Introduction
AXL, a member of the Tyro-AXL-Mer receptor tyrosine
kinases family, is not only involved in mesenchymal and neural
development, but is also associated with various high-grade cancers
and can independently predict poor overall survival of patients
with breast cancer (1,2). AXL is present predominately in
triple-negative breast cancer (TNBC) (3). It diversifies epidermal growth factor
receptor (EGFR) signaling and limits the response to EGFR-targeted
inhibitors (4). However, there is
limited research regarding AXL gene regulation in TNBC.
Protein kinase C α (PKCα), which is a member of the
PKC family, is correlated with carcinogenesis and breast cancer
development. A previous study indicated that PKCα expression is
higher in TNBC than that in non-TNBC (5). Its overexpression in breast cancer
cells has been shown to be associated with invasive growth in two
genetic models of epithelia-mesenchymal transition (EMT) (6), increased molecular potential for
anti-estrogen resistance and cell growth (7), and endocrine resistance in the clinic
(8–10). Furthermore, decreased breast cancer
cell malignancy and increased anticancer drug sensitivity can be
achieved through PKCα inhibition (10–12).
Other studies have indicated that AXL gene
expression induced by the PKC activator phorbol 12-myristate
13-acetate in leukemia cells is mediated by AP-1 motifs (13), and its downregulation in chronic
myeloid leukemia cells can be achieved by transfection with PKCα/β
small interfering RNA (14). Thus,
as PKCα and AXL have similar functions in breast cancer, there is a
possible correlation between PKCα and the AXL signaling pathway in
the induction of cell malignancy in certain types of cancer. In
this study, the involvement of PKCα was determined in AXL
expression in human TNBC cells. Results from the microarray and
tissue array analysis, and comparative analysis with PKCα inhibitor
Go6976 demonstrated that PKCα was significantly correlated with AXL
expression in human TNBC cells. Furthermore, an MZF-1 acidic domain
fragment (MZF-1 peptide) that was designed to downregulate PKCα
expression also inhibited AXL expression. This study proposes a
novel therapeutic strategy for TNBC involving the use of PKCα-AXL
signaling.
Materials and methods
Materials
The following antibodies were used in the present
study: Polyclonal mouse anti-PKCα (cat. no. 610108; BD Biosciences,
San Jose, CA, USA), polyclonal rabbit anti-AXL (cat. no. GTX108560;
GeneTex, Inc., Irvine, CA, USA), anti-vimentin (cat. no. 5741; Cell
Signaling Technologies Inc., Beverly, MA, USA) and monoclonal mouse
anti-β-actin (cat. no. sc-47778; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-labeled
anti-mouse (cat. no. W4021) and anti-rabbit (cat. no. W4011)
secondary antibodies were obtained from Promega Corporation
(Madison, WI, USA). Go6976 was obtained from Calbiochem (La Jolla,
CA, USA) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich,
St. Louis, MO, USA).
Microarray data searching
The microarray raw data were searched in
ArrayExpress (http://www.ebi.ac.uk/arrayex-press/) with accession
number E-TABM-157 for 26 TNBC cell lines.
Tissue array
The array slides (BR1503b) were purchased from US
Biomax Inc. (Rockville, MD, USA). Detailed information for this
array can be viewed at http://www.biomax.us/tissue-arrays/. There were seven
breast intraductal carcinoma and 60 breast invasive ductal
carcinoma slides, which contained 30 TNBC duplicate cores per case.
Each specimen was represented by two cores, and prepared from
paraffin-embedded specimens, deparaffinized in xylene, and
rehydrated through an alcohol series. The sections were then
incubated with 3% H2O2 for 5 min. After
washing with phosphate-buffered saline (PBS), the sections were
heated to boiling in EDTA solution (1 mM EDTA, 0.1% NP-40; pH 8.0)
for 5 min in a microwave oven. This non-competitive inhibition
procedure was repeated once after an interruption of 10 min.
After cooling for 20 min, the sections were washed
three times in PBS for 5 min and then incubated in PBS with 3%
Invitrogen fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 25 min. The sections were washed with PBS and
incubated with purified polyclonal antibodies against PKCα or AXL
[10 ng/ml PBS plus 0.2% bovine serum albumin; BSA (Sigma-Aldrich)]
at room temperature for 1 h. After washing three times in PBS for 5
min, the sections were incubated with biotinylated-labeled goat
anti-rabbit IgG (dilution, 1:2,000; Sigma-Aldrich) at room
temperature for 30 min. The sections were then washed with PBS and
incubated with ABC reagent (Avidin/Biotin kit; Vector Laboratories,
Inc., Burlingame, CA, USA) conjugated with peroxidase at room
temperature for 30 min. PKCα or AXL antigen staining was visualized
by adding 3,3′-diaminobenzidine substrate (Sigma-Aldrich). The
reaction was terminated by rinsing the sections in distilled
water.
The sections were counterstained with Gill's
hematoxylin V (Mute Pure Chemicals Ltd., Tokyo, Japan), dehydrated
in graded alcohol, and cleared with xylene prior to mounting with
Malinol (Mute Pure Chemicals Ltd.). Immunoreactivity was examined
with a BX40 system microscope (Olympus, Tokyo, Japan) with a CCD
DPII Camera (Olympus, Tokyo, Japan). Images were analyzed by
Image-Pro Plus software version 4.5 (Media Cybernetics, Silver
Spring, MD, USA). The intensity was scored as '1+,' '2+,' '3+,' and
'4+' for none, weak, moderate, and strong staining,
respectively.
