Introduction
Osteoarthritis (OA) is a common joint disease, which
is characterized by progressive articular cartilage injury with
fibrillation, rhagades, anabrosis and attrition of the cartilage,
accompanied by different levels of synovitis (1). OA is a major cause of joint
disability in the middle aged and elderly. With aging populations,
the morbidity of OA is increasing year by year (2), and OA has attracted research
attention. Epidemiology data demonstrates that the incidence of OA
in middle aged and elderly population >40 years reaches 46.3%,
of these 80% demonstrate limited joint mobility (3). Effective therapy to prevent and cure
OA requires understanding of the pathogenesis, however, the
etiological agent of osteoarthritis remains to be elucidated
despite previous studies suggesting that OA is associated with
numerous factors, including senility, obesity, inflammation, trauma
and heredity (4,5).
Oxidation results in superoxides, hydrogen peroxide,
hydroxyl radicals and other metabolic products. Excessive oxidation
mediates cellular damage, oxidation of cellular lipids and
proteins, and degeneration of DNA (6). Oxidation may be a pathophysiological
process of OA, the disorder of antioxidant defensive system in OA
patients is a critical factor of joint oxidation. Osteoarticular
inflammatory diseases refer to inflammation in joints, which has a
complicated pathogenesis and varying course (7). Over the last century, with the
development of research in biochemistry, genetics, immunology,
molecular biology and pain iconography, comprehensive studies of
osteoarticular inflammatory disease have been conducted to
elucidate epidemiology, etiology, clinical features, pathogenesis
and differential diagnoses, and develop treatment strategies
(8). Progress has been made in
understanding, particularly on ankylosing spondylitis, rheumatoid
arthritis (RA) and osteoarthritis.
Isoflavanones are the major components of the root
of the kudzu vine, these are predominantly daidzin and puerarin.
Pharmacological uses of puerarin are considered to be wide, and it
may be useful in the treatment of cardiovascular and
cerebrovascular diseases with further development (9). Puerarin does not result in marked
proliferation of rat osteoblasts, however, it promotes the
synthesis and secretion of alkaline phosphatase from osteoblasts
(10). Furthermore, it may reduce
bone resorption lacunae (11).
Furthermore, contents of Ca2+ are reduced in the
supernatant of culture solution following puerarin treatment. Via
the inhibition of bone resorption, puerarin may stimulate bone
formation to regulate bone metabolism (12). The aim of the present study was to
improve the existing understanding of the effect of puerarin to
attenuate inflammation and oxidation in mice with collagen
antibody-induced arthritis via toll-like receptor 4 (TLR4)/nuclear
factor-κB (NF-κB).
Materials and methods
Mice and experimental groups
Male DBA1/J mice (26 mice; age, 8 weeks; weight,
30–40 g) were acquired from The Second Affiliated Hospital of
Zhejiang Chinese Medical University (Hangzhou, China). The mice
were housed in a 22°C temperature controlled room with 50–60%
humidity, a 12 h light/dark cycle, and free access to food and
water. The mice were randomly divided into three groups: Control
group (n=6), model group (n=10) and puerarin group (n=10). In the
model and puerarin groups, DBA1/J mice were injected with 4 mg
collagen antibody (clones D1, F10, A2 and D8 to collagen type II;
Chemicon; EMD Millipore, Billerica, MA, USA). Mice with collagen
antibody-induced arthritis in the puerarin group were injected with
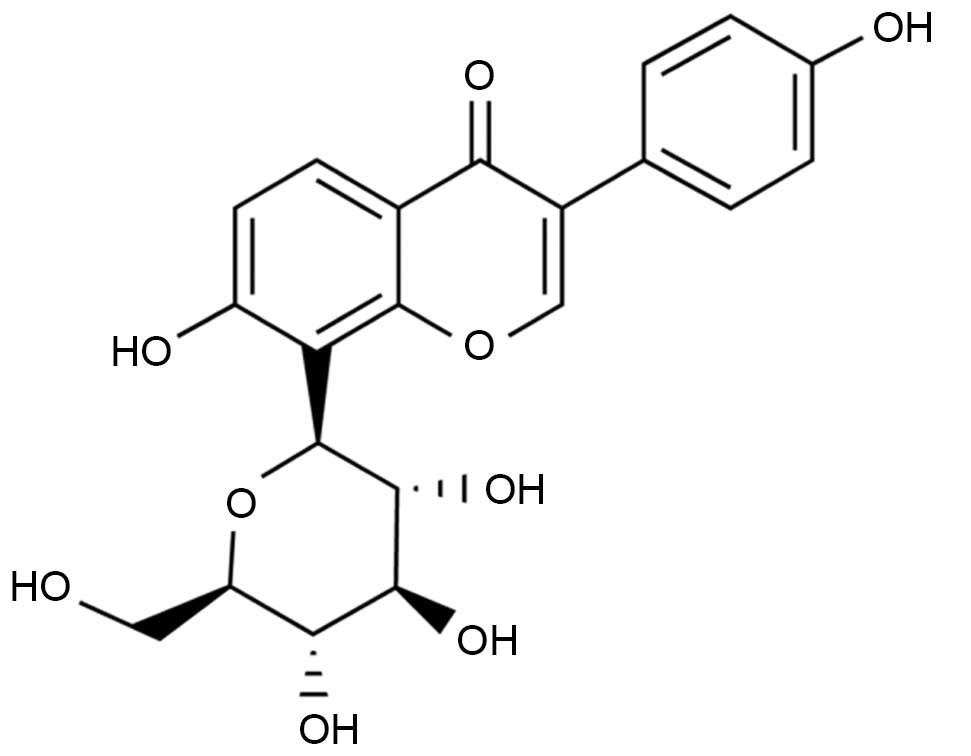
100 mg/kg puerarin (chemical structure presented in Fig. 1; Sigma-Aldrich, St. Louis, MO, USA)
in 20% propanediol via the tail vein for each day for 7 days. Mice
