Introduction
Gastric cancer is the third leading type of cancer
and has the third leading cancer-associated mortality in China. The
prognosis of gastric cancer is generally poor and, due to the
development pattern and the characteristics of gastric cancer, the
majority of patients exhibit peritoneal metastasis upon diagnosis
or develop peritoneal carcinomatosis following surgery (1–3).
Targeted prevention and treatment of peritoneal carcinomatosis from
gastric cancer is important to improve patients' quality of life
and prognosis. Recent studies have demonstrated that hyperthermic
intraperitoneal chemotherapy (HIPEC) exhibits increased efficacy in
prevention and treatment of peritoneal carcinomatosis from gastric
cancer (4,5), however, the underlying molecular
mechanisms remain to be elucidated.
Due to the lower heat resistance of tumor cells
compared with normal cells, thermo-chemotherapy eradicates tumor
cells with increased efficacy using high temperature and increased
drug sensitivity in the cancer cell population. Autophagy is
important in apoptosis induced by thermo-chemotherapy and studies
have demonstrated that, under different conditions, autophagy may
either result in tumor cell death or promote tumor cell survival
(6). Following physicochemical
damage, including radiation therapy and chemotherapy, increased
levels of basal autophagy improves the ability of tumor cells to
endure and repair damage (6).
However, excessive autophagy in tumor cells promotes autophagic
cell death, resulting in an effective treatment of cancer (7). Thus, in recent years, induction of
autophagic cell death has been considered an important cancer
therapeutic strategy.
Studies have demonstrated that reactive oxygen
species (ROS) induce the dissociation of autophagy molecules,
Beclin 1 and B-cell lymphoma 2 (Bcl-2), which activates Beclin 1
and stimulates autophagy pathways, thus leading to the inhibition
of mammalian target of rapamycin (mTOR) and an increased expression
of the autophagy marker, LC3 II. The subsequent activation of
autophagy-associated signaling pathways induce autophagic cell
death (8). A recent study
demonstrated that thermo-chemotherapy induces oxidative stress and
increases ROS levels in tumor cells (9). A combination of thermo-chemotherapy
and oxidative stress inducer, tert-butyl hydroperoxide increases
intracellular ROS levels and improves the cytotoxicity against
tumor cells (10). Thus, the
stimulation of ROS production and the induction of tumor cell
apoptosis may be a major anti-tumor mechanism of
thermo-chemotherapy.
The present study hypothesizes that one of the major
mechanisms of thermo-chemotherapy is to increase ROS levels and
stimulate oxidative stress in tumor cells, leading to autophagic
death in gastric cancer cells. To investigate this hypothesis, cell
culture experiments and animal models were used.
Thermo-chemotherapy was simulated, and the correlation between the
production of ROS and the expression of autophagy-associated genes
in gastric cancer cells was examined. In addition, the effect of
thermo-chemotherapy-induced ROS production on autophagic cell death
was investigated. The role of ROS-induced autophagic cell death in
the cytotoxic effect of thermo-chemotherapy in gastric cancer cells
was further investigated. The present study may elucidate the
mechanisms of HIPEC in the treatment and prevention of gastric
cancer peritoneal metastasis.
Materials and methods
Identification of the half-maximal
inhibitory concentration (IC50) of oxaliplatin
(L-OHP)
The SGC-7901 human gastric carcinoma cell line was
treated with L-OHP. When the SGC-7901 cells entered the exponential
phase, the L-OHP was added to Dulbecco's modified Eagle's medium
(Hyclone, Logan, UT, USA) at various concentrations (10, 20, 40,
80, 160 and 320 µg/ml). Following L-OHP treatment for 24 h,
cell viability was determined using the MTS assay to determine the
most effective concentration of L-OHP The protocol for the MTS
assay was performed according to a previous study (10). MTS and dimethyl sulfoxide used in
the MTS assay were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The IC50 was obtained from three independent
experiments, and was used in the following studies.
Treatment of cells with L-OHP and
hyperthermia (HT)
SGC-7901 cells were treated with the most effective
L-OHP concentration for 1 h, then the cells were exposed to
different temperatures (39, 41, 42, 43 and 45°C) for different
assays. Following incubation for 24 h, cell viability was monitored
using the MTS assay to determine the most effective
temperature.
Grouping and treatment of the cells
To ensure the most effective concentration of L-OHP
and use of the optimum temperature, SGC-7901 cells were randomly
divided into four groups, including: Group 1, the control group;
group 2: the HT group, treated with HT at the optimum temperature;
group 3, chemotherapy group, treated with L-OHP using the most
effective concentration; and group 4, chemotherapy + HT group,
treated with most effective concentration of L-OHP and HT. The
cells cultured at 37°C served as the negative control group.
Establishment of gastric cancer
model
To establish a gastric cancer model, 30 BALB/c-nu
nude mice (pathogen-free grade; age, 4–6 weeks old; weight, 230–280
g; purchased from the Animal Center of the Guangzhou Medical
University, Guangzhou, China) were used in the present study. The
mice were maintained in a pathogen-free environment with a 12 h
light/dark cycle, and were given adequate nutrition and water. This
study was approved by the ethics committee of Southern Medical
University (Guangzhou, China). SGC-7901 cells were subcutaneously
injected into each side of the posterior flank groin region of the
mouse. The nude mice were randomly divided into four groups (n=3):
Group 1, the control group, in 37°C, injected intraperitoneally
with glucose; group 2, HT group, in 43°C, injected
intraperitoneally with glucose (6 mg/kg, Sigma-Aldrich); group 3,
chemotherapy group, local intraperitoneal injection to the tumor at
a dose of 6 mg/kg L-OHP; and group 4, chemotherapy + HT group,
injected intraperitoneally at a dose of 6 mg/kg L-OHP and exposed
to 43°C. The drug sensitivity of cells to L-OHP was determined
using the Eppendorf BioSpectrometer (Eppendorf, Hamburg, Germany).
The optical density value represents the sensitivity of the cells.
Following administration of chemotherapy or glucose for 1 h, the
mice were fixed in a water box. The water was preheated to 43°C and
the depth of the water in the box kept the tumor region immersed in
the water. The box was placed into a biochemical incubator
(JYSP-450; Hefei Jayon Instrument Equipment Co. Ltd., Hefei, China)
at 43°C for 1.5 h, to ensure the tumor cells were heated for at
least 1 h.
Western blot analysis
The expression levels of autophagy-associated
proteins, Beclin 1, LC3B and mTOR were detected by western
blotting. The treatment of trigeminal ganglion and the western blot
processes were performed according to the previous study (10). In this study, mouse anti-Beclin 1
(cat. no. sc-48341), anti-LC3β (cat. no. sc-271625) and anti-mTOR
(cat. no. sc-293089) monoclonal antibodies were used Santa Cruz
Biotechnology, Inc. (Santa Cruz, Dallas, TX, USA; 1:2,000). The
horseradish peroxidase-conjugated rabbit anti-mouse antibody (cat.
no. sc-358920; Santa Cruz Biotechnology, Inc.; 1:1,000) was used as
the secondary antibody.
Measurement of parameters
The mice used in the gastric cancer models were
sacrificed by cervical dislocation following treatment according to
the conditions of each group for 24 h. Prior to sacrifice, mice
were anaesthetized with pentobarbital sodium (1 mg/kg, Tiangen
Biotech (Beijing) Co., Ltd., Beijing, China). The tumor tissue
(n=10) was surgically removed and part of the tumor tissue was
incubated in collagenase IV (Sigma-Aldrich) to isolate single tumor
cells to assess the level of ROS in the cells by flow cytometry.
