Introduction
Ovarian cancer is termed 'the silent killer' due to
its lack of obvious symptoms. Annually, >225,000 ovarian cancer
cases are diagnosed and it causes 125,000 mortalities (1). Ovarian cancer is the most lethal
gynecological malignancy in the United States (2). Of the newly diagnosed cases, 70% are
diagnosed at a late stage, for which the 5-year survival rate is
9–35% (3). For patients with
advanced ovarian cancer, metastasis is one of the major causes of
treatment failure and mortality (1–3).
Thus, researching invasive and metastatic mechanisms is important
for improving ovarian cancer-associated survival and cure
rates.
Tumor invasion and metastasis are complex biological
processes that depend on the matrix-degrading proteolytic system
(4,5). Matriptase (also termed suppression of
tumorigenicity 14) is a protease that has attracted considerable
interest among cancer biologists (6–8). It
contains a trans-membrane domain, two CUB domains, four low-density
lipoprotein-receptor domains and a serine protease domain (7,9). It
is expressed in a wide range of epithelial tissues, including the
epidermis, gastrointestinal tract and respiratory tract, and in
endothelial, neural and white blood cells (10–12).
Currently, few published studies have attempted to
address the importance of this protein in ovarian carcinoma.
However, there are different conclusions regarding the function of
matriptase in ovarian cancer. Tanimoto et al (10) reported that increased matriptase
expression is associated with early-stage ovarian cancer and longer
patient survival, suggesting that matriptase is a favorable
prognostic marker for ovarian cancer. Conversely, Jin et al
(13) reported that elevated
expression of matriptase in serous adenocarcinoma was significantly
associated with tumor aggressiveness. The effect of matriptase in
ovarian carcinoma remains unclear, and the conflicting conclusions
regarding matriptase may be associated with the varying expression
of its endogenous inhibitor, hepatocyte growth factor activator
inhibitor-1 (HAI-1) (14). Oberst
et al (15) demonstrated
that an imbalance of matriptase and HAI-1 is observed in advanced
ovarian cancer tissues. Furthermore, Vogel et al (16) reported that the matriptase mRNA
level was lower in cancer tissues compared with normal tissue from
healthy individuals, whereas the ratio of matriptase/HAI-1 mRNA was
higher in colorectal cancer adenomas and carcinomas compared with
corresponding tissue from control individuals. These previous
investigations indicate that the ratio of matriptase/HAI-1 involved
in the biological behavior of cancer cell. A previous study
demonstrated that the in vitro invasive and metastatic
abilities of ovarian cancer cells are correlated with the
expression level of matriptase (17). The current study aimed to determine
whether this correlation is associated with the expression of
matriptase or HAI-1, or with the ratio of matriptase/HAI-1.
Furthermore, the study aimed to demonstrate the potential effect of
matriptase inhibition as an adjuvant therapeutic.
Materials and methods
Cells culture
The homologous ovarian cancer cell lines, HO-8910
and HO-8910PM, were purchased from the Type Culture Collection
Center of Chinese Academic of Science (Shanghai, China). HO-8910
cells were established by Mou et al (18), and HO-8910PM cells were established
by Xu et al (19).
HO-8910PM cells are highly metastatic compared with HO-8910 cells.
All cells were cultured in 90% Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin and 1% streptomycin (100 IU/ml) in
a 37°C incubator with 5% CO2. The study was approved by
the ethics committee of Fujian Maternity and Children Health
Hospital (Fujian, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated according to the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Only mRNA
samples with an optical density 260/280 ratio >1.8 were used in
the experiments, this was determined using a Nanodrop 2000 (Thermo
Fisher Scientific, Inc.). The Access RT-PCR system (Promega
Corporation, Madison, WI, USA) was used according to the
manufacturer's protocol. The cDNA (2 µl) was used for qPCR.
RT-qPCR was performed using LightCycler 480 SYBR Green I Master Mix
(Roche Diagnostics GmbH, Mannheim, Germany) according to the
instructions. The cycling protocol for qPCR was as follows: 95°C
for 15 sec; 95°C for 5 sec and 60°C for 20 sec, 45 cycles; 95°C for
1 min, cooling to 55°C. The following primers were synthesized by
Takara Biotechnology Co., Ltd. (Dalian, China): Matriptase, sense
5′-TCGTCACTTGTACCAAACACACCTA-3′, antisense
5′-GAGCCTGTCTCGTGAATGACC-3′; HAI-1, sense
5′-GGCAACAAGAACAACTTTGAGGA-3′, anti-sense
5′-CAATGCAGATGACCAGGAACAC-3′. The GAPDH primers were purchased from
Takara Biotechnology Co., Ltd. (cat. no. HA067812; GenBank
accession no. NM_002046). The PCR products were 150 bp in length
for matriptase, 154 bp for HAI-1 and 138 bp for GADPH. The relative
mRNA levels were calculated using the comparative cycle threshold
(Cq) method (ΔΔCq) (20).
Fluorescent immunocytochemistry
Cells were seeded at 1.0×105 cells/well
were seed on the glass coverslips then placed in a 12-well-plate,
and cultured overnight. The cells were rinsed with
phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde
for 10 min, followed by goat serum (OriGene Technologies, Inc.,
Beijing, China)blocking for 30 min. Next, the cells were incubated
with rabbit-anti-matripase polyclonal antibody (cat. no. ab28266;
1:500; Abcam, Cambridge, UK) at 4°C for overnight. Subsequent to
incubation, cells were rinsed with PBS twice and incubated with
FITC-labeled goat-anti-rabbit antibody (cat. no. BA1110; 1:50;
Boster Bio, Wuhan, China) for 1 h. To detect the nucleus of cells,
they were washed by PBS for 5 min, and cell nuclei were stained
with 4′,6-diamidino-2phenylindole at 1:1000 dilution for 5 min.
Serum-free DMEM medium was used as a negative control. Subsequent
to the discarding of the medium, the cells were analysed using a
confocal scanning microscope (Leica Microsystems GmbH, Solms,
Germany). The image was analysed by LAS AF lite software (21). For the slides, each quadrant
section and the middle section (3 fields in total) were observed at
random at magnification, × 100, 20 cells/field were used to
calculate the intensity of green fluorescence signal and recorded
as the mean ± standard deviation (21).
Cellular scratch assay
The horizontal migration of cells was assessed by a
scratch assay (22). Cells were
seeded at a density of 5.0×105 cells/well, then imaged
at ×40 magnification with an Olympus IX70 inverted fluorescence
microscope (Olympus Corporation, Tokyo, Japan) at 0 and 24 h
post-scratching. Image ProExpress C software 5.1 (Olympus
Corporation) was used to measure the change of the cell distance
between the scratches. The average horizontal migration rate was
calculated using the following formula: (Width0
h-width24 h)/width0 h × 100.
