Introduction
Inflammatory bowel diseases (IBDs) are idiopathic
inflammatory gastrointestinal (GI) disorders, which consist of
Crohn's disease (CD) and ulcerative colitis (UC). It is currently
accepted that alterations in the gut microbiota, genetic
susceptibility, dysfunction of immunological regulation and defects
in the intestinal barrier are involved in the etiopathogenesis of
IBD (1,2). In general, UC is solely restricted to
the colon, whereas CD may affect the entire GI tract. Abdominal
pain, weight loss and bloody diarrhea are the most common symptoms
of IBD (3). IBDs are lifelong
diseases, for which patients often require lifelong pharmacological
treatment and may require surgical interventions.
The conventional medications used for the treatment
of IBD include corticosteroids, immunosuppressive drugs,
antibiotics and biological agents, including anti-tumor necrosis
factor-α. However, the use of these drugs is only effective for
temporary symptomatic relief, and is associated with serious
complications and toxic side effects (3–5).
Thus, the use of complementary and alternative medicine (CAM),
including acupuncture, homeopathy and herbal medicines, for the
treatment of patients with IBD is increasing worldwide, and the
percentage of patients with IBD using CAM in North America and
Europe has been estimated at up to 70% (6–9).
Traditional Chinese medicine (TCM) is one of the most developed
branches of CAM in the world (10). Several scientific reports and
clinical studies have characterized the effectiveness of herbal
preparations in IBD, showing promising results and providing an
insight into the mechanisms, which are beyond their therapeutic
activity (11–14).
Modified Pulsatilla decoction, containing seven
commonly used plants (Radix Pulsatillae, Cortex Phellodendri,
Rhizoma Coptidis, Cortex Fraxini, Sanchi, Radix Paeoniae Rubra and
Radix Glycyrrhizae), is formulated using the TCM formulation of
Pulsatilla decoction, and adding another three herbal plants
(Sanchi, Radix Paeoniae Rubra and Radix Glycyrrhizae). Pulsatilla
decoction possesses a variety of pharmacological effects, and the
active ingredients from these herbal plants have shown hepatic
protective, anti-inflammatory, antibacterial, antitumor and
anti-oxidant effects (15–19). Modified Pulsatilla decoction has
been used previously to treat UC in clinical practice in China,
showing promising effects against inflammation and diarrhea
(20). Although modified
Pulsatilla decoction is an effective TCM formula for the
pharmacological effects indicated above, the possible mechanism in
the treatment of UC remains to be elucidated. Thus, the present
study aimed to investigate the therapeutic efficacy of modified
Pulsatilla decoction against IBD and to elucidate the underlying
molecular mechanisms.
Materials and methods
Induction of colitis
A total of 40 male BALB/c mice (age, 6 weeks;
weight, 21–24 g) were obtained from Shanghai Laboratory Animal
Center (Shanghai, China) and housed in a specific-pathogen-free
environment under normal conditions of humidity (50±5%) and
temperature (25±2°C), with a 12-h light/dark cycle and access to
food and water ad libitum. The mice were randomly divided
into 4 groups of 10, the control, PBS, modified Pulsatilla and
sulfasalazine (SASP; Pfizer, Inc., New York, NY, USA). Colitis was
induced, as previously described, with modifications (21). Briefly, a 2×2 cm field of the
abdominal skin was shaved, and 200 µl 3% oxazolone
(Sigma-Aldrich, St. Louis, MO, USA) in 100% ethanol was applied to
pre-sensitize the BALB/c mice, and this was repeated the following
day. Subsequently, a solution of oxazolone (150 µl; 1%
oxazolone dissolved in 50% ethanol) was administered into the colon
via the anus using a 3.5-F polyurethane catheter. Following
injection of the oxazolone solution, the catheter was removed and
the mouse was held vertically for 60 sec. In the control group, the
same volume of ethanol was injected, instead of the oxazolone
solution. Following treatment, all mice were fed a standard chow
diet and tap water ad libitum. The present study was
approved by the ethics committee of Shanghai University of
Traditional Chinese Medicine (Shanghai, China) and performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (22).
Preparation and intragastric
administration of water-soluble extracts of modified Pulsatilla
decoction
The modified Pulsatilla decoction consisted of Radix
Pulsatillae (30 g), Cortex Phellodendri (12 g), Rhizoma Coptidis (5
g), Cortex Fraxini (9 g), Sanchi (3 g), Radix Paeoniae Rubra (12 g)
and Radix Glycyrrhizae (6 g). To obtain the water-soluble extracts,
10-fold higher volumes of water was added to these herbal plants,
and the resulting mixtures were then heated at 90°C for 1 h. This
procedure was repeated three times, following which all the
extraction liquids were collected, dried and stored at 4°C.
The extracts of the decoction (10 mg/g body weight)
or SASP (20 mg/g body weight) were administered intragastrically to
the oxazolone-induced colitis mice once a day for 7 days. SASP
acted as a positive control. As a negative control, an equal volume
of water was administered to the oxazolone-induced colitis mice.
The PBS group were oxazolone-induced colitis mice administered ~1
ml PBS.
Assessment of clinical colitis in
mice
The seventy of colitis was assessed using a disease
activity index (DAI) scoring system and loss in body weight. The
DAI score was calculated as the sum of the bloody stool score (0,
normal colored stool; 1, brown stool; 2. reddish stool; 3, bloody
stool) and diarrheal stool score (0, normal stool; 1, mildly soft
stool; 2, very soft stool; 3, watery stool). The body weight change
in each mouse was calculated using the following formula: Weight
loss (%) = [(weight on day 1) − (weight each day)] / weight on day
1.