MMP and apoptosis were also analyzed by flow cytometry (17548;
Beckman Coulter, Brea, CA, USA). The protocol of the flow
cytometetry was performed according to a previous study (10). Another part of the tumor tissue was
fixed with 10% neutral-buffered formalin (Sigma-Aldrich), embedded
in paraffin and cut into 5-µm sections with a microtome
(M3500; Labway Science Development Ltd., Beijing, China). The
expression levels of autophagy-associated proteins, Beclin 1, LC3B
and mTOR, were examined by immunohistochemistry according to a
previous study (10). The same
antibodies were used as in the western blot analysis section.
Transmission electron microscopy
analysis
The cells grew on the microcarriers and observed
before treatment. Microcarriers were placed in a mixture of (1:1)
propylene oxide/Epon resin (Sigma-Aldrich). Then, the microcarriers
were left overnight in pure resin for impregnation of the cells.
The microcarriers were embedded in the Epon resin (Sigma-Aldrich).
The ultra-thin sections were obtained with a Leica EM UC7
ultramicrotome (Leica, Wetzlar, Germany). Sections were deposited
on the formvar/carbon-coated nickel grids and stained with 5%
uranyl acetate and 5% lead citrate. The cells were observed using a
JEOL 1011 transmission electron microscope (TEM; JEOL Ltd., Tokyo,
Japan).
Statistical analysis
Statistical analysis was performed using Origin 6.0
and SPSS 19.0 software package (IBM SPSS, Armonk, NY, USA).
Continuous data were expressed as the mean ± standard deviation.
One-way analysis of variance (ANOVA) was used to examine
statistically significant differences between groups. Least
significant difference was used for multiple comparisons and
factorial design ANOVA was used for factorial design data. The
effect of combination therapy of L-OHP and HT on SGC-7901 cell
viability was analyzed using the q-value method (11). P<0.05 (two-tailed) was used to
indicate a statistically significant difference.
Results
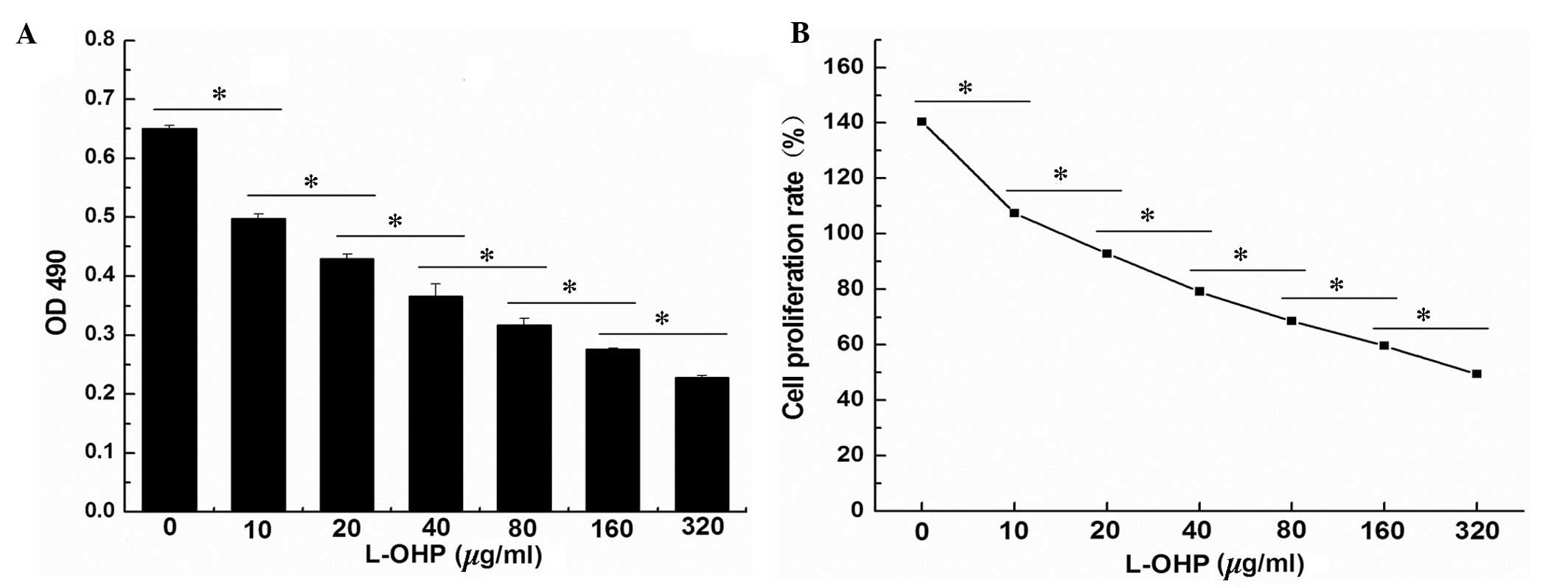
L-OHP treatment inhibits SGC-7901 cell
viability
The result of the MTS assay indicated that the
different concentrations of L-OHP inhibited SGC-7901 cell
viability. As the L-OHP concentration increased, the SGC-7901 cell
viability was significantly decreased, and there was a positive
correlation between L-OHP concentration and inhibition of cell
viability (Fig. 1A). Statistical
analysis of the results demonstrated that the cell viability in the
eight groups was significantly different (F=1,598.325, P=0.05). The
cell viability was also significantly different between any two
groups (Fig. 1B; P<0.05).
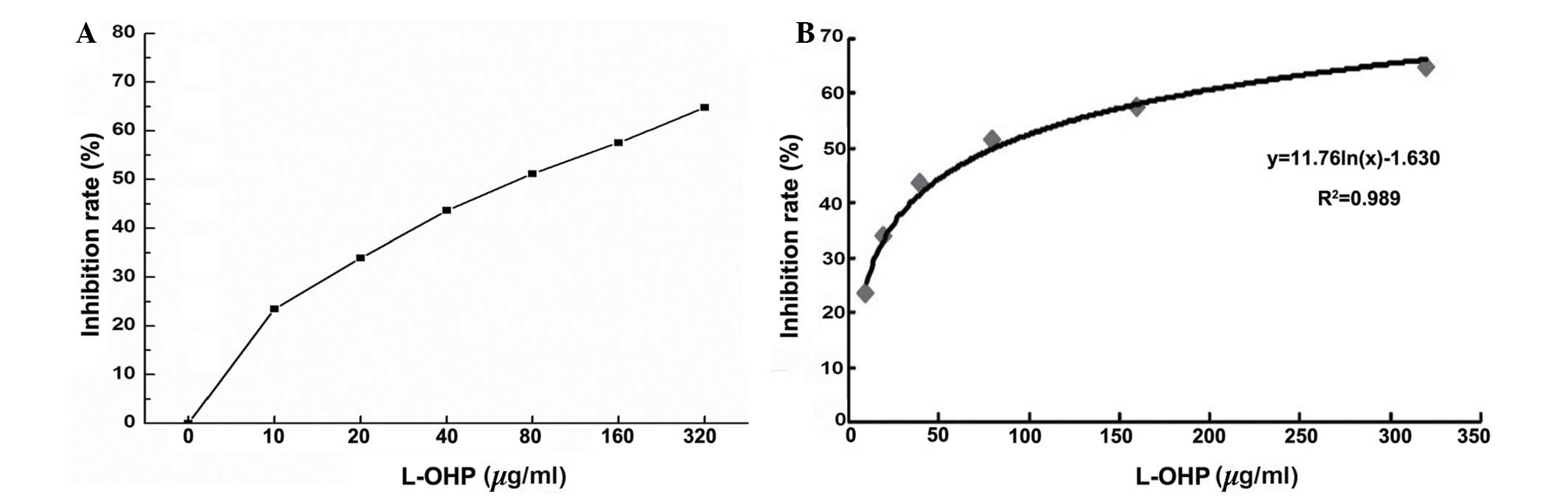
Sensitivity of SCG-7901 cells to L-OHP
correlates with IC50 regression
According to the results of the MTS assay, the
inhibitory rate was obtained from different concentrations of
L-OHP. These results indicated that the drug sensitivity of
SGC-7901 cells was positively correlated with the L-OHP inhibition
rate (Fig. 2A). Furthermore, the
drug sensitivity of the SCG-7901 cells was also correlated with the
IC50 regression curve (Fig.
