Introduction
Osteoblasts, which are formed from the
differentiation of preosteoblasts derived from mesenchymal cells,
are responsible for the formation of bone matrix and
mineralization. At the early stages of osteoblast differentiation,
gene expression associated with the formation of bone matrix, such
as type I collagen (Col I), osteocalcin (OCN) and alkaline
phosphatase (ALP) are increased. Secreted extracellular matrix is
mineralized by calcium deposition at later stages of osteoblast
differentiation (1).
Non-collagenous proteins, such as dentin sialoprotein (DSP) and
dentin phosphoprotein (DPP), which are cleaved from dentin
sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP-1), and
bone sialoprotein (BSP) are important in osteoblast differentiation
and mineralization (2).
Osseointegrated implants have been used for a long
time in orthopedics and prosthetic dentistry for the restoration of
damaged bone and loss of function. These implant materials are made
of titanium (Ti) and its alloy due to its mechanical properties and
good biocompatibility (3).
Successful dental implants undergo osseointegration properly around
the Ti surface; however, the release the metal ions from the
implant, as well as patients suffering from underlying diseases,
such as osteoporosis, diabetes, and periodontitis, can cause the
implant to fail (4). Therefore,
several studies have been performed to increase osteoblast adhesion
and differentiation through chemical and physical changes in Ti
surface structure or by producing alloys with other metals.
Ti-zirconia or Ti-niobium alloys increase the expression of genes
associated with the differentiation of osteoblasts. In addition,
oxidization of the Ti surface promotes the differentiation of
osteoblasts (5). Furthermore, bone
morphogenic protein-2 (BMP-2) increases the ALP activity with the
secretion of OCN and osteoprotegerin, and promotes the
differentiation of osteoblasts on the Ti surface (6).
SLPI is an 11.7-kDa cysteine-rich protein. It is an
epithelial cell product found in the uterine cervix, nasal mucus,
bronchial mucus, saliva, and seminal plasma (7). SLPI acts as an anti-inflammatory
factor in the early inflammatory response of odontoblasts (8). A recent study reported that SLPI
accelerates the adhesion and migration of MC3T3-E1 cells on Ti
surfaces by increasing the formation of actin stress fibers,
paxillin expression and focal adhesion kinase phosphorylation
(9). Conversely, there are no
studies regarding the effect of SLPI on the differentiation and
mineralization of osteoblasts on a Ti surface. Therefore, the aim
of the present study was to determine the function of SLPI on the
gene expression associated with the differentiation and
mineralization of osteoblasts on Ti surfaces.
Materials and methods
Ti samples
Two types of Ti discs, 20 and 48 mm in diameter and
2 mm in thickness, were used. Commercially pure titanium (Cp-Ti)
discs were kindly provided by Professor Han-Cheol Choe (Department
of Dental Materials, School of Dentistry, Chosun University,
Gwangju, Republic of Korea). Polished Cp-Ti discs were prepared
using a method described previously (9).
Cell culture and differentiation with
SLPI
MC3T3-E1 cells (American Type Culture Collection,
Manassas, VA, USA), an osteoblastic cell line derived from mouse
calvaria, were cultured in α-modified Eagle's medium (α-MEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (WelGENE, Daegu, Korea) and 1%
antibiotic-antimycotic solution (containing penicillin,
streptomycin and amphotericin B; WelGENE) according to the
manufacturer's recommendations. The cells were transferred to a Ti
surface and changed to a differentiation medium (α-MEM supplemented
with 5% FBS, 10 mM β-glycerol phosphate and 50 µg/ml
ascorbic acid) with or without 1 µg/ml recombinant human
(rh)SLPI (R&D Systems, Minneapolis, MN, USA) after 24 h. The
cells were placed into a humidified chamber and maintained in an
atmosphere containing 5% CO2 at 37°C.
Cell viability assay
Cell viability was assessed using a 3-(4,5
dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid (MTT) assay.
The MC3T3-E1 cells plated on the Ti discs (6×105
cells/ml) were incubated for 4, 7 and 10 days in differentiation
medium with or without rhSLPI. An MTT assay (Sigma-Aldrich, St.
Louis, MO, USA) was performed to examine the cell viability as
described previously (9).
Extraction of total RNA and
semi-quantitative reverse-transcription polymerase chain reaction
(RT-PCR)
The MC3T3-E1 cells plated on the Ti discs
(1×106 cells/ml) were incubated for 4, 7 and 10 days in
differentiation medium with or without rhSLPI. The total RNA was
extracted from the cells using TRI reagent (MRC Inc., Houston, TX,
USA) according to the manufacturer's instructions. Total RNA was
quantified using an ultraviolet (UV) spectrophotometer (Ultrospec
2000; GE Healthcare, Little Chalfont, UK). A 1-µg sample of
total RNA was used to synthesize the cDNA. The mRNA was incubated
at 50°C for 1 h and at 70°C for 10 min using Hyperscript RT premix
(GeneAll Biotechnology Co. Ltd., Dongnam-ro, Korea). The PCR
reaction was conducted in a thermocycler (TP600; Takara Bio Inc.,
Otsu, Japan) after adding 1 µl of cDNA and the gene specific
primers to the α-Taq premix (2U Taq DNA polymerase, 200 µM
deoxynucleotide triphosphate mixture and 2.5 mM MgCl2;
GeneAll Biotechnology). The mouse gene specific primers were
designed using the nucleotide sequences of SLPI, ALP, DSPP, DMP1,
BSP, Col I, and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH).
