Introduction
Anxiety-associated disorders constitute the largest
class of human psychopathologies and represent a major public
health problem (1,2). While the causes of these disorders
largely remain elusive, interactions between environmental and
genetic factors are thought to be crucial for their pathogenesis
and progression (2,3). In addition, various complexities of
the central nervous system make anxiety-associated disorders
exceptionally difficult to diagnose and treat. At present,
serotonin-norepinephrine re-uptake inhibitors, selective serotonin
re-uptake inhibitors and pregabalin are the first-line drugs used
to clinically treat anxiety-associated disorders (4,5).
However, side effects can limit their long-term use (5) and therefore, substantial effort has
been made to develop novel drugs to treat anxiety-associated
disorders.
Traditional Chinese Medicine (TCM) is widely used
for treating a variety of conditions, including anxiety-associated
disorders (6,7). Xiao-yao-san and its modified
formulations, including Dan-zhi-xiao-yao-san, are most commonly
used in China and other Asian countries. According to the theories
of TCM, Xiao-yao-san formulations repair the liver, promote liver
qi circulation, nourish the blood in the liver and fortify the
spleen, and these formulations are used clinically to treat a
variety of conditions, including menopausal syndrome, anemia,
functional uterine bleeding, hepatitis, chronic gastritis, pelvic
inflammatory disease, anxiety and depression (8–10).
Dan-zhi-xiao-yao-san is composed of Atractylodis
macrocephale rhizoma, Bupleuri radix, Angelicae
sinensis, poria, Glycyrrihizae radix, tree peony bark,
Gardenia jasminoides, Paeonia lactiflora Pall, mint and
roasted ginger. Although Dan-zhi-xiao-yao-san is widely used
clinically in China, its underlying mechanisms of action have
largely remained elusive. Previous studies have suggested that
Dan-zhi-xiao-yao-san prevents dimethylnitrosamine-induced hepatic
fibrosis by functioning as an antioxidant (11), and exerts antidepressive effects by
modulating neurosteroids (12,13).
However, the anxiolytic and neuroprotective effects of
Dan-zhi-xiao-yao-san and their underlying molecular mechanisms
remain to be determined.
The present study demonstrated the anxiolytic and
neuroprotective effects of Dan-zhi-xiao-yao-san in an animal model
of chronic stress. Furthermore, mechanistic investigation suggested
that the mechanism of action of this formulation may involve the
modulation of expression levels of protein phosphatase 2A (PP2A),
α-synuclein and corticosterone.
Materials and methods
Drugs and chemicals
The commercially available formulation
Dan-zhi-xiao-yao-san was obtained from E-FONG Pharmaceutical Corp.
(Guangzhou, China). The manufacturer performed quality control
testing of individual formulation components to ensure their
uniformity. The formulation contained the following 10 medicinal
herbs: Atractylodis macrocephale rhizoma, Bupleuri
radix, Angelicae sinensis, poria, Glycyrrihizae radix, tree
peony bark, Gardenia jasminoides, Paeonia lactiflora Pall,
mint and roasted ginger. The powder was dissolved in 0.9% NaCl
prior to use. Mifepristone was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Monoclonal mouse anti-α-synuclein (ab27766),
polyclonal rabbit anti-PP2A (ab137825) and monoclonal mouse
anti-glyc-eraldehyde-3-phosphate dehydrogenase (GAPDH; ab8245) were
obtained from (Abcam, Cambridge, UK). Unless otherwise indicated,
all other chemicals were purchased from Sigma-Aldrich.
Animals and drug intervention
Female Sprague-Dawley rats (n=50; aged, 2–3 months;
weight, 200–250 g) were bred in the Laboratory Animal Care Facility
at Guangzhou University of Chinese Medicine (Guangzhou, China) and
maintained under constant conditions of 22±1°C, 60±10% relative
humidity and a 12 h light/dark cycle. All animal studies were
performed in accordance with international ethical standards, and
the study protocols were approved by the Animal Care and Use
Committee of Guangzhou University of Chinese Medicine (Guangzhou,
China). The rats were randomly assigned to one of five groups
(n=10/group), which included a control group, chronic stress group
(Model), chronic stress + low-dose Dan-zhi-xiao-yao-san group (Low;
5.256 g/kg Dan-zhi-xiao-yao-san), chronic stress + high-dose
Dan-zhi-xiao-yao-san group (High; 52.56 g/kg Dan-zhi-xiao-yao-san),
and a chronic stress + mifepristone group (25 mg/kg mifepristone as
a positive control). The chronic stress model was generated, as
previously described with certain modifications (14). Briefly, the rats were subjected to
a stress-inducing condition once daily over a period of 21 days.
The order of stressor used was as follows: i) Food deprivation for
24 h, ii) water deprivation for 24 h; iii) electric foot shocks for
30 min (1 mA; duration, 1 sec; average frequency, 1 shock/min), iv)
forced swimming at 4°C for 5 min and v) immobilization for 6 h. The
rats in the drug treatment groups were administered the specified
dose of Dan-zhi-xiao-yao-san or mifepristone by intragastric
administration each day, 4 h prior to exposure to the stressful
condition, whereas rats in the control group were administered the
same volume of distilled water. The rats in all five groups were
sacrificed 24 h after their final exposure to stressful
conditions.
Elevated plus maze test
The elevated plus maze test was performed, as
previously described (15).
Briefly, the elevated plus maze consisted of two closed arms, each
49 cm long, 10 cm wide and 30 cm high, and two open arms. The arms
of the apparatus are elevated 50 cm above the ground. Each rat is
placed at the junction of the open and closed arms with the head
pointed towards one open arm. The data regarding the rat
performance in the maze test were automatically recorded using the
Any-maze video tracking system (Stoelting Company; Wood Dale, IL,
USA). Any-Maze can be programmed to automatically record all
possible indices in each task and digitally records a video of a
rat's performance in each test. The number of entries and the
duration spent on the open arms are recorded for periods of 5 min.
An increase in open arm activity (duration and/or number of
entries) reflects anti-anxiety behavior.
Hematoxylin and eosin (HE) staining of
tissue sections
The neuroprotective effects of Dan-zhi-xiao-yao-san
were examined by observing the histological integrity and damage to
tissue in the hippocampus of each rat brain following staining with
HE. Briefly, each rat brain was isolated and cut into 5 µm
sections. The sections were subsequently stained using a
combination of Mayer's hematoxylin and 0.5% aqueous eosin and
observed under a FV1000 light microscope (Olympus, Tokyo, Japan).
When using this method, the nucleus and other acidic structures in
the cells were stained blue, while the cytoplasm was stained
red.