Transwell chamber assay
The cellular invasive capacity was determined using
the Matrigel invasion chamber assay as previous reported (23). Cells were seeded at a density of
5.0×105 cells/well. The number of cells on the underside
of the filter was determined by counting cells in 5 random fields
from 3 filters for each treatment at ×200 magnification with an
inverted microscope (Olympus Corporation).
Western blotting
Whole-cell protein was extracted according to the
protocol provided with the Pierce BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). The protein content was determined using
an enzyme-linked immunosorbent assay. Whole-cell protein (100
µg) was loaded on an 8% polyacrylamide gel. Proteins were
blotted onto nitrocellulose membranes. The blots were washed in
phosphate-buffered saline (PBS) and incubated in blocking buffer
[1X PBS, 0.1% Tween-20, 5% I-Block (Thermo Fisher Scientific,
Inc.)] at 20°C for 1 h. Membranes were incubated overnight at 20°C
with a polyclonal rabbit matriptase antibody (1:1,000 dilution;
cat. no. ab28266; Abcam) or a monoclonal rabbit HAI-1 antibody
(1:2,000 dilution; cat. no. ab189511; Abcam) in blocking buffer,
followed by incubation with an alkaline phosphatase-conjugated
anti-rabbit secondary antibody (cat. no. BA0632; 1:1,000; Boster
Bio) at room temperature for 10 min. Bands were visualized using
the CDP-Star luminescence system (Sigma-Aldrich, St. Louis, MO,
USA).
Lentivirus-mediated small interfering RNA
(siRNA) construction and infection
Three pairs of complementary oligonucleotides target
matriptase gene (GenBank accession no. NM_021978) were designed as
follows: Ma-siRNA-1, CCGGCTTCTTAGCTGAATA; Ma-siRNA-2,
TGTCCAGAAGGTCTTCAAT; and Ma-siRNA-3, ACGAGAAAGTGGAATGGCTT. These
stem-loop oligonucleotides were synthesized and cloned into a
lentivirus-based vector carrying the green fluorescent protein
(GFP) gene (cat. no. GV115; GeneChem Co., Ltd., Shanghai, China). A
universal sequence (TTCTCCGAACGTGTCACGT) was used as a negative
control (NC) for RNA interference. The siRNA and NC lentiviral
constructs were prepared as previously described (24) and used to infect HO-8910PM cells at
multiplicities of infection (MOIs) of 20 (low) and 80 (high). The
siRNA with the highest silencing efficiency was used for subsequent
experiments.
Cellular DNA and apoptosis analysis
For flow cytometric analysis, the harvested cell
pellets of HO-8910PM, and transfected HO-8910PM-NC and HO-8910PM-SI
were fixed with pre-cooled 70% ethanol and stained with propidium
iodide (100 µg/ml RNase in PBS) at 37°C for 30 min. The cell
cycle distribution was then determined as previously described
(25). Apoptosis was detected
using the Annexin-V-FLUOS staining kit (Roche Diagnostics GmbH)
according to the manufacturer's protocol. Fluorescein was measured
using a FACSCanto II flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
Statistical analysis
All the experiments were performed in triplicate.
Statistical analyses were performed using the average results of
three repeated experiments under identical conditions. Numerical
data are presented as the mean ± standard deviation. The
differences between two means were compared by Student's t-test and
related parameters were analyzed using Pearson's correlation
analysis. A one-way analysis of variance was used for multiple
comparisons of groups. Data were analyzed using SPSS software 15.0
for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
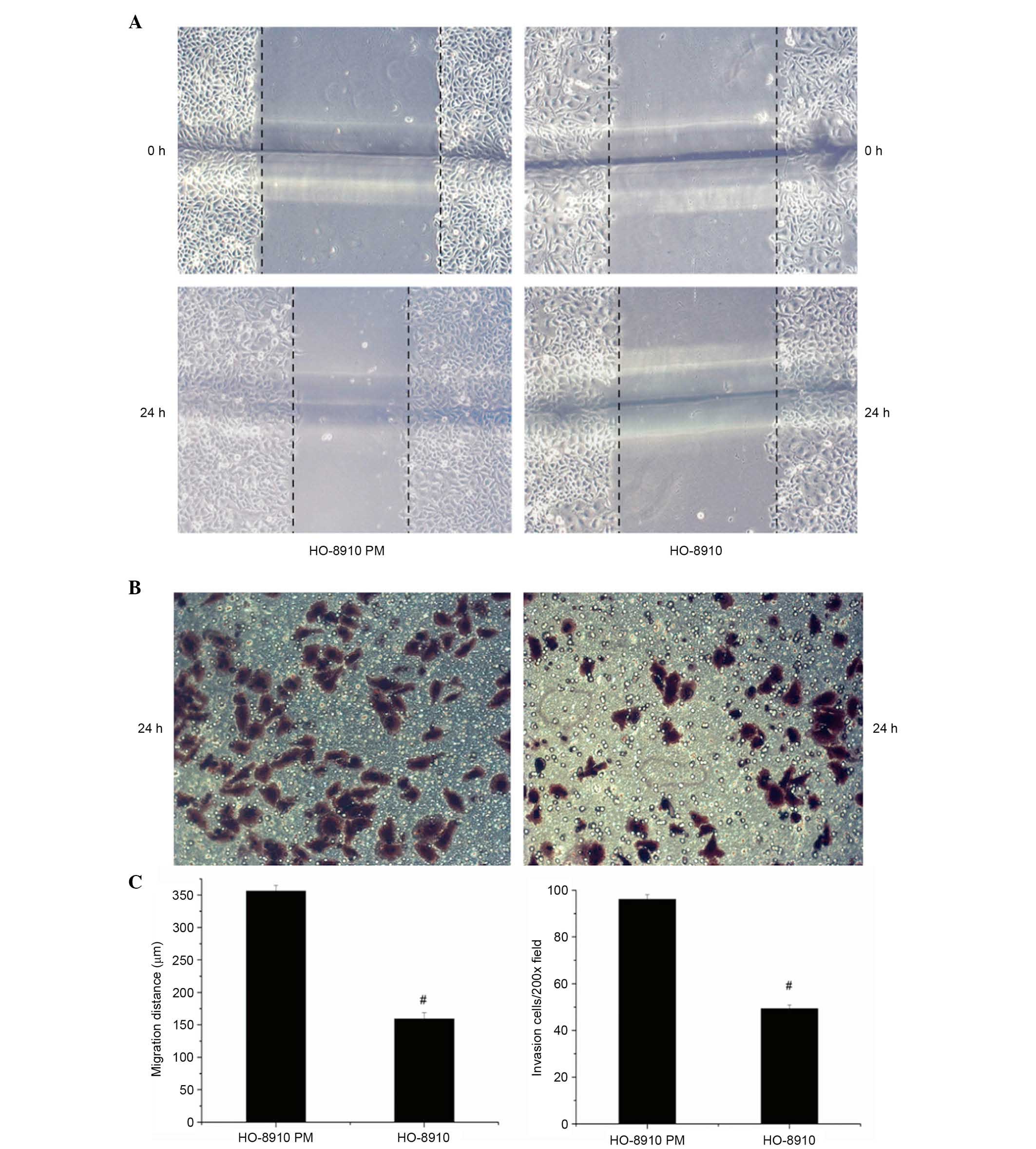
Different invasive and metastatic
activity in HO-8910 and HO-8910PM cells
A pair of syngeneic human epithelial ovarian cancer
cells, HO-8910 and HO-8910PM, were used as in vitro cellular
models of ovarian cancer. Following scratching and incubation for
24 h, the mean migration distance of HO-8910PM cells was
significantly higher compared with HO-8910 cells (347.23±8.41
µm vs. 153.95±9.56 µm; P<0.01; Fig. 1A). Additionally, in the invasion
assay, after incubation for 24 h, the number of cells that migrated
through the Transwell membrane was significantly higher for
HO-8910PM cells compared with HO-8910 cells (90.67±2.08 vs.
