Introduction
Hepatocellular carcinoma (HCC), the dominant form of
primary liver cancer, is one of the most prevalent and
life-threatening types of cancer worldwide (1–6).
Progress has been made in the investigation of the pathogenesis and
treatment of HCC, however, recurrence and metastasis remain key
challenges for effective treatment of HCC, thus limiting the
prognosis and quality of life of patients with HCC.
Typically, HCC is a hypervascular tumor. Microvessel
formation is essential for the growth and metastasis of HCC.
Microvessel density (MVD) is positively-associated with the growth
and metastasis of HCC (7).
Previous studies have reported that activated hepatic stellate
cells (aHSCs) promote the growth and metastasis of HCC by
accelerating the formation of microvessels in HCC tissues (8–11).
However, the mechanism underlying the importance of aHSCs in
microvessel formation in HCC remains to be fully elucidated.
Angiopoietin-1 (Ang-1) is a growth factor involved in angiogenesis.
Previous studies have reported that Ang-1 activates angiogenesis
and promotes tumor growth and metastasis by regulating the
proliferation and migration of endothelial cells in tumor tissues
(12–15).
The association between Ang-1 and aHSC has been
previously described. The transcription and production of vascular
endothelial growth factor (VEGF) and Ang-1 in aHSCs were increased
under hypoxic conditions, and VEGF and Ang-1 promoted aHSC
proliferation and extracellular matrix deposition, increasing the
migration and chemotaxis of cells (16). Taura et al (17) demonstrated that aHSCs promote
angiogenesis in HCC by secreting Ang-1, suggesting that aHSC and
Ang-1 are important for the development of HCC (17). However, it remains unclear whether
aHSC and Ang-1 are associated with microvessel formation in
HCC.
The present study compared the expression of Ang-1,
α smooth muscle actin (α-SMA) and MVD (CD34) between HCC,
HCC-adjacent tissues and normal liver tissues to determine the
association of Ang-1 and aHSC in the development, in particular in
angiogenesis, of HCC.
Materials and methods
Ethical approval of the study
protocol
The current study was approved by the Ethics
Committee of Sun Yat-sen University Cancer Center (Guangzhou,
China). Signed, informed consent was obtained from each patient
prior to the beginning of the current study.
Patients and tumor tissue samples
A total of 25 patients with HCC, 21 males and 4
females, aged between 29 and 75 years old, were enrolled in the
present study. Tissue samples, including HCC tumor tissues and
adjacent non-cancerous tissues (n=25), were obtained from patients
that underwent resection of primary HCC at the Third Affiliated
Hospital, Sun Yat-sen University (Guangzhou, China) between July
and October 2013. The patients had not received preoperative
chemotherapy or radiotherapy. Control samples were collected from a
total of 21 healthy individuals using the same sample collection
protocol. Samples were placed in sterile vials and immediately
stored at −80°C.
Immunohistochemistry (IHC)
Isolated tissues were fixed with 4% paraformaldehyde
for 24 h and embedded in paraffin. Tissue sections (5 µm)
were deparaffinized and rehydrated through an ethanol gradient.
Antigen retrieval was performed by boiling in a 10 mM sodium
citrate buffer (pH 6) for 15 min. Non-specific binding was blocked
by the addition of 5% bovine serum albumin (Beyotime Institute of
Biotechnology, Haimen, China). The sections were then incubated
with monoclonal rabbit anti-Ang-1 (1:200 dilution; Abcam,
Cambridge, UK; cat. no. ab8451), monoclonal rabbit anti-α-SMA
(rabbit anti-α-SMA monoclonal antibody; 1:500 dilution; Gene Tech
Co., Ltd., Shanghai, China; cat. no. GM085101) and monoclonal
rabbit anti-CD34 (1:100 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; cat. no. sc-19621) antibodies overnight at 4°C.
Detection was conducted using an horseradish peroxidase (HRP)
Detection System (Cell Marque; Sigma-Aldrich, St. Louis, MO, USA)
and 3,3′-Diaminobenzidene Substrate kit (Forevergen Biosciences,
Guangzhou, China), following the manufacturer's protocol.
Subsequent to counterstaining with hematoxylin, the slides were
dehydrated, mounted and observed under a light microscope (Olympus
Corporation, Tokyo, Japan). Quantitative analysis was performed on
the IHC images using Image-Pro Plus software (version 4.5; Media
Cybernetics, Inc., Rockville, MD, USA), and the mean optical
density (MOD) was determined from five randomly selected areas.