Immunohistochemical staining
Expression levels of α-synuclein and PP2A in the
hippocampus region of the sections of rat midbrain were examined by
immunohistochemical staining, as previously described (16). Briefly, the paraffin sections of
rat midbrain were deparaffinized and hydrated. The antigenic sites
were exposed by incubation in 10 mM citrate buffer (pH 6.0) at
90°C, and endogenous peroxidase activity was quenched. Following
blocking with 5% bovine serum albumen in phosphate-buffered saline
with Tween-20, the tissue sections were incubated with primary
antibodies, followed by incubations with goat anti-rabbit IgG
conjugated to horseradish peroxidase (HRP; ab97051, Abcam)and
3,3-N-diaminobenzidine tertrahydrochloride (Solarbio, Beijing,
China) to reveal α-synuclein and PP2A expression. The sections were
subsequently re-stained with HE and observed under a microscope
(FV1000; Olympus).
Western blotting analysis
Protein was extracted using radioimmuniprecipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
and centrifuged at 1,000 × g for 5 min at 4°C. The protein
concentration in the resulting supernatant was quantified using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Samples of denatured protein (20 µg) were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto a polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). Following blocking with non-fat milk for 2 h
at room temperature, the membrane was incubated overnight at 4°C
with antibodies against α-synuclein, PP2A, and GAPDH (1:500).
Following primary antibody incubation, the membrane was incubated
with the goat anti-rabbit HRP-conjugated secondary antibody
(ab97051, Abcam) for 1 h at room temperature. Enhanced
chemiluminescence Advance Western blotting detection reagents (GE
Healthcare, Buckinghamshire, UK) were used for detection. The
relative expression of α-synuclein and PP2A, relative to GAPDH,
were quantified using ImageJ software (National Institutes of
Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Samples of brain tissue from the hippocampus region
were analyzed for the mRNA expression of α-synuclein, PP2A and an
internal control, β-actin, by RT-qPCR. Briefly, the total RNA was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). cDNA synthesis was performed
using 2 µg RNA and a High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). The mRNA
expression of α-synuclein, PP2A and β-actin were detected and
amplified by real-time PCR using a Bestar™ Real time PCR Master Mix
kit (Biomart, Beijing, China). The final expression levels were
calculated following normalization against the expression of
β-actin. A total of three independent experiments were performed,
and each analysis was performed in triplicate. The forward and
reverse primers (Sangon Biotech, Shanghai, China) used are shown in
Table I.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Gene | GenBank accession
no. | Primer sequence
(5′→3′) |
|---|
| α-Synuclein | NM_019169.2 | F:
CCCACAAGAGGGAATCCTGG |
| | R:
AAGCCTCACTGCTAGGGTCC |
| PP2A | NM_053999.2 | F:
GAGGCGAGCCACATGTCACT |
| | R:
CCATTAGGTCAACAGACGGTGTT |
| β-actin | NM_031144.3 | F:
GGAGATTACTGCCCTGGCTCCTA |
| | R:
GACTCATCGTACTCCTGCTTGCTG |
Determination of corticosterone contents
by enzyme-linked immunosorbent assay (ELISA)
Plasma samples were analyzed for corticosterone
levels using an ELISA kit (cat. no. K014-H1; Arbor Assays, Ann
Arbor, MI, USA) and following instructions provided by the
manufacturer. All analyses were performed in triplicate.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistically significant differences between the groups
were identified by one-way analysis of variance, followed by the
paired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
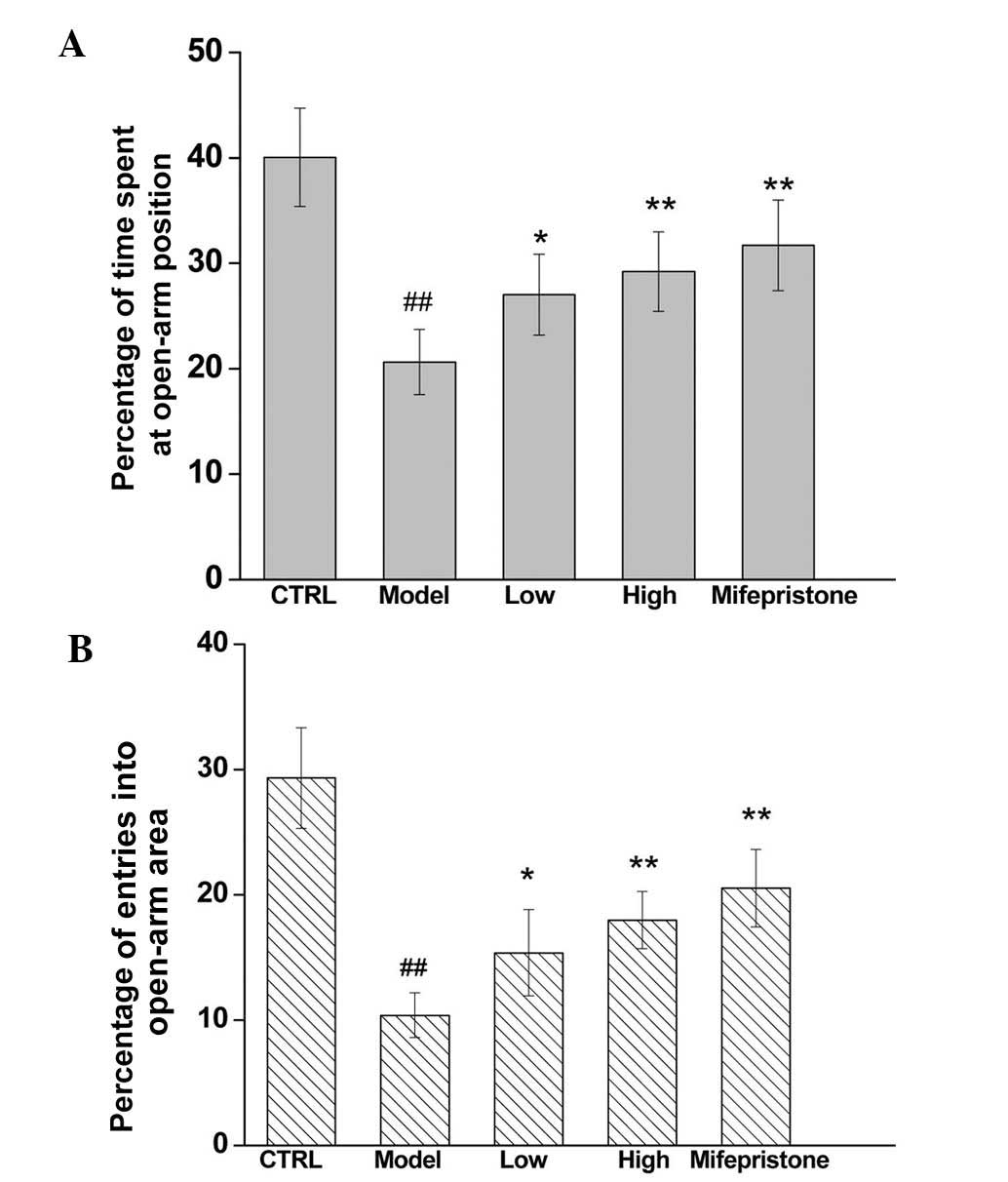
Antianxiety effect of
Dan-zhi-xiao-yao-san
The elevated plus maze test is a behavioral assay
widely used to measure the effects of antianxiety agents in rodents
(15), and the percentage of time
spent in open arms and the number of entries into open arms in this
test are sensitive biomarkers for levels of anxiety (15). In the present study, both open arm
durations and open arm entries were significantly decreased in the
chronic stress rat model (Fig. 1),
suggesting that the model can be used to produce an anxiety-like
effect. Rats treated with either Dan-zhi-xiao-yao-san or
mifepristone for 21 consecutive days by intragastric administration
exhibited significantly decreased levels of anxiety as evidenced by
their lesser degrees of decrease in open arm durations and open arm
entries (Fig. 1), indicating that
both Dan-zhi-xiao-yao-san and mifepristone exerted dose-dependent
antianxiety effects.