63.33±1.52; P<0.01; Fig.
1B).
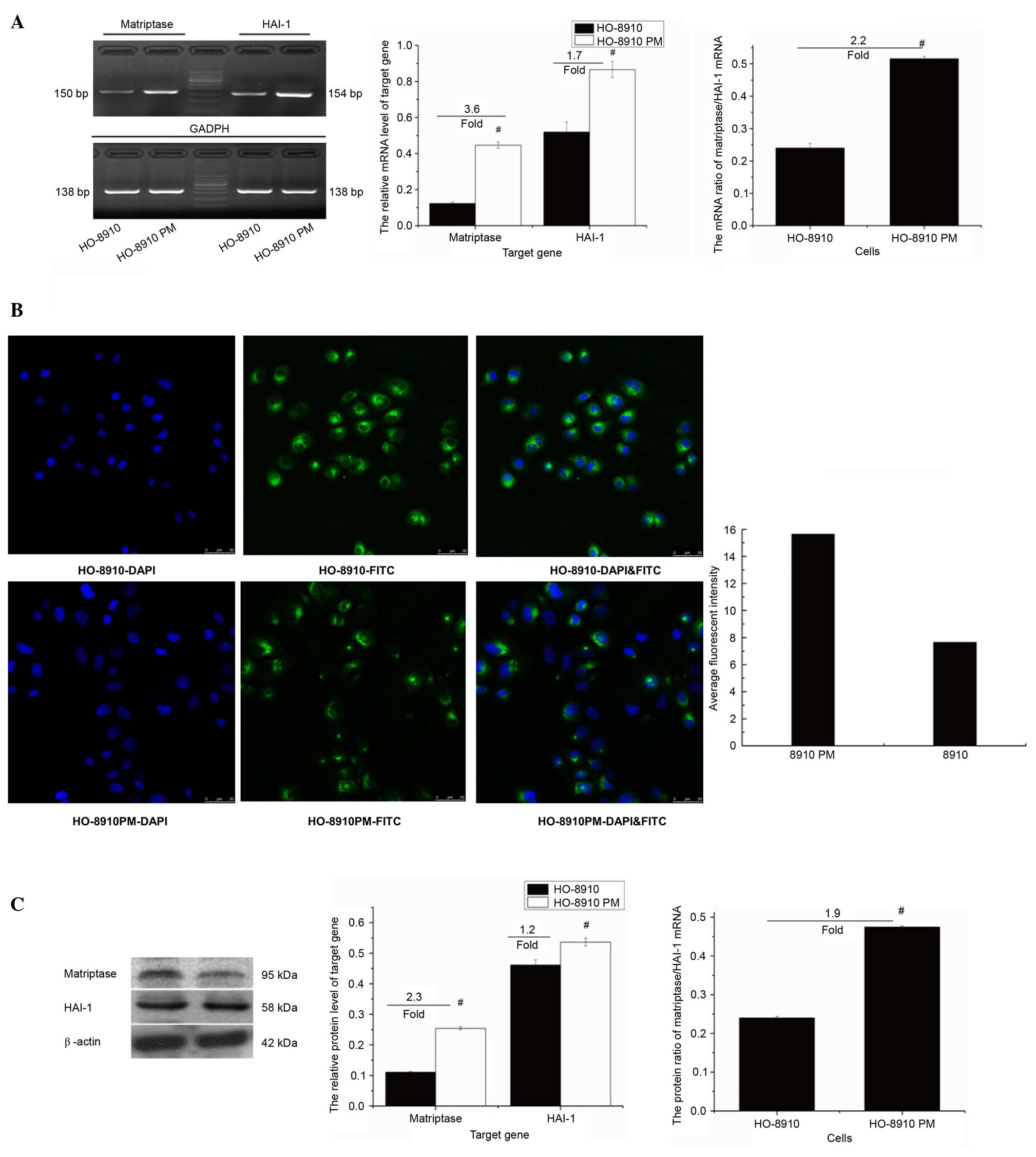
Expression of matriptase and HAI-1, and
the ratio of matriptase/HAI-1 in HO-8910 and HO-8910PM cells
The mRNA expression levels of matriptase and HAI-1
were higher in HO-8910PM cells compared with HO-8910 cells
(0.446±0.03 vs. 0.124±0.03, P<0.01 and 0.863±0.03 vs.
0.519±0.03, P<0.01, respectively; Fig. 2A) as measured by RT-qPCR. The mRNA
level of matriptase was increased by ~3.6 fold and the mRNA level
of HAI-1 was increased by ~1.7 fold in HO-8910PM compared with
HO-8910 cells. The mRNA ratio of matriptase/HAI-1 was increased
from 0.24 in HO-8910 cells to 0.52 in HO-8910PM (P<0.01; ~2.2
fold increase). Immunocytochemistry demonstrated that the
matriptase protein is predominantly localized in the cytoplasm and
at the cell membrane (Fig. 2B).
Additionally, compared with HO-8910 cells, stronger signal
intensity (15.63±0.83 vs. 7.65±1.30; P<0.01; Fig. 2B) and higher protein expression
levels of matriptase and HAI-1 (0.25±0.13 vs. 0.11±0.02, and
0.54±0.16 vs. 0.46±0.12; P<0.01; Fig 2C) were detected in HO-8910PM cells.