Cell culture
Hs578T and MDA-MB-231 breast cancer cell lines were
included in the present study. The cells were purchased from the
Bioresources Collection and Research Center, Food Industry Research
and Development Institute (Hsinchu, Taiwan), cultured with
Dulbecco's modified Eagle's medium (DMEM) and DMEM/F12,
respectively Gibco/Invitrogen; Thermo Fisher Scientific, Inc.), and
supplemented with 10% FBS, 100 U/ml penicillin G, and 100
µg/ml streptomycin (Sigma-Aldrich) in a humidified
atmosphere containing 5% CO2 at 37°C.
Transfection
Lipofectin (Sigma-Aldrich) was used to perform
transfection. Cells were cultured in a 60-mm dish containing 10%
FBS-DMEM at 37°C, incubated for 24 h before rinsing with serum-free
DMEM, and transferred into 1 ml serum-free DMEM containing 15
µg/ml Lipofectamine 2000 Transfection Reagent (Thermo Fisher
Scientific, Inc.) and 5 or 10 µg of the indicated plasmid.
After a 6-h incubation, 1 ml DMEM supplemented with 10% FBS was
added to the medium. After incubation for another 18 h, the medium
was replaced with fresh 10% FBS-DMEM before 48 h of incubation. The
cells were then lysed for western blot analysis.
Western blot analysis
The cultured cells were washed twice with PBS and
lysed with a lysing buffer containing 50 mM Tris/HCl (pH 7.4), 2 mM
EDTA, 2 mM EGTA, 150 mM NaCl, 1 mM PMSF, 1 mM NaF, 1 mM sodium
orthovanadate, 1% (v/v) 2-mercaptoethanol, 1% (v/v) Nonidet P40 and
0.3% sodium deoxycholate. The cell lysates were centrifuged at
12,000 × g and 4°C for 15 min. The supernatant was collected and
the protein concentration was determined by the Brad-ford method.
Equal amounts of protein extracts (50 µg) were subjected to
12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(Sigma-Aldrich) and blotted onto a polyvinylidene fluoride membrane
(Amersham Pharmacia Biotech, Piscataway, NJ, USA). After blocking
with BSA, the membrane was incubated with specific anti-PKCα
(1:5,000), anti-AXL (1:1,000) and anti-β-actin (1:10,000)
antibodies. The blots were then washed three times in 50 ml buffer
for 10 min and incubated with HRP-conjugated anti-mouse or
anti-rabbit antibody (1:3,000) at room temperature for 2 h.
Proteins were detected using an enhanced chemiluminescent detection
system (Amersham Pharmacia Biotech).
Cell proliferation assay
Cell proliferation was determined by an MTT assay
(Sigma-Aldrich). The cells were seeded in 24-well plates at
1×104 cells/well, and cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% FBS at 37°C overnight. These
cells were treated with various doses of Go6976 and incubated for
24 or 48 h. After incubation, the medium was replaced with fresh
medium, and the cells were incubated with 5 mg/ml MTT
(Sigma-Aldrich) for 4 h prior to dissolving in 1 ml isopropanol for
10 min. The optical density at 570 nm was then measured using a
spectrophotometer (Synergy 2 multi-mode reader; BioTek Instruments,
Inc., Winooski, VT, USA).
Plasmid construction
Vectors containing myc-MZF-1 (amino acids 60–72)
were constructed by expressing MZF-1 (amino acids 60–72;
SDLRSEQDPTDED encoded from 1,268 to 1,306 bp) in a pcDNA3.1/myc-His
vector. DNA fragments of MZF-1 (forward:
5′-GGAATTCCAAGCGATCTGAGGAGTGAACAGGACCCCACGGACGAGGATCCCAAGCTTGGG-3′
and reverse:
5′-CCCAAGCTTGGGATCCTCGTCCGTGGGGTCCTGTTCACTCCTCAGATCGCTTGGAATTCCA-3′)
were synthesized by MDBio, Inc. (Rockville, MD, USA) and cloned
through ligation into the EcoRI-HindIII sites of the
pcDNA3.1/myc-His vector.
Vector containing full length PKCα-c-myc was
constructed by expressing PKCα (28-2,046 bp) in a pcDNA3.1/myc-His
vector. The open reading frame of the human PKCα (GenBank accession
no. X52479.1) gene was obtained from MDA-MB-231 cells by reverse
transcription-polymerase chain reaction (RT-PCR).
RT-PCR
An aliquot of total RNA (0.5 µg) was reverse
transcribed using 0.5 µM oligo d(T) primers in a reaction
solution (50 µl) containing 75 mM KCl, 50 mM Tris-HCl (pH
8.3), 3 mM MgCl2, 10 mM DTT, 10 units RNase inhibitor
(Promega Corporation), 0.8 mM total dNTPs, and 200 units of Maloney
murine leukemia virus reverse transcriptase (Promega Corporation).
The sample was incubated at 42°C for 1 h and at 99°C for 5 min
before cooling on ice for 10 min.
The RT product (2 µl) was diluted with the
PCR buffer (50 mM KCl, 10 mM Tris-HCl and 2 mM MgCl2) to
a final volume of 50 µl, containing 0.5 µM dNTPs
(final concentration, 0.8 mM) and 0.5 units of Super-Therm Taq DNA
polymerase [Southern Cross Biotechnology (Pty) Ltd., Cape Town,
South Africa]. PCR was performed on a GeneAmp PCR system 2400
(Applied Biosystems; Thermo Fisher Scientific, Inc.). For each
experiment, up to 40 cycles were performed to avoid reaching the
PCR plateau values. The PCR products were analyzed by 1.2% agarose
gel electrophoresis and direct visualization using SYBR Green I
(Cambrex Bio Science Rockland Ltd., Rockland, ME, USA) staining.
The agarose gels were scanned and analyzed using the Kodak
Scientific 1D Imaging system (Kodak, Rochester, NY, USA). The
specificity of the cDNA was also evaluated via DNA sequence
analysis (data not shown).
