Introduction
Tuberculosis (TB) is a leading cause of morbidity
and mortality worldwide, with 8.7 million new cases and 1.4 million
cases of mortality attributed to TB in 2011 (1). Multi-drug-resistant TB (MDR-TB) has
emerged as a novel challenge, particularly in developing countries.
Approximately 60% of newly occurring MDR-TB cases occur in
Southeast Asia and the Western Pacific region (2). The increased prevalence of MDR-TB is
predominantly associated with nonstandard chemotherapy and a lack
of funding to support the treatment of MDR-TB with second line
anti-TB drugs (3). Another
contributory factor is the long duration of chemotherapy, which may
induce functional impairment of the liver and kidneys, consequently
leading to premature cessation of treatment. China has the second
highest prevalence of TB, according to the fifth national
epidemiological sampling survey of TB in China in 2010 (4). The incidence of active pulmonary TB
was 459 per 100,000 inhabitants in China, and the incidence of
smear-positive or culture-positive pulmonary TB was 66 or 119 per
100,000 inhabitants, respectively (4). TB control presents a major challenge
in China, due to the high incidence of MDR-TB and the lack of
funding for treatment with second-line anti-TB drugs. The treatment
of patients with MDR-TB or serious liver injury is complex;
therefore, a novel approach is required and therapeutic vaccination
may be helpful. Previous studies have demonstrated that DNA
vaccines exert profound therapeutic effects in mice with TB
(5,6). In addition, immunotherapy with
plasmid DNA encoding mycobacterial antigens combined with
conventional chemotherapy presents a more rapidly effective form of
treatment for TB reactivation and reinfection (7,8).
DNA vaccination has been pursued for the treatment
of tuberculosis due to its ability to enhance cellular immune
responses, including T helper (Th) type 1 and cytotoxic T
lymphocyte immune responses, which are essential for the control of
TB (9,10). Lowrie et al (11) demonstrated that DNA vaccines,
initially designed to prevent infection, may also have a pronounced
therapeutic action. In animal models, immunotherapy with plasmid
DNA is a valuable adjunct to anti-TB chemotherapy, which has been
shown to shorten the duration and improve the treatment of latent
TB infection (12). Heat shock
protein 65 DNA is able to switch the immune response from one that
is relatively inefficient and produces bacterial stasis, to one
that kills bacteria in heavily infected mice (6). Ha et al (5) demonstrated that immunotherapy using a
plasmid DNA vaccine encoding mycobacterial antigen 85A (Ag85A),
combined with conventional chemotherapy, was highly effective for
the prevention of Mycobacterium tuberculosis (M. tb)
reactivation and reinfection in mice. Another strong T-cell antigen
of M. tb is 6 kDa early secretory antigenic target (ESAT-6),
which is absent from the current vaccine Bacillus Calmette-Guérin
(BCG) (13), and is considered an
attractive candidate for the development of novel TB vaccines
(14). ESAT-6 is strongly
recognized during the early phase of M. tb infection, as
well as in patients with active TB, and is a potent inducer of
long-lasting memory immunity against TB (14,15).
Both Ag85A and ESAT-6 represent components of TB vaccines that were
tested in early clinical trials (16,17).
To date, ESAT-6 has been used to construct several
types of TB vaccine, including recombinant BCG, DNA vaccines and
subunit vaccines. Lowrie et al (11) reported that bacterial numbers in
the lungs and spleen of mice were decreased, and T-cell profiles
were altered towards a Th1-type dominant response following
treatment with an ESAT-6 DNA vaccine, thus indicating that an ESAT
6-DNA vaccine may exert beneficial effects in mice with TB. Since
the immunotherapeutic effects of single-antigen Ag85A DNA or ESAT-6
DNA vaccines are limited, the use of a DNA plasmid that expresses
both as a chimeric protein is an attractive vaccine strategy
(18). Chimeric DNA vaccines may
exert stronger immunotherapeutic effects and reduce vaccine costs,
as compared with a mixture of two vaccines. Furthermore, a fusion
protein of the closely related Ag85B and ESAT-6 has been shown to
generate good protective immunity in macaques (16). Accordingly, the present study
constructed an Ag85A/ESAT-6 chimeric DNA vaccine, which consisted
of one ag85a gene into which two copies of the esat-6
gene were inserted (19). Our
previous study demonstrated that the immunotherapeutic effects of
an Ag85A/ESAT-6 chimeric DNA vaccine containing one copy of the
esat-6 gene were reduced compared with those of the Ag85A
DNA vaccine in MDR-TB infected mice (20). The present study extended those
observations, and demonstrated that immunotherapy with a chimeric
DNA vaccine containing two copies of the esat-6 gene
unexpectedly enhanced mortality of mice infected with either MDR-TB
or drug sensitive M. tb.
Materials and methods
Plasmid DNA
Plasmid vector pVAX1 DNA, Ag85A DNA, and
Ag85A/ESAT-6 chimeric DNA were purified by Shanghai H&G
Biotechnology Company (Shanghai, China) using the EndoFree plasmid
purification kit (Qiagen, Hilden, Germany).
Mice
A total of 160 pathogen-free female BALB/c mice
(age, 6–8 weeks) were purchased from the Academy of Military
Medical Sciences (Beijing, China). The mice were maintained in a
level-2 negative pressure biosafety biohazard laboratory at the
309th Hospital of Chinese PLA (Beijing, China) with -40 Pa room
pressure, -10 Pa cage pressure, 22±2°C temperature, 55±5% relative
humidity and a 12 h light/dark cycle. The mice were fed a sterile
commercial mouse diet (Beijing Keaoxieli Feed Co., Ltd., Beijing,
China). The study was approved by the Ethics Committee of the 309th
Hospital of the Chinese PLA (Beijing, China).