The protein level of HAI-1 was increased by ~2.3 fold in HO-8910PM
compared with HO-8910 cells, and the protein of HAI-1 was increased
by ~1.2 fold. The protein ratio of matriptase/HAI-1 was increased
from 0.24 in HO-8910 cells to 0.47 in HO-8910PM cells (~1.9 fold
increase).
Association of invasive and metastatic
activity with the expression of matriptase
To evaluate the potential association of matriptase
expression with the invasive and metastatic activity of ovarian
cancer cells, Pearson's correlation analysis was performed. The
metastasis and invasiveness of the ovarian cancer cells were
positively correlated with matriptase mRNA expression levels
(r=0.994 and r=0.965, respectively; P<0.01) and
matriptase protein expression levels (r=0.976 and
r=0.961, respectively; P<0.05). The metastasis and
invasiveness were also positively correlated with the ratio of
matriptase/HAI-1 (r=0.929 and r=0.912, respectively;
P<0.01). However, metastasis and invasion were not significantly
correlated with the expression of HAI-1 (P>0.05).
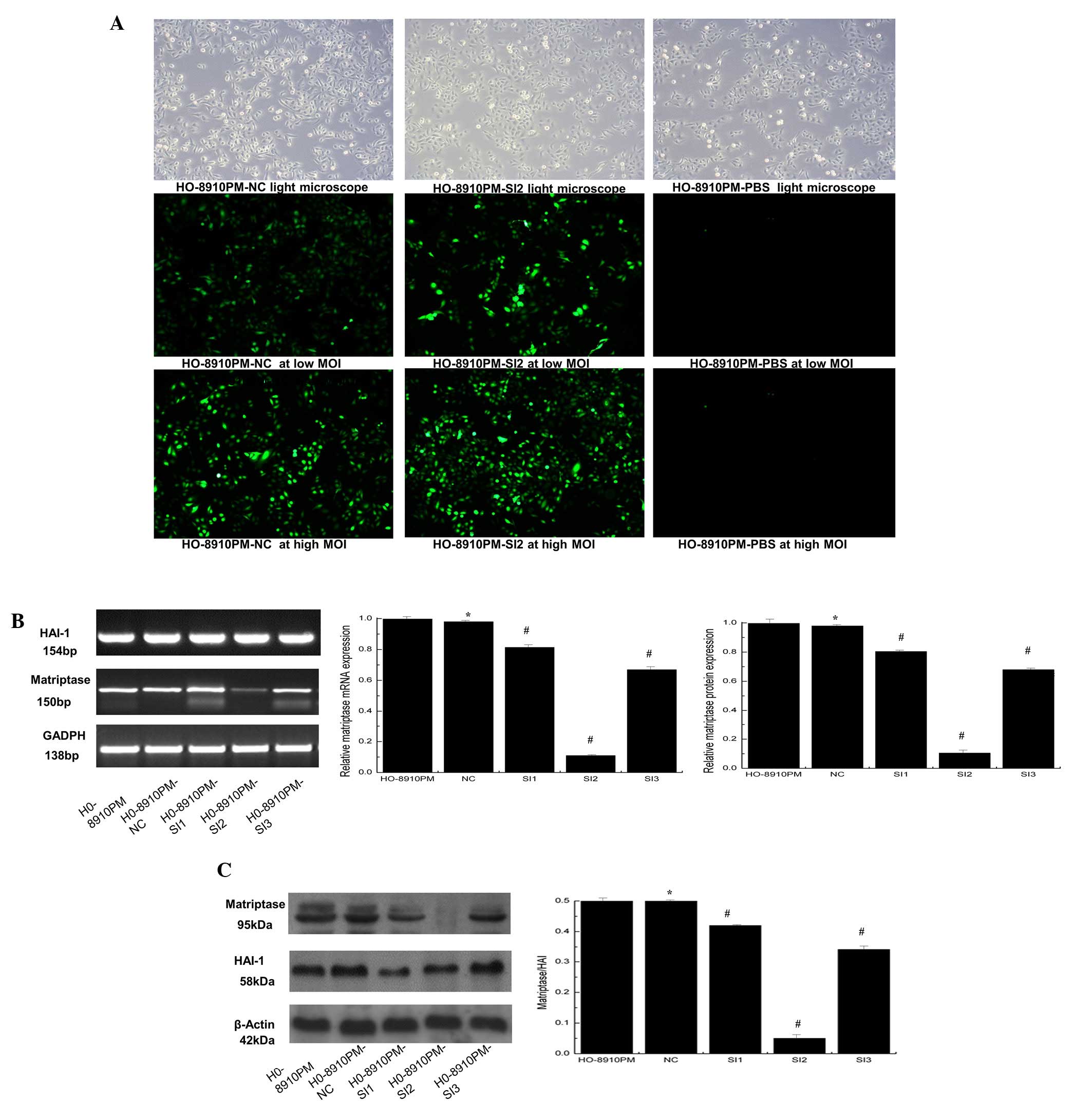
Suppression of matriptase expression in
HO-8910PM cells
Three lentivirus-mediated siRNAs targeting
matriptase were constructed and used to infect HO-8910PM cells at
high and low MOIs (Fig. 3A).
Compared with NC, matriptase mRNA levels in HO-8910PM cells were
decreased by 19.63, 89.72 and 32.43% by Ma-siRNA-1, Ma-siRNA-2, and
Ma-siRNA-3, respectively (Fig.
3B). Additionally, western blot analysis demonstrated that
Ma-siRNA-2 significantly reduced the matriptase protein expression
level compared with NC (P<0.01; Fig. 3C). The siRNA inhibited matriptase
expression but did not affect HAI-1 levels. The HO-8910PM cells
infected with Ma-siRNA-2, which induced the greatest inhibition of
matriptase levels, were termed HO-8910PM-SI2 and selected for
further analysis.
Inhibition of the invasiveness and
metastatic ability of HO-8910PM cells by downregulation of
matriptase
The invasiveness and metastatic ability of
HO-8910PM-SI2 cells were compared with HO-8910PM, HO-8910PM-NC, and
HO-8910 cells. The number of HO-8910PM-SI2 cells (38.33±3.51) that
penetrated the Transwell chamber membrane was significantly reduced
compared with HO-8910PM (100.00±4.36), HO-8910PM-NC (92.67±2.52),
and HO-8910 cells (62.33±3.06, all P<0.01, Fig. 4A). Additionally, the 24-h migration
distances of HO-8910PM-SI2 cells (104.33±7.07 µm) were
significantly reduced compared with HO-8910PM (394.08±8.20
µm), HO-8910PM-NC (387.44±2.76 µm) and HO-8910 cells
(198.80±10.46 µm; all P<0.01; Fig. 4B).