in the control and model groups were injected with same volume of
saline. Observational clinical scores was determined on a scale
from 0 to 3, using a digital Vernier caliper (VWR International,
Radnor, PA, USA) as follows: 0= normal paws, no swelling or
redness; 1= swelling and/or redness in one digit or in the ankle;
2= swelling and/or redness in one or two digits and ankle, and 3=
entire paw is swollen or red.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Mice were anaesthetized and sacrificed using
amobarbital (80 mg/kg; Sigma-Aldrich), then arthritis tissue
samples from the knee joint were obtained and washed with
phosphate-buffered saline. Total RNA was isolated from arthritis
tissue samples using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Total RNA was used for cDNA synthesis by
incubation with M-MLV Reverse Transcriptase (Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h at 37°C. qPCR was conducted using
ABI 7500 Real-Time PCR System with SYBR Green Master mix (Takara
Bio, Inc., Otsu, Japan). Primers used were as follows: Forward,
5′-CACTGTAACTGGGGGCAACT-3′ and reverse, 5′-CACTTCTTGTCAGCGTCGAA-3′
for matrix metalloproteinase 9 (MMP-9), and forward,
5′-GGCATTGCTCTCAATGACAA-3′ and reverse, 5′-TGTGAGGGAGATGCTCAGTG-3′
for GAPDH. Cycling conditions were as follows: 94°C for 10 min;
followed by 40 cycles at 95°C for 30 sec, 58°C for 45 sec and 72°C
for 45 sec; and 72°C for 7 min. The mRNA expression of MMP-9 was
calculated using the formula 2−ΔΔCq method (13).
Western blot analysis
Tissue samples from the knee joint (50 mg) were
harvested and homogenized, and protein extracted with
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Haimen, China). The protein was quantified using BCA
Protein assay kit (Sangon Biotech Co., Ltd., Shanghai, China).
Equal quantities of proteins (50–100 µg) were separated on
12% SDS-PAGE gels for 20 min and transferred onto polyvinylidene
difluoride membranes (EMD Millipore) at 100 V for 1 h. The membrane
was then blocked with 5% non-fat milk in Tris-buffered saline
containing 0.1% Tween 20 (TBST) for 2 h. The membrane was incubated
with antibodies against TLR4 (1:2,000; cat. no. 14358; Cell
Signaling Technology, Inc., Danvers, MA, USA), phosphorylated Janus
kinase 2 (p-JAK2; 1:2,000; cat. no. 8082; Cell Signaling
Technology, Inc.), p-signal transducer and activator of
transcription 3 (p-STAT3; 1:2,000; cat. no. 9145; Cell Signaling
Technology, Inc.) and β-actin (1:2,000; cat. no. BB-2101-2;
BestBio, Shanghai, China) overnight at 4°C with agitation.
Following washing with TBST and incubated with anti-rabbit
immunoglobulin G secondary antibodies (1:5,000; cat. no. BB-2202-1;
BestBio) for 2 h. The membrane was visualized with an enhanced
chemiluminescence kit (cat. no. BB-3501-3; BestBio) according to
the manufacturer's protocols and exposed to X-ray film. Protein
levels were assessed using MacBiophotonics Image J software
(version 1.41a; imagej.net/mbf)
Activitiy of malondialdehyde (MDA),
superoxide dismutase (SOD), tumor necrosis factor-α (TNF-α),
interleukin-6 (IL-6), caspase-3 and NF-κB
Commercial ELISA kits were used to determine the
levels of MDA (cat. no. BB-4709-1; BestBio), SOD (cat. no.
BB-4710-2; BestBio), TNF-α (cat. no. H052; Nanjing Jiancheng
Biological Engineering Institute, Nanjing, China), IL-6 (cat. no.
H007; Nanjing Jiancheng Biological Engineering Institute) and
caspase-3 (cat. no. BB-4106-2; BestBio) in knee joint arthritis
samples, according to the manufacturer's protocols.
Statistical analyses
Data are presented as the mean ± standard deviation.
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA) was used to
perform one-way analysis of variance and Fisher's protected least
significant difference test. P<0.05 was considered to indicate a
statistically significant result.