The following primers were used for PCR
amplification: SLPI forward, 5′-TGCTTAACCCTCCCAATGTC-3′ and
reverse, 5′-AATGCTGAGCCAAAAGGAGA-3′; ALP forward,
5′-AAGACGTGGCGGTCTTTGC-3′ and reverse, 5′-GGGAATCTGTGCAGTCTGTG-3′;
DSPP forward, 5′-CGACCCTTGTCCAGGA-3′ and reverse,
5′-CATGGACTCGTCATCGAA-3′; DMP1 forward, 5′-CGAGTCTCAGGAGGACA-3′ and
reverse, 5′-CTGTCCTCCTCACTGGA-3′; BSP forward,
5′-ACCGGCCACGCTACTTTCTTTAT-3′ and reverse,
5′-TCCTCGTCGCTTTCCTTCACTTT-3′; Col I forward,
5′-ATTCGGAGCTCAAGATGTAA-3′ and reverse, 5′-CAGTCAAGTCCTAGCCAAAC-3′;
GAPDH forward, 5′-CCATGGAGAAGGCTGGG-3′ and reverse,
5′-CAAAGTTGTCATGGATGACC-3′. All primers were synthesized by Bioneer
(Daejeon, Korea). Each PCR reaction protocol consisted of an
initial denaturation at 95°C for 2 min followed by three-step
cycling: Denaturation at 95°C for 20 sec, annealing for 10 sec at a
temperature optimized for each primer pair and extension at 72°C
for 30 sec. The gene-specific conditions were as follows: SLPI,
60°C and 30 cycles; ALP, 62°C and 30 cycles; DSPP, 56°C and 35
cycles; DMP1, 55°C and 35 cycles; BSP, 60°C and 30 cycles; Col I,
50°C and 35 cycles; and GAPDH, 56°C and 30 cycles respectively,
After the required number of cycles (30–35), the reactions
underwent a final extension at 72°C for 5 min. GAPDH was used as
the internal control.
All PCR products were electrophoresed on 1.5% or 2%
agarose gels (Takara Bio Inc.) buffered with 0.5X
Tris-borate-ethylenediamine tetraacetate and stained with ethidium
bromide (GeNet Bio, Daejeon, Korea) after amplification. The
staining bands were visualized using Gel-Doc (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The intensities of the bands were
measured and quantified using Science Lab Image Gauge software
(version 3.12; Fuji Film, Tokyo, Japan) as described previously
(10).
Western blot analysis
Differentiated MC3T3-E1 cells (1×106
cells/ml) with or without SLPI for 4, 7 and 10 days were harvested
and the protein was extracted using Nonidet P (NP)-40 lysis buffer
[150 mM NaCl, 1% NP-40, 50 mM Tris-HCl (pH 7.4), 2 mM
Na3VO4, 2 mM Na4P2O, 50
mM NaF, 2 mM ethylenediamine tetraacetic acid, 0.1 µg/ml
leupeptin and 1 µg/ml aprotinin; BioShop Co., Burlington,
ON, Canada]. The extracted protein was incubated using a Dc protein
assay kit (Bio-Rad Laboratories, Inc.) and the protein
concentration was determined using a UV spectrophotometer (EL311;
BioTek Instruments, Winooski, VT, USA). A total of 20 µg of
protein per lane was subjected to 15% SDS-polyacrylamide gel
(Bio-Rad Laboratories, Inc.) electrophoresis and separated proteins
were then transferred to a polyvinylidene difluoride membrane
(Merck Millipore, Darmstadt, Germany). Following blocking with 5%
non-fat dry milk in 20 mM Tris (pH 7.4), 150 mM NaCl and 0.1%
Tween-20 (TBS-T) for 1 h at room temperature, the membrane was
probed with the following primary antibodies at 1:1,000 dilution at
4°C for 16 h: Mouse anti-β-actin (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or rabbit anti-mouse SLPI
antibody provided by Takara Bio Inc. via their custom antibody
service (order no. ARP4093; selective for the peptide sequence
EGGKNDAIKIGAC in the polypeptide region of the mouse SLPI protein)
(8). The membrane was then blotted
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or
goat anti-mouse (cat. no. sc-2004 or sc-2005, respectively; 1:5,000
dilution; Santa Cruz Biotechnology Inc.). The membrane was washed
four times for 10 min each at room temperature in TBS-T after
treatment with the primary and secondary antibodies, respectively.
β-actin was used as the internal control. Blots were developed by
treatment with an enhanced chemiluminescence solution (Luminata
Crescendo Western HRP substrate; Merck Millipore) and images were
captured on X-ray film (Fuji Film, Tokyo, Japan). The density of
the expressed bands was measured using a Science Lab Image Gauge
(version 3.12; Fuji Film).
Alizarin Red S staining
To identify the formation of mineralized nodules
following MC3T3-E1 cell differentiation on Ti surfaces, the cells
were stained with 2% Alizarin Red S (Sigma-Aldrich). Mineralized
nodules were observed using a stereoscopic microscope (Stemi
2000-C, Carl Zeiss, Oberkochen, Germany). The Alizarin Red
S-stained MC3T3-E1 cells were incubated with cetylpyridinium
chloride (Acros Organics; Thermo Fisher Scientific, Inc.) to
dissolve and release calcium-bound Alizarin Red S into the
solution. The absorbance of Alizarin Red S released was measured at
562 nm using a microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Statistical analysis
All experiments were conducted in triplicate. All
data is reported as the mean ± standard deviation determined using
Excel 2010 statistical software (Microsoft, Redmond, WA, USA).