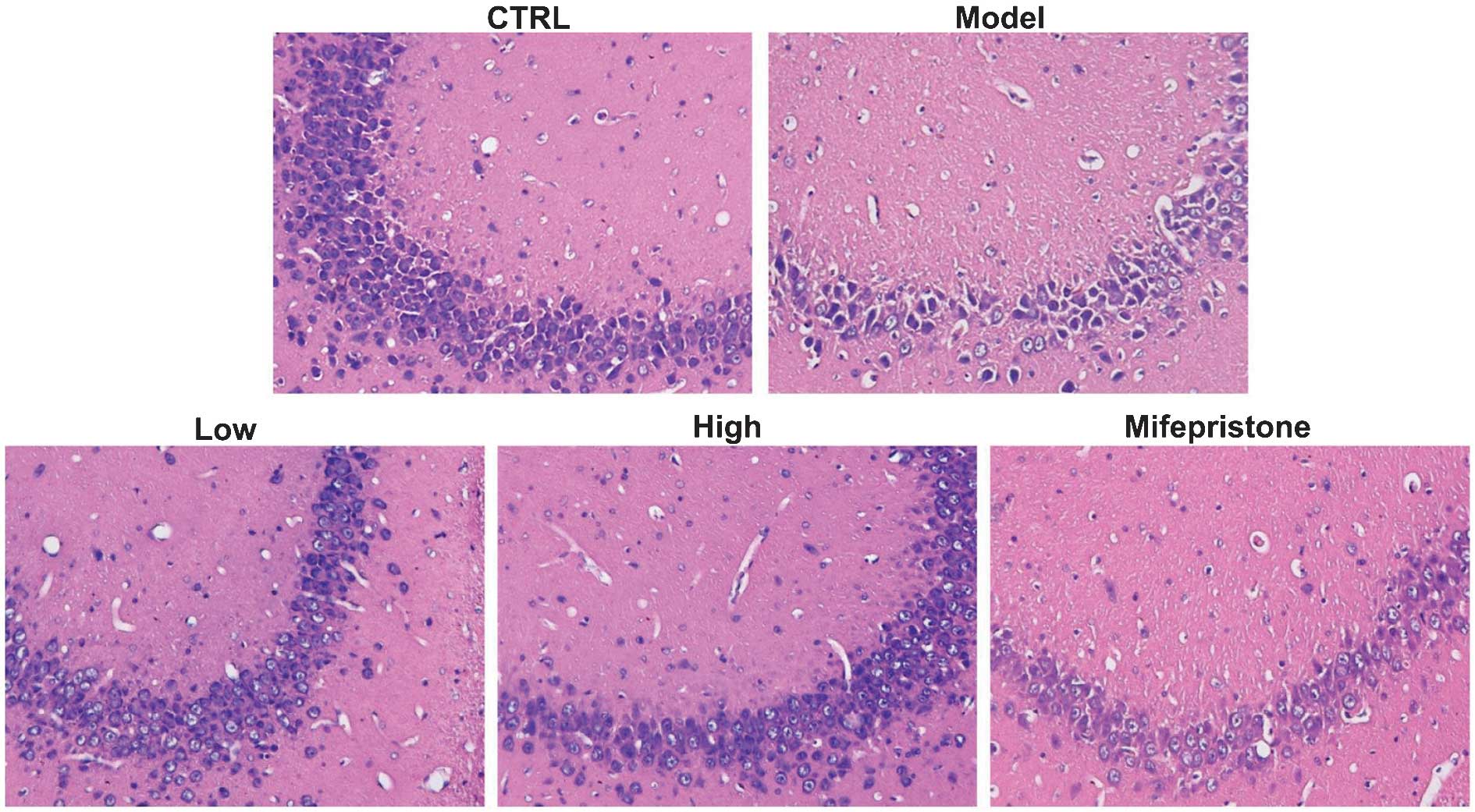
Neuroprotective effect of
Dan-zhi-xiao-yao-san
Microscopic examinations of rat mid-brain areas
stained with HE revealed that chronic stress induced degenerative
changes in the hippocampus region of stressed rats when compared
with non-stressed rats (Fig. 2).
Rats in the model group exhibited neuronal cells with pyknotic
nuclei, which were clearly distinguishable from viable neuronal
cells in the control group that displayed round and pale stained
nuclei. However, following intragastric administration of either
Dan-zhi-xiao-yao-san or mifepristone for 21 consecutive days,
neuronal cell death was significantly inhibited. These results
demonstrated that Dan-zhi-xiao-yao-san exerted a neuroprotective
effect in animals subjected to chronic stress.