M. tb strains
M. tb standard strain H37Rv and the clinical
MDR-TB HB361 strain were used to infect the mice. The M. tb
H37Rv strain was provided by National Academy for the Control of
Pharmaceutical and Biological Products (Beijing, China). The HB361
was a clincal strain isolated from a TB patient sputum sample at
the Tuberculosis Department, Thorax Disease Hospital (Shijiazhuang,
China). The status was confirmed by conventional species
identification, and conventional drug susceptibility testing using
the absolute concentration method on Lowenstein-Jensen medium, in
line with the Chinese Laboratory Science Procedure of Diagnostic
Bacteriology in tuberculosis (21). The HB361 strain was resistant to
rifampin (RFP), isoniazid and streptomycin, but was sensitive to
pyrazinamide (PZA).
Generation and treatment of a mouse model
of TB
In the first experiment, in order to evaluate the
adjunctive therapeutic effects of Ag85A/ESAT-6 chimeric DNA, 70
female BALB/c mice (age, 6–8 weeks), were infected intravenously
with MDR-TB HB361 [220,000 colony-forming units (CFU)]. The mice
were randomly divided into seven groups (n=10/group) and were
treated as follows: i) Plasmid vector pVAX1 treatment as a negative
control; ii) RFP (Shenyang Red Flag Pharmacy Co., Ltd., Shenyang,
China) treatment as a negative control; iii) PZA (Chengdu Jinghua
Biological Product Co., Ltd., Chengdu, China) treatment as a
positive control; iv) Ag85A DNA treatment as a positive control; v)
Ag85A/ESAT-6 chimeric DNA treatment; vi) RFP + Ag85A/ESAT-6
chimeric DNA treatment; vii) PZA + Ag85A/ESAT-6 chimeric DNA
treatment. Treatment of the mice began on the 2nd day
post-infection. RFP (0.4 mg/20 g) and PZA (0.6 mg/20 g) were orally
administered every day for 3 months. Ag85A DNA and Ag85A/ESAT-6
chimeric DNA were injected intramuscularly at a dosage of 100
µg in 100 µl saline five times at 2-week
intervals.
In the second experiment, in order to further prove
the therapeutic effects of Ag85A/ESAT-6 chimeric DNA, and to
evaluate the effects of Ag85A/ESAT-6 chimeric protein enhancement,
90 female BALB/c mice (age, 6–8 weeks) were infected intravenously
with M. tuberculosis H37Rv (520,000 CFU). Subsequently, the
mice were randomly divided into five groups (n =16/group) and were
treated as follows: i) Saline treatment as a negative control; ii)
plasmid vector pVAX1 treatment as a negative control; iii)
Mycobacterium vaccae vaccine (Anhui Longcom Biological
Pharmacy Co., Ltd., Anhui, China) treatment as positive control,
iv) Ag85A DNA treatment as a positive control; v) Ag85A/ESAT-6
chimeric DNA plus Ag85A/ESAT-6 chimeric protein enhancement
treatment. Ag85A DNA and Ag85A/ESAT-6 chimeric DNA were purified
using the EndoFree plasmid purification kit (Qiagen) and were
prepared by Shanghai H&G Biotechnology Company (19). Treatment began at the
3rd day post-infection. The Vaccae vaccine (22.5
µg) was injected intramuscularly three times at 2-week
intervals. Ag85A DNA (100 µg) was injected intramuscularly
three times at 2-week intervals. Ag85A/E SAT-6 chimeric DNA (100
µg) was injected intramuscularly three times at 2-week
intervals, and was then boosted once on the 10th day
after the final DNA injection by intraperitoneal injection of 90
µg Ag85A/ESAT-6 chimeric protein.
Bacterial counts
In the first experiment, the mice were sacrificed by
cervical dislocation under anesthetic with 5 ml dethyl ether
(Beijing Chemical Reagents Company, Beijing, China) 3 months
post-infection, whereas in the second experiment 10 mice were
sacrificed on the 3rd day post-infection, prior to
treatment; and 80 mice were sacrificed 7 weeks post-infection. The
lungs and spleens of the mice were collected and homogenized in
saline. The lungs and spleens from mice in the Ag85A/ESAT-6 DNA
group and the lungs from mice in the Ag85A/ESAT-6 chimeric DNA plus
Ag85A/E SAT-6 chimeric protein boost group were harvested from mice
that had succumbed prior to sacrifice (n=10). The tissue suspension
was serially diluted 10-fold in saline, and 0.1 ml tissue dilutions
were plated in duplicate onto Lowenstein-Jensen plates and
incubated at 37°C for 4 weeks. Colonies on each plate were
enumerated and the results were expressed as bacterial
load/organ.
Pulmonary histopathological
examination
The lungs were fixed in 10% (v/v) buffered formalin
for 24 h, dehydrated in ethanol and paraffin-embedded. The
paraffin-embedded tissue sections (3 µm) were prepared and
stained with hematoxylin and eosin. Images were obtained using a
Huahai Pathological Diagnosis System (Xi'an Huahai Medical
Information Technology Co., Ltd., Xi'an, China) and analyzed by a
certified pathologist using a light microscope (BX51; Olympus
Corporation, Tokyo, China).
Statistical analyses
Data are expressed as the mean ± standard deviation.