2B; P<0.05, r=0.7832).
The results indicated that the IC50 was
80.66 µg/ml (Fig. 2B), and
therefore a concentration of L-OHP of 80 µg/ml was used in
the subsequent experiments.
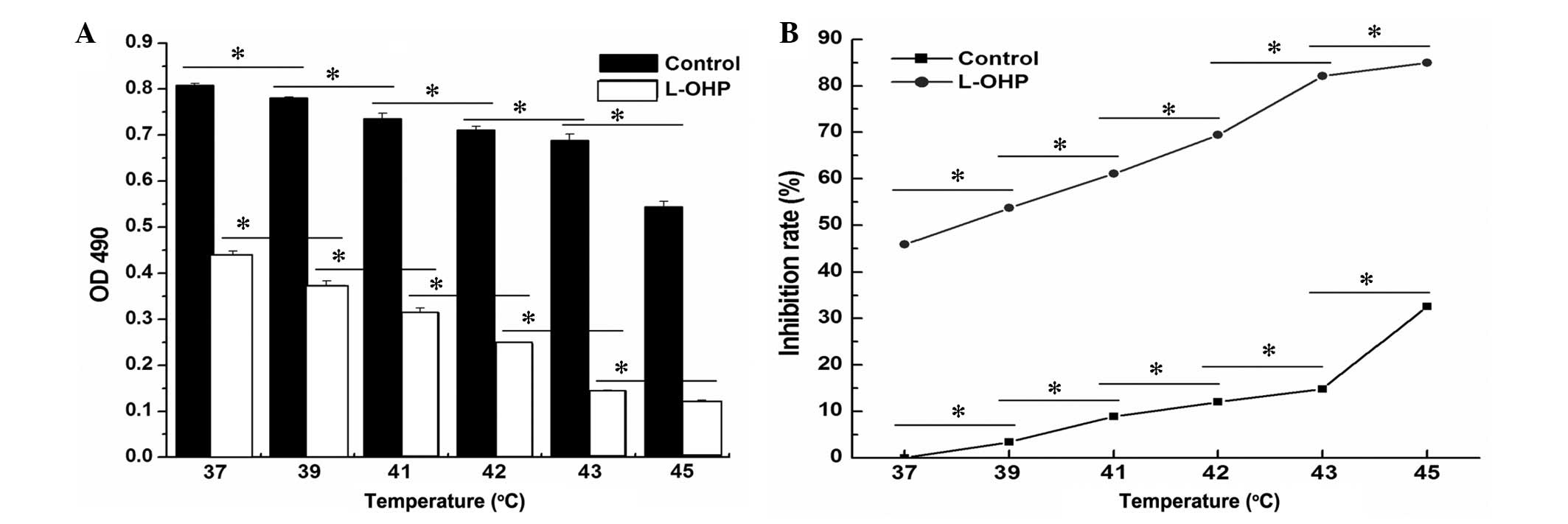
HT treatment inhibited SGC-7901 cell
viability
To investigate the effect of temperature on the
SGC-7901 cell viability, the SGC-7901 cells were treated with
different temperatures (37, 39, 41, 42, 43 and 45°C) when the cells
entered the exponential phase. Following treatment with 80
µg/ml L-OHP for 24 h, the SGC-7901 cells were heated for 1 h
and cultured for a further 24 h. Cell viability was monitored using
the MTS assay (Fig. 3A). The
results indicate that the cell viability was gradually decreased
following the increase in temperature and that high temperature
resulted in cell death in an increased number of the tumor cells.
Statistical analysis of the results demonstrated that the
interaction between L-OHP and temperature was significant in the
suppression of cell proliferation (Fig. 3B; F=25,201.956, P=0.05; F=993.630,
P=0.05).
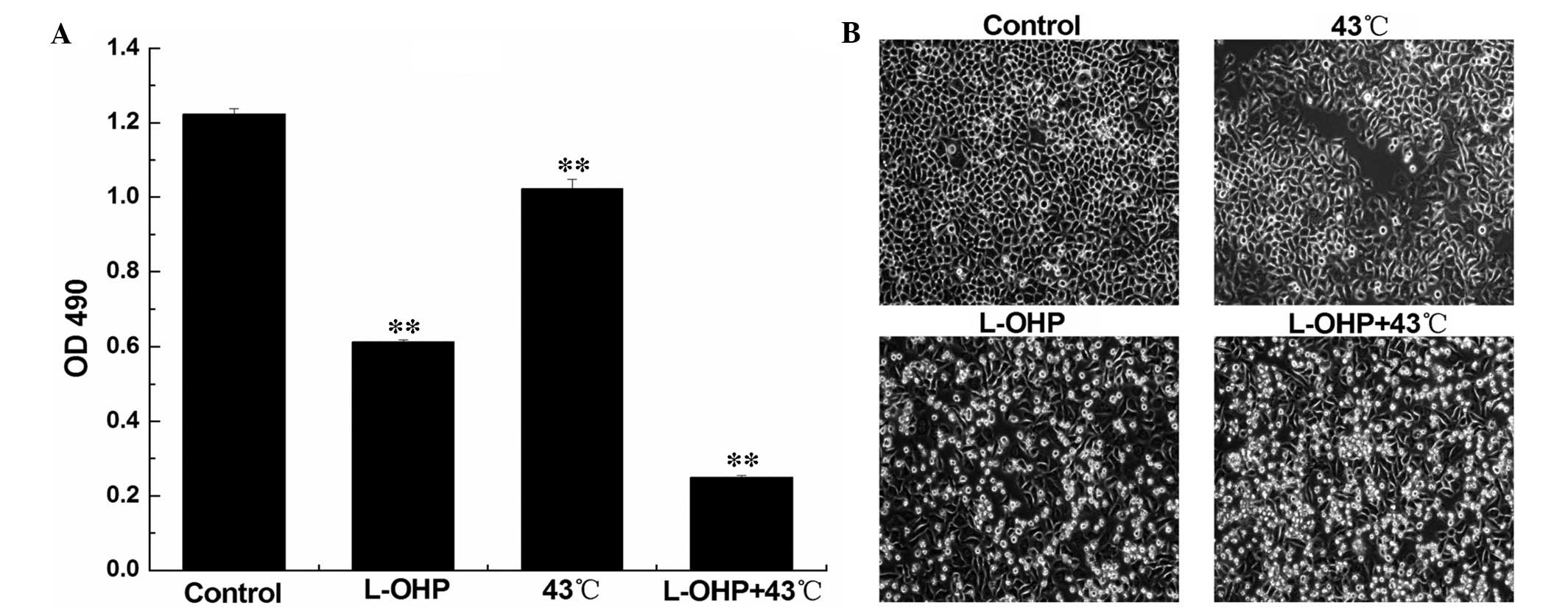
L-OHP and HT inhibit SGC-7901 cell
viability
According to the results of the IC50
determination and the optimum temperature experiments, a
concentration of 80 µg/ml L-OHP and 43°C were selected for
the incubation of the SGC-7901 cells, and to demonstrate the
synergistic effect. The cell viability was analyzed using the MTS
assay. Compared with the control group, the cell viability of the
HT group, chemotherapy group and chemotherapy + HT group were
significantly decreased (P<0.05). The reduced cell viability in
the chemotherapy + HT group was the highest (P<0.01; Fig. 4A).