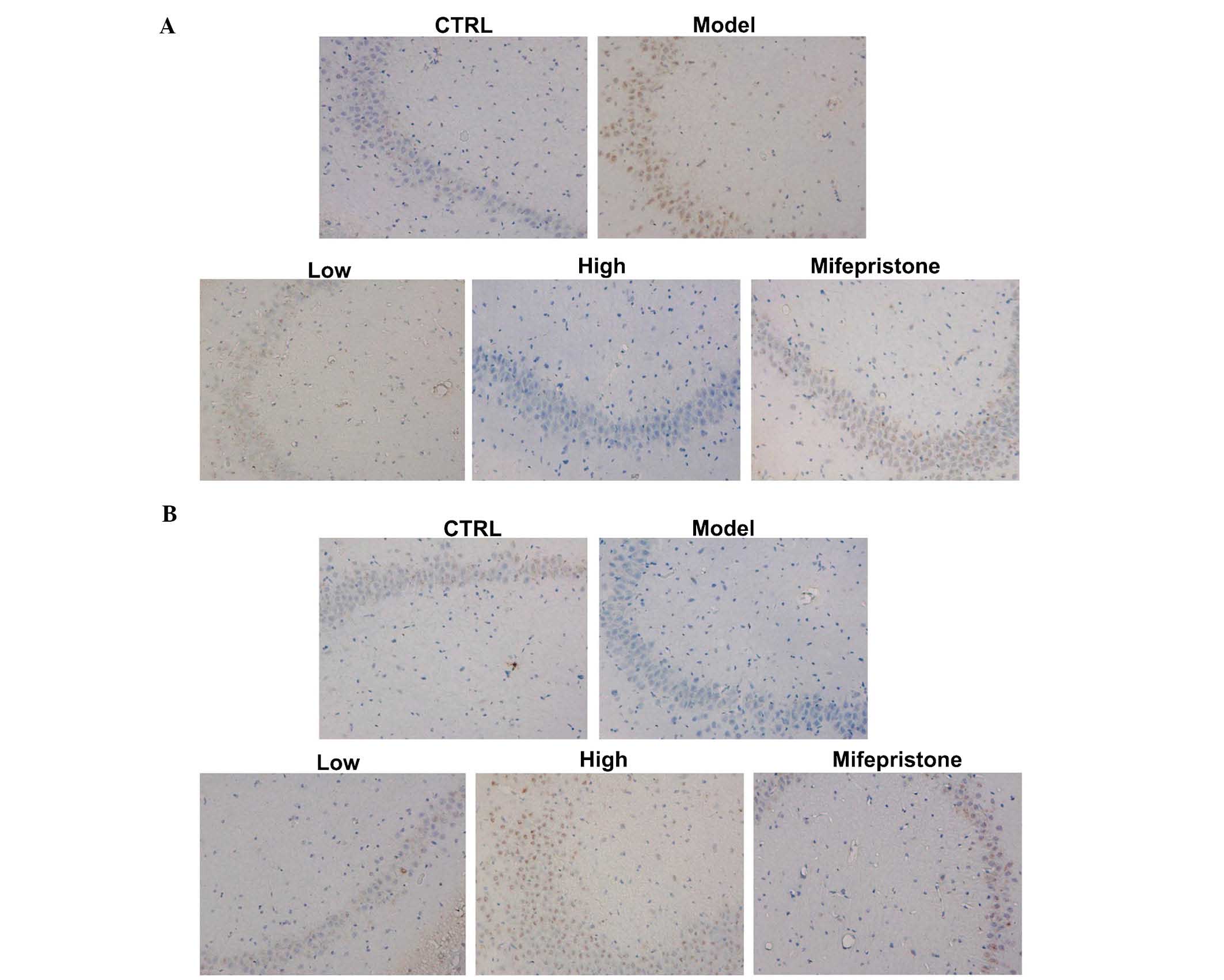
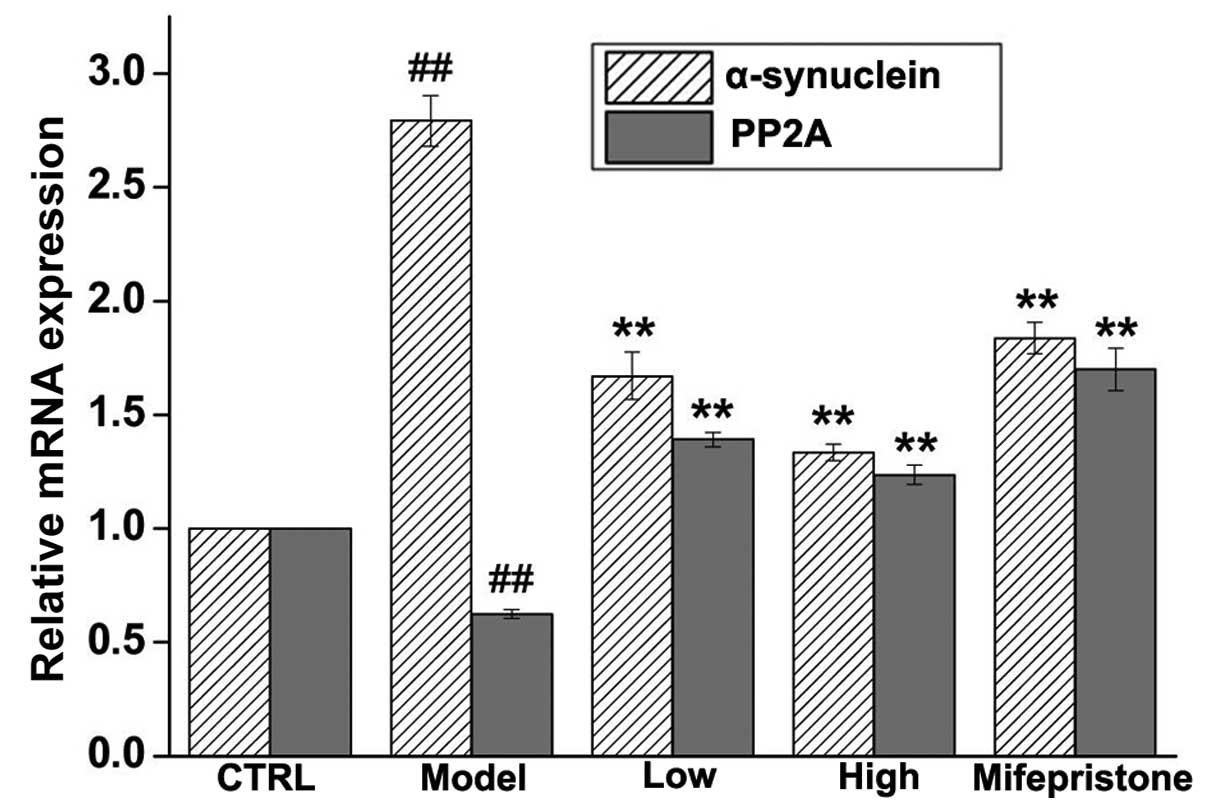
Dan-zhi-xiao-yao-san modulates the
expression of α-synuclein and PP2A
The effects of our chronic stress model on the
expression of α-synuclein and PP2A proteins were assessed in the
hippocampus region by immunohistochemical methods. As shown in
Fig. 3A and B, chronic stress
induced increased expression of α-synuclein and decreased
expression of PP2A, when compared with these expression levels in
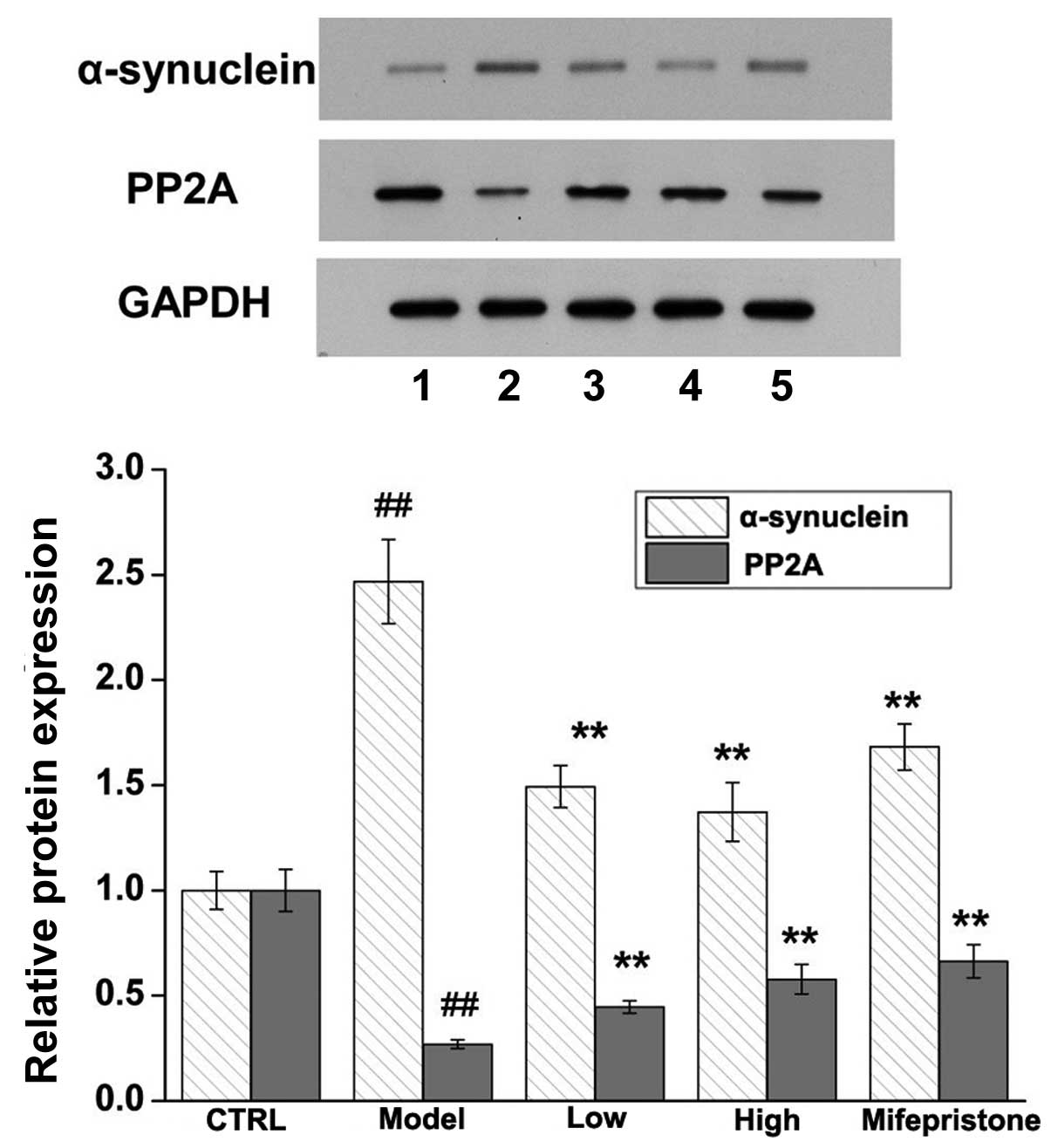
the control group. Next, the protein expression levels of
α-synuclein and PP2A were assessed by western blotting. Similar to
the immunohistochemical staining, it was revealed that chronic
stress significantly upregulated the expression of α-synuclein and
downregulated the expression of PP2A in the hippocampus region when
compared with these expression levels in the control group
(Fig. 4). Whether the induced
changes in the protein expression levels of α-synuclein and PP2A
were modulated via expression of their respective mRNAs was next
determined. RT-qPCR revealed that exposure to chronic stress
induced increased expression of α-synuclein and decreased
expression of PP2A, by modulating their respective mRNA levels
(Fig. 5). The effects of
Dan-zhi-xiao-yao-san and mifepristone on chronic stress-induced
changes in α-synuclein and PP2A were determined by
immunohistochemical, western blotting and RT-qPCR methods. As shown
in Figs. 3Figure 4–5, Dan-zhi-xiao-yao-san markedly inhibited
chronic stress-induced upregulation of α-synuclein. Additionally,
Dan-zhi-xiao-yao-san markedly inhibited chronic stress-induced
downregulated of PP2A. Finally, as a positive control, mifepristone
reversed the chronic stress-induced changes in α-synuclein and PP2A
expression. Collectively, these results suggested that
Dan-zhi-xiao-yao-san modulated changes in the expression levels of
α-synuclein and PP2A, induced by chronic stress.