P<0.05 and P<0.01 were considered to indicate a statistically
significant difference. The significant differences were determined
using an unpaired Student's t-test.
Results
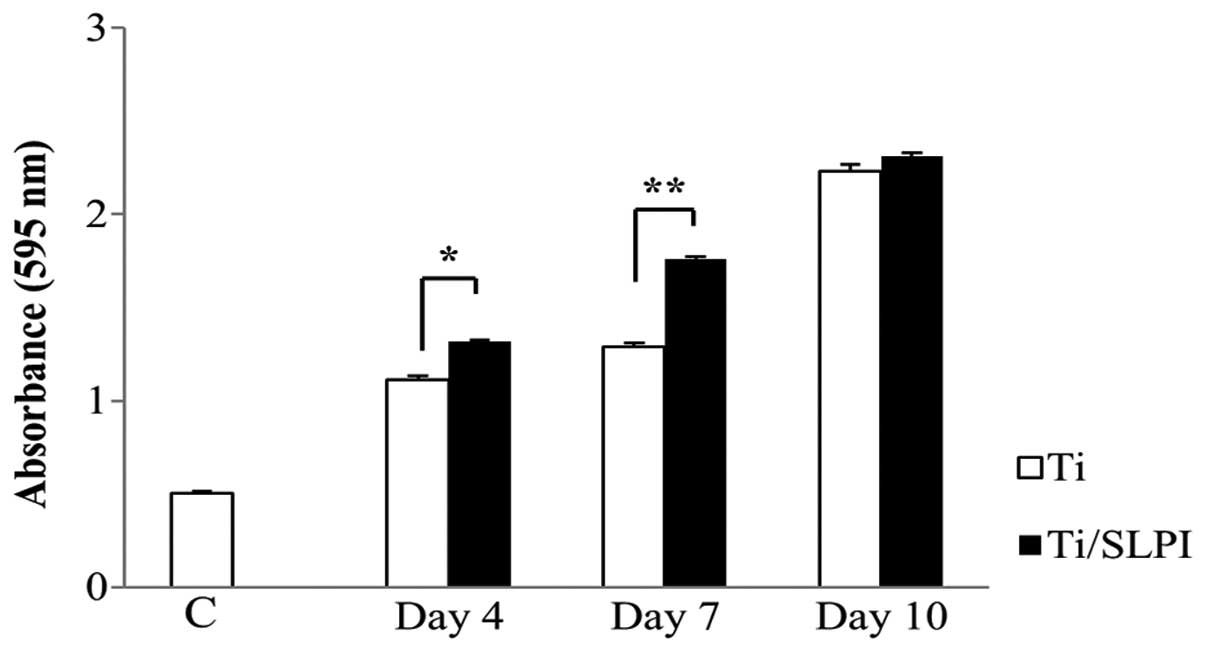
Proliferation of SLPI-treated MC3T3-E1
cells during differentiation on Ti discs
The viability of SLPI-treated MC3T3-E1 cells was 1.1
and 1.4 times higher than that of the untreated cells at 4 and 7
days, respectively, during differentiation on a Ti disc (P<0.05
and P<0.01; Fig. 1). However,
no difference in cell viability between untreated and SLPI-treated
cells was observed at day 10 of differentiation on the Ti disc.
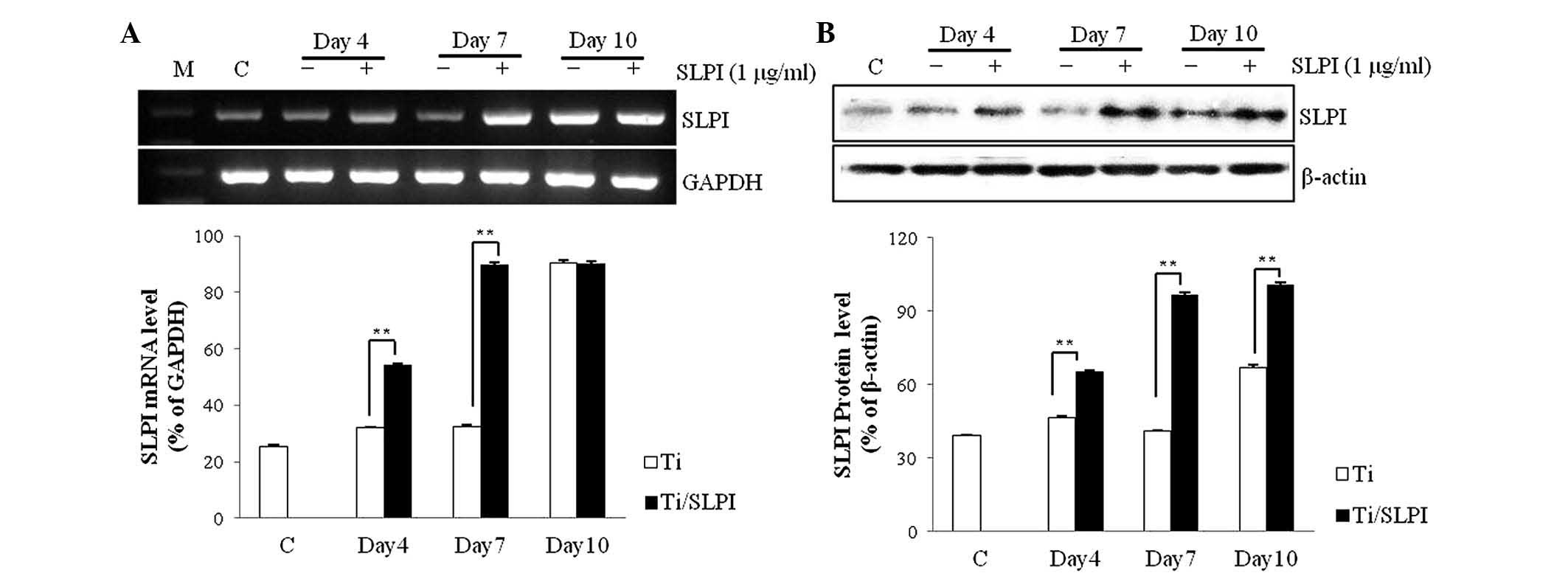
SLPI mRNA and protein expression in
SLPI-treated MC3T3-E1 cells during differentiation on Ti discs
SLPI mRNA expression in the SLPI-treated MC3T3-E1
cells was 1.7 and 2.8 times higher than that of the untreated cells
at 4 and 7 days, respectively, during differentiation on a Ti disc
(P<0.01; Fig. 2A). However, no