Statistical significance between the groups was calculated using
one-way analysis of variance followed by Tukey's test, or by
Kruskal-Wallis H test for qualitative data. Statistical analyses
were performed by SAS version 9.2 (SAS Institute, Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Experiment 1
In the first experiment, in which the mice were
infected with the MDR clinical isolate HB361, one mouse succumbed
in the RFP treatment group 84 days post-infection (mortality 10%);
one mouse succumbed 89 days post-infection in the plasmid vector
group (mortality 10%); two mice succumbed 91 days post-infection in
the Ag85A/ESAT-6 DNA + RFP group (mortality 20%); in the
Ag85A/ESAT-6 chimeric DNA group all 10 mice succumbed between 63
and 64 days post-infection (24–48 h after the 5th
Ag85A/ESAT-6 DNA vaccination) (mortality 100%); whereas all of the
mice in the other treatment groups survived (mortality 0%)
(Table I). Mice infected with the
RFP-resistant clinical isolate of M. tb suffered a marked
increase in mortality when treated with the DNA vaccine; however,
this increase was ameliorated if they were also treated with
chemotherapy, not only with PZA (to which the bacteria were
sensitive) but also with RFP (to which the bacteria were clinically
defined as resistant).
 | Table IMortality and time of death of mice
with multi-drug-resistant tuberculosis within 3 months of infection
with the Mycobacterium tuberculosis clinical isolate
HB361. |
Table I
Mortality and time of death of mice
with multi-drug-resistant tuberculosis within 3 months of infection
with the Mycobacterium tuberculosis clinical isolate
HB361.
| Group (n=10) | Mortality (no.
mice) | Mortality (%) | Time of
death
(days post-infection) | P-valuea |
|---|
| Vector | 1 | 10 | 89 | 1.191xE-4 |
| RFP | 1 | 10 | 84 | 1.191xE-4 |
| PZA | 0 | 0 | – | 1.083xE-5 |
| Ag85A DNA | 0 | 0 | – | 1.083xE-5 |
| Ag85A/ESAT-6
chimeric DNA | 10 | 100 | 63 64 | |
| RFP + Ag85A/ESAT-6
chimeric DNA | 2 | 20 | 91 | 7.145xE-4 |
| PZA + Ag85A/ESAT-6
chimeric DNA | 0 | 0 | – | 1.083xE-5 |
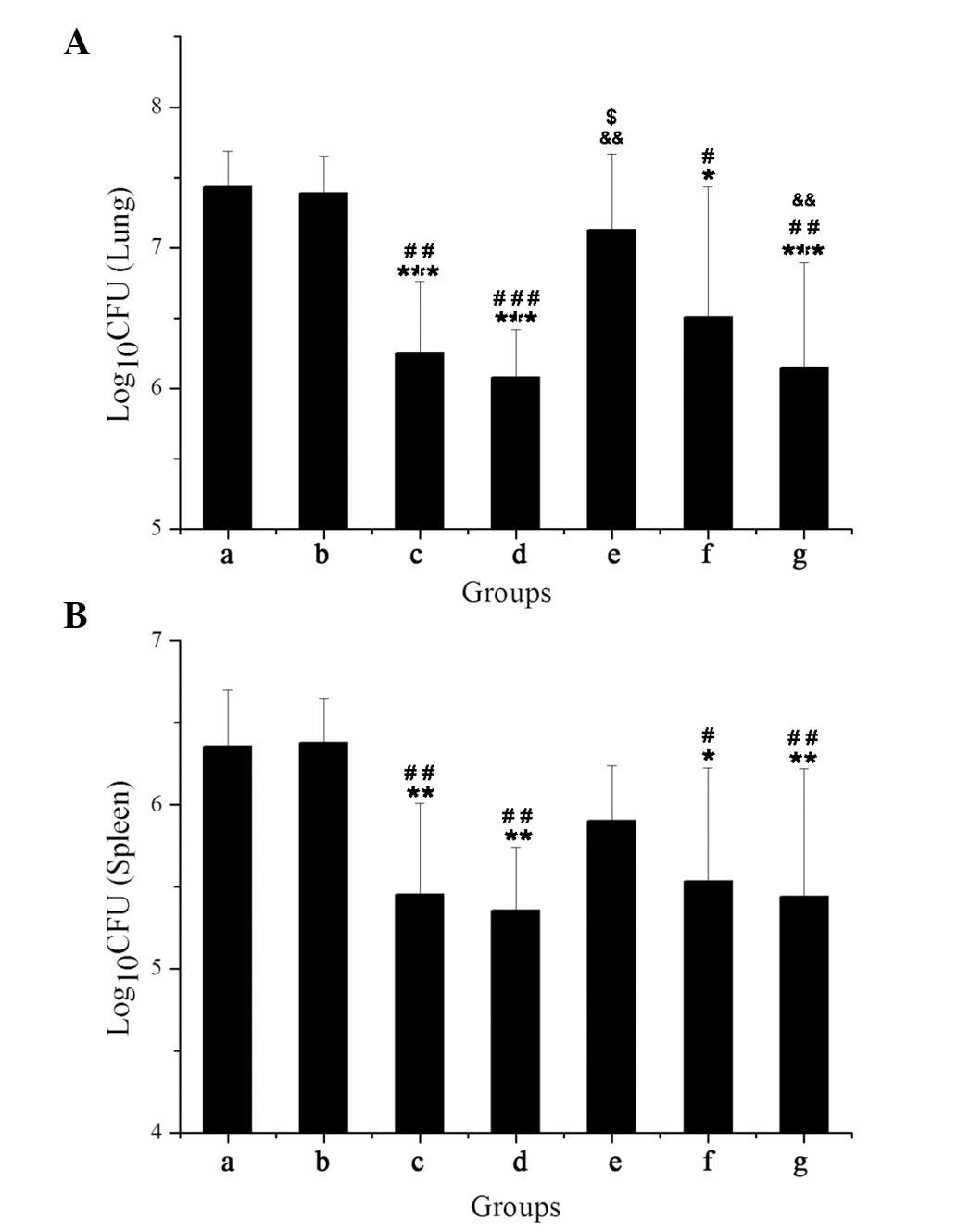
A total of 3 months post-infection with HB361,
substantial bacterial loads were present in the lungs and spleens
of the control mice and the mice treated with RFP, as well as in
the lungs and spleens of the mice that succumbed following
treatment with Ag85A/ESAT-6 chimeric DNA (Fig. 1). Bacterial loads were ~10-fold
lower in the tissues from mice treated with PZA, Ag85A DNA, or
Ag85A/ESAT-6 chimeric DNA plus either PZA or RFP.