The primers were as follows:
5′-GGAATTCCAATGGCTGACGTTTTCCCGGGC-3′ (containing an EcoRI site) and
5′-CCCAAGCTTTACTGCACTCTGTAAGATGGG-3′ (containing a HindIII site).
The PKCα fragment was constructed with EcoRI and
HindIII, and the fragment was isolated and cloned through
ligation into the EcoRI-HindIII sites of the
mammalian expression system vector pcDNA3.1/myc-His(−)B
vector (Invitrogen; Thermo Fisher Scientific, Inc.).
TAT-fused peptide design
The TAT-fused peptides were designed such that the
TAT moiety corresponded to amino acid residues 48–57 of the HIV TAT
protein (15), The TAT-fused
peptide MZF-1 fragment (60–72; YGRK-KRRQRRRGGGDEDTPDQESRLDS) was
purchased from MDBio, Inc. (Rockville, MD, USA).
Statistical analysis
Microarray data analyses were performed using a
linear regression test. Tissue array data analyses were performed
by the Mann-Whitney U tests and Fisher's exact test, and data are
expressed as the mean ± standard error of the mean. Cell
proliferation and western blotting results were analyzed by
analysis of variance and Student's t-test was used for the
two-group comparisons. Analyse-it software (http://analyse-it.com/) was used for performing
statistical analysis and P<0.05 was considered to indicate a
statistically significant difference.
Results
PKCα is correlated with AXL expression in
TNBC
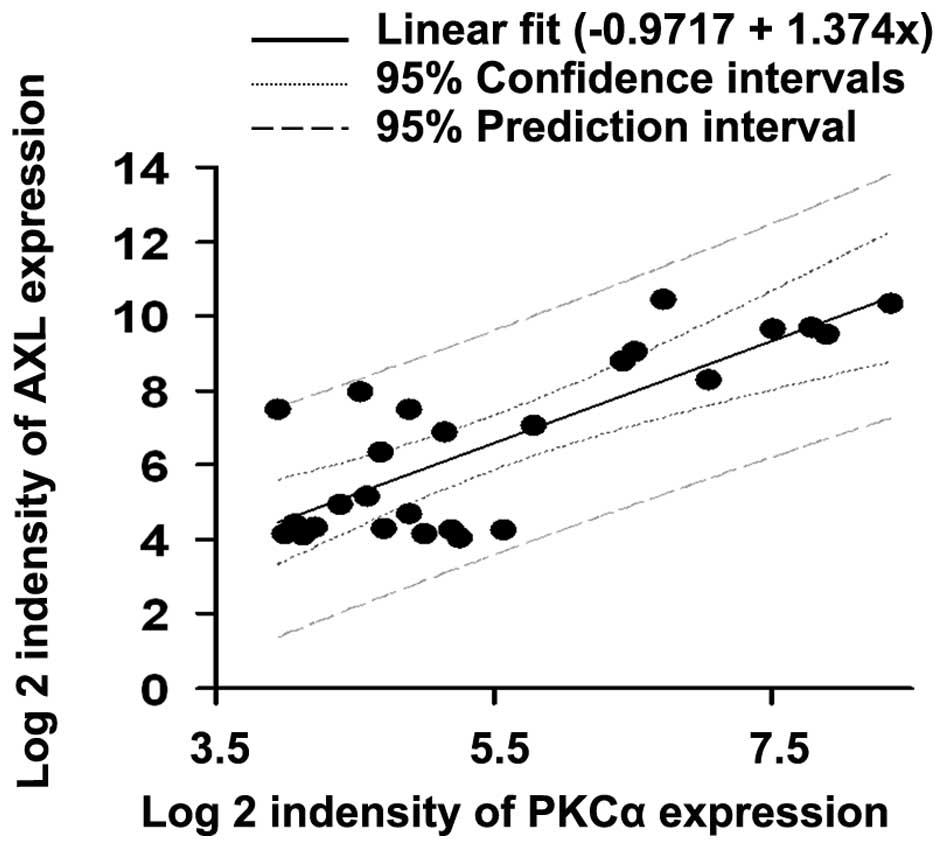
Previous microarray data was analyzed and a
significant correlation between PKCα and AXL expression in human
TNBC cell lines was demonstrated (Fig.
1). Tissue array results confirmed the same phenomenon in TNBC
(Fig. 2 and Tables I and II). Data showed that the higher
expression of AXL was correlated with elevated PKCα expression in
TNBC. No significant association was identified between AXL
expression and all clinicopathological features and biomarkers.
