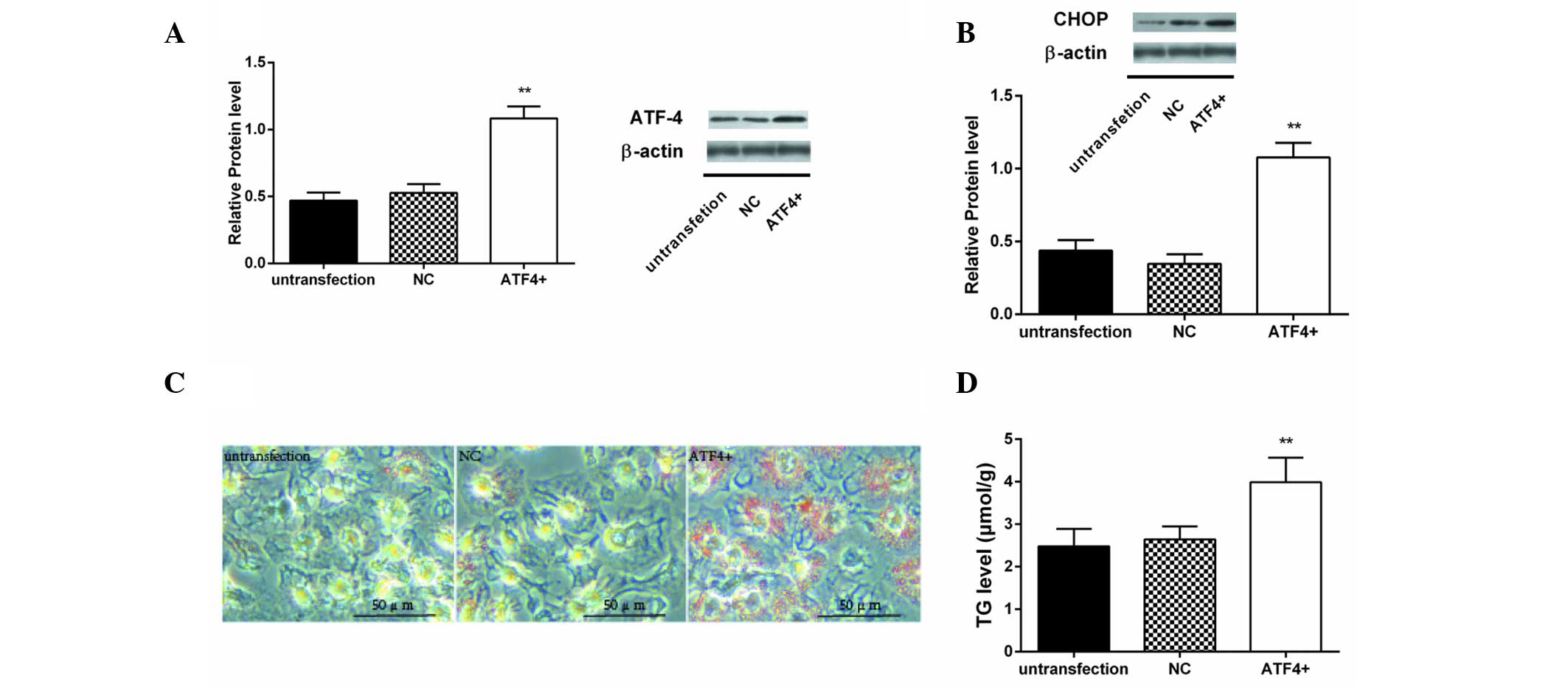
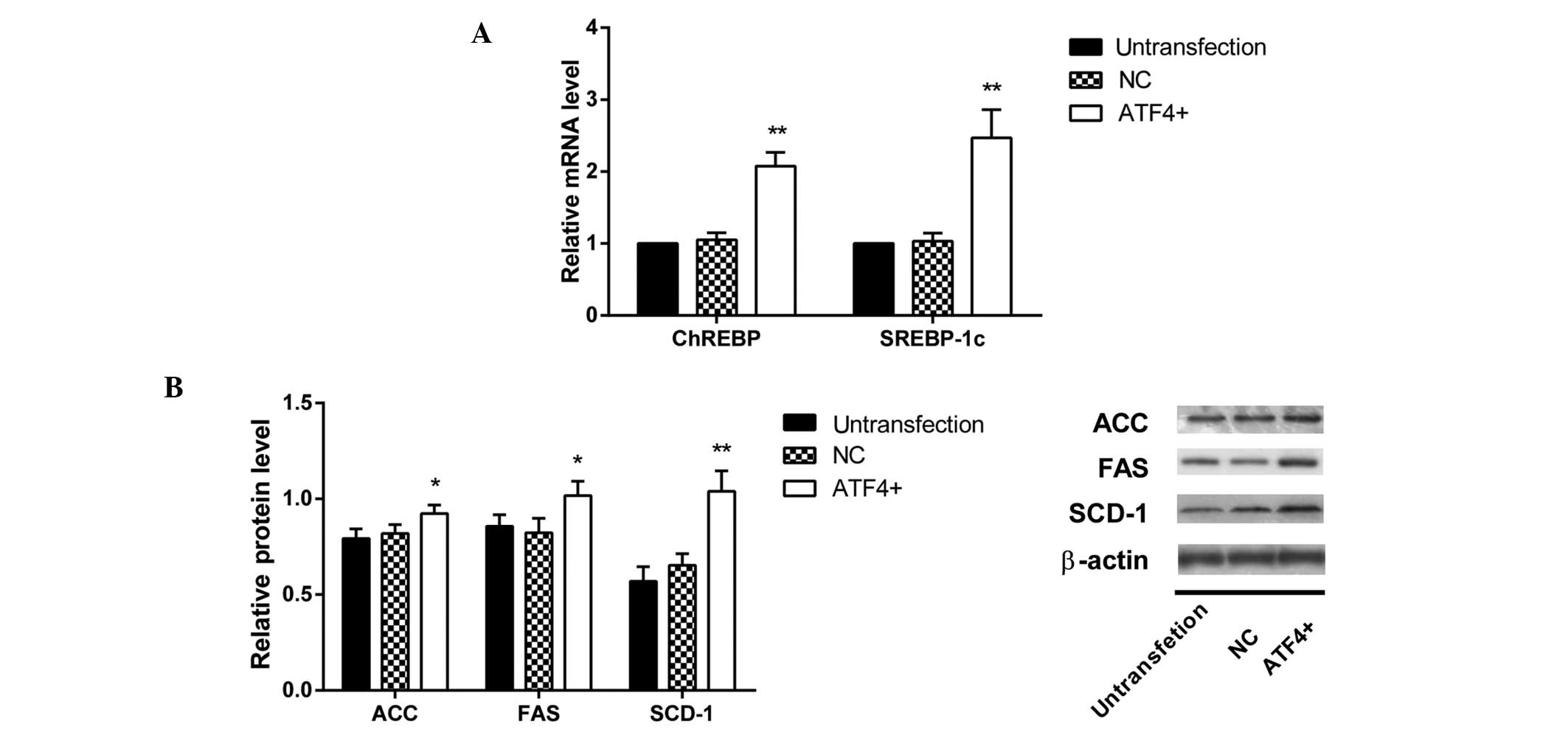
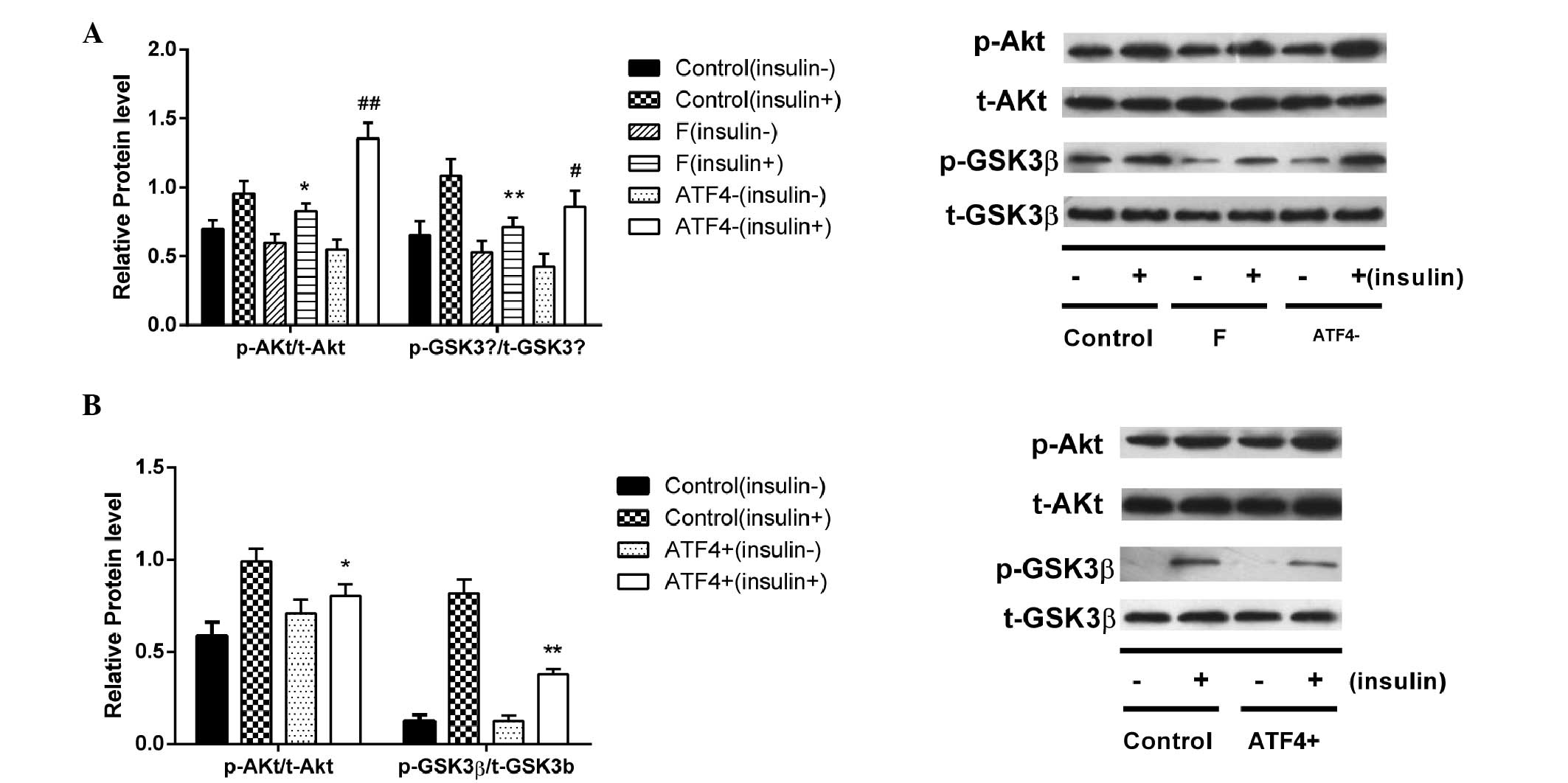
|
1
|
Malavolti M, Battistini NC, Miglioli L,
Bagni I, Borelli L, Marino M, Scaglioni F and Bellentani S:
Influence of lifestyle habits, nutritional status and insulin
resistance in NAFLD. Front Biosci (Elite Ed). 4:1015–1023. 2012.
View Article : Google Scholar
|
|
2
|
Firneisz G: Non-alcoholic fatty liver
disease and type 2 diabetes mellitus: The liver disease of our age?
World J Gastroenterol. 20:9072–9089. 2014.PubMed/NCBI
|
|
3
|
Leite NC, Villela-Nogueira CA, Cardoso CR
and Salles GF: Non-alcoholic fatty liver disease and diabetes: From
physiopathological interplay to diagnosis and treatment. World J
Gastroenterol. 20:8377–8392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perry RJ, Samuel VT, Petersen KF and
Shulman GI: The role of hepatic lipids in hepatic insulin
resistance and type 2 diabetes. Nature. 510:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren LP, Chan SM, Zeng XY, Laybutt DR,