difference in expression of SLPI mRNA between untreated and
SLPI-treated cells was observed at day 10 of differentiation on the
Ti disc. SLPI protein expression in the SLPI-treated MC3T3-E1 cells
was 1.4, 2.4 and 1.5 times higher than that of the untreated cells
at 4, 7 and 10 days, respectively, during differentiation on a Ti
disc (P<0.01; Fig. 2B).
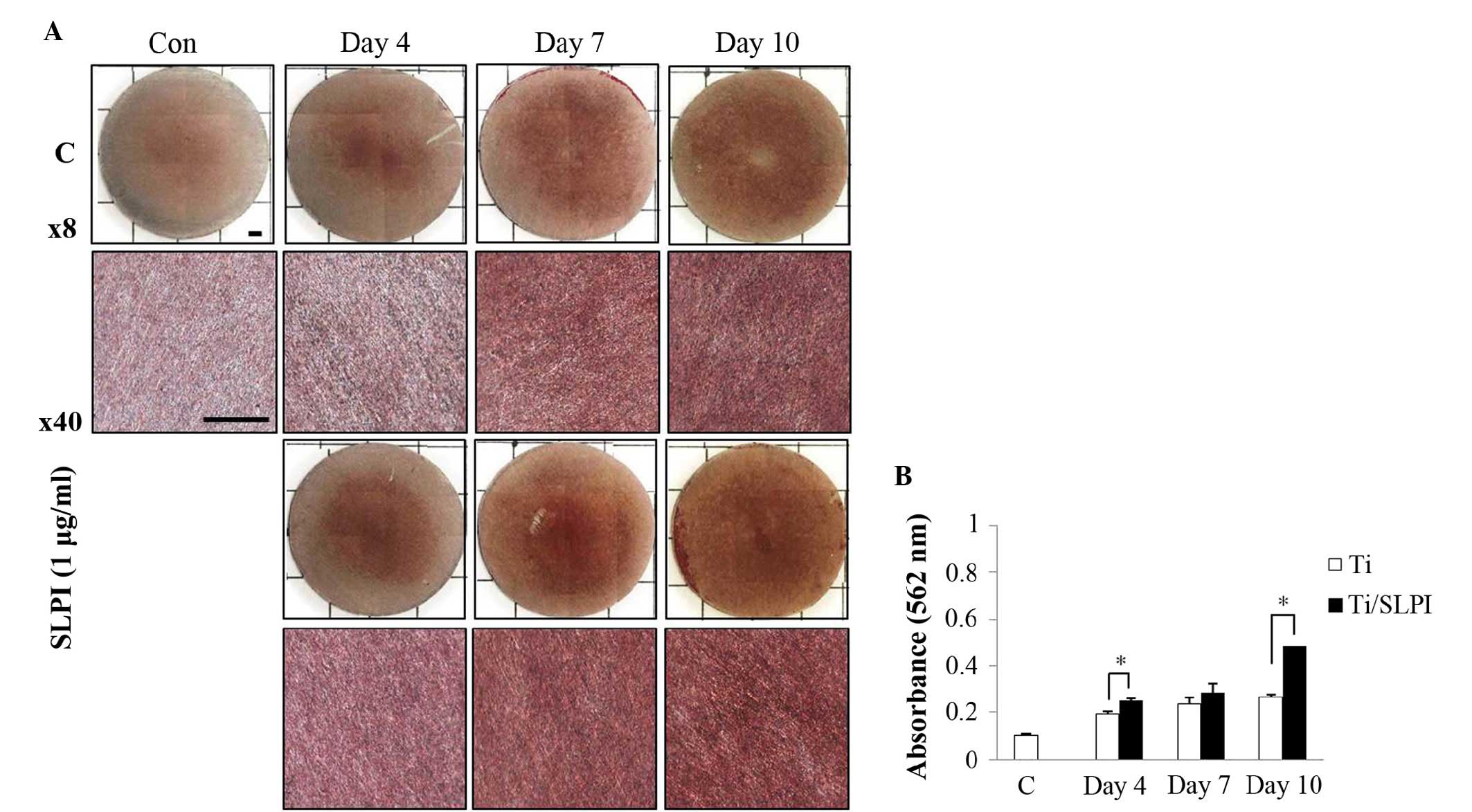
Mineralization of SLPI-treated MC3T3-E1
cells during differentiation on a Ti disc
Alizarin Red S staining showed that the SLPI-treated
MC3T3-E1 cells increased the level of mineral deposition compared
with that of the untreated cells during differentiation on a Ti
disc (Fig. 3A). The mineralized
nodule formation in the SLPI-treated MC3T3-E1 cells was 1.3 and 1.8
times higher than that of the untreated cells at 4 and 10 days,
respectively, during differentiation on a Ti disc (P<0.05;
Fig. 3B). However, at day 7 of
differentiation, no difference in the mineralized nodule formation
was observed between untreated and SLPI-treated cells.