Histological examination of colon
After 7 days, the mice were sacrificed using 0.1 ml
2% sodium pentobarbital and a 10 cm portion of the distal colon was
removed from 4 mice in each group, washed with saline and fixed in
10% natural buffered formalin. The fixed tissues were then embedded
in paraffin (Sigma-Aldrich), cut into tissue sections (5 µm
thick) and stained with hematoxylin and eosin (Leica Microsystems,
Ltd., Milton Keynes, UK) and examined with a microscope (Leica
Microsystems, Ltd.) and qualitatively evaluated with Leica QWin
software 3.1 using the following parameters: Transmural
infiltrating of mononuclear cells, thickening of the colon wall,
mucosal hyperplasia, erosions, depletion of goblet cells and crypt
architectural distortion.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Frozen colon samples were homogenized in 1 ml cold
buffer containing 0.1 M phosphate buffer (pH 7.4) and 1% protease
inhibitor cocktail (Sigma-Aldrich). The homogenized samples were
centrifuged at 12,000 × g for 15 min at 4°C and the supernatants
were collected and stored at −80°C. Total RNA was isolated from the
colon tissues using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1.5 µg RNA was
reverse transcribed into cDNA using a GoScript™ Reverse
Transcription system (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. The RT-qPCR analysis was
performed to determine the expression levels of interleukin (IL)-5
and IL-13 by using SYBR green PCR master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The primers for IL-5 were: Sense
5′-AAG GAT GCT TCT GCA CTTGA-3′ and antisense
5′-GGAAGCCTCATCGTCTCATT-3′; the primers for IL-13 were: Sense
5′-AGCATGGTATGGAGTGTGGA-3′ and antisense
5′-TTGCAATTGGAGATGTTGGT-3′; and the primers for GAPDH were: Sense
5′-AGGTCGGTGTGAACGGATTTG-3′ and antisense
5′-TGTAGACCATGTAGTTGAGGTCA-3′. The PCR reaction was performed on an
iCycler Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with thermocycling conditions as follows: 95°C
for 5 min; 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C
for 20 sec. The fluorescence intensity was determined by the CFX96
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.) The
relative expression ratio of cytokines was calculated using the
2−ΔΔCq method (23).
ELISA
The protein levels of IL-5 and IL-13 in the
supernatants of the colon tissue lysates were measured using Mouse
IL-13 Quantikine ELISA kit and Mouse IL-5 Quantikine ELISA kit
(R&D Systems, Minneapolis, MN, USA), according to the
manufacturer's protocol. All samples were analyzed in duplicate and
normalized to the total protein content of the sample, expressed as
pg/mg protein.
Western blot analysis
Frozen colonic tissues were mechanically homogenized
(20% w/v) in lysis buffer (pH 7.4) containing 0.1 mM EDTA, 20 mM
HEPES, 12.5 mM MgCl2, 150 mM 0.1% Nonidet P40, NaCl, 1
mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, and 1
µg/ml leupeptin, aprotinin, and pepstatin. Homogenates were
sonicated and then centrifuged for 10 min and 12,000 × g. Protein
concentration was assessed using the Bradford protein assay.
Equivalent quantities (20 µg) of protein from the cells
lysates were loaded onto SDS-PAGE gels (Beyotime Institute of
Biotechnology, Haimen, China) and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% non-fat milk in Tris-buffered
saline, and then incubated with primary antibodies overnight at
4°C, followed by incubation at room temperature for 2 h with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(Beyotime Institute of Biotechnology; cat. no. A0208; dilution,
1:5,000). Signals were detected using enhanced chemiluminesecence
reagent (EMD Millipore). rabbit polyclonal ZO-1 (cat. no. 40-2200;
dilution, 1:1,000), rabbit polyclonal claudin-2 (cat. no.
PA5-13335; dilution, 1:1,000) and rabbit monoclonal occludin
antibodies (cat. no. 710192; dilution, 1:1,000) were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.), rabbit monoclonal
inhibitor of NF-κB-α (IκB-α) antibody was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA; cat. no. 4812;
dilution, 1:1,000) and the rabbit polyclonal GAPDH antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA;
cat. no. sc-25778; dilution, 1:1,000).