Results
Observational clinical scores were
decreased in puerarin treated mice
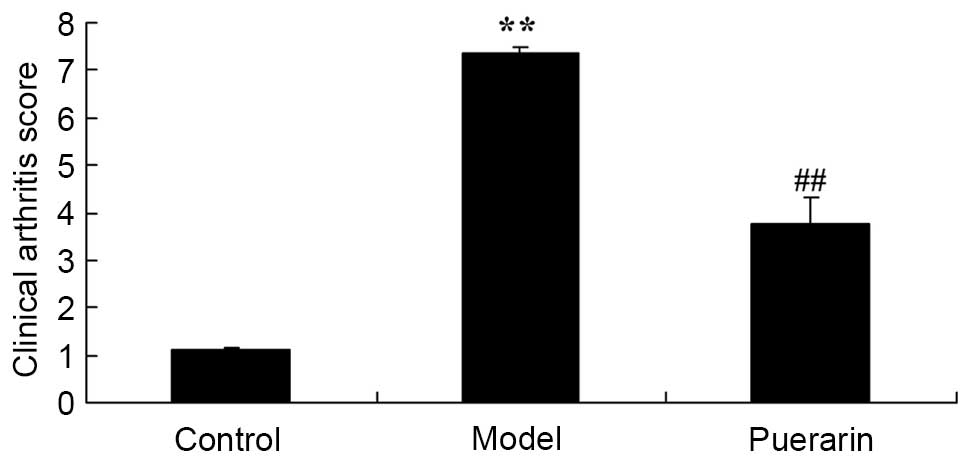
The effect of puerarin on observational clinical
scores in mice with collagen antibody-induced arthritis was
examined. As presented in Fig. 2,
observational clinical scores were significantly increased in the
collagen antibody-induced arthritis group compared with the
non-arthritis control mice (P<0.05). Treatment of arthritis mice
with 100 mg/kg puerarin for 7 days significantly reduced the
observational clinical scores in collagen antibody-induced
arthritis mice compared with the model mice (P<0.05; Fig. 2).
Treatment with puerarin decreases the
oxidative stress observed in mice with collagen antibody-induced
arthritis
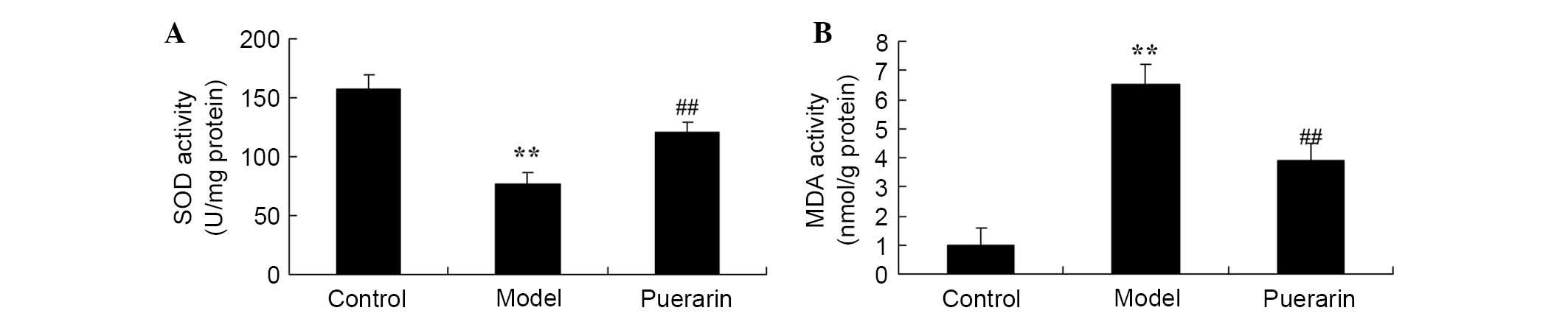
The effect of puerarin on oxidative stress damage in
arthritis was also investigated. As presented in Fig. 3, there was a significant increase
in oxidative stress, indicated by decreased SOD level and increased
MDA level in collagen antibody-induced arthritis mice as compared
with normal rats (P<0.05). However, treatment with 100 mg/kg
puerarin for 7 days significantly decreased the suppression of the
SOD level and increase in MDA level observed in collagen
antibody-induced arthritis mice (P<0.05; Fig. 3).