Furthermore, the cells were observed under an
inverted microscope (model, IX73; Olympus, Tokyo, Japan). Compared
with control group, the cell density of the HT and chemotherapy
groups were reduced and the cells were observed. Cell growth in the
chemotherapy + HT group was slowest, the cells were observed to be
round and floating in the medium, and cell apoptosis occurred to
the greatest extent in this group (Fig. 4B).
The effect of combination therapy of L-OHP and HT
for SGC-7901 cell viability was calculated by the q-value method.
The q-value was determined to be 1.370, suggesting that the
combination therapy of L-OHP and HT had a synergistic effect to
inhibit cell viability.
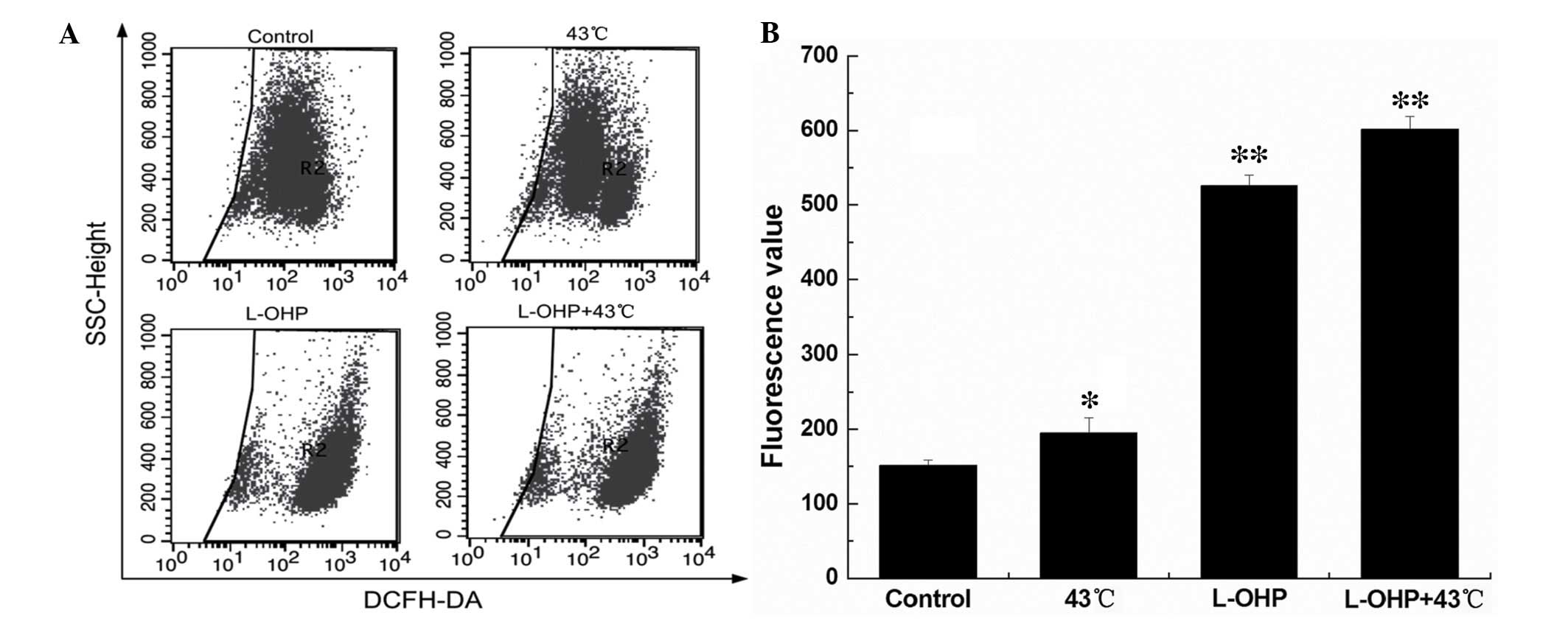
Combination therapy of L-OHP and HT
promotes expression of ROS
The ROS level was detected by flow cytometry.
Compared with the control group, the ROS level of the HT,
chemotherapy and chemotherapy + HT groups were significantly
increased (Fig. 5A; P<0.01).
The increase in ROS level was highest in the chemotherapy + HT
group (Fig. 5B; P<0.01).