Data analysis
Results are presented as the mean ± standard
deviation. All data presented are representative of three or more
experiments, each with similar results. Statistical analysis was
conducted using SPSS 19.0 (IBM SPSS, Armonk, NY, USA) and
significance was determined using Student's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Modified Pulsatilla decoction treatment
attenuates the severity of oxazolone-induced colitis in mice
To investigate the therapeutic effect of modified
Pulsatilla decoction on IBD, the present study induced experimental
colitis in mice via skin pre-sensitization and colonic
administration of oxazolone. The results demonstrated that the mice
injected with oxazolone developed experimental colitis with severe
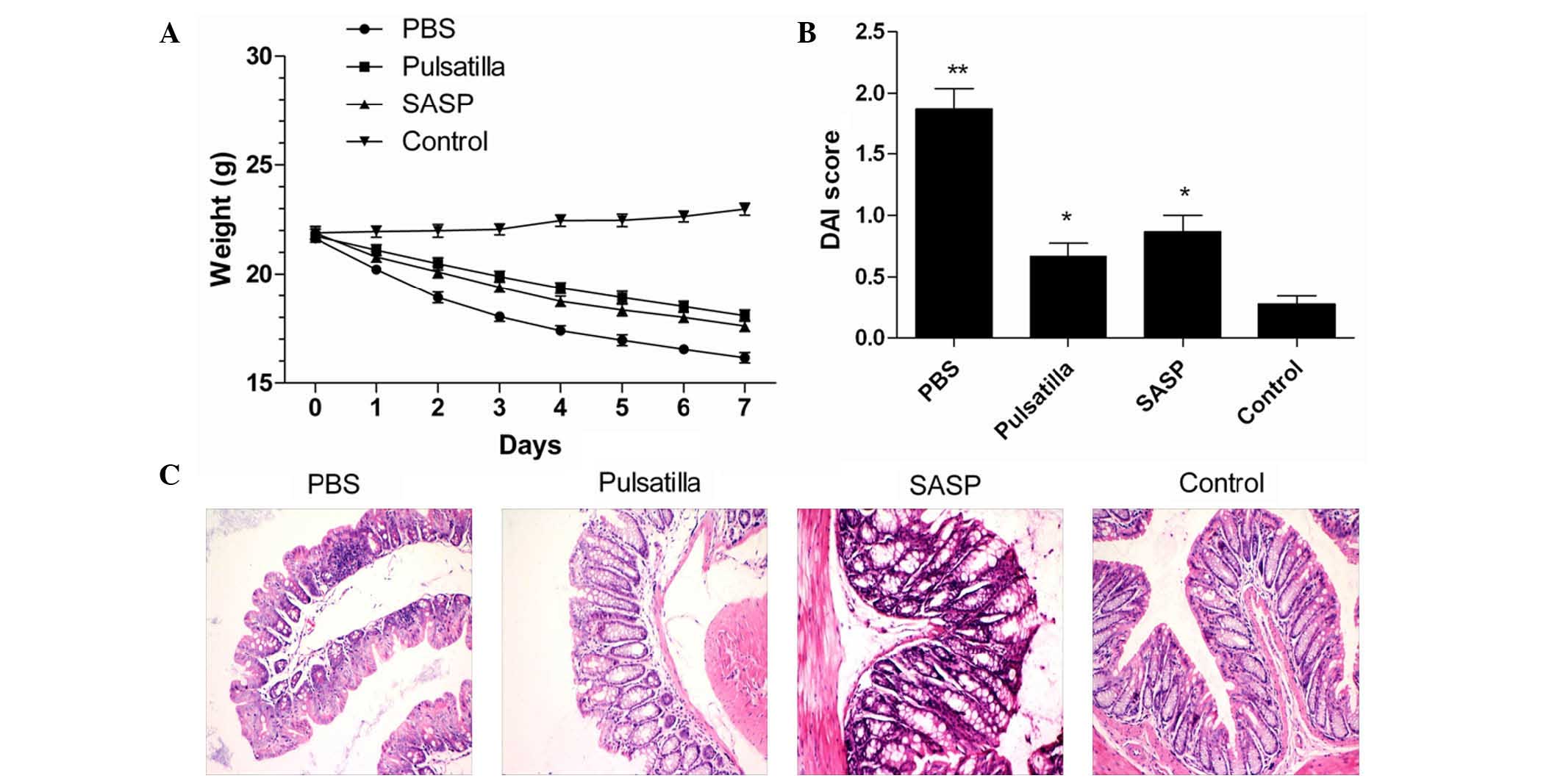
weight loss (Fig. 1A), severe
hunching, lethargy, piloerection and diarrhea, and showed a
significantly increased DAI score, compared with the normal group
(P<0.01). Although there were no differences between the
Pulsatilla decoction-treated mice and the SASP-treated mice, oral
administration of modified Pulsatilla decoction and SASP
significantly attenuated the severity of oxazolone-induced colitis,
which showed decreased DAI scores, compared with the untreated
group (P<0.01; Fig. 1B).
The severity of the histological changes in the
colon were then qualitatively evaluated in the mice treated with or
without modified Pulsatilla decoction or SASP. As shown in Fig. 1C, the colonic histological changes
were characterized by transmural infiltrating of mononuclear cells,
thickening of the colon wall, mucosal hyperplasia, erosions,
depletion of goblet cells and crypt architectural distortion.
Compared with the modified Pulsatilla decoction-treated mice and
SASP-treated mice, the severity of damage was more marked in the
untreated mice. However, the colonic tissues in the Pulsatilla
decoction-treated and SASP-treated mice showed marked improvements
in inflammation. Together, these findings indicated that the
modified Pulsatilla decoction treatment attenuated the severity of
oxazolone-induced colitis in the mice.