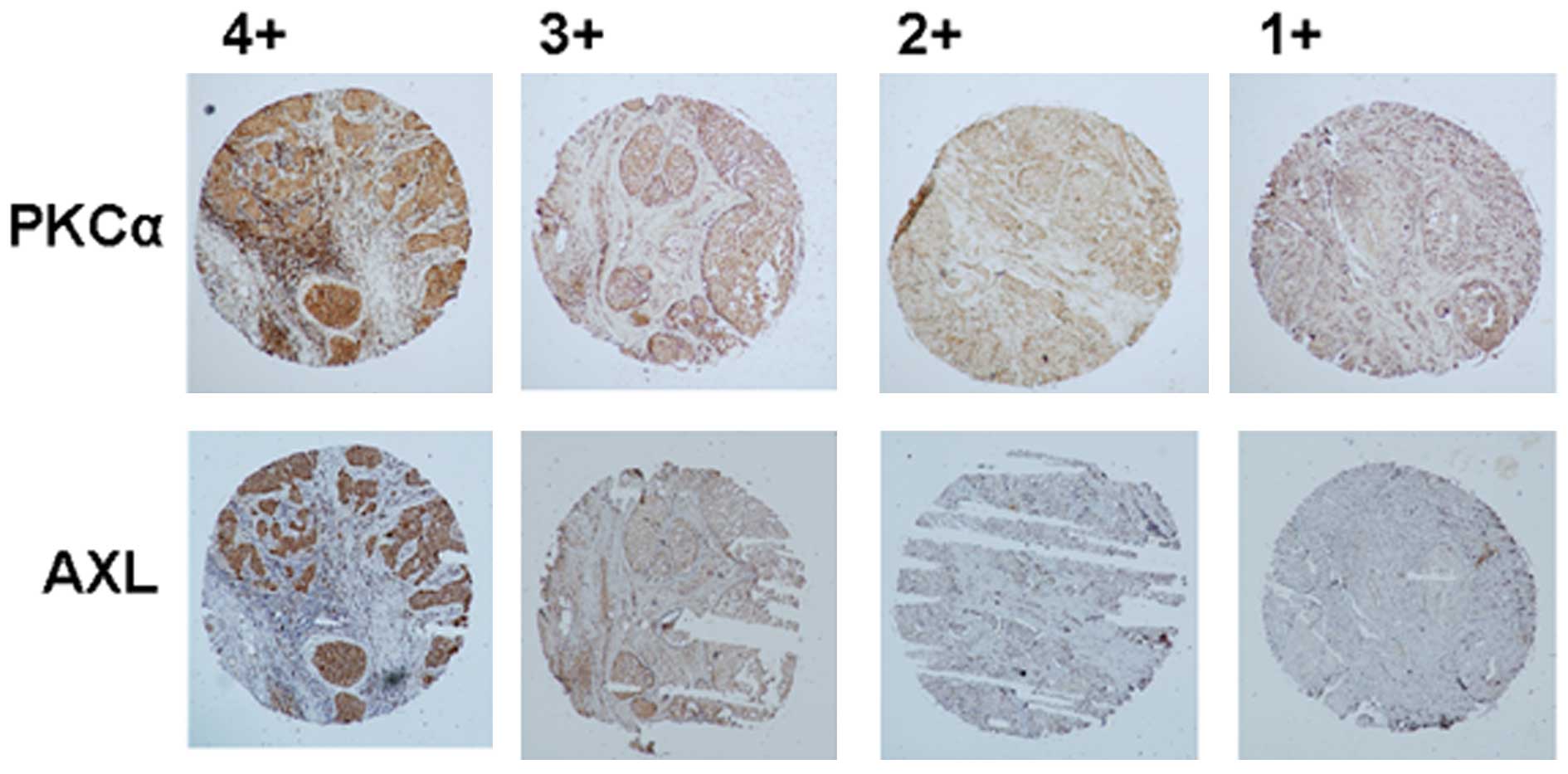 | Figure 2Expression of PKCα and AXL in TNBC
(magnification, ×40). The array slides (BR1503b) were purchased
from US Biomax Inc. (Rockville, MD, USA). The slides were prepared
and stained with antibodies against PKCα and AXL. The intensity was
scored as '1+,' '2+,' '3+,' and '4+' for no, weak, moderate and
strong staining, respectively. PKCα, protein kinase C α; TNBC,
triple-negative breast cancer. |
 | Table IExpression of AXL in breast cancer
tissue with high and low expression of PKCα (n=30). |
Table I
Expression of AXL in breast cancer
tissue with high and low expression of PKCα (n=30).
| Level of PKCαa | No. of patients | AXL
expressionb |
|---|
| Low | 8 | 1.50±0.19c |
| High | 22 | 2.68±0.12 |
 | Table IIAssociation between the expression
levels of AXL and the clinical characteristics of patients with
breast cancer (n=30). |
Table II
Association between the expression
levels of AXL and the clinical characteristics of patients with
breast cancer (n=30).
| Clinical