ALP, non-collagenous and collagenous gene
expression in SLPI-treated MC3T3-E1 cells during differentiation on
Ti discs
RT-PCR analysis showed that ALP, DSPP, DMP-1, BSP,
and Col I mRNA expression was higher in the SLPI-treated MC3T3-E1
cells compared with that of the untreated cells during
differentiation on a Ti disc (Fig.
4A). ALP mRNA expression in the SLPI-treated MC3T3-E1 cells was
higher than that of the untreated cells at 4, 7 and 10 days during
differentiation on a Ti disc. The DSPP and Col I mRNA expression in
the SLPI-treated MC3T3-E1 cells were higher than that of the
untreated cells at 4 and 7 days during differentiation on a Ti
disc. The DMP1 and BSP mRNA expression in the SLPI-treated MC3T3-E1
cells was higher than that of the untreated cells at 7 days during
differentiation on a Ti-disc (P<0.05 and P<0.01,
respectively; Fig. 4B).
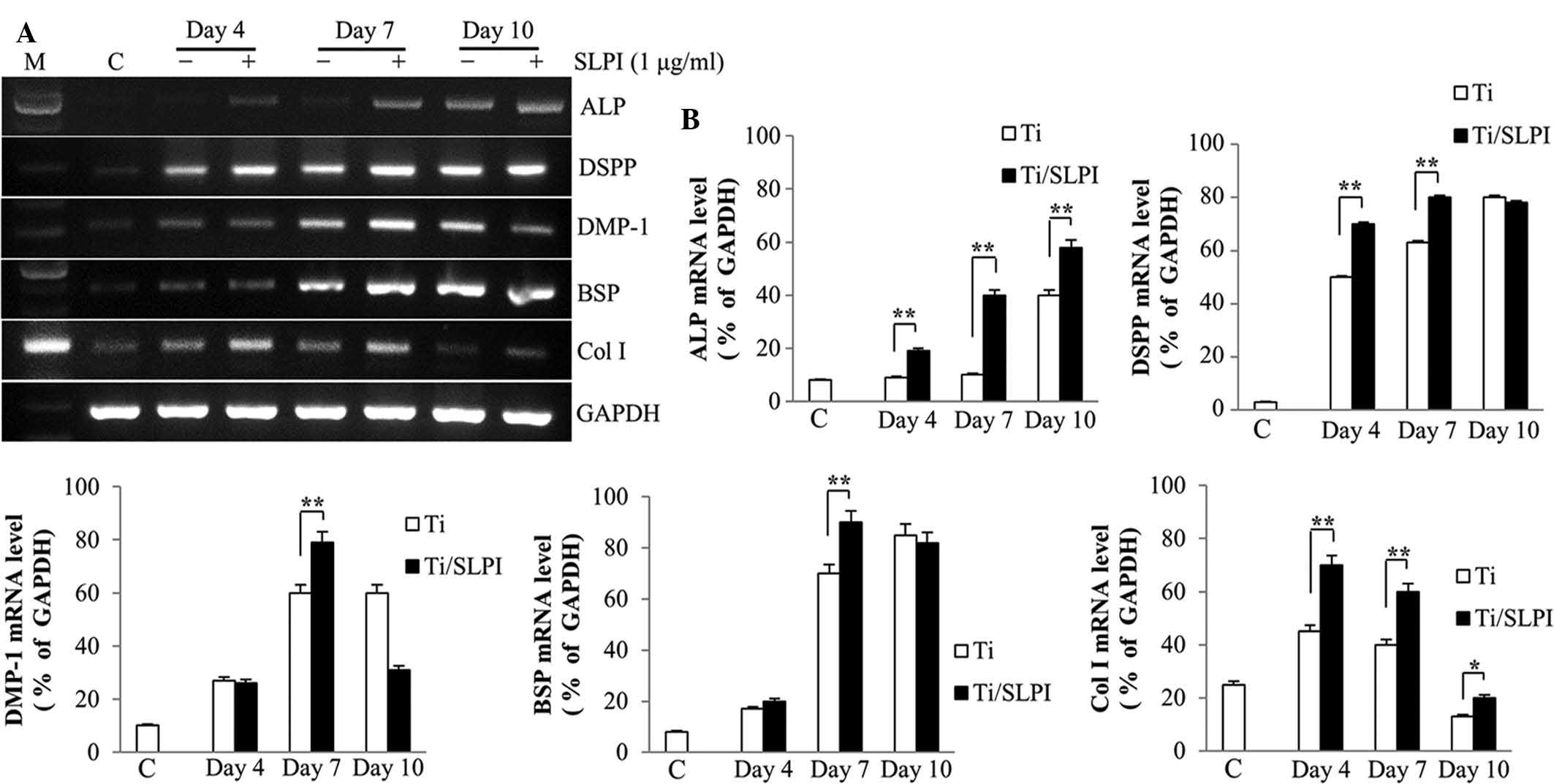 | Figure 4Expression of non-collagenous and
collagenous genes in SLPI-treated MC3T3-E1 cells during
differentiation on Ti discs. (A) SLPI increased the ALP, DSPP,
DMP-1, BSP and Col I mRNA expression in MC3T3-E1 cells compared
with that of the control during differentiation on Ti discs. (B)
Quantification of polymerase chain reaction results
(**P<0.01). SLPI, secretory leukocyte protease
inhibitor; Ti, titanium; ALP, alkaline phosphatase; DSPP, dentin
sialophosphoprotein; DMP-1, dentin matrix protein 1; BSP, bone
sialoprotein; Col I, collagen I; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; C, undifferentiated MC3T3-E1 cells without SLPI
treatment on Ti discs (control). |
Discussion
The cytotoxicity of the ions released from various
metallic implant materials, including Ti, results in decreases in
the proliferation of osteoblasts (11). In addition, Ti-particles decrease
the cell viability through the promotion of apoptosis (12). A recent study reported that SLPI
increases the proliferation of oral keratinocytes and it was
reported to be an effective nanomolecule for successful
implantation through an increase in osteoblast adhesion and
proliferation on a Ti surface (13). In addition, SLPI increases the cell
viability of pancreatic cancer cells by inhibiting apoptosis
(14). In this study, the level of
SLPI-treated MC3T3-E1 cell proliferation was higher than that of
the control during differentiation, and SLPI mRNA and protein
expression was significantly higher in the SLPI-treated MC3T3-E1
cells. Therefore, SLPI increases the viability of osteoblasts
during differentiation on a Ti surface.
Bone morphogenic protein, fibroblast growth factor 2
(FGF2) and transforming growth factor-β1 (TGF-β1) stimulate
mineralization and bone marrow mesenchymal stem cell (BMSC)
differentiation into osteoblasts. BMP-2 increases the ALP activity
in BMSCs and also stimulates differentiation into osteoblasts and
mineralization (15). In addition,
BMP-2 increases DSPP, BSP and DMP1 mRNA expression and