Treatment with puerarin decreases the
inflammatory response observed in mice with collagen
antibody-induced arthritis
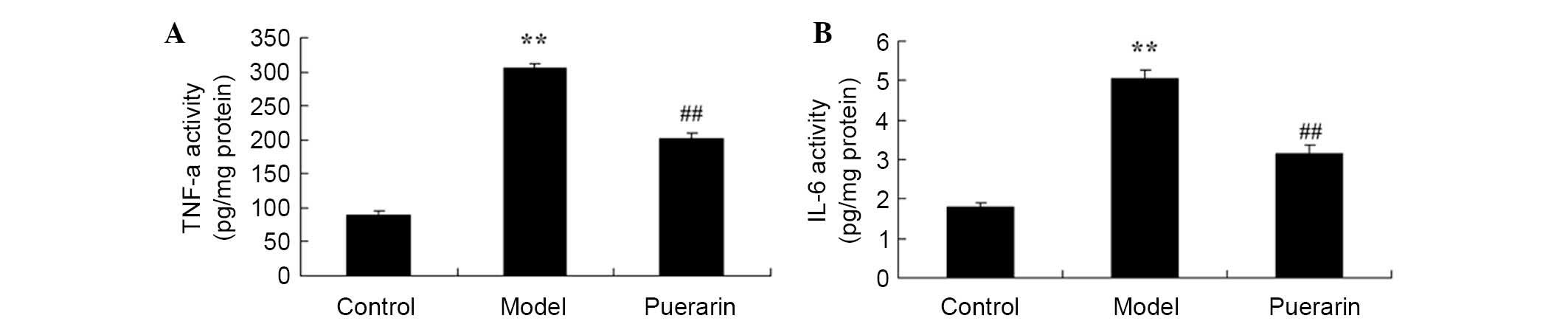
The effect of puerarin on the inflammatory response
in mice with collagen antibody-induced arthritis was analyzed. As
presented in Fig. 4, there was a
significant increase in TNF-α (P<0.05) and IL-6 levels
(P<0.05) in collagen antibody-induced arthritis mice compared
with the control mice. Treatment of arthritis model mice with 100
mg/kg puerarin for 7 days significantly inhibited the promotion of
TNF-α (P<0.05) and IL-6 (P<0.05) levels in collagen
antibody-induced arthritis mice (Fig.
4).
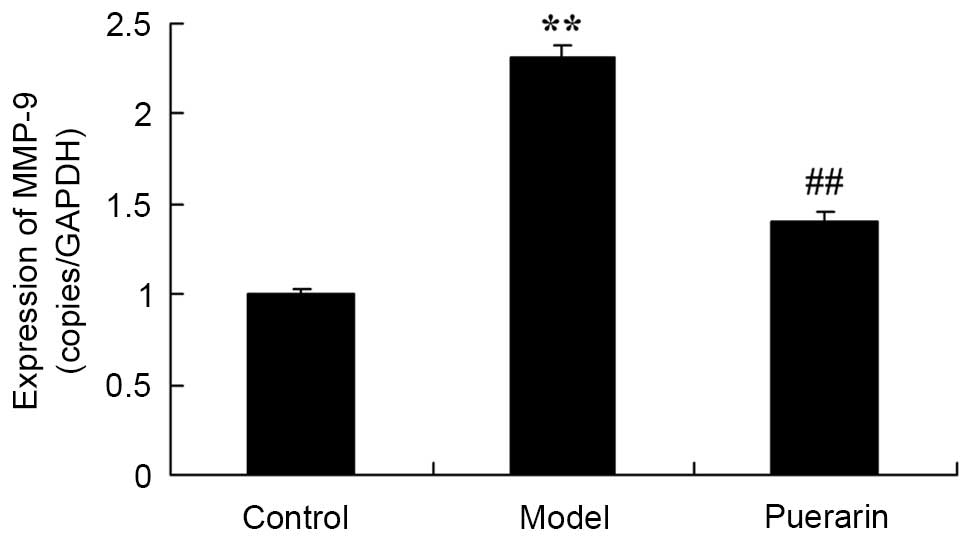
Treatment with puerarin decreases MMP-9
mRNA expression in mice with collagen antibody-induced
arthritis
The potential mechanism underlying mechanism for the
effect of puerarin on collagen antibody-induced arthritis was
investigated. As presented in Fig.
5, the mRNA expression of MMP-9 was significatntly increased in
collagen antibody-induced arthritis mice as compared with control
mice (P<0.05). However, the mRNA expression of MMP-9 was
significantly suppressed following treatment with 100 mg/kg
puerarin for 7 days in collagen antibody-induced arthritis mice
(P<0.05; Fig. 5).
Treatment with puerarin decreases TLR4
protein expression in mice with collagen antibody-induced
arthritis
Western blot analysis demonstrated that the protein
expression of TLR4 was significantly increased in mice with
collagen antibody-induced arthritis, compared with the control
group (P<0.05; Fig. 6).
Treatment with 100 mg/kg puerarin for 7 days significantly
suppressed the increase in TLR4 protein expression levels in
collagen antibody-induced arthritis mice (P<0.05; Fig. 6).