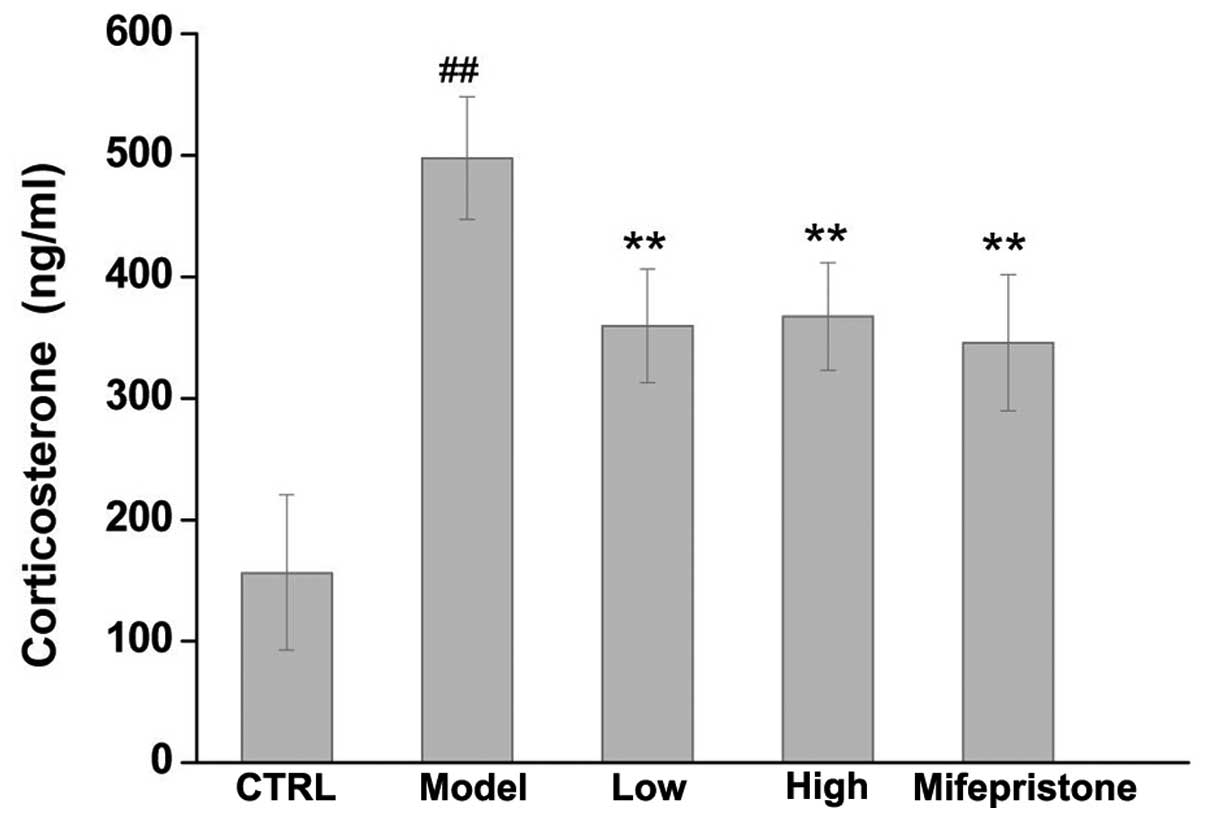
Dan-zhi-xiao-yao-san modulates the
expression of corticosterone
The present study next investigated the effects of
Dan-zhi-xiao-yao-san on chronic stress-induced expression of
corticosterone using ELISA to determine the levels of
corticosterone in rat serum. As shown in Fig. 6, the levels of corticosterone in
serum samples from the model group were significantly higher
compared with those in the control group. By contrast, both
Dan-zhi-xiao-yao-san and mifepristone significantly reversed the
chronic stress-induced changes in corticosterone levels.
Discussion
Dan-zhi-xiao-yao-san is one of several herbal
medicines commonly used for treating various diseases in China,
including anxiety-associated diseases, including menopausal
syndrome, insomnia and depression (17–19).
However, the underlying mechanisms for its clinical effects are
poorly understood. In the present study, Dan-zhi-xiao-yao-san
exhibited antianxiety and neuroprotective effects in a rat model of
chronic stress. It was demonstrated that Dan-zhi-xiao-yao-san
significantly inhibited stress-induced upregulation of α-synuclein
and downregulation of PP2A in the hippocampus region of rat brains.
Additionally, Dan-zhi-xiao-yao-san also significantly attenuated
stress-induced upregulation of corticosterone. It was hypothesized
that these findings may also explain the clinical effects of
Dan-zhi-xiao-yao-san in patients with anxiety-associated diseases.
It was noted that the antianxiety effects of Dan-zhi-xiao-yao-san
were comparable to those shown by mifepristone, suggesting that
Dan-zhi-xiao-yao-san and traditional anxiolytics, including
mifepristone, may exhibit similar clinical efficacies.
When exposed to conditions of stress, the body makes
adaptive changes to maintain homeostasis. However, high intensity
stressors may disrupt the body's adaptive abilities (20), resulting in organ damage and
disease. In fact, stress has an important role in the
pathophysiology of several neurological diseases, including
Parkinson's disease, Alzheimer's disease, post-traumatic stress
disorder, depression and schizophrenia (20–22).
In addition, increasing evidence indicates that stressful
experiences, which occur throughout life, are crucial for the
development and pathogenesis of several psychiatric disorders,
including anxiety and depression (23). In animal models, chronic stressors
cause behavioral changes associated with anxiety disorders
(24,25); for example, foot shock, exposure to
cold water and immobilization have been reported to induce
anxiety-like effects in rodents (14,26).
In our animal model, stress was produced by exposing
rats to a stress-inducing condition (either electric foot shocks,
swimming in ice-cold water or immobilization) once daily, at
randomly selected times, for 21 consecutive days. The present study
subsequently used the elevated plus maze test to determine whether
the rats exhibited anxiety-associated symptoms and behaviors. When
using the elevated plus maze test, the percentage of time spent by
a rat in open arms and the number of open arm entries are sensitive
biomarkers for anxiety (15). As
shown in Fig. 1, the open arm
activity (duration and entries) by rats exposed to our chronic
stress model were significantly decreased, suggesting that the
stress model caused anxiety-like effects in rats. Additionally,
this chronic stress model was used to test the antianxiety effects
of Dan-zhi-xiao-yao-san. The results revealed that
Dan-zhi-xiao-yao-san significantly attenuated chronic
stress-induced anxiety in a dose-dependent manner, when compared
with results shown by control rats. Mifepristone is a well
established antianxiety agent (27), and in the present study, this
compound was used as a positive control to assist with confirming
the pharmacological effects of Dan-zhi-xiao-yao-san. It was
revealed that a high dose of Dan-zhi-xiao-yao-san produced effects
similar to those produced by mifepristone in the elevated plus maze
test, further confirming the marked antianxiety effect of
Dan-zhi-xiao-yao-san.
Several neurodegenerative diseases, including
Parkinson's and Alzheimer's disease, are accompanied by neuronal
death and feelings of anxiety (28,29).
Previous studies with rats have shown that extreme chronic stress
may cause neuronal injuries, which selectively affect hippocampus
structure (30). Therefore, in the
present study, HE staining was used to investigate the effects of
Dan-zhi-xiao-yao-san on stress-induced neuronal death, and
particularly in the hippocampus region. Rats exposed to chronic
stress exhibited hippocampus neurons with pyknotic nuclei,
indicative of neuronal death, while rats in the
Dan-zhi-xiao-yao-san-treated groups displayed viable neurons with
round and pale stained nuclei. These results indicated that
Dan-zhi-xiao-yao-san attenuated stress-induced neuronal death.
Additionally, the positive control compound, mifepristone, also
attenuated stress-induced neuronal death in the hippocampus
region.