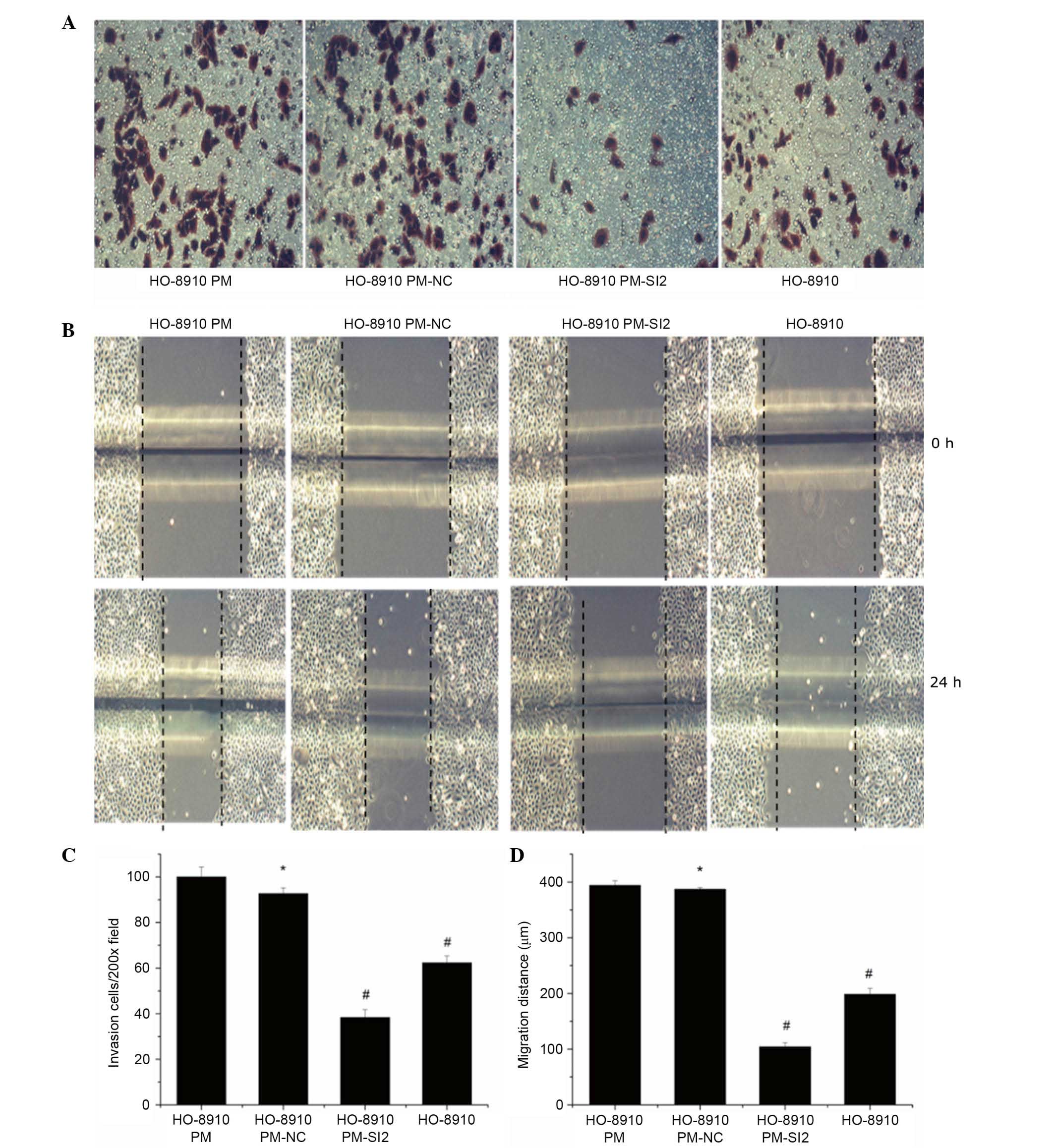 | Figure 4Inhibition of the invasiveness and
metastatic ability of ovarian cancer cells via matriptase
suppression. (A) Invasion and (B) migration were measured by
Transwell and scratch assays, respectively. Magnification, ×200.
(C) The number of HO-8910PM-SI2 cells that migrated through the
Transwell membrane was significantly lower (38.33±3.51) compared
with HO-8910PM (100.00±4.36, P<0.01), HO-8910PM-NC (92.67±2.52,
P<0.01), and HO-8910 cells (62.33±3.06, P<0.01). The number
of HO-8910PM-NC cells and HO-8910PM cells that migrated through the
Transwell membrane was not significantly different (P=0.185). (D)
Scratch assay results indicated that matriptase depletion
significantly decreased the migratory ability of HO-8910PM cells
(P<0.01). Compared with that of HO-8910PM-SI2 cells (104.33±7.07
µm), the 24-h migration distances of HO-8910PM (394.08±8.20
µm, P<0.01), HO-8910PM-NC (387.44±2.76 µm,
P<0.01) and HO-8910 cells (198.80±10.46 µm, P<0.01)
were significantly increased. *P>0.05 vs. HO-8910PM;
#P<0.05 vs. HO-8910PM. NC, negative control; SI,
small interfering RNA. |
Matriptase suppression induces apoptosis
in HO-8910 and HO-8910PM cells
The percentage of G0/G1, S and
G2/M phase HO-8910PM-SI2 cells were 54.81, 41.03 and
4.16%, respectively. The number of matriptase-knockdown
HO-8910PM-SI2 cells in the G0/G1 phase
(54.81±0.34%) was significantly increased compared with
HO-8910PM-NC (43.08±0.47%) and HO-8910PM cells (42.73±0.39%; both
P<0.01; Fig. 5A). Whereas, the
number of G2/M phase HO-8910PM-SI2 cells (4.16±0.74%)
was significantly reduced compared with HO-8910PM-NC (17.65±0.63%)
and HO-8910PM (8.35±0.65%; both P<0.01). However, no significant
difference in S phase content was observed between the
HO-8910PM-SI2, HO-8910PM-NC and HO-8910PM cells (41.03±1.02,
39.27±0.97, and 38.92±1.12%, respectively). Furthermore, matriptase
suppression decreased the percentage of surviving cells and
increased the percentage of early apoptotic cells (Fig. 5B). Compared with HO-8910PM-NC
(88.7±0.41%) and HO-8910PM cells (86.2±0.41%), the number surviving
HO-8910PM-SI2 cells of was significantly lower (75.10±0.41%;
P<0.01). Additionally, the number of early apoptotic
HO-8910PM-SI2 cells (15.10±0.81%) was significantly increased
compared with HO-8910PM-NC (5.2±0.39%) and HO-8910PM cells
(5.3±0.33%; both P<0.01). The percentages of late apoptotic and
necrotic cells were not significantly different between the
cells.