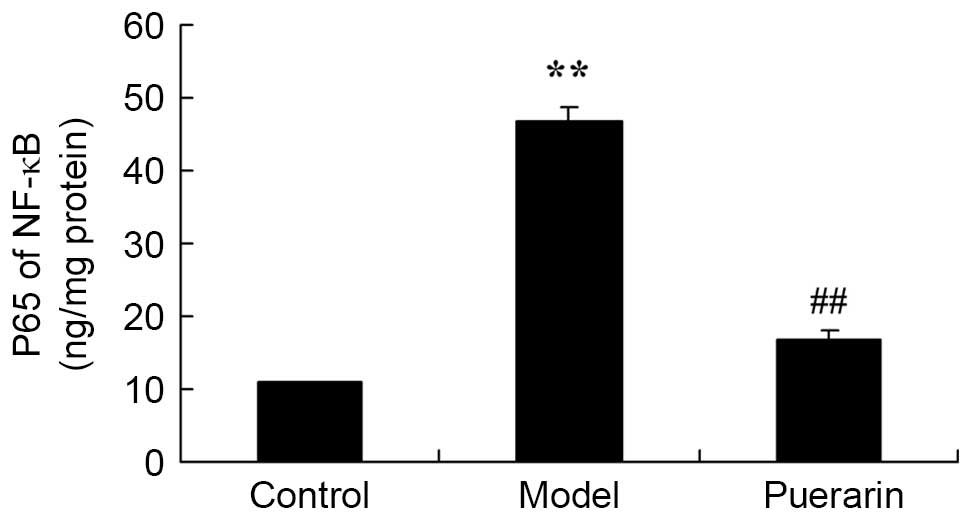
Treatment with puerarin decreases the
activity of NF-κB in mice with collagen antibody-induced
arthritis
Compared with the control group, collagen
antibody-induced arthritis increased the activity of NF-κB
(P<0.05; Fig. 7). Treatment
with 100 mg/kg puerarin for 7 days significantly reduced the
activity of NF-κB in mice with collagen antibody-induced arthritis
(P<0.05; Fig. 7).
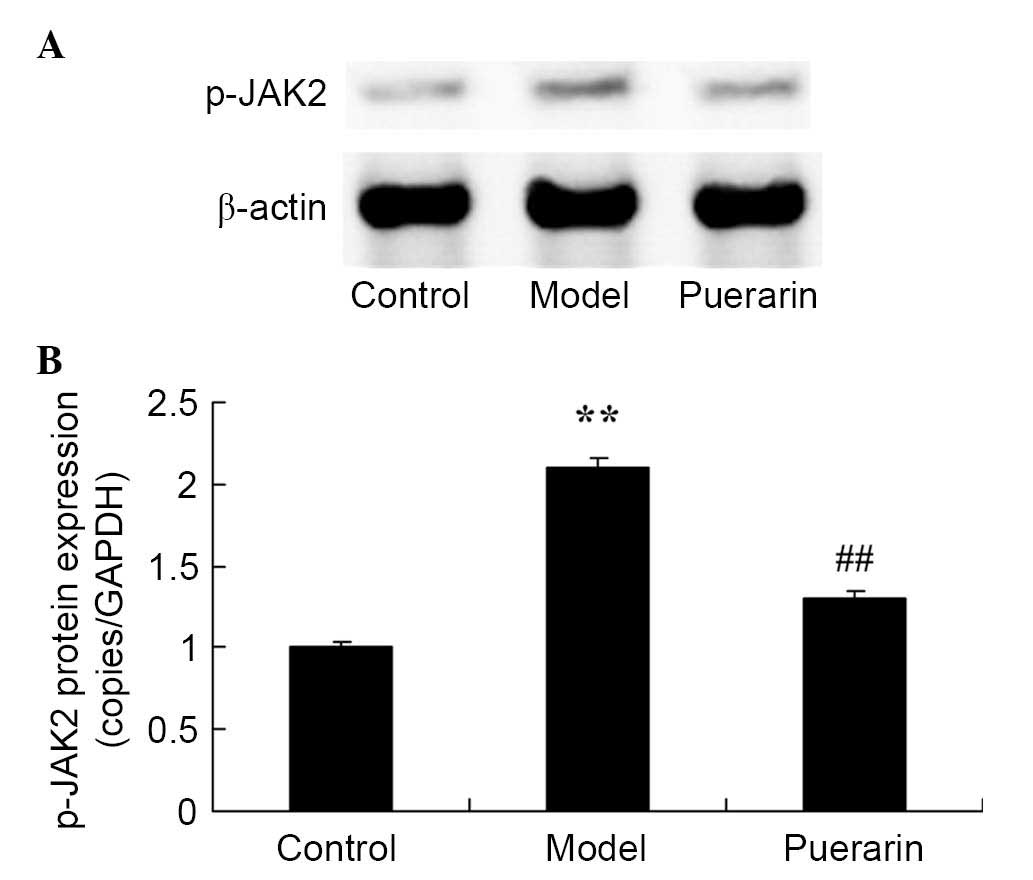
Treatment with puerarin decreases the
protein expression levels of p-JAK2 in mice with collagen
antibody-induced arthritis
A significant increase in the protein expression of
p-JAK2 was observed in mice with collagen antibody-induced
arthritis (P<0.05; Fig. 8).
Administration of 100 mg/kg puerarin for 7 days significantly
suppressed the increase in p-JAK2 protein expression levels in mice
with collagen antibody-induced arthritis (P<0.05; Fig. 8).