After finding that Dan-zhi-xiao-yao-san exerted
marked antianxiety and neuroprotective effects, the present study
examined the possible mechanisms for those effects. α-synuclein,
the predominant component of Lewy bodies, serves an important role
in the pathogenesis of Parkinson's and several other diseases
(31). In addition, anxiety is a
well-known feature of anxiety-associated diseases, including
Parkinson's disease (32), and it
is believed that α-synuclein may be important in anxiety. Results
of previous studies have suggested that a genomic region located
close to the α-synuclein gene may be associated with
anxiety-associated behaviors in animals (33). Additionally, the levels of
α-synuclein are known to be elevated in the hippocampus of innate
anxiety rats (34). In the present
study, immunohistochemistry, western blotting and RT-qPCR were used
to show that exposure to chronic stress upregulated the mRNA and
protein expression levels of α-synuclein in the rat hippocampus
region. However, treatment with either Dan-zhi-xiao-yao-san or
mifepris-tone significantly inhibited these stress-induced
increases. Furthermore, Dan-zhi-xiao-yao-san inhibited the
expression of α-synuclein in a dose-dependent manner, and a high
dose of Dan-zhi-xiao-yao-san was more effective than mifepristone
for downregulating the expression of α-synuclein. PP2A is important
for the regulation of MAP kinases, including extracellular
signal-regulated kinases 1/2, c-Jun N-terminal kinases and p38
(35), and also serves important
roles in regulating tyrosine hydroxylase synthesis and α-synuclein
expression (36). In addition,
α-synuclein reduces the expression and activity of PP2A (37). In the present study, it was
revealed that exposure to chronic stress significantly decreased
PP2A expression, whereas Dan-zhi-xiao-yao-san and mifepristone
reversed this effect at both the mRNA and protein levels. The
neuroprotective effects of Dan-zhi-xiao-yao-san may be partially
attributable to its regulation of the expression levels of
α-synuclein and PP2A. Taken together, these results showed that the
antianxiety and neuroprotective effects of Dan-zhi-xiao-yao-san may
involve regulation of α-synuclein and PP2A expression.
Finally, the effects of Dan-zhi-xiao-yao-san on
corticosterone expression in the rat model of chronic stress were
assessed. Corticosterone is the predominant glucocorticoid produced
in the cortex of rodent adrenal glands (38). It is well established that
conditions which produce stress and anxiety induce expression of
corticosterone, which subsequently induces anxiety (39–41).
It was revealed that rats in our chronic stress model exhibited
high levels of corticosterone in their blood serum (Fig. 6). By contrast, treatment with
either Dan-zhi-xiao-yao-san or mifepristone significantly decreased
these stress-induced elevated levels of corticosterone.
In conclusion, the present results showed that
Dan-zhi-xiao-yao-san had both antianxiety and neuroprotective
effects in the rat model of chronic stress. The underlying
mechanisms for these effects may involve regulation of α-synuclein
and PP2A expression, as well as modulation of corticosterone
expression. The present study assists with elucidating possible
mechanisms for the antianxiety effect of Dan-zhi-xiao-yao-san, and
may provide evidence supporting the clinical use of this
formulation. However, the individual herbs and other predominant
components responsible for the antianxiety and neuroprotective
effects of Dan-zhi-xiao-yao-san remain to be clearly identified,
and the molecular pathways and underlying mechanisms responsible
for those effects require further study.
Acknowledgments
The present study was supported by the National
Natural Science foundation of China (no. 81403304), the Shenzhen
Municipal Science and Technology Innovation Council (no.
JCYJ20130328154910812) and the Xinhuo Program in Guangzhou
University of Chinese Medicine (no. XH20140106). The authors would
like to thank Professor Zhi-yu Wang (Guangzhou University of
Chinese Medicine, Guangzhou, China) for english language editing of
the manuscript.
References
|
1
|
Demyttenaere K, Bruffaerts R, Posada-Villa
J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de
Girolamo G, Morosini P, et al: Prevalence, severity, and unmet need
for treatment of mental disorders in the World Health Organization
World Mental Health Surveys. JAMA. 291:2581–2590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hettema JM, Neale MC and Kendler KS: A
review and meta-analysis of the genetic epidemiology of anxiety
disorders. Am J Psychiatry. 158:1568–1578. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sachser N and Lesch KP: The interplay of
genotype and environment in the development of fear and anxiety.
e-Neuroforum. 4:57–62. 2013. View Article : Google Scholar
|
|
4
|
Bandelow B, Sher L, Bunevicius R,
Hollander E, Kasper S, Zohar J and Möller HJ; WFSBP Task Force on
Mental Disorders in Primary Care; WFSBP Task Force on Anxiety
Disorders, OCD and PTSD: Guidelines for the pharmacological
treatment of anxiety disorders, obsessive-compulsive disorder and
post-traumatic stress disorder in primary care. Int J Psychiatry
Clin Pract. 16:77–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farach FJ, Pruitt LD, Jun JJ, Jerud AB,
Zoellner LA and Roy-Byrne PP: Pharmacological treatment of anxiety
disorders: Current treatments and future directions. J Anxiety
Disorders. 26:833–843. 2012. View Article : Google Scholar
|
|
6
|
Bystritsky A, Hovav S, Sherbourne C, Stein
MB, Rose RD, Campbell-Sills L, Golinelli D, Sullivan G, Craske MG
and Roy-Byrne PP: Use of complementary and alternative medicine in
a large sample of anxiety patients. Psychosomatics. 53:266–272.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarris J, McIntyre E and Camfield DA:
Plant-based medicines for anxiety disorders, part 2: A review of
clinical studies with supporting preclinical evidence. CNS Drugs.
27:301–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen WF, Xu L, Yu CH, Ho CK, Wu K, Leung
GC and Wong MS: The in vivo therapeutic effect of free wanderer
powder (xiāo yáo sǎn, Xiaoyaosan) on mice with 4T1 cell induced
breast cancer model. J Tradit Complement Med. 2:67–75.
2012.PubMed/NCBI
|
|
9
|
Qin F, Wu XA, Tang Y, Huang Q, Zhang ZJ
and Yuan JH: Meta-analysis of randomized controlled trials to
assess the effectiveness and safety of free and easy wanderer plus,
a poly-herbal preparation for depressive disorders. J Psychiatr
Res. 45:1518–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fratkin JP and Dharmananda S: Chinese
herbal patent medicines: The clinical desk reference. Shya
Publications; Boulder, CO: 2001
|
|
11
|
Chien SC, Chang WC, Lin PH, Chang WP, Hsu
SC, Chang JC, Wu YC, Pei JK and Lin CH: A Chinese herbal medicine,
Jia-Wei-Xiao-Yao-San, prevents dimethylnitrosamine-induced hepatic
fibrosis in rats. Scientific World Journal. 2014:2175252014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Han M, Liu Z, Wang J, He Q and
Liu J: Chinese herbal formula Xiao yao san for treatment of
depression: A systematic review of randomized controlled trials.