Modified Pulsatilla decoction treatment
significantly reduces the secretion of pro-inflammatory
cytokines
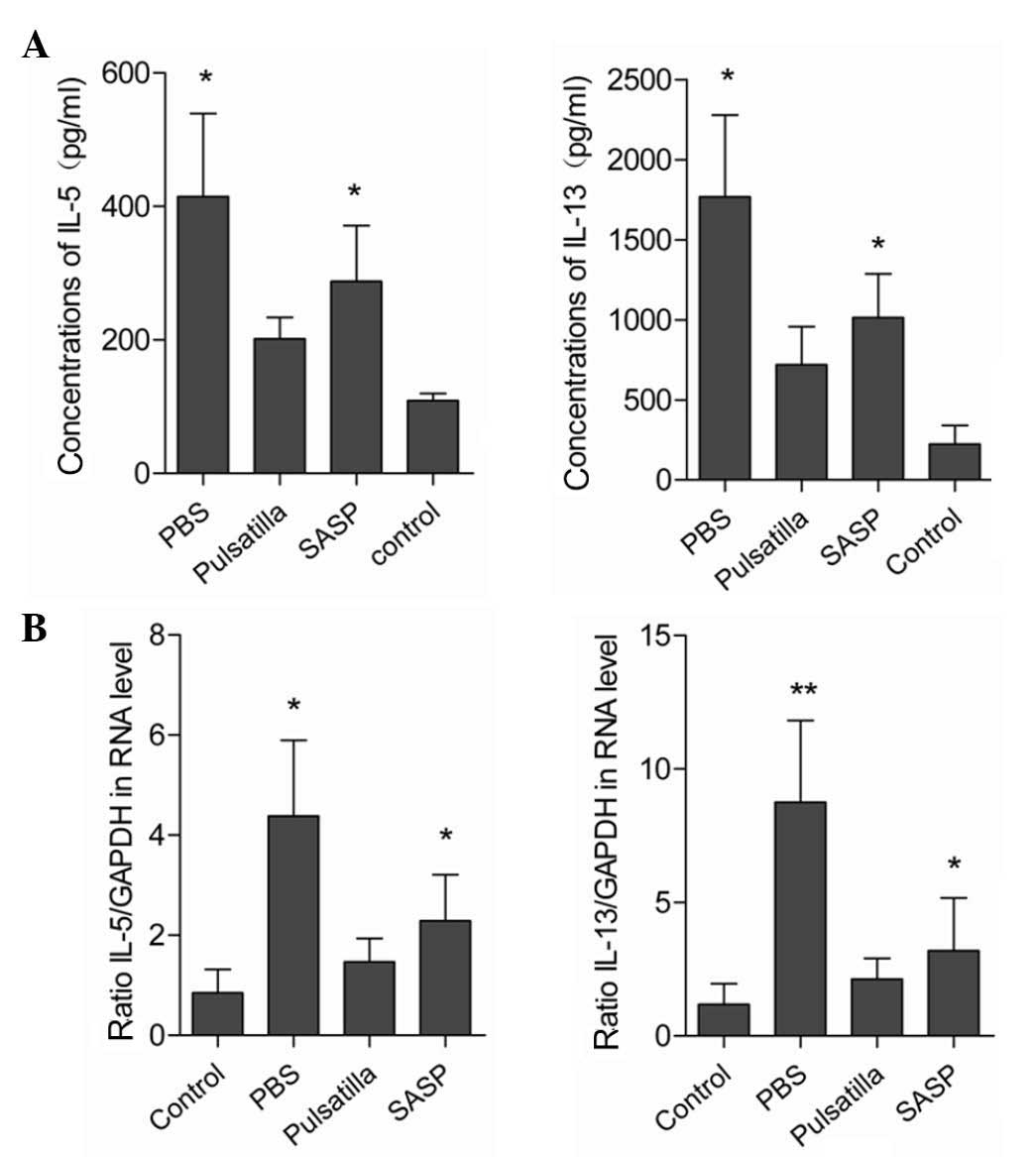
As the excessive production of pro-inflammatory
cytokines is closely correlated with tissue damage and is critical
in the pathogenesis of IBD (24),
the present study characterized the expression levels of
inflammatory cytokines in the colonic mucosal tissues. As shown in
Fig. 2A and B, the expression
levels of IL-5 and IL-13 were low in the colon tissues of the
normal mice, and markedly upregulated in the colon tissues of the
oxazolone-induced colitis mice. Following the administration of
modified Pulsatilla decoction or SASP, the elevated expression
levels of colonic IL-5 and IL-13 were significantly attenuated.
However, no significant differences were detected in the expression
levels of colonic IL-5 and IL-13 between the modified Pulsatilla
decoction and SASP-treated mice. Therefore, these results suggested
that Pulsatilla decoction exerted anti-inflammatory effects in
oxazolone-induced colitis.