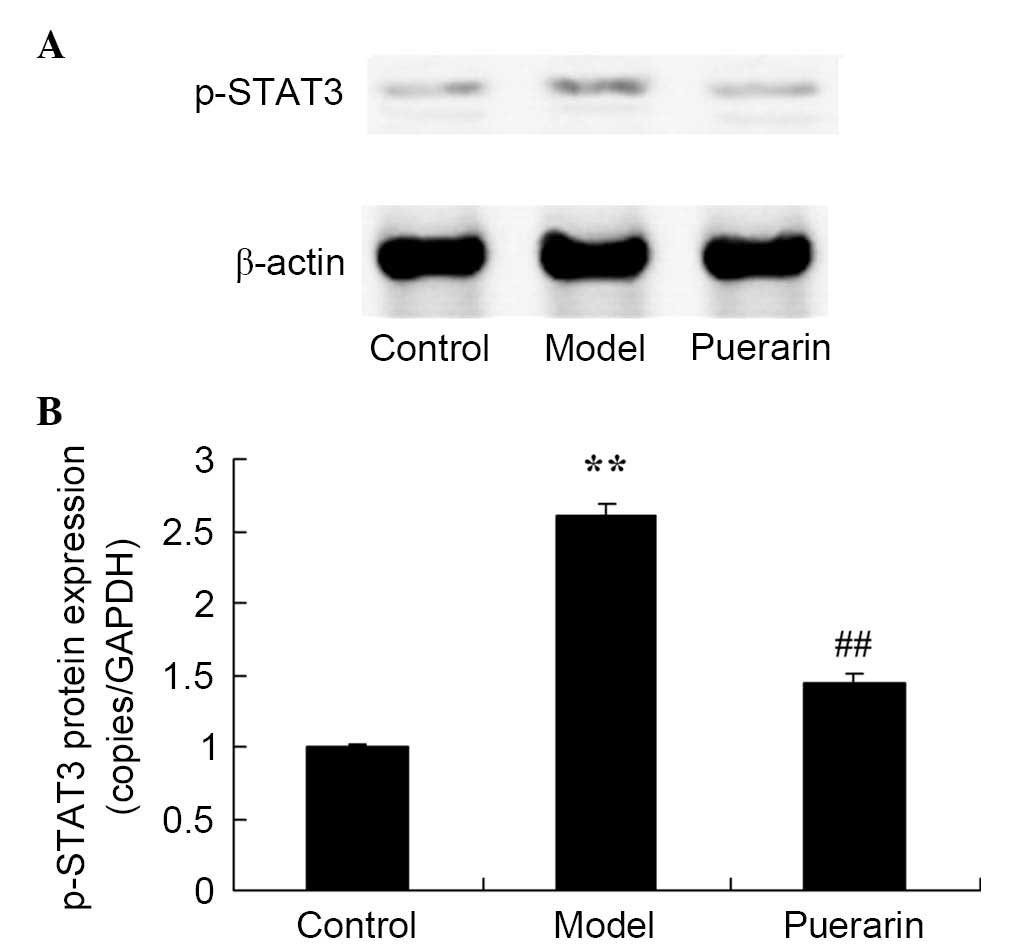
Treatment with puerarin decreases protein
expression of p-STAT3 in mice with collagen antibody-induced
arthritis
Furthermore, as presented in Fig. 9, the protein expression of p-STAT3
in mice with collagen antibody-induced arthritis was increased
compared with the control group (P<0.05). However, puerarin
significantly suppressed the increase in p-STAT3 protein expression
levels in mice with collagen antibody-induced arthritis (P<0.05;
Fig. 9).
Discussion
RA is a chronic autoimmune disease with pathological
features that include proliferation and invasion of synovial
membranes, which may form invasive pannus and result in damage to
bone and cartilage (14).
Frequently used biological agents against B cells, T cells and
non-inflammatory factors are effective in 70% of OA patients,
however, other patients continue to struggle disability (15). In the current study, puerarin
significantly decreased the observed clinical scores of collagen
antibody-induced arthritis in the model mice (P<0.05).
As an important product of lipid oxidation, MDA is
widely used to indicate levels of reactive oxygen species (ROS)
lipid oxidation (16). A previous
study demonstrated that ROS lipid oxidation in serum and synovial
fluid of OA patients is abnormal. SOD is an important enzyme that
acts against ROS to decrease oxidative stress (17). Conversion of ROS super-oxide anion
into hydrogen peroxide, an organism can reduce oxidative damage to
the articular cartilage. The glutathione (GSH) system is a key
antioxidant (18). The GSH system
decreases ROS levels and constitutes glutathione peroxidase,
glutathione transferase and glutathione (18). It was observed that puerarin
significantly suppressed the decrease in SOD level and increase in
MDA level in collagen antibody-induced arthritis mice (each
P<0.05).
Inflammatory cytokines are important in the
apoptosis of cartilage cells. The genesis and progression of OA is
closely associated with cartilage injuries as a result of arthritis
(19). When cells are under
inflammatory stress or injured, abnormal mechanical stress results
in activation of cartilage cells to stimulate macrophages to
produce a series of inflammatory mediators. TNF-α is an important
in inflammation of whole body and apoptosis of cartilage cells
(20). During the pathogenesis of
OA, IL-1β is an important cytokine for the degradation of cartilage
matrix and the destruction of articular cartilage. IL-1β is also
key in the synthesis and degradation of extracellular matrix of
articular chondrocytes. The results of the present study suggest
that puerarin significantly inhibits the increase in TNF-α and IL-6
levels in collagen antibody-induced arthritis mice (P<0.05).