characteristica | Level of AXLb
| P-valuec |
|---|
| Low (n) | High (n) |
|---|
| Age, years |
| ≤60 | 13 | 13 | NS |
| >60 | 3 | 1 | |
| TNM-staging |
| I, IIa and IIb | 14 | 11 | NS |
| IIIa and IIIb | 2 | 3 | |
| Histopathological
grading |
|
Well-differentiated | 3 | 4 | NS |
| Moderately- or
poorly-differentiated | 13 | 10 | |
| Ki67 |
| Negative | 10 | 9 | NS |
| Positive | 6 | 5 | |
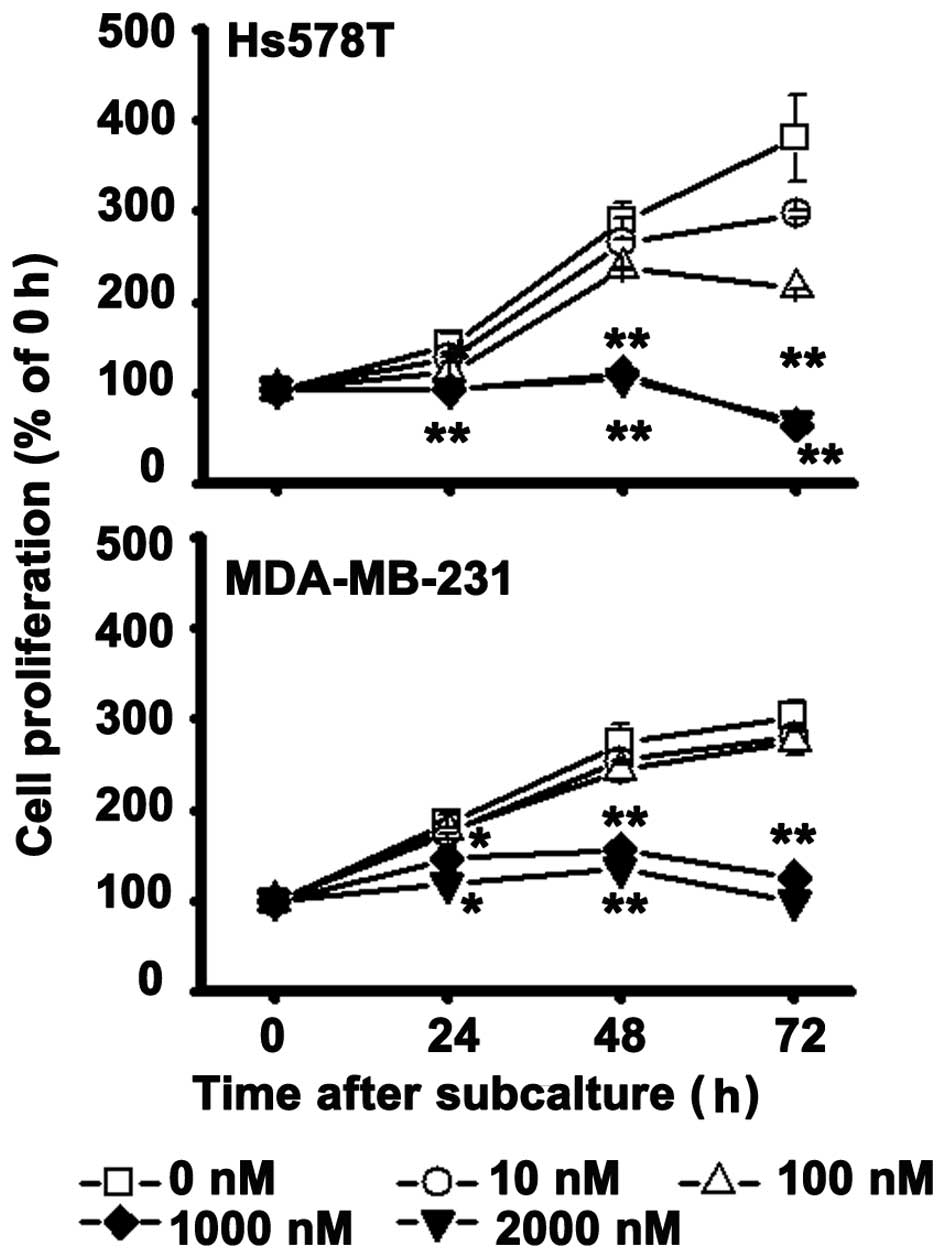
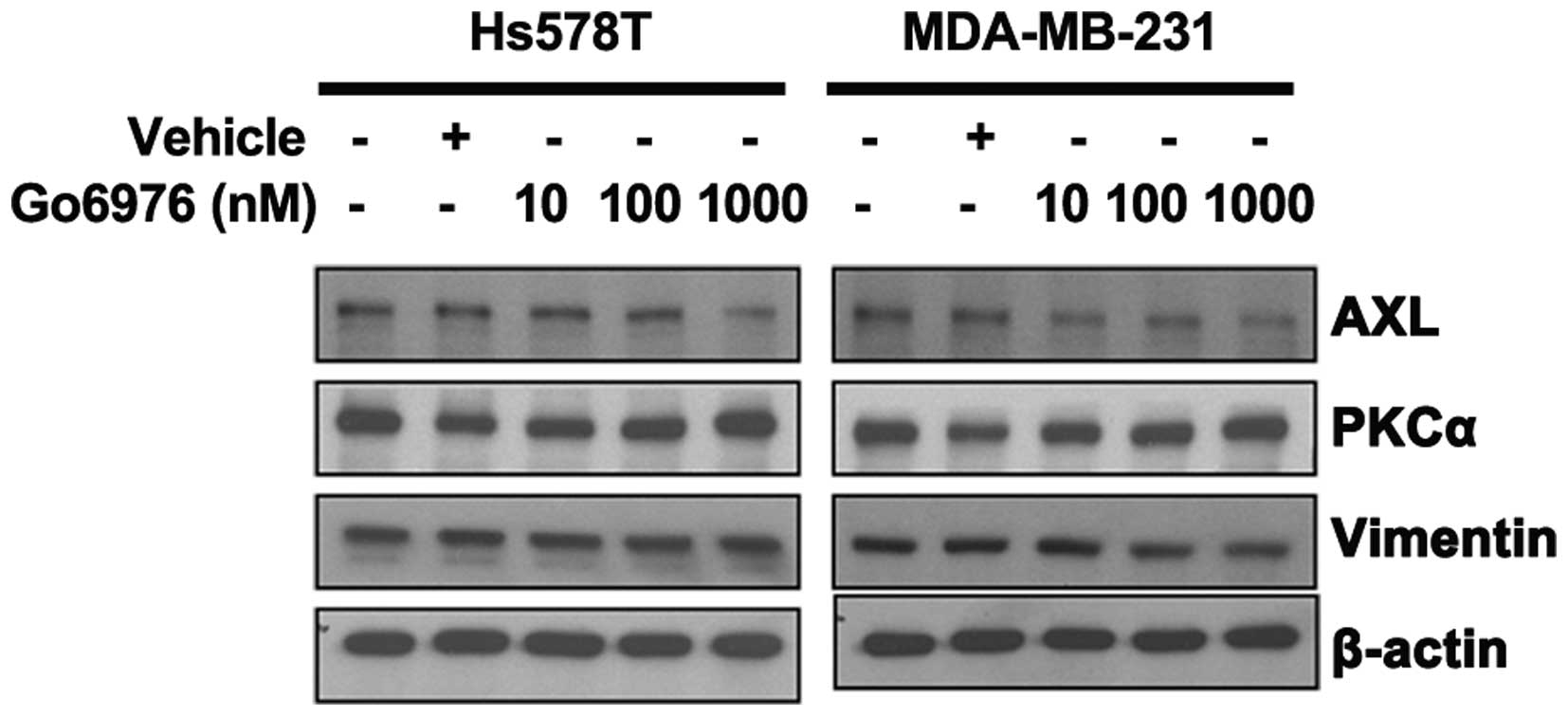
AXL expression is inhibited by Go6976 in
TNBC cells
To further determine the correlation between PKCα
and AXL expression, a selective PKCα/β inhibitor, Go6976, was used
to treat the breast cancer cells. The results show that Go6976, at
a gradient concentration of 10–1,000 nM, significantly inhibited
TNBC MDA-MB-231 cell proliferation from 92 to 41% of the control
group (Fig. 3), and TNBC Hs578T
cell proliferation from 78 to 29%. AXL and vimentin expression also
decreased in a dose-dependent manner (Fig. 4), but there were no changes in PKCα
expression.