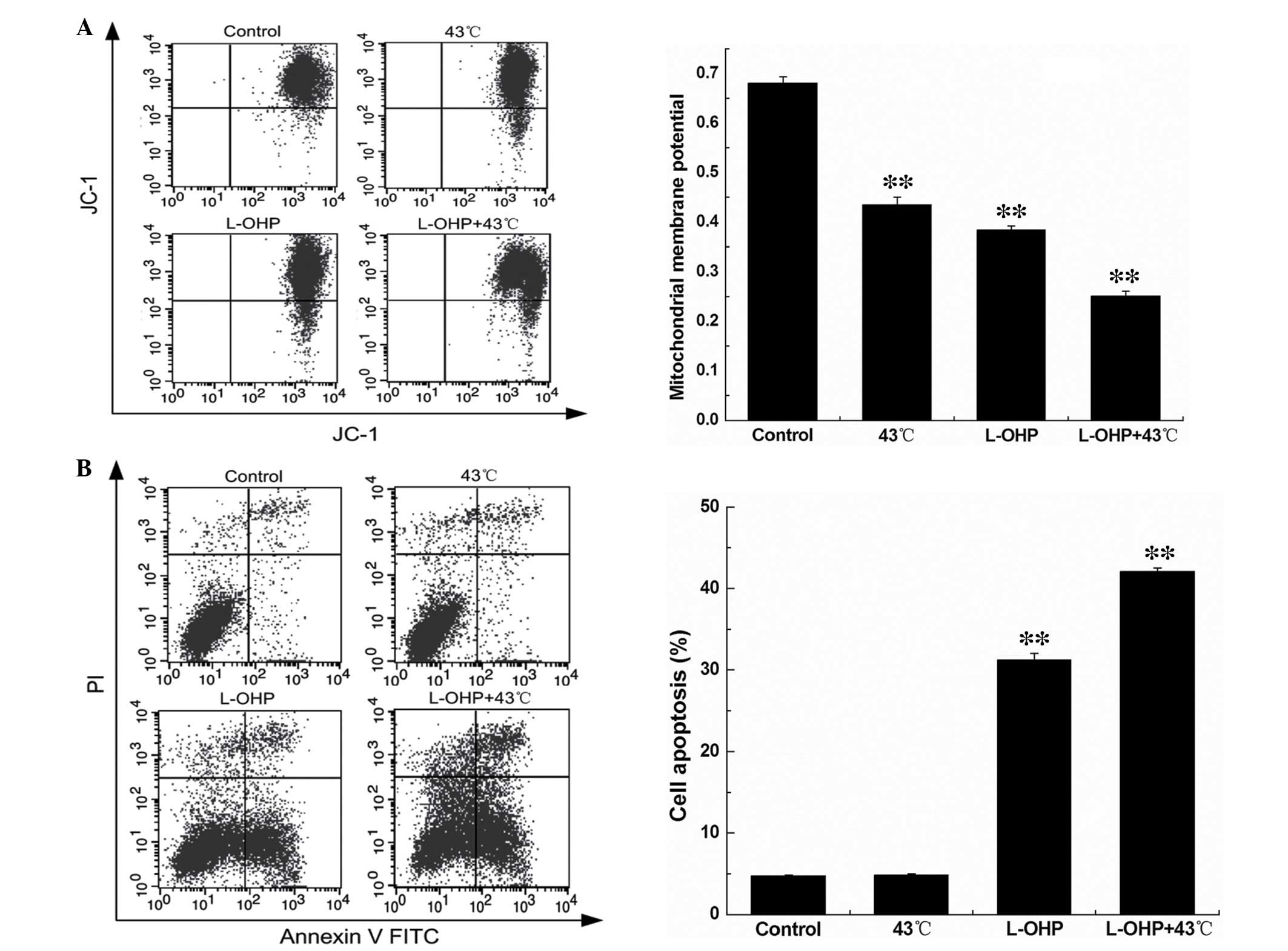
Combination of L-OHP and HT reduces
SGC-7901 cell mitochondrial membrane potential (MMP) and promotes
SGC-7901 cell apoptosis
The MMP was analyzed by flow cytometry. Compared
with the control group, the MMP of the HT, chemotherapy and
chemotherapy + HT groups were decreased. The MMP level of the
chemotherapy group and the chemotherapy + HT group were
significantly decreased (Fig. 6A;
P<0.01).
The apoptosis of SGC-7901 cells was also detected by
flow cytometry. Compared with the control group, the HT group had
no significant effect on the apoptosis of SGC-7901 cells, which
demonstrated that HT only inhibited the cell viability, with no
effect on the apoptosis of SGC-7901 cells. However, treatment with
L-OHP or L-OHP + HT significantly increased apoptosis in SGC-7901
cells (Fig. 6B; P<0.01).
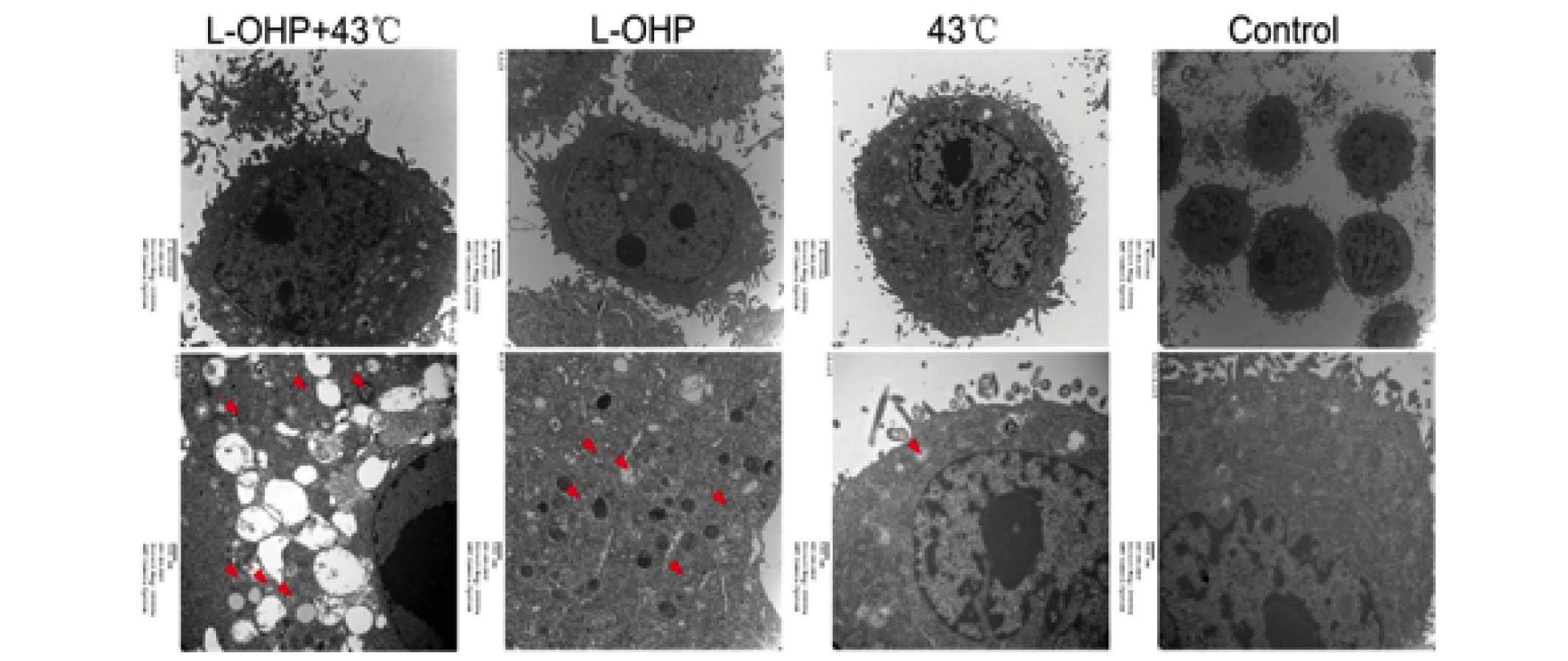
Combination therapy effect of L-OHP + HT
resulted in autophagic cell death
In order to investigate whether the apoptosis
induced by the L-OHP + HT treatment was associated with autophagy,
the autophagosome produced was observed under a transmission
electron microscope (5656AG; FEI, Eindhoven, The Netherlands)
(Fig. 7). The results demonstrate
that the cells exhibited greater chromatin pyknosis and
autophagosomes in the L-OHP and chemotherapy + HT groups, and the
cell membrane appeared incomplete and the cells appeared close to
cell death in the chemotherapy + HT group, suggesting the lethal
effect of combination therapy with chemotherapy + HT were
associated with autophagy.