TLRs are transduction receptors of transmembrane
signals, they have been observed to be highly conserved in living
organisms. They recognize invasive pathogenic microorganisms as a
component of the natural immune system and are involved in the
initiation and regulation of the immune response (21). Endogenous ligands from pathogenic
microorganisms activate and initiate downstream TLR signal
transduction pathways in synovial fibroblasts (22). Thus, inflammation-associated
cytokines are produced. Previous studies have demonstrated that the
expression of TLRs is increased at areas of damage to the cartilage
and synovium (23). The signal
transduction pathway of TLRs is involved in damage to articular
cartilage and synovium, thus, OA may be associated with TLRs and
the innate immune response, which is mediated by its downstream
signaling pathways (21). In the
present study, puerarin significantly suppressed the increase of
TLR4 protein expression in collagen antibody-induced arthritis mice
(P<0.05).
Cytokines are micromolecule polypeptides, which
transmit signals for, mediate and regulate the immune system and
inflammation. The regulation of the immune system depends on the
equilibrium of pro-inflammatory cytokines and suppression of
inflammatory cytokines. Furthermore, it also depends on the
regulation of its signal transduction pathway. JAK/STAT is an
important signaling pathway to mediate cytokine signal
transduction. The regulation of the JAK/STAT signaling pathway is
predominantly manifested by the phosphorylation of its target
proteins (24). The
phosphorylation of associated proteins and the subsequent signaling
cascade magnifies signals, and increased spread and signal
mediation is observed. During the development of RA, STAT3 remains
in an activated state. Expression levels of p-STAT3 are increased
and transmitted into the cell nucleus, which induces transcription
of target genes. Via biological effects, including inhibition of
apoptosis of fibroblast-like synoviocytes and the promotion of T
cell survival, the inflammatory response may worsen (25). Consequently, a therapy targeting
the activity of p-STAT3 may achieve an anti-inflammatory effect
(25). In the current study,
puerarin significantly reduced NF-κB activity, and suppressed
p-JAK2 and p-STAT3 protein expression levels in mice with collagen
antibody-induced arthritis (all P<0.05). This is consistent with
previous studies that demonstrated puerarin attenuates NF-κB
activity in rats with chronic alcohol-induced liver injury
(26), and suppressed the
JAK2/STAT3 signaling pathway in rat non-alcoholic fatty liver
disease (27).
In conclusion, the effect of puerarin decreases
clinical scoring of arthritis, and inhibits inflammation and
oxidative damage in mice with collagen antibody-induced arthritis.
A possible underlying mechanism is the inhibition of inflammatory
processes via suppressed TLR4/NF-κB and JAK2/STAT3 signaling
pathways. The present study also suggests that puerarin may be
useful as a novel agent for the treatment of arthritis.
Acknowledgments
The present study was supported by Zhejiang Chinese
Medicine Supporting Project for Young Scientists (grant no.
2008YA010) and the Natural Science Foundation of Zhejiang Province
(grant no. LY13H270007).
References
|
1
|
Zhong Y, Huang Y, Santoso MB and Wu LD:
Sclareol exerts anti-osteoarthritic activities in
interleukin-1β-induced rabbit chondrocytes and a rabbit
osteoarthritis model. Int J Clin Exp Pathol. 8:2365–2374. 2015.
|
|
2
|
Laufer Y and Dar G: Effectiveness of
thermal and athermal short-wave diathermy for the management of
knee osteoarthritis: A systematic review and meta-analysis.
Osteoarthritis Cartilage. 20:957–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burrows NJ, Booth J, Sturnieks DL and
Barry BK: Acute resistance exercise and pressure pain sensitivity
in knee osteoarthritis: A randomised crossover trial.
Osteoarthritis Cartilage. 22:407–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaz MA, Baroni BM, Geremia JM, Lanferdini
FJ, Mayer A, Arampatzis A and Herzog W: Neuromuscular electrical
stimulation (NMES) reduces structural and functional losses of
quadriceps muscle and improves health status in patients with knee
osteoarthritis. J Orthop Res. 31:511–516. 2013. View Article : Google Scholar
|
|
5
|
Brouwers H, von Hegedus J, Toes R,
Kloppenburg M and Ioan-Facsinay A: Lipid mediators of inflammation
in rheumatoid arthritis and osteoarthritis. Best Pract Res Clin
Rheumatol. 29:741–755. 2015. View Article : Google Scholar
|
|
6
|
Yu H, Ye WB, Zhong ZM, Ding RT and Chen
JT: Effect of advanced oxidation protein products on articular
cartilage and synovium in a rabbit osteoarthritis model. Orthop
Surg. 7:161–167. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin J, Shang L, Ping AS, Li J, Li XJ, Yu
H, Magdalou J, Chen LB and Wang H: TNF/TNFR signal transduction
pathway-mediated anti-apoptosis and anti-inflammatory effects of
sodium ferulate on IL-1β-induced rat osteoarthritis chondrocytes in
vitro. Arthritis Res Ther. 14:R2422012. View Article : Google Scholar
|
|
8
|
Benedetti S, Canino C, Tonti G, Medda V,
Calcaterra P, Nappi G, Salaffi F and Canestrari F: Biomarkers of
oxidation, inflammation and cartilage degradation in osteoarthritis
patients undergoing sulfur-based spa therapies. Clin Biochem.