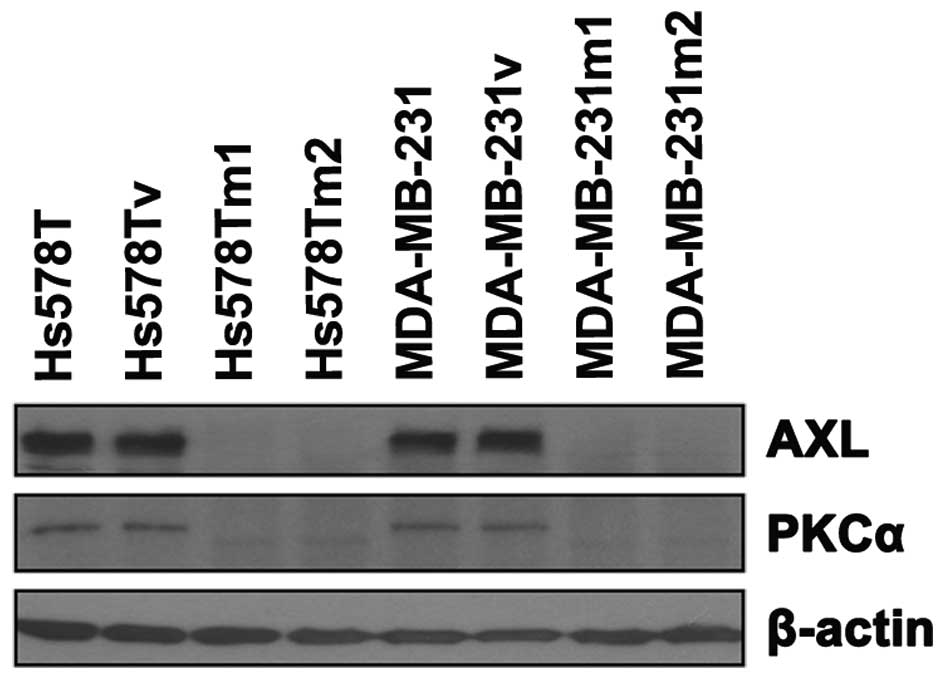
PKCα and AXL are downregulated by an
MZF-1 peptide in TNBC cells
In a previous study, it was demonstrated that PKCα
is regulated by MZF-1 and Elk-1 (16). Recently, data has shown that MZF-1
and Elk-1 form a heterodimer and a peptide (MZF-1 peptide) matching
one of their binding domains (Lee et al, unpublished data),
which is the binding site for Elk-1. When saturation of the Elk-1
binding site was induced by transfection with this peptide into the
MDA-MB-231 and Hs578T cells, PKCα and AXL expression was observed
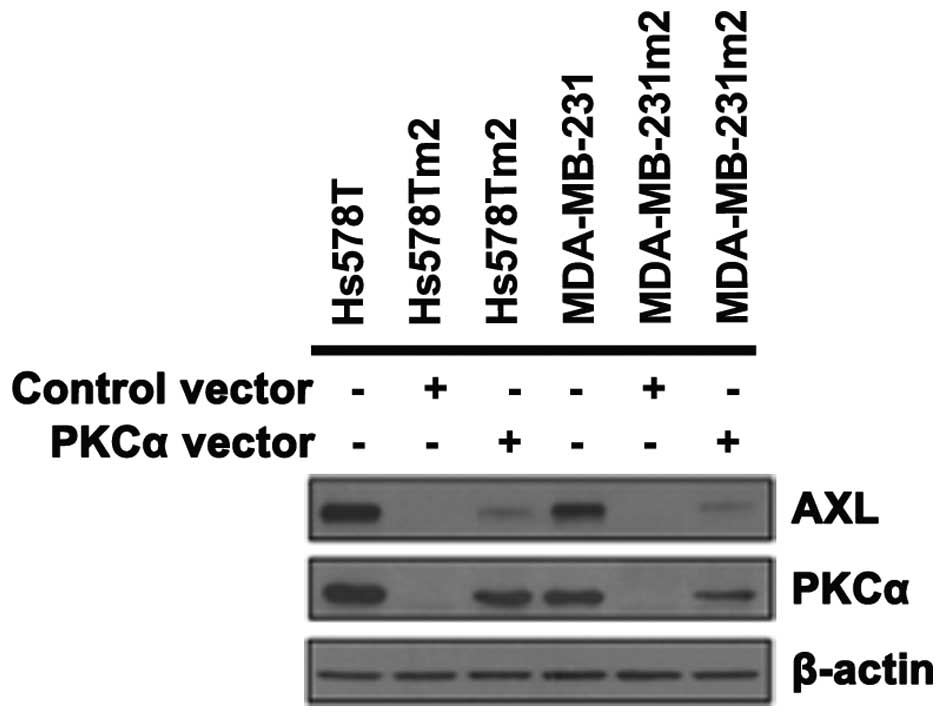
to be decreased (Fig. 5). This was
reversed by co-treatment with full length of PKCα (Fig. 6). When the same cells were treated
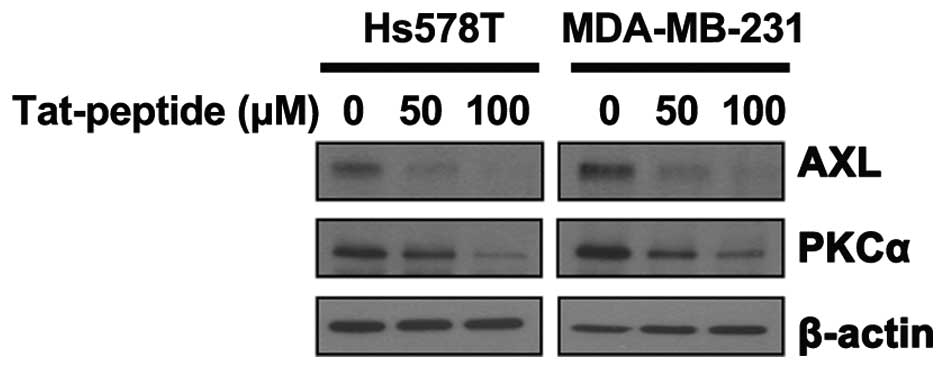
with a TAT-fused peptide, which is a TAT peptide fused to the
fragment of the MZF-1 acidic domain, inhibition was also observed
(Fig. 7), confirming the
correlation between PKCα and AXL.
