Hasegawa R and Inoue T: Change
of calibration method for enzyme assay in clinical biochemistry
using automatic analyzer - comparison of calibration methods using
K factor and human standard serum. Eisei Shikenjo Hokoku. 99–101.
1996.In Japanese.
|
|
25
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao K, Chen C, Shi Q, Deng W, Zuo T, He
X, Liu T, Zhao L and Wang W: Inhibition of glycogen synthase
kinase-3β attenuates acute kidney injury in sodium
taurocholateinduced severe acute pancreatitis in rats. Mol Med Rep.
10:3185–3192. 2014.PubMed/NCBI
|
|
28
|
Jin X, Zhang Y, Li X, Zhang J and Xu D:
C-type natriuretic peptide ameliorates ischemia/reperfusion-induced
acute kidney injury by inhibiting apoptosis and oxidative stress in
rats. Life Sci. 117:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Viñas JL, Sola A, Genescà M, Alfaro V, Pí
F and Hotter G: NO and NOS isoforms in the development of apoptosis
in renal ischemia/reperfusion. Free Radic Biol Med. 40:992–1003.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Falschlehner C and Boutros M: Innate
immunity: Regulation of caspases by IAP-dependent ubiquitylation.
EMBO J. 31:2750–2752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kischkel FC, Hellbardt S, Behrmann I,
Germer M, Pawlita M, Krammer PH and Peter ME:
Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a
death-inducing signaling complex (DISC) with the receptor. EMBO J.
14:5579–5588. 1995.
|
|
32
|
Hill MM, Adrain C, Duriez PJ, Creagh EM
and Martin SJ: Analysis of the composition, assembly kinetics and
activity of native Apaf-1 apoptosomes. EMBO J. 23:2134–2145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashemi M: The study of pentoxifylline
drug effects on renal apoptosis and BCL-2 gene expression changes
following ischemic reperfusion injury in rat. Iran J Pharm Res.
13:181–189. 2014.PubMed/NCBI
|
|
34
|
Chen X, Ji B, Han B, Ernst SA, Simeone D
and Logsdon CD: NF-kappaB activation in pancreas induces pancreatic
and systemic inflammatory response. Gastroenterology. 122:448–457.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng Y, Gallagher SF, Haines K, Baksh K
and Murr MM: Nuclear factor-kappaB mediates Kupffer cell apoptosis
through transcriptional activation of Fas/FasL. J Surg Res.
130:58–65. 2006. View Article : Google Scholar
|
|
36
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thompson JE, Phillips RJ,
Erdjument-Bromage H, Tempst P and Ghosh S: I kappa B-beta regulates
the persistent response in a biphasic activation of NF-kappa B.
Cell. 80:573–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fecher LA, Amaravadi RK and Flaherty KT:
The MAPK pathway in melanoma. Curr Opin Oncol. 20:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|