mineralization in dental pulp stem cells (16). Furthermore, BMP-2 treatment
increases the differentiation of osteoblasts on a Ti surface by
increasing the ALP activity (6).
FGF2 stimulates proliferation and differentiation and increases Col
I and BSP mRNA expression and differentiation of BMSCs (17). Calcium deposition is increased
during osteoblast differentiation on the Ti surface and the
expression of ALP and Col I is increased at the early stages of
differentiation by TGF-β1 treatment (18). In a recent study, SLPI mRNA and
protein expression was increased in the odontoblast layer of the
tooth germ cell during dentinogenesis, and the formation of
mineralized nodules was increased compared with that of the control
in MDPC-23 cells (an odontoblastic cell line) by an SLPI treatment
(19).
ALP is an essential enzyme at the early stages of
osteoblast differentiation and it increases gene expression
associated with osteoblast differentiation, such as Col I and
osteopontin (20). DSPP, an
odontoblast-specific gene, is secreted by odontoblasts and then
processed proteolytically into DSP and DPP proteins during the
formation of predentin (21). DPP
activates the initiation of hydroxyapatite formation during
dentinogenesis, and DSP regulates the initiation of dentin
mineralization (22,23). In addition, the expression of DSPP
was identified in the osteogenic cell lines, such as MC3T3-E1 and
ROS 17/2.8, as well as odontoblasts (24). DMP1 increases the formation of bone
nodules and mineralization as the nucleator of hydroxyapatite
(25). Among the factors
associated with differentiation and mineralization, BSP is one of
the component proteins in mineralized tissue, such as bone, dentin,
cementum, and calcified cartilage (26). BSP acts as a nucleator of early
apatite crystal formation and regulates the direction of crystal
growth to form ribbon-like apatite crystals during the
mineralization process (27). Col
I is an important protein in bone that triggers the differentiation
of osteoblasts and the level of Col I mRNA expression is decreased
in MC3T3-E1 cells during mineralization (28). In this study, SLPI increased the
expression of ALP, DSPP, DMP-1, BSP and Col I mRNA in MC3T3-E1
cells associated with the formation of mineralized nodules compared
with that of the control during differentiation on a Ti surface. A
comparison of the present results with other studies indicates that
SLPI can promote the differentiation and mineralization of MC3T3-E1
cells on a Ti surface. In previous studies, SLPI was shown to
increase MC3T3-E1 cell adhesion to a Ti-surface as well as the
proliferation of KB human oral carcinoma cells (9,13).
In addition, SLPI was reported to increase mineralized nodule
formation and expression of BSP, DSPP, osteocalsin, osteonectin and
Col I, which are associated with odontoblast differentiation and
mineralization, thereby acting as a stimulant of these processes
(19).