Discussion
The present microarray data show that PKCα was
significantly correlated with AXL expression in the TNBC cell
lines. Tissue array data showed that AXL was correlated with
elevated PKCα expression in TNBC, suggesting that AXL and PKCα
synergize to promote breast cancer malignant progression. These
results were consistent with the findings that AXL downregulation
in leukemia is achieved by transfection with PKCα/β small
interfering RNA (14). In
addition, inactivation of AXL caused by monoclonal antibodies and
RNA interference attenuates tumor growth and reduces cell survival
(15,17). Furthermore, treatment with the PKCα
inhibitor Go6976, demonstrated that AXL and the EMT-related gene,
vimentin, could be regulated by PKCα in TNBC cells. These findings
suggest the involvement of AXL signaling in PKCα-dependent
TNBC.
Furthermore, the decrease of AXL expression was
correlated with PKCα downregulation in MZF-1 peptide-treated TNBC
cells. Moreover, the EMT-related gene vimentin and slug expression,
cell migration, tumorigenicity, and drug-resistance of the same
cells were also previously shown to be reduced (Lee et al,
unpublished data). These findings suggest that PKCα may promote AXL
expression and EMT in TNBC cells. By contrast, Tam et al
(18) found that encoding
EMT-related genes in non-cancer stem cells can induce PKCα
expression causing their transformation into cancer stem cells.
However the data from the present study demonstrates an opposite
effect, where the PKCα-induced EMT is mediated through the AXL
signaling pathway, can also occur in TNBC cells.
AXL has been reported to be induced by PKC through
the PKC/mitogen-activated protein kinase (MAPK)/AP-1 signaling axis
in leukemia (13). Moreover, AXL
gene expression can be regulated by MZF-1 in chronic myeloid
leukemia, as well as microRNA-34a and EMT-related genes in breast
cancer (19–21). This suggests that some of the
above-mentioned MAPK and microRNA genes, including AXL, may be
downstream of PKCα, as it also regulates claudin-1 via the Snail-
and MAPK-dependent pathways during EMT (22), and are associated with invasive
growth in two genetic models of EMT (6). Thus, although the molecular mechanism
of AXL expression regulated by PKCα in TNBC cells remains unclear,
it was suggested that PKCα-induced AXL expression may be mediated
through multiple signaling pathways.
Gjerdrum et al (2) did not identify any significant
association between AXL expression and important
clinicopathological features, such as tumor diameter, histological
grade, expression of estrogen and progesterone receptors, and
auxiliary lymph node status. Moreover, AXL expression was not
significantly associated with Her2 status, E-cadherin expression,
markers of basal differentiation (cytokeratin 5/6 or P-cadherin),
or tumor cell proliferation by Ki-67 staining (2). The present data confirmed the absence
of significant associations between AXL expression and all
clinicopathological features and biomarkers.
In lung and bladder cancer, like breast cancer, AXL
has been correlated with poor diagnosis and its inhibition has been
demonstrated to be an important mechanism in inhibiting cancer
progression (23). Moreover, AXL
and vimentin expression levels and cell proliferation in A549 and
HT5637 cell lines can also be inhibited by Go6976 (data not shown).
In conclusion, these results confirm the correlation between AXL
and PKCα, and suggest PKCα-AXL signaling may be a treatment target,
particularly in malignant cancer cells. Furthermore, the present
findings provide the basis for additional research into the
treatment of TNBC.
Acknowledgments
The authors would like to thank Mr. Andy Chang for
critical reading of the manuscript. This study was supported by
grants from the China Medical University Hospital (grant no.
DMR-104-019), the Ministry of Science and Technology, Republic of
China (grant no 104-2320-B-039-032) and the Taiwan Ministry of
Health and Welfare Clinical Trial and Research Center of Excellence
(grant no. MOHW105-TDU-B-212-133019), Taiwan, R.O.C.