43:973–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Wu T, Chen X, Ni J, Duan X, Zheng
J, Qiao J, Zhou L and Wei J: Puerarin injection for unstable angina
pectoris. Cochrane Database Syst Rev. CD0041962006.PubMed/NCBI
|
|
10
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar
|
|
11
|
Wu J, Zhang X and Zhang B: Efficacy and
safety of puerarin injection in treatment of diabetic peripheral
neuropathy: A systematic review and meta-analysis of randomized
controlled trials. J Tradit Chin Med. 34:401–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Chin A, Zhang L, Lu J and Wong RW:
The role of traditional Chinese medicines in osteogenesis and
angiogenesis. Phytother Res. 28:1–8. 2014. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Choa RM and Giele HP: Inter- and
intrarater reliability of osteoarthritis classification at the
trapeziometacarpal joint. J Hand Surg Am. 40:23–26. 2015.
View Article : Google Scholar
|
|
15
|
Figueroa D, Calvo R, Villalón IE, Meleán
P, Novoa F and Vaisman A: Clinical outcomes after arthroscopic
treatment of knee osteoarthritis. Knee. 20:591–594. 2013.
View Article : Google Scholar
|
|
16
|
Firuzi O, Spadaro A, Spadaro C, Riccieri
V, Petrucci R, Marrosu G and Saso L: Protein oxidation markers in
the serum and synovial fluid of psoriatic arthritis patients. J
Clin Lab Anal. 22:210–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura S, Akagi M, Yoshida K, Hayakawa
S, Sawamura T, Munakata H and Hamanishi C: Oxidized low-density
lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1
(LOX-1) in cultured bovine articular chondrocytes increases
production of intracellular reactive oxygen species (ROS) resulting
in the activation of NF-kappaB. Osteoarthritis Cartilage.
12:568–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Guo X, Duan C, Wu S, Yu H and
Lammi M: Identification of differentially expressed genes and
pathways between primary osteoarthritis and endemic osteoarthritis
(Kashin-Beck disease). Scand J Rheumatol. 42:71–79. 2013.
View Article : Google Scholar
|
|
19
|
Kaufman GN, Zaouter C, Valteau B, Sirois P
and Moldovan F: Nociceptive tolerance is improved by bradykinin
receptor B1 antagonism and joint morphology is protected by both
endothelin type A and bradykinin receptor B1 antagonism in a
surgical model of osteoarthritis. Arthritis Res Ther. 13:R762011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levinger I, Levinger P, Trenerry MK,
Feller JA, Bartlett JR, Bergman N, McKenna MJ and Cameron-Smith D:
Increased inflammatory cytokine expression in the vastus lateralis
of patients with knee osteoarthritis. Arthritis Rheum.
63:1343–1348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arranz A, Gutiérrez-Cañas I, Carrión M,
Juarranz Y, Pablos JL, Martínez C and Gomariz RP: VIP reverses the
expression profiling of TLR4-stimulated signaling pathway in
rheumatoid arthritis synovial fibroblasts. Mol Immunol.
45:3065–3073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klawitter M, Hakozaki M, Kobayashi H,
Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T,
Meier U, et al: Expression and regulation of toll-like receptors
(TLRs) in human intervertebral disc cells. Eur Spine J.
23:1878–1891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takakubo Y, Barreto G, Konttinen YT, Oki H
and Takagi M: Role of innate immune sensors, TLRs, and NALP3 in
rheumatoid arthritis and osteoarthritis. J Long Term Eff Med
Implants. 24:243–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BH, Lee JM, Jung YG, Kim S and Kim TY:
Phytosphingosine derivatives ameliorate skin inflammation by
inhibiting NF-kB and JAK/STAT signaling in keratinocytes and mice.
J Invest Dermatol. 134:1023–1032. 2014. View Article : Google Scholar
|
|
25
|
Wang X, Liu Q, Ihsan A, Huang L, Dai M,
Hao H, Cheng G, Liu Z, Wang Y and Yuan Z: JAK/STAT pathway plays a
critical role in the proinflammatory gene expression and apoptosis
of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin.
Toxicol Sci. 127:412–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li R, Liang T, He Q, Guo C, Xu L, Zhang K
and Duan X: Puerarin, isolated from Kudzu root (Willd), attenuates
hepatocellular cytotoxicity and regulates the GSK-3β/NF-kB pathway
for exerting the hepatoprotection against chronic alcohol-induced
liver injury in rats. Int Immunopharmacol. 17:71–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng P, Ji G, Ma Z, Liu T, Xin L, Wu H,
Liang X and Liu J: Therapeutic effect of puerarin on non-alcoholic
rat fatty liver by improving leptin signal transduction through
JAK2/STAT3 pathways. Am J Chin Med. 37:69–83. 2009. View Article : Google Scholar : PubMed/NCBI
|