Evid Based Complement and Alternat Med. 2012:9316362012. View Article : Google Scholar
|
|
13
|
Mizowaki M, Toriizuka K and Hanawa T:
Anxiolytic effect of Kami-Shoyo-San (TJ-24) in mice: Possible
mediation of neurosteroid synthesis. Life Sci. 69:2167–2177. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campos AC, Fogaca MV, Aguiar DC and
Guimarães FS: Animal models of anxiety disorders and stress. Rev
Bras Psiquiatr. 35(Suppl 2): S101–S111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walf AA and Frye CA: The use of the
elevated plus maze as an assay of anxiety-related behavior in
rodents. Nat Protoc. 2:322–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volk H, Potschka H and Löscher W:
Immunohistochemical localization of P-glycoprotein in rat brain and
detection of its increased expression by seizures are sensitive to
fixation and staining variables. J Histochem Cytochem. 53:517–531.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HY, Lin YH, Wu JC, Chen YC, Yang SH,
Chen JL and Chen TJ: Prescription patterns of Chinese herbal
products for menopausal syndrome: Analysis of a nationwide
prescription database. J Ethnopharmacol. 137:1261–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeung WF, Chung KF, Poon MM, Ho FY, Zhang
SP, Zhang ZJ, Ziea ET and Wong Taam V: Prescription of chinese
herbal medicine and selection of acupoints in pattern-based
traditional chinese medicine treatment for insomnia: A systematic
review. Evid Based Complement Alternat Med. 2012:9025782012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeung WF, Chung KF, Ng KY, Yu YM, Ziea ET
and Ng BF: A systematic review on the efficacy, safety and types of
Chinese herbal medicine for depression. J Psychiatr Res.
57:165–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McEwen BS: Central effects of stress
hormones in health and disease: Understanding the protective and
damaging effects of stress and stress mediators. Eur J Pharmacol.
583:174–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saavedra JM, Sánchez-Lemus E and Benicky
J: Blockade of brain angiotensin II A T1 receptors ameliorates
stress, anxiety, brain inflammation and ischemia: Therapeutic
implications. Psychoneuroendocrinology. 36:1–18. 2011. View Article : Google Scholar
|
|
22
|
Djamshidian A and Lees AJ: Can stress
trigger Parkinson's disease? J Neurosurg Psychiatry. 85:878–881.
2014. View Article : Google Scholar
|
|
23
|
Heim C and Nemeroff CB: The impact of
early adverse experiences on brain systems involved in the
pathophysiology of anxiety and affective disorders. Biol
Psychiatry. 46:1509–1522. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adriaan Bouwknecht J, Olivier B and Paylor
RE: The stress-induced hyperthermia paradigm as a physiological
animal model for anxiety: A review of pharmacological and genetic
studies in the mouse. Neurosci Biobehav Rev. 31:41–59. 2007.
View Article : Google Scholar
|
|
25
|
Haller J and Alicki M: Current animal
models of anxiety, anxiety disorders, and anxiolytic drugs. Curr
Opin Psychiatry. 25:59–64. 2012. View Article : Google Scholar
|
|
26
|
Jaggi AS, Bhatia N, Kumar N, Singh N,
Anand P and Dhawan R: A review on animal models for screening
potential anti-stress agents. Neurol Sci. 32:993–1005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeBattista C and Belanoff J: The use of
mifepristone in the treatment of neuropsychiatric disorders. Trends
Endocrinol Metab. 17:117–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leentjens AF, Dujardin K, Marsh L,
Martinez-Martin P, Richard IH and Starkstein SE: Anxiety and motor
fluctuations in Parkinson's disease: A cross-sectional
observational study. Parkinsonism Relat Disord. 18:1084–1088. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gallagher D, Coen R, Kilroy D, Belinski K,
Bruce I, Coakley D, Walsh B, Cunningham C and Lawlor BA: Anxiety
and behavioural disturbance as markers of prodromal Alzheimer's
disease in patients with mild cognitive impairment. Int J Geriatr
Psychiatry. 26:166–172. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Radley JJ and Morrison JH: Repeated stress
and structural plasticity in the brain. Ageing Res Rev. 4:271–287.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goedert M: Alpha-synuclein and
neurodegenerative diseases. Nat Rev Neurosci. 2:492–501. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morris EP, Stewart SH and Ham LS: The
relationship between social anxiety disorder and alcohol use
disorders: A critical review. Clin Psychol Rev. 25:734–760. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramos A, Moisan MP, Chaouloff F, Mormede C
and Mormède P: Identification of female-specific QTLs affecting an
emotionality-related behavior in rats. Mol Psychiatry. 4:453–462.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiavegatto S, Izidio GS, Mendes-Lana A,
Aneas I, Freitas TA, Torrão AS, Conceição IM, Britto LR and Ramos
A: Expression of alpha-synuclein is increased in the hippocampus of
rats with high levels of innate anxiety. Mol Psychiatry.
14:894–905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Shepherd EG and Nelin LD: MAPK
phosphatases-regulating the immune response. Nat Rev Immunol.
7:202–212. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toska K, Kleppe R, Armstrong CG, Morrice
NA, Cohen P and Haavik J: Regulation of tyrosine hydroxylase by
stress-activated protein kinases. J Neurochem. 83:775–783. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu J, Lou H, Alerte TN, Stachowski EK,
Chen J, Singleton AB, Hamilton RL and Perez RG: Lewy-like
aggregation of α-synuclein reduces protein phosphatase 2A activity
in vitro and invivo. Neuroscience. 207:288–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wittmann W, Schunk E, Rosskothen I,
Gaburro S, Singewald N, Herzog H and Schwarzer C:
Prodynorphin-derived peptides are critical modulators of anxiety
and regulate neurochemistry and corticosterone.
Neuropsychopharmacology. 34:775–785. 2009. View Article : Google Scholar
|
|
39
|
Shepard JD, Barron KW and Myers DA:
Corticosterone delivery to the amygdala increases
corticotropin-releasing factor mRNA in the central amygdaloid
nucleus and anxiety-like behavior. Brain Res. 861:288–295. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vallée M, Mayo W, Dellu F, Le Moal M,
Simon H and Maccari S: Prenatal stress induces high anxiety and
postnatal handling induces low anxiety in adult offspring:
Correlation with stress-induced corticosterone secretion. J
Neurosci. 17:2626–2636. 1997.PubMed/NCBI
|
|
41
|
Mitra R and Sapolsky RM: Acute
corticosterone treatment is sufficient to induce anxiety and
amygdaloid dendritic hypertrophy. Proc Nat Acad Sci. 105:5573–5578.
2008. View Article : Google Scholar : PubMed/NCBI
|