References
|
1
|
Asiedu MK, Beauchamp-Perez FD, Ingle JN,
Behrens MD, Radisky DC and Knutson KL: AXL induces
epithelial-to-mesenchymal transition and regulates the function of
breast cancer stem cells. Oncogene. 33:1316–1324. 2014. View Article : Google Scholar
|
|
2
|
Gjerdrum C, Tiron C, Høiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
et al: Axl is an essential epithelial-to-mesenchymal
transition-induced regulator of breast cancer metastasis and
patient survival. Proc Natl Acad Sci USA. 107:1124–1129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer AS, Miller MA, Gertler FB and
Lauffenburger DA: The receptor AXL diversifies EGFR signaling and
limits the response to EGFR-targeted inhibitors in triple-negative
breast cancer cells. Sci Signal. 6:ra662013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tonetti DA, Gao W, Escarzaga D, Walters K,
Szafran A and Coon JS: PKCα and ERβ Are Associated with
Triple-Negative Breast Cancers in African American and Caucasian
Patients. Int J Breast Cancer. 2012:7403532012. View Article : Google Scholar
|
|
6
|
Ouelaa-Benslama R, De Wever O, Hendrix A,
Sabbah M, Lambein K, Land D, Prévost G, Bracke M, Hung MC, Larsen
AK, et al: Identification of a GαGβγ, AKT and PKCα signalome
associated with invasive growth in two genetic models of human
breast cancer cell epithelial-to-mesenchymal transition. Int J
oncol. 41:189–200. 2012.PubMed/NCBI
|
|
7
|
Frankel LB, Lykkesfeldt AE, Hansen JB and
Stenvang J: Protein Kinase C alpha is a marker for antiestrogen
resistance and is involved in the growth of tamoxifen resistant
human breast cancer cells. Breast Cancer Res Treat. 104:165–179.
2007. View Article : Google Scholar
|
|
8
|
Tonetti DA, Morrow M, Kidwai N, Gupta A
and Badve S: Elevated protein kinase C alpha expression may be
predictive of tamoxifen treatment failure. Br J Cancer.
88:1400–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Assender JW, Gee JM, Lewis I, Ellis IO,
Robertson JF and Nicholson RI: Protein kinase C isoform expression
as a predictor of disease outcome on endocrine therapy in breast
cancer. J Clin Pathol. 60:1216–1221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lønne GK, Cornmark L, Zahirovic IO,
Landberg G, Jirström K and Larsson C: PKCalpha expression is a
marker for breast cancer aggressiveness. Mol Cancer. 9:762010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim J, Thorne SH, Sun L, Huang B and
Mochly-Rosen D: Sustained inhibition of PKCα reduces intravasation
and lung seeding during mammary tumor metastasis in an in vivo
mouse model. Oncogene. 30:323–333. 2011. View Article : Google Scholar
|
|
12
|
Li Z, Wang N, Fang J, Huang J, Tian F, Li
C and Xie F: Role of PKC-ERK signaling in tamoxifen-induced
apoptosis and tamoxifen resistance in human breast cancer cells.
Oncol Rep. 27:1879–1886. 2012.PubMed/NCBI
|
|
13
|
Mudduluru G, Leupold JH, Stroebel P and
Allgayer H: PMA up-regulates the transcription of Axl by AP-1
transcription factor binding to TRE sequences via the MAPK cascade
in leukaemia cells. Biol Cell. 103:21–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dufies M, Jacquel A, Belhacene N, Robert
G, Cluzeau T, Luciano F, Cassuto JP, Raynaud S and Auberger P:
Mechanisms of AXL overexpression and function in Imatinib-resistant
chronic myeloid leukemia cells. Oncotarget. 2:874–885. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keating AK, Kim GK, Jones AE, Donson AM,
Ware K, Mulcahy JM, Salzberg DB, Foreman NK, Liang X, Thorburn A
and Graham DK: Inhibition of Mer and Axl receptor tyrosine kinases
in astrocytoma cells leads to increased apoptosis and improved
chemosensitivity. Mol Cancer Ther. 9:1298–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh YH, Wu TT, Tsai JH, Huang CY, Hsieh
YS and Liu JY: PKCalpha expression regulated by Elk-1 and MZF-1 in
human HCC cells. Biochem Biophys Res Commun. 339:217–225. 2006.
View Article : Google Scholar
|
|
17
|
Song X and Wang H, Logsdon CD, Rashid A,
Fleming JB, Abbruzzese JL, Gomez HF, Evans DB and Wang H:
Overexpression of receptor tyrosine kinase Axl promotes tumor cell
invasion and survival in pancreatic ductal adenocarcinoma. Cancer.
117:734–743. 2011. View Article : Google Scholar
|
|
18
|
Tam WL, Lu H, Buikhuisen J, Soh BS, Lim E,
Reinhardt F, Wu ZJ, Krall JA, Bierie B, Guo W, et al: Protein
kinase C α is a central signaling node and therapeutic target for
breast cancer stem cells. Cancer Cell. 24:347–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mudduluru G, Vajkoczy P and Allgayer H:
Myeloid zinc finger 1 induces migration, invasion and in vivo
metastasis through Axl gene expression in solid cancer. Mol Cancer
Res. 8:159–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH,
Ambs S and Caplen NJ: Identification of the receptor tyrosine
kinase AXL in breast cancer as a target for the human miR-34a
microRNA. Breast Cancer Res Treat. 130:663–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar
|
|
22
|
Kyuno D, Kojima T, Yamaguchi H, Ito T,
Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K and
Sawada N: Protein kinase Cα inhibitor protects against
downregulation of claudin-1 during epithelial-mesenchymal
transition of pancreatic cancer. Carcinogenesis. 34:1232–1243.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paccez JD, Vogelsang M, Parker MI and
Zerbini LF: The receptor tyrosine kinase Axl in cancer: Biological
functions and therapeutic implications. Int J Cancer.
134:1024–1033. 2014. View Article : Google Scholar
|