The present study was the first to report the
function of SLPI in osteoblast differentiation and mineralization
on a Ti surface. Previous studies have shown that SLPI regulates
the formation of dentin and mineralization of odontoblasts, and
that SLPI increases the adhesion and viability of pre-osteoblasts
on a Ti surface. The present study revealed that SLPI increases the
viability and differentiation of pre-osteoblasts during
osteoblastogenesis and stimulates mineralization through the
upregulation of ALP, DSPP, DMP1, BSP and Col I expression on a Ti
surface (Fig. 5). At present, the
most important challenge in implant fixation is the
osseointegration between osteoblasts and a Ti surface. Therefore,
the action of SLPI during mineralization and differentiation of
pre-osteoblasts can reduce failure of osseointegration and
consequently increase the success rate of implantation. This
suggested that SLPI may be an effective adjuvant for clinically
successful implantation by increasing the osseointegration on a Ti
surface after implant placement. The findings of the present study
strongly indicated that SLPI regulates the expression of factors
involved in the formation of bone matrix in osteoblasts. While the
signal transduction pathways of the involvement of SLPI in the
differentiation and mineralization in osteoblastic cells may be
further elucidated, the evidence provided by the present study
suggests that an in vivo study may be due to test the
efficacy of SLPI in improving dental implantation in an animal
system.
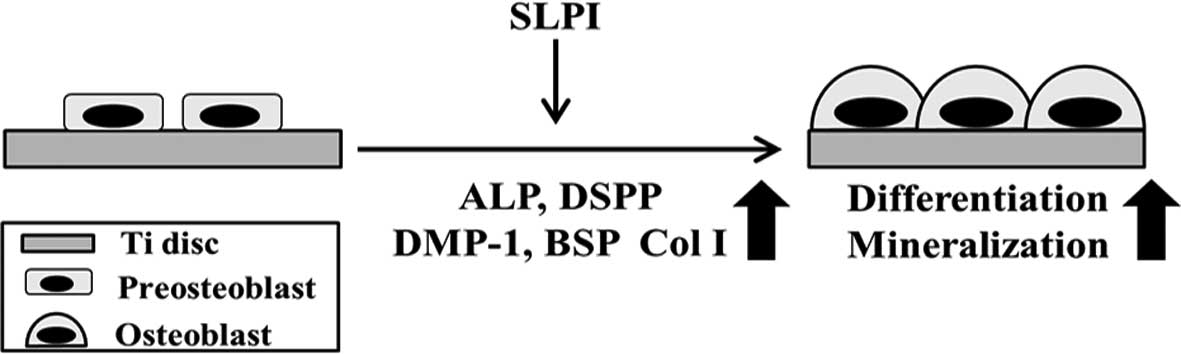 | Figure 5Schematic diagram illustrating the
beneficial effects of SLPI on osteoblastogenesis and mineralization
on Ti discs. SLPI increases the cell viability and differentiation
of preosteoblasts during osteoblastogenesis, and increases the
expression of ALP, DSPP, DMP-1, BSP, and Col I during the
mineralization of osteoblasts. SLPI, secretory leukocyte protease
inhibitor; ALP, alkaline phosphatase; DSPP, dentin
sialophosphoprotein; DMP-1, dentin matrix protein 1; BSP, bone
sialoprotein; Col I, collagen I. |
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) funded by the Ministry of Science, ICT
& Future Planning (grant no. R13-2008-010-01001-0).
References
|
1
|
Klumpers DD, Zhao X, Mooney DJ and Smit
TH: Cell mediated contraction in 3D cell-matrix constructs leads to
spatially regulated osteogenic differentiation. Integr Biol (Camb).
5:1174–1183. 2013. View Article : Google Scholar
|
|
2
|
Narayanan K, Ramachandran A, Hao J, He G,
Park KW, Cho M and George A: Dual functional roles of dentin matrix
protein 1. Implications in biomineralization and gene transcription
by activation of intracellular Ca2+ store. J Biol Chem.
278:17500–17508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kokubo T, Pattanayak DK, Yamaguchi S,
Takadama H, Matsushita T, Kawai T, Takemoto M, Fujibayashi S and
Nakamura T: Positively charged bioactive Ti metal prepared by
simple chemical and heat treatments. J R Soc Interface. 7(Suppl 5):
S503–S513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anner R, Grossmann Y, Anner Y and Levin L:
Smoking, diabetes mellitus, periodontitis and supportive
periodontal treatment as factors associated with dental implant
survival: A long-term retrospective evaluation of patients followed
for up to 10 years. Implant Dent. 19:57–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandrovcova M, Jirka I, Novotna K, Lisa V,
Frank O, Kolska Z, Stary V and Bacakova L: Interaction of human
osteoblast-like Saos-2 and MG-63 cells with thermally oxidized
surfaces of a titanium-niobium alloy. PLoS One. 9:e1004752014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olivares-Navarrete R, Hyzy SL, Pan Q, Dunn
G, Williams JK, Schwartz Z and Boyan BD: Osteoblast maturation on
microtextured titanium involves paracrine regulation of bone
morphogenetic protein signaling. J Biomed Mater Res A.