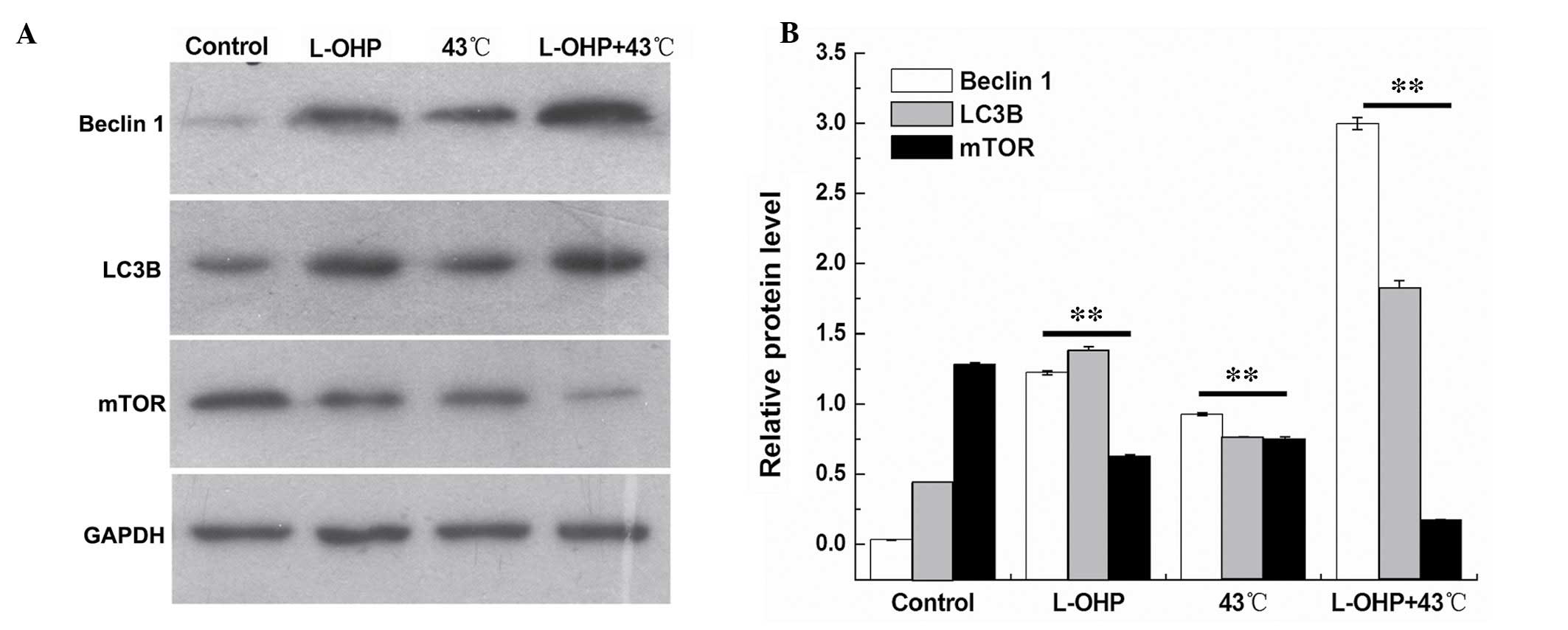
Combination therapy with L-OHP + HT
promotes expression of autophagy-associated proteins
The expression levels of autophagy-associated
proteins, Beclin 1, LC3B and mTOR were detected by western
blotting. The expression of Beclin 1 and LC3B was demonstrated to
be significantly increased in the L-OHP and the L-OHP + HT groups
(P<0.01), and the expression of mTOR exhibited a negative
correlation with the degree of autophagy, which demonstrated that
autophagy-associated protein, LC3, was activated by type I
translation into type II, the expression of Beclin 1 was
significantly increased, and the expression of mTOR was
significantly decreased in the L-OHP and the chemotherapy + HT
groups. The results demonstrate that cell death was associated with
autophagy. Statistical analysis of the results indicates that the
interaction between L-OHP and temperature was significant in the
suppression of cell proliferation (Fig. 8; P<0.01).
Discussion
Gastric cancer is important as it has a high
incidence and the early symptoms of gastric cancer are easily
confused with gastritis or other benign diseases of stomach, making
it difficult to diagnose at an early stage when tumors are confined
to the mucosa or submucosa. In China, the detection rate of early
gastric cancer is only ~5–10%, and the majority of patients are
diagnosed at an advanced stage (2,3,12).
Diffuse peritoneal carcinomatosis often occurs in patients with
serosal invasion. Patients who undergo radical resection may still
develop local recurrence, and peritoneal carcinomatosis with
malignant ascites is often developed quickly following surgery,
leading to poor quality of life for the patients and low long-term
survival rates (13). Improving
the long-term survival in patients with advanced-stage gastric
cancer, and increasing their quality of life, are urgent clinical
issues requiring attention.
HIPEC is a novel adjunct treatment for peritoneal
carcinomatosis. A large volume of thermo-chemotherapy liquid
increases the exposure of peritoneal micrometastases to the
chemotherapeutic agents, and maintains high, constant and lasting
therapeutic agent concentrations in the abdominal cavity. Thus,
HIPEC improves the effect of treatment of peritoneal
micrometastases from gastric cancer and peritoneal
carcinomatosis-induced ascites (14). Previous clinical studies have
demonstrated the effectiveness of HIPEC in treating refractory
gastric cancer with malignant ascites (15–17).
Currently, HIPEC has been widely used in the treatment of malignant
ascites, but the treatment protocol remains under debate. The
underlying molecular mechanisms of its therapeutic activity
requires elucidation, thus, thermo-chemotherapy cannot be optimized
according to these mechanisms. The overall treatment outcomes of
peritoneal carcinomatosis from gastric cancer is poor, initiating
controversy over the continued use of HIPEC in clinical
practice.
In the present study, by establishing an in
vitro model for assessing the effect of thermo-chemotherapy
using the human gastric cancer cell line SGC-7901, the synergistic
effect between the L-OHP and HT was demonstrated. In order to
determine the optimal concentration of L-OHP, SGC-7901 cells were
treated with different concentrations of L-OHP, and the cell
viability in each group was assessed by MTS assay. The results
demonstrated that different concentrations of L-OHP inhibited the
proliferation of SGC-7901 cells. The inhibition of the cell
viability was positively correlated with the concentration of
L-OHP, as an increased L-OHP concentration led to increased
inhibition of cell viability.
The cell viability in each group (37, 39, 41, 42, 43
and 45°C) was assessed using the MTS assay. The results
demonstrated decreased viability with increasing temperature,
indicating a cytotoxic effect of hyperthermia. The q-value method
(11) was used to determine
whether there was a synergistic effect between the chemotherapy and
hyperthermia. The results demonstrated that at 41°C, hyperthermia
acted synergistically with chemotherapy, and with the rising
temperature, the inhibitory effect on cell proliferation increased;
however, from 43–45°C, the inhibitory effect of hyperthermia on
cell proliferation did not change markedly, indicating that
thermo-chemotherapy exerted a notable cytotoxic effect on SGC-7901
human gastric carcinoma cells at high temperatures of 43–45°C. High
temperature in the peritoneal cavity may result in thermal damage
to the bowel, or intestinal necrosis in severe cases. It also
increases the risk of adhesive small bowel obstruction (18). Cui et al (18) used domestic swine as experimental
animals and established an animal model of HIPEC using the BR-TRG-I
body cavity hyperthermic perfusion extracorporeal circulatory
system. Settings of 44 and 45°C were used, and the result indicated
that HIPEC at 44°C for 1.5 h had no significant effect on the mice
vital signs, liver and renal function, but resulted in mild damage
to the liver, kidney, small intestine and other organs. However, at
45°C, HIPEC for 1.5 h had a marked impact on the animal's vital
signs, resulting in serious damage to liver and kidney function,
and notable pathological injuries to the liver, kidney, small
intestine and other organs. To adjust for the difference in heat
tolerance between humans and animals and the safety of clinical
procedures, 43°C was selected as the temperature for hyperthermia
therapy.