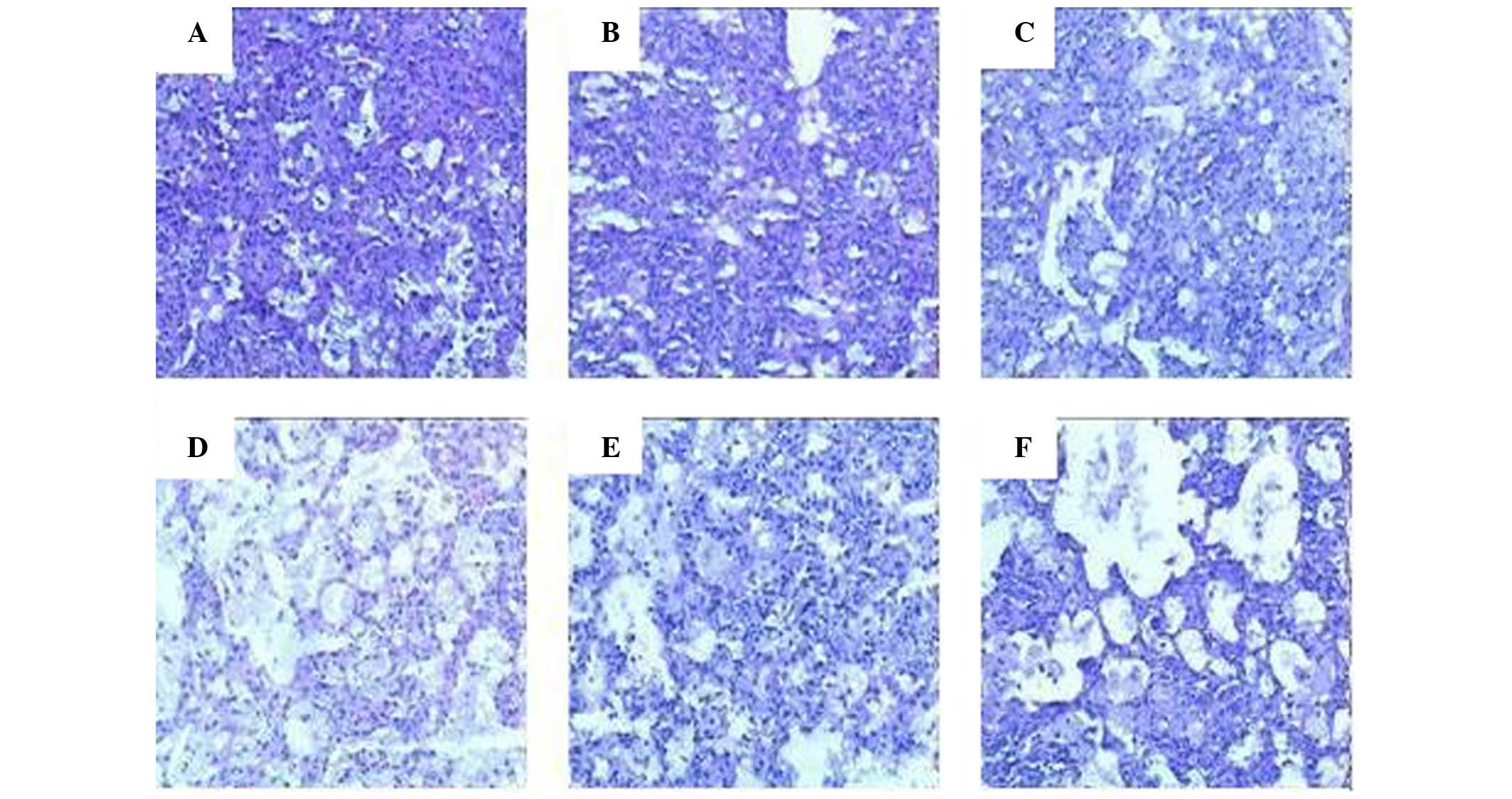
Microscopic examination of the lung sections from
mice in the plasmid vector and RFP-treated groups detected little
alteration from the expected histopathological characteristics of
murine tuberculosis. Mice in the other groups exhibited more foam
cells and multi-nucleated giant cells, but fewer lymphocytes in the
lung sections, and the alveolar profiles detected relatively clear
and normal structures. The percentage of the total section area
that was deemed pathological was 100, 90, 65–75, 30–40, 50–60 and
50–60% in the plasmid vector group, RFP group, PZA group, Ag85A DNA
group, RFP + Ag85A/ESAT-6 chimeric DNA group and PZA + Ag85A/ESAT-6
chimeric DNA group, respectively. Representative histopathological
images of each group are presented in Fig. 2.