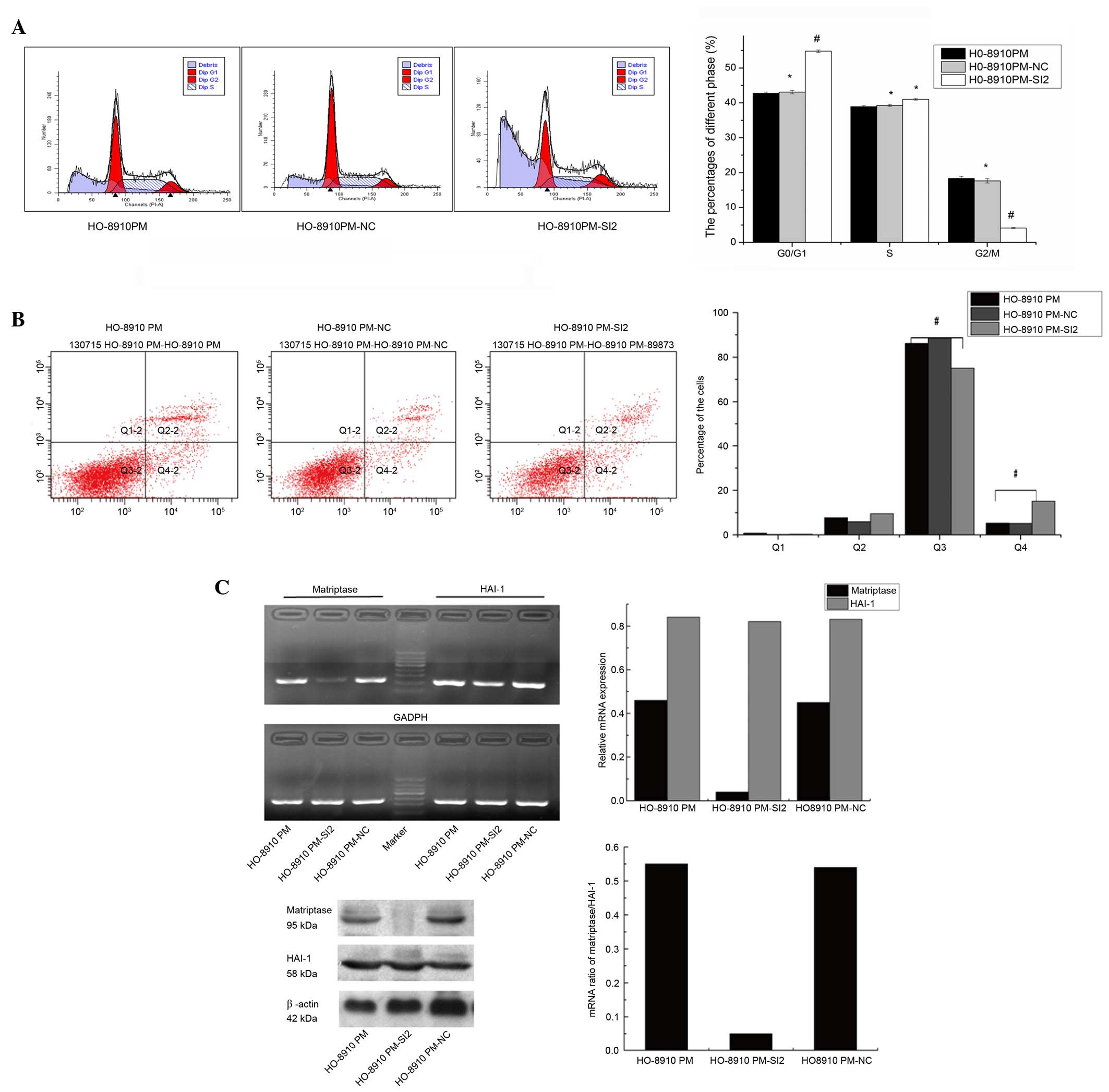 | Figure 5Downregulation of matriptase results
in cell cycle arrest and increased apoptosis in HO-8910PM cells.
(A) Flow cytometry analysis demonstrated that the percentage of
cells in the G1/G0 phase was significantly higher for
matriptase-depleted HO-8910PM-SI2 cells than for HO-8910PM-NC and
HO-8910PM cells (42.73±0.39%, P<0.01). Tthe percentage of
HO-8910PM-SI2 cells in the G2/M phase was significantly lower
(4.16±0.74%) than that of HO-8910PM-NC (17.65±0.63%, P<0.01) and
HO-8910PM cells (18.35±0.65%, P<0.01). Conversely, no
differences in S phase content were noted among the 3 cell lines.
(B) Matriptase depletion significantly decreased the percentage of
surviving cells and increased the percentage of early apoptotic
cells in HO-8910PM-SI2 cells compared with the negative control and
wild type cells. The percentages of late apoptotic and necrotic
cells were not significantly different. (C) Relative mRNA and
protein expression levels of matriptase and HAI-1 were detected in
HO-8910PM, HO-8910PM-SI2 and HO-8910PM-NC cells. The highest
silencing efficiency was achieved in HO-8910PM-SI2 cells using
matriptase-SI2. The mRNA and protein expression levels of
matriptase and HAI-1 were comparable in HO-8910 and HO-8910-NC
cells.#compared with HO-8910PM-NC and HO-8910PM cells,
significantly fewer HO-8910PM-SI2 cells survived and the number of
early apoptotic HO-8910PM-SI2 cells was significantly higher.
*P>0.05 vs. HO-8910PM; #P<0.05 vs.
HO-8910PM. Q1, cellular debris, Q2, late apoptotic and necrotic
cells, Q3, surviving cells, Q4, early apoptotic cells. NC, negative
control; SI, small interfering RNA; HAI-1, hepatocyte growth factor
activator inhibitor-1. |
Discussion
Despite clinical effort, metastatic ovarian cancer
often inevitably progresses to eventually cause mortality (25). Several previous reports suggest
that matriptase is involved in the initiation of epithelial cell
carcinogenesis (12,26–28).
Furthermore, matriptase may also be important for cell invasiveness
and metastasis (29,30). In the current study, the human
ovarian cancer cell HO-8910 and its homologous highly metastatic
clone HO-8910PM were used to assess the potential association
between the invasiveness and metastatic ability, and the mRNA and
protein expression levels of matriptase and HAI-1. As demonstrated
in multiple previous reports, HO8910-PM cells are more invasive and
metastatic compared with HO-8910 cells (17,19,31,32).