According to the above-mentioned results, the L-OHP
concentration of 80 µg/ml and the optimum temperature of
43°C were selected to treat each group of cells. Results of the MTS
assay demonstrated that, compared with the cell viability in the
control group, cell viabilities in the HT, chemotherapy and
chemotherapy + HT groups were significantly decreased (P<0.05),
with the chemotherapy + HT group exhibiting the largest difference.
The combined effect of L-OHP and hyperthermia was evaluated by the
calculation of the q-value and the q-value was 1.370. The
experimental results further verified the synergistic effect of
L-OHP and HT. In addition, the cell growth in each group was
observed under an inverted microscope. Compared with cells in the
control group, cells in the hyperthermia and chemotherapy groups
exhibited significantly lower density and marked rounded morphology
(P<0.05). Among all the groups, cells in the chemotherapy + HT
group demonstrated the slowest growth and the highest level of cell
death, indicated by the majority of the cells becoming rounded and
floating in the media. The results demonstrated synergistic
cytotoxicity between HT and chemotherapy, and the combined therapy
improved the L-OHP sensitivity in tumor cells, suggesting a
clinical application for a reduced dose of chemotherapeutic agents
in order to decrease short- and long-term side-effects, but also to
maintain a cytotoxic effect with HT exposure.
ROS are a class of oxygen-containing compounds with
potent biological activities generated by exogenous oxidants, or
from intracellular aerobic metabolism. In health, ROS production
and clearance is maintained in a dynamic balance. When ROS
production exceeds clearance and intracellular antioxidants cannot
effectively degrade and remove them, ROS accumulate in cells,
resulting in oxidative stress (19,20).
This alters the opening of mitochondrial membrane channels by
oxidizing unsaturated fatty acids in mitochondrial and cell
membranes, resulting in increased membrane permeability (21). The increase in membrane
permeability reduces the mitochondrial ATP and Ca2+
contents, which in turn results in the release of cytochrome
c and mitochondrial swelling. Studies suggest that severe
damage to the mitochondria results in autophagy, autophagic cell
death, apoptosis and necrosis (22,23).
ROS-induced tumor cell apoptosis is important in the association
between ROS and apoptosis. Studies have demonstrated that ROS is
associated with various types of apoptosis (21–23).
In the present study, flow cytometry was used to detect
intracellular ROS levels following treatment in each group of
cells, and it was observed that the level of ROS was significantly
higher in cells in the thermo-chemotherapy group than that in cells
in the control group (P<0.05). Furthermore, using flow cytometry
to assess rates of cell apoptosis in each group, it was observed
that the proportion of apoptotic cells in the chemotherapy + HT
group was significantly higher than in the chemotherapy,
hyperthermia, and control groups (P<0.05), suggesting that
thermo-chemotherapy induces apoptosis in SGC-7901 cells. The
apoptosis may be mediated by ROS, and the current study
hypothesizes that thermo-chemotherapy induces an accumulation of
ROS in SGC-7901 cells, and the consequent oxidative stress induces
tumor cell apoptosis and may be one of the major mechanisms that
underlie the anti-tumor effects of thermo-chemotherapy.
Increasing evidence has demonstrated that ROS are
involved in cell proliferation, differentiation and apoptosis, but
also act as signaling molecules in the process of autophagy
activation (24,25). The process of autophagy is often
accompanied by changes in ROS levels. Studies have observed that
when autophagy is induced by therapeutic agents, the intracellular
ROS levels change at the same time. For example, when
lipopolysaccharides and the pan-caspase inhibitor, Z-VAD induce
autophagy in macrophages, ROS levels in macrophages increase at the
same time (22,23,26,27).
Studies have demonstrated that excessive accumulation of ROS leads
to cytotoxic effects, which induce excessive autophagy and lead
directly to autophagic cell death (22,23,27).
Beclin 1 and LC3, the autophagy markers, are involved in the
process of autophagy. Quinsay et al (28) identified that the expression of
Beclin 1 and LC3 is associated with ROS, and peroxides, a type of
ROS, induce autophagy by upregulating Beclin 1 and LC3. Few studies
have investigated the association between thermo-chemotherapy and
autophagy. In order to further assess whether HT and
chemotherapy-induced apoptosis is associated with autophagy, the
formation of autophagic bodies were observed in each group by
transmission electron microscopy. In cells of the chemotherapy and
chemotherapy + HT groups, chromatin condensation and increased
autophagic bodies were observed, and in the thermo-chemotherapy
group, cells exhibited incomplete cell membranes and appeared close
to cell death, indicating an association between autophagy and the
cytotoxicity of L-OHP and HT in SGC-7901 cells.
Following the observation of autophagy by
transmission electron microscopy, the underlying molecular
mechanisms of autophagy induction by L-OHP or HT were investigated.
When autophagy is induced, the expression of Beclin 1 increases
markedly. Beclin 1 promotes the process of autophagy by serving as
a “platform”, forming complexes with a variety of proteins, and
allowing the relocalization of autophagic proteins to
autophagosomal membranes. mTOR, as a sensor of ATP, amino acids,
growth factors and insulin, is important in the regulation of cell
growth. mTOR inhibits autophagy by regulating the upstream and
downstream signaling pathways, acting as a cell “doorman”.
Therefore, the present study focused on autophagy-associated
proteins, LC3B, Beclin 1 and mTOR, for the investigation of the
underlying mechanisms.
The current study investigated the mechanism of
thermo-chemotherapy-induced cytotoxicity. Thermo-chemotherapy
increased the level of intracellular ROS, and decreased the MMP,
thereby reducing cell viability and inducing apoptosis. To assess
whether chemotherapy, HT or thermo-chemotherapy-induced apoptosis
is associated with autophagy, intracellular structures were
observed using transmission electron microscopy. It was observed
that, following chemotherapy or thermo-chemotherapy, a large number
of autophagic bodies were produced, particularly in the
thermo-chemotherapy group, where the highest number were observed,
demonstrating that the L-OHP and HT result in autophagic cell
death, and thermo-chemotherapy has the greatest cytotoxic effect.
The molecular mechanisms of thermo-chemotherapy-induced
cytotoxicity were also investigated. Western blot analysis
demonstrated changes in protein levels of autophagy-associated
genes, LC3B, Beclin 1 and mTOR, in each group of cells, with the
change in the chemotherapy + HT group being the most marked,
suggesting that chemotherapy or thermo-chemotherapy-induced cell
death is mediated by autophagy, and thermo-chemotherapy exerts an
increased effect. The results suggest that thermo-chemotherapy may
upregulate the expression of autophagy-associated proteins, Beclin
1 and LC3B via ROS, and induce autophagy in SGC-7901 human gastric
cancer cells, leading to autophagic cell death. This process may be
one of the major underlying mechanisms of the anti-tumor effects of
thermo-chemotherapy.
The finding that ROS affects the level of autophagy
is important for cancer research and therapeutic strategies.
Although ROS were observed to be signaling molecules involved in
the regulation of autophagy, the specific mechanisms by which they
induce autophagy and the complete signal transduction pathways
remain to be elucidated. In addition, whether various ROS work
together or individually to regulate autophagy remains to be
determined. The association between the ROS-induced
autophagy-associated cell survival and cell death requires further
investigation.