Experiment 2
In the second experiment, in which mice were
infected with M. tb strain H37Rv, three mice succumbed
between 12 and 14 days post-infection in the saline group
(mortality 19%); one mouse succumbed 14 days post-infection in the
plasmid vector group (mortality 6%); one mouse succumbed 40 days
post-infection in the Vaccae vaccine group (mortality 6%);
in the Ag85A/ESAT-6 chimeric DNA vaccine plus Ag85A/ESAT-6 chimeric
protein boost group, 13 of the 16 mice succumbed 40 days
post-infection (48 h after Ag85A/ESAT-6 protein boosting; mortality
81%); and all of the mice in the Ag85A DNA group survived
(mortality 0%) (Table II).
 | Table IIMortality and time of death of mice
with tuberculosis within 7 weeks of infection with Mycobaterium
tuberculosis H37Rv. |
Table II
Mortality and time of death of mice
with tuberculosis within 7 weeks of infection with Mycobaterium
tuberculosis H37Rv.
| Group (n=10) | Mortality (no.
mice) | Mortality (%) | Time of
death
(days post-infection) | P-valuea |
|---|
| Saline | 3 | 19 | 12 14 | 1.10xE-3 |
| Vector | 1 | 6 | 14 | 3.852xE-5 |
| Vaccae | 1 | 6 | 40b | 3.852xE-5 |
| Ag85A DNA | 0 | 0 | | 3.224xE-6 |
| Ag85A/ESAT-6
chimeric | 13 | 81 | 40 | |
| DNA + Ag85A/ESAT-6
chimeric protein boost | | | | |
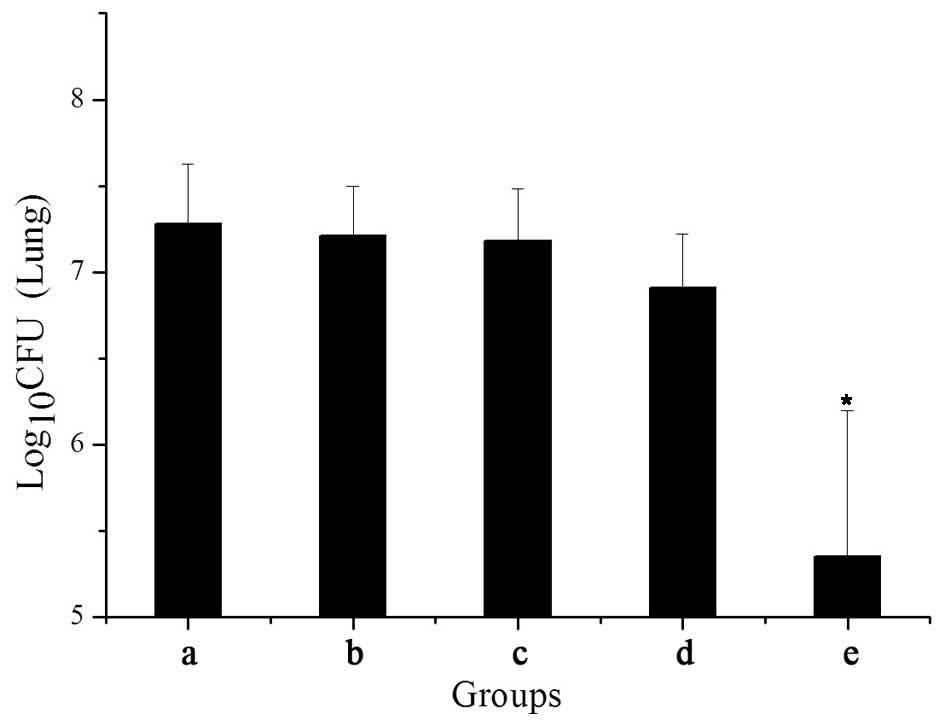
The log10 bacterial load in the lungs was
determined at 3 days (data not shown) or 7 weeks post-infection
(Fig. 3). At 3 days post-infection
with H37Rv (prior to treatment), the log10 bacterial
load in the lungs from 10 mice was 7.04. At 7 weeks post-infection,
compared with the CFU in the control groups treated with saline
(7.31) or empty DNA vaccine vector (7.20), treatment with
Vaccae had no effect on CFU (7.21). The load was slightly
but significantly lower (6.93; P<0.05) following treatment with
Ag85A DNA, and was almost 2 log10 lower in the mice that
succumbed following treatment with Ag85A/ESAT-6 chimeric DNA plus
Ag85A/ESAT-6 chimeric protein boost (5.37 log10; P<
0. 0 01).