Tanimoto et al (10)
reported that the matriptase expression level is frequently
elevated in early-stage ovarian cancer and declines as the disease
progresses. However, Jin et al (13) demonstrated elevated matriptase
immunostaining scores in serous adenocarcinoma were significantly
correlated with the TNM and FIGO staging.
An explanation for these differences may be that
ovarian carcinoma subtypes exhibit different biological behaviors
(3,33). Notably, breast cancer also exhibits
a similar diversity in matriptase expression and function (34,35).
Additionally, the variation may be associated with the expression
of HAI-1. Oberst et al (15) reported that the ratio of
matriptase/HAI-1 was increased in advanced-stage ovarian cancers. A
similar increased ratio of matriptase/HAI-1 was also reported in
advanced stage color cancer (16,36).
Upregulation of the ratio is dependent on increased expression of
matriptase or decreased the expression of HAI-1. To maximize
control of the other potential factors that may affect invasiveness
and metastatic ability, the current study used a pair of homologous
ovarian cell lines with similar genetic backgrounds. The results of
the present study demonstrated that the increasing ratio of
matriptase/HAI-1 (~2 fold in HO-8910PM) was predominantly dependent
on the relative increased expression of matriptase (~3.6 fold in
mRNA and ~2.3 fold in protein), not the decreased expression of
HAI-1, as the mRNA and protein levels of the latter were also
increased by ~1.7 and 1.2 fold, respectively. Correlation analyses
demonstrated that the different metastatic and invasive abilities
of the ovarian cancer cells were positively correlated with the
ratio of matriptase/HAI-1 and the expression level of matriptase,
but not with the expression level of HAI-1. Furthermore, RT-qPCR
and western blotting demonstrated that siRNA infection
significantly decreased the matriptase expression level in
HO-8910PM cells, resulting in significantly decreased in the
HO-8910PM cell invasion and migration. Thus, it is concluded that
the ovarian cancer cell metastasis and invasion was dependent on
the activation of matriptase, but not on HAI-1.
The present study concluded that the ratio of
matriptase/HAI-1 is directly and positively correlated with
cellular invasion and metastasis, and the ratio is predominantly
altered by changes in the expression level of matriptase. Thus,
matriptase may be a potential therapeutic target. Results from flow
cytometric analysis demonstrated that HO-8910PM-SI2 cells exhibited
a greater proportion of G0/G1 phase cells and
smaller proportion of G2/M phase cells compared with
HO-8910PM and HO-8910PM-NC cells, indicating that matriptase
down-regulation results in ovarian cancer cell cycle arrest.
Although the cellular apoptosis percentage in HO-8910PM-SI2 cells
was significantly increased compared with HO-8910PM and
HO-8910PM-NC cells, the apoptosis induced by siRNA is limited
compared with cytotoxic drugs, such as cisplatin, which typically
induce apoptosis in ~30% of cells at 10 µM (37). Other previous studies demonstrated
that inhibition of matriptase enhances the treatment of
hepatocellular carcinoma, breast cancer and prostate cancer
(38,39). In ovarian cancer, matriptase
downregulation results in the inhibition of intraperitoneal tumor
growth in nude mice and prolongs the mean survival of tumor-bearing
mice (30). In the current study,
as a candidate therapeutic target, matriptase suppression resulted
in cell cycle arrest in the G0/G1 phase and
induced limited apoptosis. Thus, targeting this protein may slow
the progression of metastatic ovarian cancer, rather than providing
a cure. Matriptase may be useful as target for adjuvant therapy for
classical cytotoxic chemotherapy to limit cellular invasion and
metastasis.
In summary, the findings of the current study
suggest that the increased ratio of matriptase/HAI-1 is
predominantly dependent on the increased expression level of
matriptase, and it is a reliable indicator that reflects the
aggressive nature of ovarian cancer cells. Matriptase may
potentially be an adjuvant therapeutic target for inhibiting
ovarian cancer invasion and metastasis.
Acknowledgments
This study was supported by Fujian Provincial
Natural and Scientific Foundation (grant no. 2012J01310) and Key
Program of Fujian Provincial Department of Science & Technology
(grant no. 2009Y0007).
References
|
1
|
Marczak A and Denel M: Trabectedin as a
single agent and in combination with pegylated liposomal
doxorubicin-activity against ovarian cancer cells. Contemp Oncol
(Pozn). 18:149–152. 2014.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karst AM and Drapkin R: The new face of
ovarian cancer modeling: Better prospects for detection and
treatment. F1000 Med Rep. 3:222011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang C and Werb Z: The many faces of
metalloproteases: Cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanimoto H, Underwood LJ, Wang Y,
Shigemasa K, Parmley TH and O'Brien TJ: Ovarian tumor cells express
a transmembrane serine protease: A potential candidate for early
diagnosis and therapeutic intervention. Tumour Biol. 22:104–114.
2001. View Article : Google Scholar
|
|
7
|
Takeuchi T, Shuman MA and Craik CS:
Reverse biochemistry: Use of macromolecular protease inhibitors to
dissect complex biological processes and identify a membrane-type
serine protease in epithelial cancer and normal tissue. Proc Natl
Acad Sci USA. 96:11054–11061. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szabo R and Bugge TH: Type II
transmembrane serine proteases in development and disease. Int J
Biochem Cell Biol. 40:1297–1316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CY, Anders J, Johnson M, Sang QA and
Dickson RB: Molecular cloning of cDNA for matriptase, a
matrix-degrading serine protease with trypsin-like activity. J Biol
Chem. 274:18231–18236. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanimoto H, Shigemasa K, Tian X, Gu L,
Beard JB, Sawasaki T and O'Brien TJ: Transmembrane serine protease
TADG-15 (ST14/Matriptase/MT-SP1): Expression and prognostic value
in ovarian cancer. Br J Cancer. 92:278–283. 2005.
|
|
11
|
Nakamura K, Hongo A, Kodama J, Abarzua F,
Nasu Y, Kumon H and Hiramatsu Y: Expression of matriptase and
clinical outcome of human endometrial cancer. Anticancer Res.