Iseli TJ, Sun RQ, Kraegen EW, Cooney GJ, Turner N and Ye JM:
Differing endoplasmic reticulum stress response to excess
lipogenesis versus lipid oversupply in relation to hepatic
steatosis and insulin resistance. PLoS One. 7:e308162012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Basseri S and Austin RC: Endoplasmic
reticulum stress and lipid metabolism: Mechanisms and therapeutic
potential. Biochem Res Int. 2012:8413622012. View Article : Google Scholar
|
|
8
|
Zhou H, Zhang K, Janciauskiene S and Li X:
Endoplasmic reticulum stress and lipid metabolism. Biochem Res Int.
2012:2575282012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volmer R and Ron D: Lipid-dependent
regulation of the unfolded protein response. Curr Opin Cell Biol.
33:67–73. 2015. View Article : Google Scholar :
|
|
10
|
Lee AH and Glimcher LH: Intersection of
the unfolded protein response and hepatic lipid metabolism. Cell
Mol Life Sci. 66:2835–2850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren LP, Song GY, Hu ZJ, Zhang M, Peng L,
Chen SC, Wei L, Li F and Sun W: The chemical chaperon
4-phenylbutyric acid ameliorates hepatic steatosis through
inhibition of de novo lipogenesis in high-fructose-fed rats. Int J
Mol Med. 32:1029–1036. 2013.PubMed/NCBI
|
|
13
|
Oyadomari S, Harding HP, Zhang Y,
Oyadomari M and Ron D: Dephosphorylation of translation initiation
factor 2alpha enhances glucose tolerance and attenuates
hepatosteatosis in mice. Cell Metab. 7:520–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Huang Z, Du Y, Cheng Y, Chen S and
Guo F: ATF4 regulates lipid metabolism and thermogenesis. Cell Res.
20:174–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohamed HA, Yao W, Fioravante D, Smolen PD
and Byrne JH: cAMP-response elements in Aplysia creb1, creb2 and
Ap-uch promoters: Implications for feedback loops modulating long
term memory. J Biol Chem. 280:27035–27043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seo J, Fortuno ER III, Suh JM, Stenesen D,
Tang W, Parks EJ, Adams CM, Townes T and Graff JM: Atf4 regulates
obesity, glucose homeostasis and energy expenditure. Diabetes.