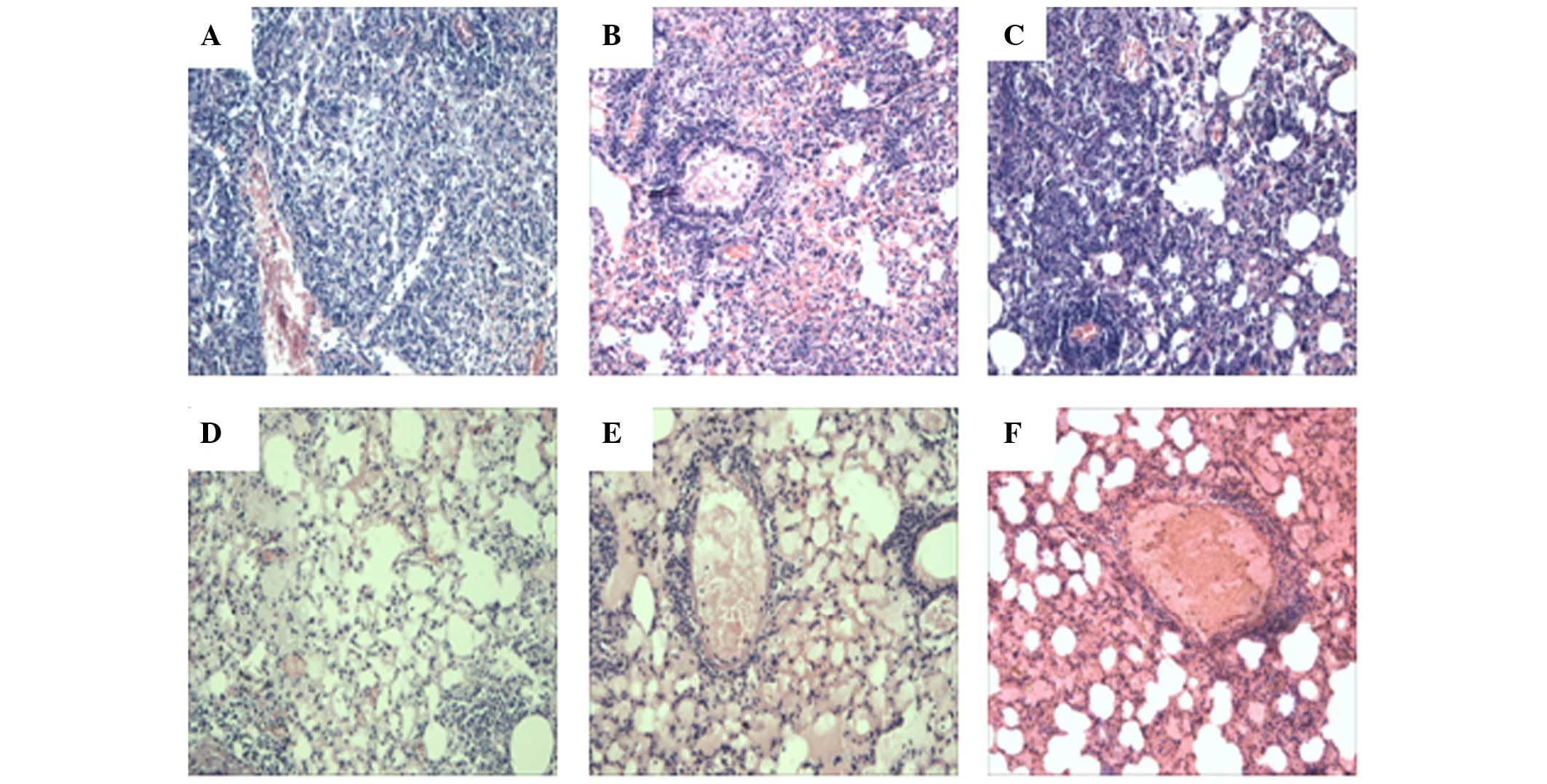
Histopathological examination of the lungs
demonstrated that in general the vaccinated groups exhibited
reduced pathology compared with in the control groups receiving
saline or plasmid vector (Fig. 4).
At 3 days post-infection, a small scope of consolidation in the
local area, scattered small lung lesions, transudate, and
hyperaemia in the alveoli were observed (data not shown). At 7
weeks post-infection, more lymphocytes, extensive lung lesions,
hyperaemia in the alveoli and damaged structures were observed in
the lung sections of the mice in the saline and vector groups. In
the Vaccae vaccine group lung lesions were limited, and
moderate lymphocyte infiltration and relatively clear and normal
structures were observed. In the Ag85A DNA group, lesions were
slight with few lymphocytes, and the alveoli exhibited relatively
clear and normal structures. Mice in the Ag85A/ESAT-6 chimeric DNA
plus Ag85A/ESAT-6 chimeric protein boost group (both alive and
dead) all exhibited reduced lesions; however, more transudatory
proteins and hyperaemia were detected in the alveoli, and dilated
capillary vessels and more lymphocytes surrounding the capillary
vessels were observed.
Discussion
The present study determined whether immunotherapy
with an Ag85A/ESAT-6 chimeric DNA vaccine combined with
conventional chemotherapy was more effective for the treatment of
TB or MDR-TB in mice, compared with treatment with a single Ag85A
DNA vaccine.
The immunotherapeutic effects of DNA vaccines
expressing Ag85A have been reported in murine TB (5–7), and
have been shown to exert action against MDR-TB (20). In addition, ESAT-6 DNA vaccines
have been reported to have immunotherapeutic and immune protective
effects in mice infected with TB (11,12,22,23).
However, the present study did not detect an enhanced protective
effect of a DNA vaccine expressing the fusion protein. It was
confirmed that repeated dosing with Ag85A DNA exerted therapeutic
effects against H37Rv infection, reducing both CFU and pathology.
Conversely, the disease worsened following vaccination with
Ag85A/ESAT-6 DNA in mice infected with either H37Rv or HB361.
Repeated dosing with the plasmid accelerated mortality without
having a marked effect on HB361 CFU; however, some granuloma
formation was replaced with vasculitis and edema (experiment 1).
Similarly, boosting immunity using the fusion protein following
Ag85A/ESAT-6 DNA vaccination (experiment 2) accelerated the lethal
outcome of infection with H37Rv, thus suggesting that the
phenomenon was protein-driven and was not dependent upon the strain
of M. tb. In addition, granulomas were not a prominent
feature of the histopathology; however, vasculitis, hyperaemia and
edema appeared likely to contribute toward mortality. Reduced
bacterial load was detected in the dead mice, thus suggesting that
there may have been a bacterial killing component to the immune
response, however this was not investigated.
The harmful effect of the vaccine in HB361-infected
mice was much reduced when the vaccine was given as an adjunct to
treatment with PZA, a chemotherapeutic agent to which the bacterium
was susceptible. This is consistent with an immunopathology that
depends upon antigen load, which was reduced by the drug. Notably,
however, the harmful effects were also slightly reduced when the
vaccine was administered alongside RFP; mortality, pathology and
CFU were all reduced, even though the bacteria were clinically
defined as resistant to RFP and monotherapy with RFP was
ineffective. Whether this finding was due to a direct modulation of
the immune response by RFP, as observed elsewhere (24,25),
or due to a greater susceptibility of the bacteria to the drug
in vivo, as implied by other studies (26) is currently unknown.