Modified Pulsatilla decoction treatment
restores the alteration of tight junction proteins in colon
epithelial cells
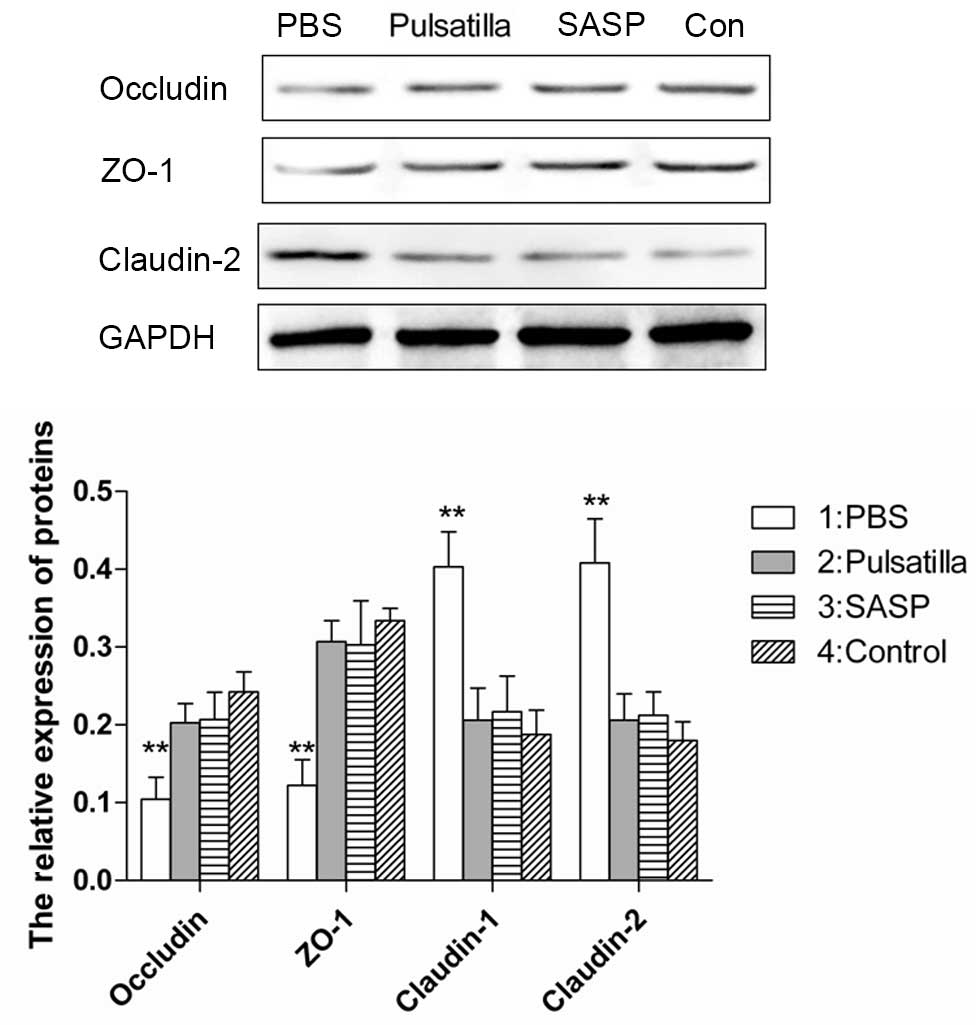
Dysregulated gut epithelial permeability, which is
determined by the intercellular tight junctions in chronic
intestinal inflammation, has been suggested as a primary defect in
IBD. To determine whether modified Pulsatilla decoction treatment
can protect the integrity of the intestinal barrier, the present
study examined the expression levels of the tight junction
proteins, occludin, ZO-1 and claudin-2 in the colonic epithelial
cells of mice treated with or without modified Pulsatilla decoction
or SASP. As expected, the intercellular tight junctions were
disrupted in the colon epithelium of the oxazolone-induced colitis
mice, which was demonstrated by decreased expression levels of the
tight junction proteins, occludin and ZO-1, and increased
expression of claudin-2. However, treatment with modified
Pulsatilla decoction or SASP restored the altered expression levels
of occludin, ZO-1 and claudin-2 in the colon epithelial cells of
the oxazolone-induced colitis mice, almost to normal levels
(Fig. 3). These results indicated
that modified Pulsatilla decoction prevented the alteration of
tight junction proteins caused by the inflammatory stimuli in colon
epithelial cells.
Modified Pulsatilla decoction treatment
inhibits the activation of the NF-κB signaling pathway
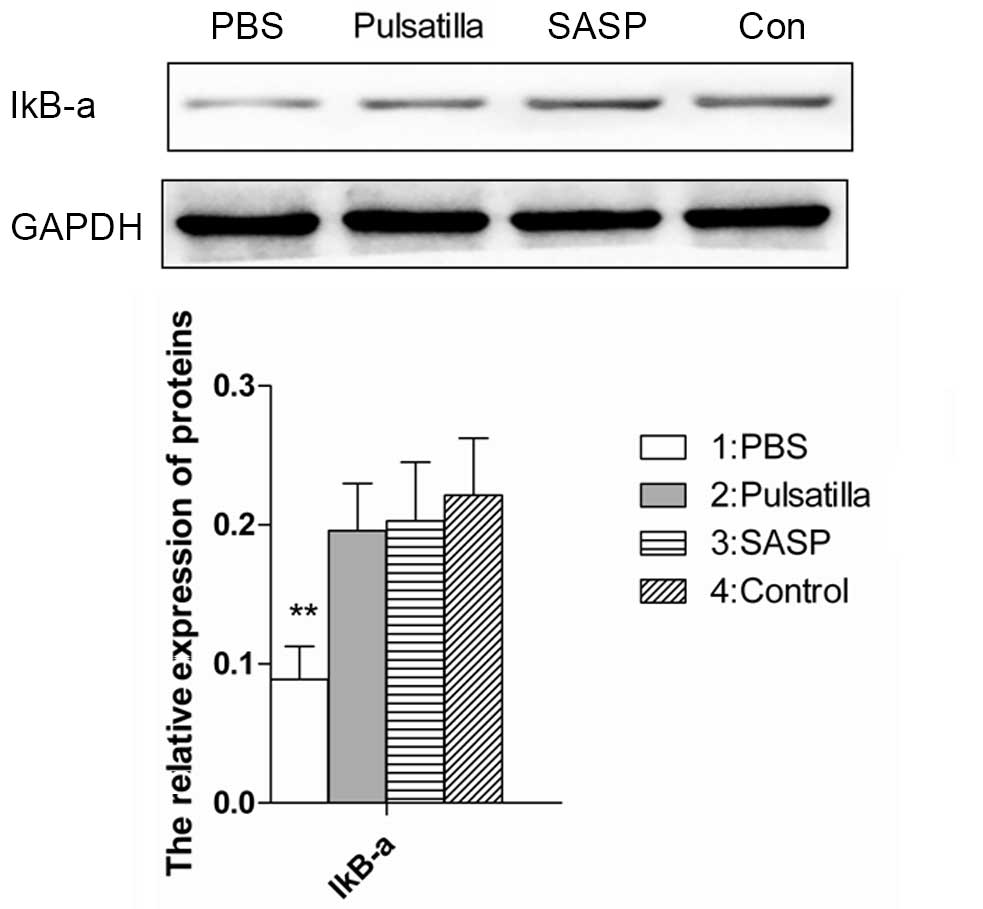
The nuclear transcription factor, NF-κB, is core in
monitoring the development and progression of IBD by regulating the
expression levels of a wide range of pro-inflammatory cytokines.