29:1685–1690. 2009.PubMed/NCBI
|
|
12
|
Szabo R, Rasmussen AL, Moyer AB, Kosa P,
Schafer JM, Molinolo AA, Gutkind JS and Bugge TH: c-Met-induced
epithelial carcinogenesis is initiated by the serine protease
matriptase. Oncogene. 30:2003–2016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin JS, Hsieh DS, Loh SH, Chen A, Yao CW
and Yen CY: Increasing expression of serine protease matriptase in
ovarian tumors: Tissue microarray analysis of immunostaining score
with clinicopathological parameters. Mod Pathol. 19:447–452. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benaud C, Dickson RB and Lin CY:
Regulation of the activity of matriptase on epithelial cell
surfaces by a blood-derived factor. Eur J Biochem. 268:1439–1447.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oberst MD, Johnson MD, Dickson RB, Lin CY,
Singh B, Stewart M, Williams A, al-Nafussi A, Smyth JF, Gabra H and
Sellar GC: Expression of the serine protease matriptase and its
inhibitor HAI-1 in epithelial ovarian cancer: Correlation with
clinical outcome and tumor clinicopathological parameters. Clin
Cancer Res. 8:1101–1107. 2002.PubMed/NCBI
|
|
16
|
Vogel LK, Saebø M, Skjelbred CF, Abell K,
Pedersen ED, Vogel U and Kure EH: The ratio of Matriptase/HAI-1
mRNA is higher in colorectal cancer adenomas and carcinomas than
corresponding tissue from control individuals. BMC Cancer.
6:1762006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang ZQ, Chen XF, Sun PM, Mao XD, Lin F
and Song YY: Expression and significance of matriptase in ovarian
cancer cells with diverse metastatic potential. Zhonghua Fu Chan Ke
Za Zhi. 48:370–374. 2013.In Chinese. PubMed/NCBI
|
|
18
|
Mou HZ, Xu SH and Zhang YY: The
establishment of human ovarian carcinoma cell line HO-8910 and its
characteristics. Zhonghua Fu Chan Ke Za Zhi. 29:162–164. 1911994.In
Chinese.
|
|
19
|
Xu S, Mou H, Lü G, Zhu C, Yang Z, Gao Y,
Lou H, Liu X, Cheng Y and Yang W: Gene expression profile
differences in high and low metastatic human ovarian cancer cell
lines by gene chip. Chin Med J (Engl). 115:36–41. 2002.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Rosnoblet C, Legrand D, Demaegd D,
Hacine-Gherbi H, de Bettignies G, Bammens R, Borrego C, Duvet S,
Morsomme P, Matthijs G and Foulquier F: Impact of disease-causing
mutations on TMEM165 subcellular localization, a recently
identified protein involved in CDG-II. Hum Mol Genet. 22:2914–2928.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa
P, Gui Y and Cai Z: Synthetic miRNA-mowers targeting miR-183-96-182
cluster or miR-210 inhibit growth and migration and induce
apoptosis in bladder cancer cells. PloS One. 7:e522802012.
View Article : Google Scholar
|
|
23
|
Qi S, Song Y, Peng Y, Wang H, Long H, Yu
X, Li Z, Fang L, Wu A, Luo W, et al: ZEB2 mediates multiple
pathways regulating cell proliferation, migration, invasion and
apoptosis in glioma. PloS One. 7:e388422012. View Article : Google Scholar
|
|
24
|
Yu LL, Chang K, Lu LS, Zhao D, Han J,
Zheng YR, Yan YH, Yi P, Guo JX, Zhou YG, et al: Lentivirus-mediated
RNA interference targeting the H19 gene inhibits cell proliferation
and apoptosis in human choriocarcinoma cell line JAR. BMC Cell
Biol. 14:262013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
List K: Matriptase: A culprit in cancer?
Future Oncol. 5:97–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanders AJ, Parr C, Davies G, Martin TA,
Lane J, Mason MD and Jiang WG: Genetic reduction of matriptase-1
expression is associated with a reduction in the aggressive
phenotype of prostate cancer cells in vitro and in vivo. J Exp Ther
Oncol. 6:39–48. 2006.
|
|
28
|
Chou FP, Xu H, Lee MS, Chen YW, Richards
OX, Swanson R, Olson ST, Johnson MD and Lin CY: Matriptase is
inhibited by extravascular antithrombin in epithelial cells but not
in most carcinoma cells. Am J Physiol Cell Physiol.
301:C1093–C1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SL, Dickson RB and Lin CY: Activation
of hepatocyte growth factor and urokinase/plasminogen activator by
matriptase, an epithelial membrane serine protease. J Biol Chem.
275:36720–36725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki M, Kobayashi H, Kanayama N, Saga Y,
Suzuki M, Lin CY, Dickson RB and Terao T: Inhibition of tumor
invasion by genomic down-regulation of matriptase through
suppression of activation of receptor-bound pro-urokinase. J Biol
Chem. 279:14899–14908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen C, Sun MZ, Liu S, Yeh D, Yu L, Song
Y, Gong L, Hao L, Hu J and Shao S: Smad4 mediates malignant
behaviors of human ovarian carcinoma cell through the effect on
expressions of E-cadherin, plasminogen activator inhibitor-1 and
VEGF. BMB Rep. 43:554–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong PX, Jia N, Xu ZJ, Liu YT, Li DJ and
Feng YJ: Silencing of IQGAP1 by shRNA inhibits the invasion of
ovarian carcinoma HO-8910PM cells in vitro. J Exp Clin Cancer Res.
27:772008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Köbel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Parr C, Watkins G, Mansel RE and Jiang WG:
The hepatocyte growth factor regulatory factors in human breast
cancer. Clin Cancer Res. 10:202–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang JY, Dolled-Filhart M, Ocal IT, Singh
B, Lin CY, Dickson RB, Rimm DL and Camp RL: Tissue microarray
analysis of hepatocyte growth factor/Met pathway components reveals
a role for Met, matriptase and hepatocyte growth factor activator
inhibitor 1 in the progression of node-negative breast cancer.
Cancer Res. 63:1101–1105. 2003.PubMed/NCBI
|
|
36
|
Kosa P, Szabo R, Molinolo AA and Bugge TH:
Suppression of tumorigenicity-14, encoding matriptase, is a
critical suppressor of colitis and colitis-associated colon
carcinogenesis. Oncogene. 31:3679–3695. 2012. View Article : Google Scholar :
|
|
37
|
Sun PM, Wei LH, Luo MY, Liu G, Wang JL,
Mustea A, Könsgen D, Lichtenegger W and Sehouli J: The telomerase
activity and expression of hTERT gene can serve as indicators in
the anti-cancer treatment of human ovarian cancer. Eur J Obstet
Gynecol Reprod Biol. 130:249–257. 2007. View Article : Google Scholar
|
|
38
|
Welman A, Sproul D, Mullen P, Muir M,
Kinnaird AR, Harrison DJ, Faratian D, Brunton VG and Frame MC:
Diversity of matriptase expression level and function in breast
cancer. PloS One. 7:e341822012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tripathi M, Potdar AA, Yamashita H, Weidow
B, Cummings PT, Kirchhofer D and Quaranta V: Laminin-332 cleavage
by matriptase alters motility parameters of prostate cancer cells.
Prostate. 71:184–196. 2011. View Article : Google Scholar
|