103:1721–1731. 2015. View Article : Google Scholar :
|
|
7
|
Thompson RC and Ohlsson K: Isolation,
properties, and complete amino acid sequence of human secretory
leukocyte protease inhibitor, a potent inhibitor of leukocyte
elastase. Proc Natl Acad Sci USA. 83:6692–6696. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi BD, Jeong SJ, Wang G, Kim HJ, Kim BO,
Hwang HK, Lim DS, Kim SH and Jeong MJ: Temporal induction of
secretory leukocyte protease inhibitor (SLPI) in odontoblasts by
lipopolysaccharide and wound infection. J Endod. 35:997–1002. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong SJ, Wang G, Choi BD, Hwang YH, Kim
BH, Ko YM and Jeong MJ: Secretory leukocyte protease inhibitor
(SLPI) increases focal adhesion in MC3T3-E1 osteoblast on titanium
surface. J Nanosci Nanotechnol. 15:200–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao Y, Geng Y and Jing H: Effect of
hirudin on the levels of acute lung injury rat tumor necrosis
factor-α and matrix metalloproteinase-12. Mol Med Rep. 5:873–875.
2012.PubMed/NCBI
|
|
11
|
Li Y, Wong C, Xiong J, Hodgson P and Wen
C: Cytotoxicity of titanium and titanium alloying elements. J Dent
Res. 89:493–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pioletti DP, Leoni L, Genini D, Takei H,
Du P and Corbeil J: Gene expression analysis of osteoblastic cells
contacted by orthopedic implant particles. J Biomed Mater Res.
61:408–420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G, Lim DS, Choi BD, Park JJ, Jeong
SJ, Kim JS, Kim JD, Park JS, Kim EK, Kim BH, et al: Effect of
secretory leukocyte protease inhibitor on migration and invasion of
human KB oral carcinoma cells. Anim Cells Syst. 15:139–146. 2011.
View Article : Google Scholar
|
|
14
|
Zuo J, Zhang C, Ren C, Pang D, Li Y, Xie
X, Tang Z and Jiang X: Secretory leukocyte protease inhibitor is a
proliferation and survival factor for pancreatic cancer cells. Clin
Transl Oncol. 17:314–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JY, Kim MR and Kim SJ: Modulation of
osteoblastic/odontoblastic differentiation of adult mesenchymal
stem cells through gene introduction: A brief review. J Korean
Assoc Oral Maxillofac Surg. 39:55–62. 2013. View Article : Google Scholar
|
|
16
|
Yang X, van der Kraan PM, Bian Z, Fan M,
Walboomers XF and Jansen JA: Mineralized tissue formation by
BMP2-transfected pulp stem cells. J Dent Res. 88:1020–1025. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh SA, Lee HY, Lee JH, Kim TH, Jang JH,
Kim HW and Wall I: Collagen three-dimensional hydrogel matrix
carrying basic fibroblast growth factor for the cultivation of
mesenchymal stem cells and osteogenic differentiation. Tissue Eng
Part A. 18:1087–1100. 2012. View Article : Google Scholar
|
|
18
|
Zhang H, Ahmad M and Gronowicz G: Effects
of transforming growth factor-beta 1 (TGF-beta1) on in vitro
mineralization of human osteoblasts on implant materials.
Biomaterials. 24:2013–2020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong JO, Wang G, Jeong SJ, Choi BD, Lee
HY and Jeong MJ: Function of secretory leukocyte protease inhibitor
(SLPI) in odontoblast during mouse tooth development. J Nanosci
Nanotechnol. 15:120–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MB, Song Y and Hwang JK: Kirenol
stimulates osteoblast differentiation through activation of the BMP
and Wnt/β-catenin signaling pathways in MC3T3-E1 cells.
Fitoterapia. 98:59–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
George A, Bannon L, Sabsay B, Dillon JW,
Malone J, Veis A, Jenkins NA, Gilbert DJ and Copeland NG: The
carboxyl-terminal domain of phosphophoryn contains unique extended
triplet amino acid repeat sequences forming ordered
carboxyl-phosphate interaction ridges that may be essential in the
biomineralization process. J Biol Chem. 271:32869–32873. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito T, Arsenault AL, Yamauchi M, Kuboki
Y and Crenshaw MA: Mineral induction by immobilized
phosphoproteins. Bone. 21:305–311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki S, Haruyama N, Nishimura F and
Kulkarni AB: Dentin sialophosphoprotein and dentin matrix
protein-1: Two highly phosphorylated proteins in mineralized
tissues. Arch Oral Biol. 57:1165–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin C, Brunn JC, Cadena E, Ridall A and
Butler WT: Dentin sialoprotein in bone and dentin
sialophosphoprotein gene expressed by osteoblasts. Connect Tissue
Res. 44(Suppl 1): S179–S183. 2003. View Article : Google Scholar
|
|
25
|
He G, Dahl T, Veis A and George A:
Nucleation of apatite crystals in vitro by self-assembled dentin
matrix protein 1. Nat Mater. 2:552–558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Macneil RL, Sheng N, Strayhorn C, Fisher
LW and Somerman MJ: Bone sialoprotein is localized to the root
surface during cementogenesis. J Bone Miner Res. 9:1597–1606. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hunter GK and Goldberg HA: Modulation of
crystal formation by bone phosphoproteins: Role of glutamic
acid-rich sequences in the nucleation of hydroxyapatite by bone
sialoprotein. Biochem J. 302:175–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: An in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|