IκBα is one member of a family of cellular proteins, which function
to inhibit the NF-κB transcription factor. The stabilization of the
IκB-α subunit in the cytoplasm indicates the NF-κB signaling has
been inhibited (25). For further
insight into the molecular mechanism underlying the therapeutic
effects of modified Pulsatilla decoction in IBD, the present study
detected the expression of IκBα in the colon tissues of mice
treated with or without modified Pulsatilla decoction. As shown in
Fig. 4, the expression of IκB-α
was decreased in the colonic tissues of the oxazolone-induced
colitis mice. However, stabilization of the IκB-α subunit in the
colonic tissues of the modified Pulsatilla decoction- and
SASP-treated mice was comparable to that of the normal mice.
Therefore, modified Pulsatilla decoction inhibited the activation
of the NF-κB signaling pathway, which contributed to its
therapeutic effects on IBD.
Discussion
TCM is one of the most developed branches of CAM in
managing various gastrointestinal disorders, and a wide range of
natural products have shown effectiveness for the treatment of IBD
(6–10). Modified Pulsatilla decoction is a
TCM formulation based on the Pulsatilla decoction, with the
addition of another three herbal plants (Sanchi, Radix Paeoniae
Rubra and Radix Glycyrrhizae). It has been shown that the active
ingredient from the herbal plants in the formulation of Pulsatilla
decoction possesses a variety of pharmacological effects, including
hepatic protective, anti-inflammatory, antibacterial, antitumor and
anti-oxidant effects (15–19). Modified Pulsatilla decoction been
used previously to treat UC in clinical practice in China, however,
the possible underlying mechanism in the treatment of UC remains to
be elucidated. The present study aimed to determine the efficiency
and the underlying mechanisms of modified Pulsatilla decoction in
experimental colitis.
Oxazolone-induced colitis is a hapten-induced murine
model of colitis, with pathologic and immunologic features similar
to UC (21). In the present study,
colitis was induced in the mice by the intrarectal administration
of a low dose of oxazolone, following skin pre-sensitization with
oxazolone. As expected, the colitis induced by oxazolone in the
mice produced a significant loss in body weight, and the DAI score
of the oxazolone-induced colitis mice was significantly higher,
compared with that of the normal mice. Oxazolone-induced colitis
has several similarities with human UC in terms of the
morphological changes, which were characterized by transmural
infiltrating of mononuclear cells, thickening of the colon wall,
mucosal hyperplasia, erosions, depletion of goblet cells and crypt
architectural distortion (21,26).
SASP is the first-line therapy for the induction and maintenance of
remission in patients with UC (27,28),
however, conventional treatment of UC is currently limited to
overcoming symptoms, and is often associated with severe adverse
effects from the drugs used (27,28).
In previous years, the efficacy of Chinese herbal medicine in the
treatment of IBD has been extensively characterized (6–10).
The present study demonstrated the therapeutic effects of modified
Pulsatilla decoction in treating oxazolone-induced colitis, which
was demonstrated by improvements in symptomatic parameters,
including body weight loss and severity of diarrhea, and
histological damage of the colon. In addition, no differences
between the therapeutic effects of SASP and modified Pulsatilla
decoction were observed in treating oxazolone-induced colitis.
Therefore, these findings indicated the efficiency of modified
Pulsatilla decoction in treating experimental IBD.
An imbalance in T helper (Th)1/Th2 cytokines is key
in triggering the intestinal mucosa immune response, and UC is
characterized by a Th2 immune response in which IL-13 and IL-5 have
been identified as important cytokines (29–32).
The Th2 cytokines, IL-13 and IL-5, are secreted at high levels by
colonic lamina propria cells in patients with UC, which cause
epithelial cell cytotoxicity (31,32).
In UC, IL-13 may impair epithelial barrier function by affecting
epithelial apoptosis, tight junctions and restitution velocity
(32). Consistent with previous
studies, the present study found that increased expression levels
of IL-5 and IL-13 were observed in the colon tissues of the
oxazolone-induced colitis mice. Following the administration of
modified Pulsatilla decoction into the oxazolone-induced colitis
mice, the elevated expression levels of colonic IL-5 and IL-13 were
significantly attenuated, which suggested the anti-inflammatory
effects of modified Pulsatilla decoction in treating IBD.
Intestinal inflammation is associated with defective
intestinal epithelial tight junction barriers, which is an
important pathogenic factor contributing to the development of IBD
(33–35). The stable complex formed by
occludin, ZO-1 and claudin-2 is critical for tight junction
assembly, and the delocalization of occludin and ZO-1 from the
tight junctions is associated with intestinal barrier dysfunction
and increased permeability (33–35).