In conclusion, ROS production in gastric cancer
cells downregulates the expression of the autophagic gene, m-TOR,
and upregulates the expression of genes, Beclin 1 and LC3B. This
indicates a positive correlation between ROS levels and the level
of autophagy in gastric cancer cells. There is a linear association
between thermo-chemotherapy-induced production of ROS and the
cytotoxic effect of thermo-chemotherapy in gastric cancer cells,
indicating an important role of thermo-chemotherapy-induced ROS
production in autophagic cell death in gastric cancer cells. The
consequent ROS-induced autophagic cell death is important to the
cytotoxic effect of thermo-chemotherapy. However, the specific
underlying mechanism of ROS accumulation and the occurrence of
autophagy remain to be elucidated. In the future, further
investigation into the specific mechanisms, and use of various
types of cell lines, will indicate whether the results are
consistent across different types of cancer.
Acknowledgments
The present study was supported by grants from the
Guangdong Province and Hong Kong SAR Breakthrough Funds in Key
Areas of Science and Technology (grant no. 2006Z1-E6041) and the
Guangdong Provincial Science and Technology Program (grant no.
2009A030301013).
References
|
1
|
Tang Y, Liu X, Su B, Zhang Z, Zeng X, Lei
Y, Shan J, Wu Y, Tang H and Su Q: microRNA-22 acts as a metastasis
suppressor by targeting metadherin in gastric cancer. Mol Med Rep.
11:454–460. 2015.
|
|
2
|
Li H, Lu P, Lu Y, Liu C, Xu H, Wang S and
Chen J: Predictive factors of lymph node metastasis in
undifferentiated early gastric cancers and application of
endoscopic mucosal resection. Surg Oncol. 19:221–226. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato M, Ono S, Mabe K, Sakamoto N and
Aaska M: After endoscopic treatment of early stage gastric cancer.
Nihon Rinsho. 71:1429–1435. 2013.In Japanese. PubMed/NCBI
|
|
4
|
Valle M, Van der Speeten K and Garofalo A:
Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy
(HIPEC) in the management of refractory malignant ascites: A
multi-institutional retrospective analysis in 52 patients. J Surg
Oncol. 100:331–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ba MC, Cui SZ, Lin SQ, Tang YQ, Wu YB,
Wang B and Zhang XL: Chemotherapy with laparoscope-assisted
continuous circulatory hyperthermic intraperitoneal perfusion for
malignant ascites. World J Gastroenterol. 16:1901–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang R and Tang D: Autophagy in pancreatic
cancer pathogenesis and treatment. Am J Cancer Res. 2:383–396.
2012.PubMed/NCBI
|
|
7
|
Jabs T: Reactive oxygen intermediates as
mediators of programmed cell death in plants and animals. Biochem
Pharmacol. 57:231–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li ZY, Yang Y, Ming M and Liu B:
Mitochondrial ROS generation for regulation of autophagic pathways
in cancer. Biochem Biophys Res Commun. 414:5–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lehmann K, Rickenbacher A, Jang JH,
Oberkofler CE, Vonlanthen R, von Boehmer L, Humar B, Graf R,
Gertsch P and Clavien PA: New insight into hyperthermic
intraperitoneal chemotherapy: Induction of oxidative stress
dramatically enhanced tumor killing in in vitro and in vivo models.
Ann Surg. 256:730–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen F, Wang CC, Kim E and Harrison LE:
Hyperthermia in combination with oxidative stress induces
autophagic cell death in HT-29 colon cancer cells. Cell Biol Int.
32:715–723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Law AS and Burt DW: qValue - a program to
calculate comparative measures of genomic reorganisation from
cytogenetic and/or linkage information. Comput Appl Biosci.
12:181–183. 1996.PubMed/NCBI
|
|
12
|
Peng J and Wang Y: Epidemiology, pathology
and clinical management of multiple gastric cancers: A mini-review.
Surg Oncol. 19:e110–e114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Shammaa HA, Li Y and Yonemura Y:
Current status and future strategies of cytoreductive surgery plus
intraperitoneal hyperthermic chemotherapy for peritoneal
carcinomatosis. World J Gastroenterol. 14:1159–1166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YP, Ling Y, Qi QF, Zhang YP, Zhang CS,
Zhu CT, Wang MH and Pan YD: Genetic polymorphisms of ERCC1-118,
XRCC1-399 and GSTP1-105 are associated with the clinical outcome of
gastric cancer patients receiving oxaliplatin-based adjuvant
chemotherapy. Mol Med Rep. 7:1904–1911. 2013.PubMed/NCBI
|
|
15
|
Zhang XL, Shi HJ, Cui SZ, Tang YQ and Ba
MC: Prospective, randomized trial comparing 5-FU/LV with or without
oxaliplatin as adjuvant treatment following curative resection of
gastric adenocarcinoma. Eur J Surg Oncol. 37:466–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu JF, Zhou XK, Chen JH, Yi G, Chen HG,
Ba MC, Lin SQ and Qi YC: Up-regulation of PIK3CA promotes
metastasis in gastric carcinoma. World J Gastroenterol.
16:4986–4991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ba M, Cui S, Lin S, Tang Y, Wu Y and Zhang
X: Resection of a giant hepatocellular carcinoma weighing over ten
kilograms. World J Gastroenterol. 16:1422–1424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui S, Ba M, Huang D, Tang Y and Wu Y:
Study and development of BR-TRG-I hyperthermic perfusion
intraperitoneal treatment system. China Medical Devices. 24:7–9.
2009.
|
|
19
|
Fleury C, Mignotte B and Vayssière JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:1005–1028. 2000.
|
|
21
|
Cui K, Luo X, Xu K and Ven Murthy MR: Role
of oxidative stress in neurodegeneration: Recent developments in
assay methods for oxidative stress and nutraceutical antioxidants.
Prog Neuropsychopharmocol Biol Psychiatry. 28:771–799. 2004.
View Article : Google Scholar
|
|
22
|
Lemasters JJ, Nieminen AL, Qian T, Trost
LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA,
Brenner DA and Herman B: The mitochondrial permeability transition
in cell death: A common mechanism in necrosis, apoptosis and
autophagy. Biochim Biophys Acta. 1366:177–196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vurusaner B, Poli G and Basaga H: Tumor
suppressor genes and ROS: Complex networks of interactions. Free
Radic Biol Med. 52:7–18. 2012. View Article : Google Scholar
|
|
24
|
Scherz-Shouval R and Elazar Z: Regulation
of autophagy by ROS: Physiology and pathology. Trends Biochem Sci.
36:30–38. 2011. View Article : Google Scholar
|
|
25
|
Scherz-Shouval R, Shvets E, Fass E, Shorer
H, Gil L and Elazar Z: Reactive oxygen species are essential for
autophagy and specifically regulate the activity of Atg4. EMBO J.
26:1749–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Y, Kim SO, Li Y and Han J: Autophagy
contributes to caspase-independent macrophage cell death. J Biol
Chem. 281:19179–19187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu HD and Lu JX: Progress in mitophagy.
Chin J Cell Biol (Henderson NV). 23:467–471. 2008.
|
|
28
|
Quinsay MN, Thomas RL, Lee Y and
Gustafsson AB: Bnip3-mediated mitochondrial autophagy is
independent of the mitochondrial permeability transition pore.
Autophagy. 6:855–862. 2010. View Article : Google Scholar : PubMed/NCBI
|