58:2565–2573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lange PS, Chavez JC, Pinto JT, Coppola G,
Sun CW, Townes TM, Geschwind DH and Ratan RR: ATF4 is an oxidative
stress-inducible, prodeath transcription factor in neurons in vitro
and in vivo. J Exp Med. 205:1227–1242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Meng Q, Xiao F, Chen S, Du Y, Yu J,
Wang C and Guo F: ATF4 deficiency protects mice from
high-carbohydrate-diet-induced liver steatosis. Biochem J.
438:283–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao G, Zhang T, Yu S, Lee S,
Calabuig-Navarro V, Yamauchi J, Ringquist S and Dong HH: ATF4
protein deficiency protects against high fructose-induced
hypertriglyceridemia in mice.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
J Biol Chem. 288:25350–25361. 2013.
View Article : Google Scholar
|
|
21
|
Kammoun HL, Chabanon H, Hainault I, Luquet
S, Magnan C, Koike T, Ferré P and Foufelle F: GRP78 expression
inhibits insulin and ER stress-induced SREBP-1c activation and
reduces hepatic steatosis in mice. J Clin Invest. 119:1201–1215.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XQ, Xu CF, Yu CH, Chen WX and Li YM:
Role of endoplasmic reticulum stress in the pathogenesis of
nonalcoholic fatty liver disease. World J Gastroenterol.
20:1768–1776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thorburn AW, Storlien LH, Jenkins AB,
Khouri S and Kraegen EW: Fructose-induced in vivo insulin
resistance and elevated plasma triglyceride levels in rats. Am J
Clin Nutr. 49:1155–1163. 1989.PubMed/NCBI
|
|
24
|
Aragno M, Tomasinelli CE, Vercellinatto I,
Catalano MG, Collino M, Fantozzi R, Danni O and Boccuzzi G:
SREBP-1c in nonalcoholic fatty liver disease induced by
Western-type high-fat diet plus fructose in rats. Free Radic Biol
Med. 47:1067–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimano H, Horton JD, Shimomura I, Hammer
RE, Brown MS and Goldstein JL: Isoform 1c of sterol regulatory
element binding protein is less active than isoform 1a in livers of
transgenic mice and in cultured cells. J Clin Invest. 99:846–854.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Denechaud PD, Bossard P, Lobaccaro JM,
Millatt L, Staels B, Girard J and Postic C: ChREBP, but not LXRs,
is required for the induction of glucose-regulated genes in mouse
liver. J Clin Invest. 118:956–964. 2008.PubMed/NCBI
|
|
27
|
Malhi H and Kaufman RJ: Endoplasmic
reticulum stress in liver disease. J Hepatol. 54:795–809. 2011.
View Article : Google Scholar
|
|
28
|
Gentile CL, Frye M and Pagliassotti MJ:
Endoplasmic reticulum stress and the unfolded protein response in
nonalcoholic fatty liver disease. Antioxid Redox Signal.
15:505–521. 2011. View Article : Google Scholar :
|
|
29
|
Sha H, He Y, Chen H, Wang C, Zenno A, Shi
H, Yang X, Zhang X and Qi L: The IRE1alpha-XBP1 pathway of the
unfolded protein response is required for adipogenesis. Cell Metab.
9:556–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sriburi R, Jackowski S, Mori K and Brewer
JW: XBP1: A link between the unfolded protein response, lipid
biosynthesis and biogenesis of the endoplasmic reticulum. J Cell
Biol. 167:35–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee AH, Scapa EF, Cohen DE and Glimcher
LH: Regulation of hepatic lipogenesis by the transcription factor
XBP1. Science. 320:1492–1496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bobrovnikova-Marjon E, Hatzivassiliou G,
Grigoriadou C, Romero M, Cavener DR, Thompson CB and Diehl JA:
PERK-dependent regulation of lipogenesis during mouse mammary gland
development and adipocyte differentiation. Proc Natl Acad Sci USA.
105:16314–16319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vallejo M, Ron D, Miller CP and Habener
JF: C/ATF, a member of the activating transcription factor family
of DNA-binding proteins, dimerizes with CAAT/enhancer-binding
proteins and directs their binding to cAMP response elements. Proc
Natl Acad Sci USA. 90:4679–4683. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mamady H and Storey KB: Coping with the
stress: Expression of ATF4, ATF6 and downstream targets in organs
of hibernating ground squirrels. Arch Biochem Biophys. 477:77–85.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C and Guo F: Effects of activating
transcription factor 4 deficiency on carbohydrate and lipid
metabolism in mammals. IUBMB Life. 64:226–230. 2012. View Article : Google Scholar : PubMed/NCBI
|