Claudin-2 is a pore-forming protein, which enhances tight junction
permeability for cations and provides the molecular basis for leak
flux diarrhea in IBD (36,37). Thus, upon stimulation by
inflammatory cytokines, the structure of tight junctions are
disrupted, which is demonstrated by decreased expression levels or
internalization of occludin and ZO-1, and increased expression of
the pore-forming, claudin-2. In the present study, the results
showed that the tight junction proteins, occludin, ZO-1 and
claudin-2, were significantly altered in the colon epithelial cells
from the oxazolone-induced colitis mice, however, modified
Pulsatilla decoction or SASP efficaciously prevented tight junction
disruption.
The nuclear transcription factor, NF-κB, is monitors
the development and progression of IBD by regulating the expression
of a wide range of pro-inflammatory cytokines (38,39).
In patients with IBD, increased NF-κB activation has been detected
in the intestinal lamina propria (38,39).
It also has been shown that the increase of intestinal tight
junction permeability is NF-κB-dependent (40,41).
In the present study, it was found that the NF-κB pathway was
activated in the colon tissues from the oxazolone-induced colitis
mice, and this activation was significantly counteracted by
modified Pulsatilla decoction, indicating the possibility of this
TCM formulation to downregulate the intestinal response to chronic
inflammatory stimuli.
In conclusion, the present study determined the
therapeutic efficiency of Pulsatilla decoction in oxazolone-induced
colitis and examined the underlying mechanisms. In addition, no
adverse effects were observed following treatment with modified
Pulsatilla decoction. Although it is widely agreed the use of
herbal medicines is safe for the treatment of various diseases in
China, the long-term safety of modified Pulsatilla decoction
requires further investigation.
Acknowledgments
This study was supported by a grant from Putuo
Hospital (grant no. 2013PT054) and the '315' Program for Reserve
Experts of Putuo Science & Technology Commission (grant no.
14Q-RC-10).
References
|
1
|
Baumgart DC and Carding SR: Inflammatory
bowel disease: Cause and immunobiology. Lancet. 369:1627–1640.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baumgart DC and Sandborn WJ: Inflammatory
bowel disease: Clinical aspects and established and evolving
therapies. Lancet. 369:1641–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nielsen OH: New strategies for treatment
of inflammatory bowel disease. Front Med (Lausanne). 1:32014.
|
|
5
|
Danese S: New therapies for inflammatory
bowel disease: From the bench to the bedside. Gut. 61:918–932.
2012. View Article : Google Scholar
|
|
6
|
Hilsden RJ, Verhoef MJ, Rasmussen H,
Porcino A and DeBruyn JC: Use of complementary and alternative
medicine by patients with inflammatory bowel disease. Inflamm Bowel
Dis. 17:655–662. 2011. View Article : Google Scholar
|
|
7
|
Langmead L and Rampton DS: Review article:
Complementary and alternative therapies for inflammatory bowel
disease. Aliment Pharmacol Ther. 23:341–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahimi R, Mozaffari S and Abdollahi M: On
the use of herbal medicines in management of inflammatory bowel
diseases: A systematic review of animal and human studies. Dig Dis
Sci. 54:471–480. 2009. View Article : Google Scholar
|
|
9
|
Jackson LN, Zhou Y, Qiu S, Wang Q and
Evers BM: Alternative medicine products as a novel treatment
strategy for inflammatory bowel disease. Am J Chin Med. 36:953–965.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sałaga M, Zatorski H, Sobczak M, Chen C
and Fichna J: Chinese herbal medicines in the treatment of IBD and
colorectal cancer: A review. Curr Treat Options Oncol. 15:405–420.
2014. View Article : Google Scholar
|
|
11
|
Li R, Alex P, Ye M, Zhang T, Liu L and Li
X: An old herbal medicine with a potentially new therapeutic
application in inflammatory bowel disease. Int J Clin Exp Med.
4:309–319. 2011.PubMed/NCBI
|
|
12
|
Cao YB, Zhang JD, Diao YY, Yan L, Wang DJ,
Jia XM, Gao PH, Cheng MH, Xu Z, Wang Y and Jiang YY: Effects of
Changtai granules, a traditional compound Chinese medicine, on
chronic trinitrobenzene sulfonic acid-induced colitis in rats.
World J Gastroenterol. 11:3539–3543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sandborn WJ, Targan SR, Byers VS, Rutty
DA, Mu H, Zhang X and Tang T: Andrographis paniculata extract
(HMPL-004) for active ulcerative colitis. Am J Gastroenterol.
108:90–98. 2013. View Article : Google Scholar :
|
|
14
|
Zhang F, Li Y, Xu F, Chu Y and Zhao W:
Comparison of Xilei-san, a Chinese herbal medicine and
dexamethasone in mild/moderate ulcerative proctitis: A double-blind
randomized clinical trial. J Altern Complement Med. 19:838–842.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Chen X, Duan H, Hu Y and Mu X:
Pulsatilla decoction and its active ingredients inhibit secretion
of NO, ET-1, TNF-alpha and IL-1 alpha in LPS-induced rat intestinal
microvascular endothelial cells. Cell Biochem Funct. 27:284–288.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Zhang M, Zhang P, Song Z, Ma Z
and Qu H: Silver complexation and tandem mass spectrometry for
differentiation of triterpenoid saponins from the roots of
Pulsatilla Chinensis (Bunge) Regel. Rapid Commun Mass Spectrom.