The present study hypothesized that the harmful
effects of DNA expressing the chimeric Ag85A/ESAT-6 antigen in
TB-infected mice may have been a consequence of the development of
hypersensitivity. Histopathological analysis detected slight
lesions, increased transudate and hyperaemia in alveoli, dilated
capillary vessels, and increased lymphocytes surrounding the
capillary vessels in both experiments. This was the case whether
the mice had naturally succumbed or were sacrificed following
Ag85A/ESAT-6 treatment.
Previous studies, which used the ESAT-6 DNA vaccine
or Ag85B/ESAT-6 fusion protein vaccine (as an adjunct to IC31) as
preventative vaccines in mice or humans, or as therapeutic vaccines
in mice (11,12,16,20,27),
did not detect any adverse reaction. However, in the present study,
inserting two esat-6 genes within the ag85a gene
created a novel entity capable of converting a partially protective
immune response into a harmful immune response. The underlying
mechanisms have not yet been explored, but may reflect the
increased expression levels of ESAT-6 relative to Ag85A. ESAT-6 is
not a biologically inert protein antigen, and has been proven to
act as a virulence factor. ESAT-6 can cause lysis of red blood
cells and macrophages by membrane pore formation (28). In addition, it can cause disruption
of membrane conductance, destruction of artificial planar bilayers
(28), formation of membrane
pores, and induction of apoptosis in macrophages by binding to
laminin and activating caspase expression (29,30).
Therefore, ESAT-6 may act as a cytolytic pore-forming toxin,
inducing lysis of red blood cells, macrophages and lung epithelial
cells, and this may be considered the primary mechanism underlying
the hypersensitivity reaction detected in the present study.
In conclusion, in the context of therapeutic
application against TB in mice, relatively high expression of
ESAT-6 antigen via multiple immunizations with Ag85A/ESAT-6
chimeric DNA induced a hypersensitivity reaction and accelerated
mortality of the mice. These results suggested that ESAT-6 may not
be suitable for use in immunotherapeutic vaccines against TB.
Acknowledgments
The present study was supported by grants from the
Serious Infectious Diseases Special Foundation of China (grant nos.
2008ZX10003013-2 and 2012ZX10003008002), the WHO IVR Steering
Committee (grant no. V25-181-202) and the National Nature and
Science Foundation of China (grant no. 30070730). The study has
been presented as part of a poster at the 3rd International
Conference on Vaccines and Vaccination.
Abbreviations:
|
MDR-TB
|
multi-drug-resistant tuberculosis
|
|
TB
|
tuberculosis
|
|
M. tb
|
Mycobacterium tuberculosis
|
|
Th
|
T helper
|
|
CFU
|
colony-forming units
|
|
RFP
|
rifampin
|
|
PZA
|
pyrazinamide
|
References
|
1
|
Glaziou P, Falzon D, Floyd K and
Raviglione M: Global epidemiology of tuberculosis. Semin Respir
Crit Care Med. 34:3–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
WHO/IUATLD Global Project on
Anti-Tuberculosis Drug Resistance Surveillance: Anti-tuberculosis
drug resistance in the world. (Third global report/the WHO/IUATLD
Global Project on Anti-Tuberculosis Drug Resistance Surveillance).
1999–2002
|
|
3
|
Mori T: MDR-TB - its characteristics and
control in Asia-Pacific rim symposium in USJCMSP 10th international
conference on emerging infectious diseases in the Pacific rim.
Tuberculosis (Edinb). 87(Suppl 1): S5–S9. 2007. View Article : Google Scholar
|
|
4
|
The Office of the Fifth National TB
Epidemiological Survey, Technical Guidance Group of the Fifth
National TB Epidemiological Survey: The Fifth National Tuberculosis
Epidemiological Survey in 2010. Chin J Antitubere. 34:485–508.
2010, 2012.
|
|
5
|
Ha SJ, Jeon BY, Youn JI, Kim SC, Cho SN
and Sung YC: Protective effect of DNA vaccine during chemotherapy
on reactivation and reinfection of Mycobacterium tuberculosis. Gene
Ther. 12:634–638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silva CL, Bonato VL, Coelho-Castelo AA, De
Souza AO, Santos SA, Lima KM, Faccioli LH and Rodrigues JM:
Immunotherapy with plasmid DNA encoding mycobacterial hsp65 in
association with chemotherapy is a more rapid and efficient form of
treatment for tuberculosis in mice. Gene Ther. 12:281–287. 2005.
View Article : Google Scholar
|
|
7
|
Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK,
Sung YC and Cho SN: Therapeutic effect of DNA vaccines combined
with chemotherapy in a latent infection model after aerosol
infection of mice with Mycobacterium tuberculosis. Gene Ther.
10:1592–1599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu D, Jiang S and Luo X: Therapeutic
effects of Ag85B and MPT64 DNA vaccines in a murine model of
Mycobacterium tuberculosis. Vaccine. 23:4619–4624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper AM, Dalton DK, Stewart TA, Griffin
JP, Russell DG and Orme IM: Disseminated tuberculosis in interferon
gamma gene-disrupted mice. J Exp Med. 178:2243–2247. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flynn JL, Chan J, Triebold KJ, Dalton DK,
Stewart TA and Bloom BR: An essential role for interferon gamma in
resistance to Mycobacterium tuberculosis infection. J Exp Med.