22:3783–3790. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan B, Ma Y, Shi R and Wang T:
Simultaneous quantification of three alkaloids of Coptidis Rhizoma
in rat urine by high-performance liquid chromatography: Application
to pharmacokinetic study. Biopharm Drug Dispos. 28:511–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan CO, Chu CC, Mok DK and Chau FT:
Analysis of berberine and total alkaloid content in cortex
phellodendri by near infrared spectroscopy (NIRS) compared with
high-performance liquid chromatography coupled with ultra-visible
spectrometric detection. Anal Chim Acta. 592:121–131. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Z, Zhu X and Zhang H: Micelle-mediated
extraction and cloud point preconcentration for the analysis of
aesculin and aesculetin in Cortex fraxini by HPLC. J Pharm Biomed
Anal. 44:867–873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muluye RA, Bian Y and Alemu PN:
Anti-inflammatory and antimicrobial effects of heat-clearing
Chinese herbs: A current review. J Tradit Complement Med. 4:93–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heller F, Fuss IJ, Nieuwenhuis EE,
Blumberg RS and Strober W: Oxazolone colitis, a Th2 colitis model
resembling ulcerative colitis, is mediated by IL-13-producing NK-T
cells. Immunity. 17:629–638. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. National Academies Press; Washington, D.C., USA: 2011
|
|
23
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
León AJ, Gómez E, Garrote JA, Bernardo D,
Barrera A, Marcos JL, Férnandez-Salazar L, Velayos B, Blanco-Quirós
A and Arranz E: High levels of proinflammatory cytokines, but not
markers of tissue injury, in unaffected intestinal areas from
patients with IBD. Mediators Inflamm. 2009:302009. View Article : Google Scholar
|
|
25
|
Imbert V, Rupec RA, Livolsi A, Pahl HL,
Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger
P, Baeuerle PA and Peyron JF: Tyrosine phosphorylation of I kappa
B-alpha activates NF-kappa B without proteolytic degradation of I
kappa B-alpha. Cell. 86:787–798. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kojima R, Kuroda S, Nakamaru K and
Hatakeyama S: Oxazolone-induced colitis in BALB/C mice: A new
method to evaluate the efficacy of therapeutic agents for
ulcerative colitis. J Pharmacol Sci. 96:307–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Svartz N: Salazopyrin a new sulfanilamide
preparation: A. Therapeutic results in rheumatic polyarthritis. B.
Therapeutic results in ulcerative colitis. C. Toxic manifestations
in treatment with sulfanilamide preparation. Acta Med Scand.
11:557–590. 1942.
|
|
28
|
Kornbluth A and Sachar DB; Practice
Parameters Committee of the American College of Gastroenterology:
Ulcerative colitis practice guidelines in adults: American college
of gastroenterology, practice parameters committee. Am J
Gastroenterol. 105:501–523; quiz 524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strober W and James SP: The immunologic
basis of inflammatory bowel disease. J Clin Immunol. 6:415–432.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fuss IJ, Neurath M, Boirivant M, Klein JS,
de la Motte C, Strong SA, Fiocchi C and Strober W: Disparate CD4+
lamina propria (LP) lymphokine secretion profiles in inflammatory
bowel disease. Crohn's disease LP cells manifest increased
secretion of IFN-gamma, whereas ulcerative colitis LP cells
manifest increased secretion of IL-5. J Immunol. 157:1261–1270.
1996.PubMed/NCBI
|
|
32
|
Heller F, Florian P, Bojarski C, Richter
J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm
M, et al: Interleukin-13 is the key effector Th2 cytokine in
ulcerative colitis that affects epithelial tight junctions,
apoptosis and cell restitution. Gastroenterology. 129:550–564.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hering NA, Fromm M and Schulzke JD:
Determinants of colonic barrier function in inflammatory bowel
disease and potential therapeutics. J Physiol. 590:1035–1044. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013. View Article : Google Scholar
|
|
35
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weber CR, Raleigh DR, Su L, Shen L,
Sullivan EA, Wang Y and Turner JR: Epithelial myosin light chain
kinase activation induces mucosal interleukin-13 expression to
alter tight junction ion selectivity. J Biol Chem. 285:12037–12046.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prasad S, Mingrino R, Kaukinen K, Hayes
KL, Powell RM, MacDonald TT and Collins JE: Inflammatory processes
have differential effects on claudins 2, 3 and 4 in colonic
epithelial cells. Lab Invest. 85:1139–1162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schreiber S, Nikolaus S and Hampe J:
Activation of nuclear factor kappa B inflammatory bowel disease.
Gut. 42:477–484. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Neurath MF, Becker C and Barbulescu K:
Role of NF-kappaB in immune and inflammatory responses in the gut.
Gut. 43:856–860. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma TY, Iwamoto GK, Hoa NT, Akotia V,
Pedram A, Boivin MA and Said HM: TNF-alpha-induced-induced increase
in intestinal epithelial tight junction permeability requires
NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol.
286:G367–G376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beutheu Youmba S, Belmonte L, Galas L,
Boukhettala N, Bôle-Feysot C, Déchelotte P and Coëffier M:
Methotrexate modulates tight junctions through NF-κB, MEK, and JNK
Pathways. J Pediatr Gastroenterol Nutr. 54:463–470. 2012.
View Article : Google Scholar
|