178:2249–2254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lowrie DB, Tascon RE, Bonato VL, Lima VM,
Faccioli LH, Stavropoulos E, Colston MJ, Hewinson RG, Moelling K
and Silva CL: Therapy of tuberculosis in mice by DNA vaccination.
Nature. 400:269–271. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan X, Gao Q and Fu R: DNA vaccine
encoding ESAT-6 enhances the protective efficacy of BCG against
Mycobacterium tuberculosis infection in mice. Scand J Immunol.
66:523–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harboe M, Oettinger T, Wiker HG,
Rosenkrands I and Andersen P: Evidence for occurrence of the ESAT-6
protein in Mycobacterium tuberculosis and virulent Mycobacterium
bovis and for its absence in Mycobacterium bovis BCG. Infect Immun.
64:16–22. 1996.PubMed/NCBI
|
|
14
|
Brandt L, Oettinger T, Holm A, Andersen AB
and Andersen P: Key epitopes on the ESAT-6 antigen recognized in
mice during the recall of protective immunity to Mycobacterium
tuberculosis. J Immunol. 157:3527–3533. 1996.PubMed/NCBI
|
|
15
|
Andersen P and Heron I: Specificity of a
protective memory immune response against Mycobacterium
tuberculosis. Infect Immun. 61:844–851. 1993.PubMed/NCBI
|
|
16
|
Langermans JA, Doherty TM, Vervenne RA,
van der Laan T, Lyashchenko K, Greenwald R, Agger EM, Aagaard C,
Weiler H, van Soolingen D, et al: Protection of macaques against
Mycobacterium tuberculosis infection by a subunit vaccine based on
a fusion protein of antigen 85B and ESAT-6. Vaccine. 23:2740–2750.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pathan AA, Minassian AM, Sander CR,
Rowland R, Porter DW, Poulton ID, Hill AV, Fletcher HA and McShane
H: Effect of vaccine dose on the safety and immunogenicity of a
candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine.
30:5616–5624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bryder K, Sbai H, Nielsen HV, Corbet S,
Nielsen C, Whalen RG and Fomsgaard A: Improved immunogenicity of
HIV-1 epitopes in HBsAg chimeric DNA vaccine plasmids by structural
mutations of HbsAg. DNA Cell Biol. 18:219–225. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Song D, Zhang H, He W, Fan X, Zhang
Y, Huang J, Wang X, Liu Q and Xiong S: Improved humoral immunity
against tuberculosis ESAT-6 antigen by chimeric DNA prime and
protein boost strategy. DNA Cell Biol. 25:25–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang Y, Wu X, Zhang J, Li N, Yu Q, Yang
Y, Bai X, Liu C, Shi Y, Liu Q, et al: The treatment of mice
infected with multi-drug-resistant Mycobacterium tuberculosis using
DNA vaccines or in combination with rifampin. Vaccine.
26:4536–4540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinese Antituberculosis Association:
Chinese laboratory science procedure of diagnostic bacteriology in
tuberculosis. Wang SM: China Education Culture Press; Beijing: pp.
46–61. 2006
|
|
22
|
Skjøt RL, Oettinger T, Rosenkrands I, Ravn
P, Brock I, Jacobsen S and Andersen P: Comparative evaluation of
low-molecular-mass proteins from Mycobacterium tuberculosis
identifies members of the ESAT-6 family as immunodominant T-cell
antigens. Infect Immun. 68:214–220. 2000. View Article : Google Scholar
|
|
23
|
Wang QM, Sun SH, Hu ZL, Yin M, Xiao CJ and
Zhang JC: Improved immunogenicity of a tuberculosis DNA vaccine
encoding ESAT6 by DNA priming and protein boosting. Vaccine.
22:3622–3627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Humber DP, Nsanzumuhire H, Aluoch JA,
Webster AD, Aber VR, Mitchison DA, Girling DJ and Nunn AJ:
Controlled double-blind study of the effect of rifampin on humoral
and cellular immune responses in patients with pulmonary
tuberculosis and tuberculosis contacts. Am Rev Respir Dis.
122:425–436. 1980.PubMed/NCBI
|
|
25
|
Ziglam HM, Daniels I and Finch RG:
Immunomodulating activity of rifampicin. J Chemother. 16:357–361.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chanwong S, Maneekarn N, Makonkawkeyoon L
and Makonkawkeyoon S: Intracellular growth and drug susceptibility
of Mycobacterium tuberculosis in macrophages. Tuberculosis.
87:130–133. 2007. View Article : Google Scholar
|
|
27
|
van Dissel JT, Arend SM, Prins C, Bang P,
Tingskov PN, Lingnau K, Nouta J, Klein MR, Rosenkrands I, Ottenhoff
TH, et al: Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and
long-lived Mycobacterium tuberculosis specific T cell responses in
naïve human volunteers. Vaccine. 28:3571–3581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith J, Manoranjan J, Pan M, Bohsali A,
Xu J, Liu J, McDonald KL, Szyk A, LaRonde-LeBlanc N and Gao LY:
Evidence for pore formation in host cell membranes by
ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape
from the vacuole. Infect Immun. 76:5478–5487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Derrick SC and Morris SL: The ESAT6
protein of Mycobacterium tuberculosis induces apoptosis of
macrophages by activating caspase expression. Cell Microbiol.
9:1547–1555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Wel N, Hava D, Houben D, Fluitsma
D, van Zon M, Pierson J, Brenner M and Peters PJ: M. tuberculosis
and M. leprae translocate from the phagolysosome to the cytosol in
myeloid cells. Cell. 129:1287–1298. 2007. View Article : Google Scholar : PubMed/NCBI
|