Western blotting
A total of 25 tissue samples were used to confirm
the expression profile of Ang-1 by western blot analysis. The
samples (~100 mg each) were homogenized and lysed in 500 µl
radioimmunoprecipitation assay buffer. Next, the samples were
centrifuged at 16,000 × g for 1 min at 4°C, the supernatant was
collected and was quantified by the Brad-ford Protein assay kit
(Beyotime Institute of Biotechnology). Denatured recombinant Ang-1
protein was electrophoresed by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The membranes were then blocked with 5% non-fat milk in
phosphate-buffered saline for 1 h at room temperature and incubated
overnight at 4°C with anti-Ang-1 monoclonal antibodies (1:200
dilution) or anti-GAPDH mouse monoclonal antibodies (1:1,000
dilution; Santa Cruz Biotechnology, Inc.; cat. no. sc-25778). The
HRP-conjugated goat anti-rabbit IgG was used as a secondary
antibody (1:3,000 dilution; Bio-Rad Laboratories, Inc., Hercules,
CA, USA; cat. no. 403005). Immunoreactive bands were detected using
an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Grayscale detection was
quantified using ImageJ software (version 1.43; National Institutes
of Health, Bethesda, MD, USA) and normalized to GAPDH.
Statistical analysis
Comparison of differential expression of the
proteins in the HCC, tumor-adjacent and normal liver tissues was
performed using one-way analysis of variance. Fisher's Least
Significant Difference test was utilized for comparisons between
groups, and Spearman's correlation coefficient was performed to
determine the association between expression of any two proteins of
interest. All calculations were performed using SPSS software,
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
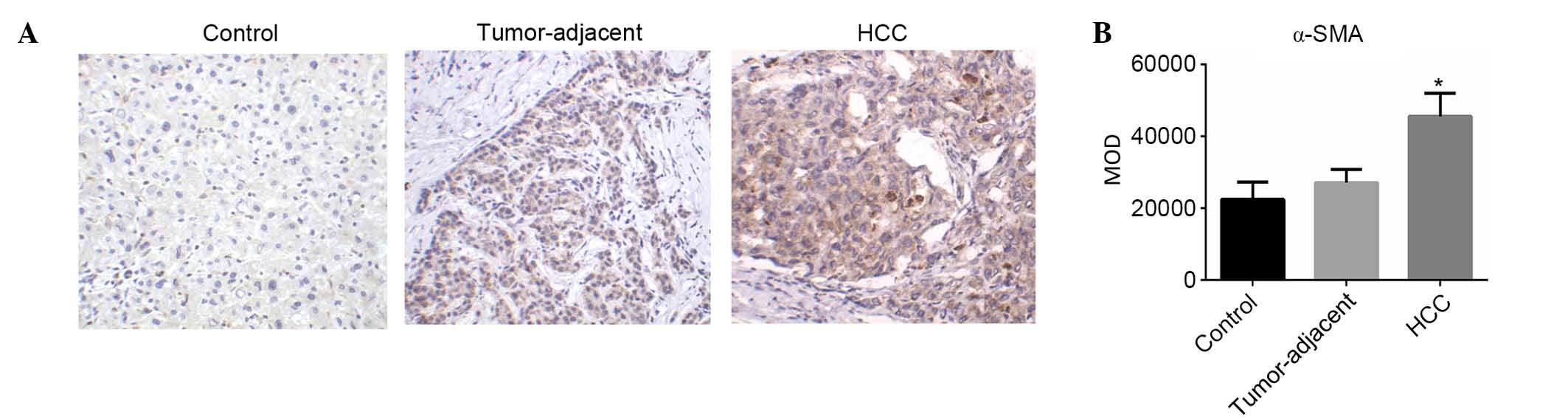
Expression levels of α-SMA in HCC,
tumor-adjacent tissues and normal liver tissues
HSCs in normal liver tissues were not activated.
Inflammation and mechanical stimulation may activate HSCs to become
aHSCs that express α-SMA (10). In
the present study, the expression levels of α-SMA in HSCs in HCC,
tumor-adjacent tissues and normal liver tissues were evaluated
using IHC. α-SMA-positive cells were detected in the cancer cell
nest and hepatic blood sinus (Fig.
1A). The MOD levels of α-SMA expression in HCC, tumor-adjacent
tissues and normal liver tissues were (4.56±0.64)×104,
(2.71±0.37)×104 and (2.25±0.48)×104,
respectively. The expression of α-SMA in HCC was significantly
higher compared with tumor-adjacent (F=7.09; P<0.05; Fig. 1B) and normal liver tissues (F=7.42;
P<0.05; Fig. 1B).
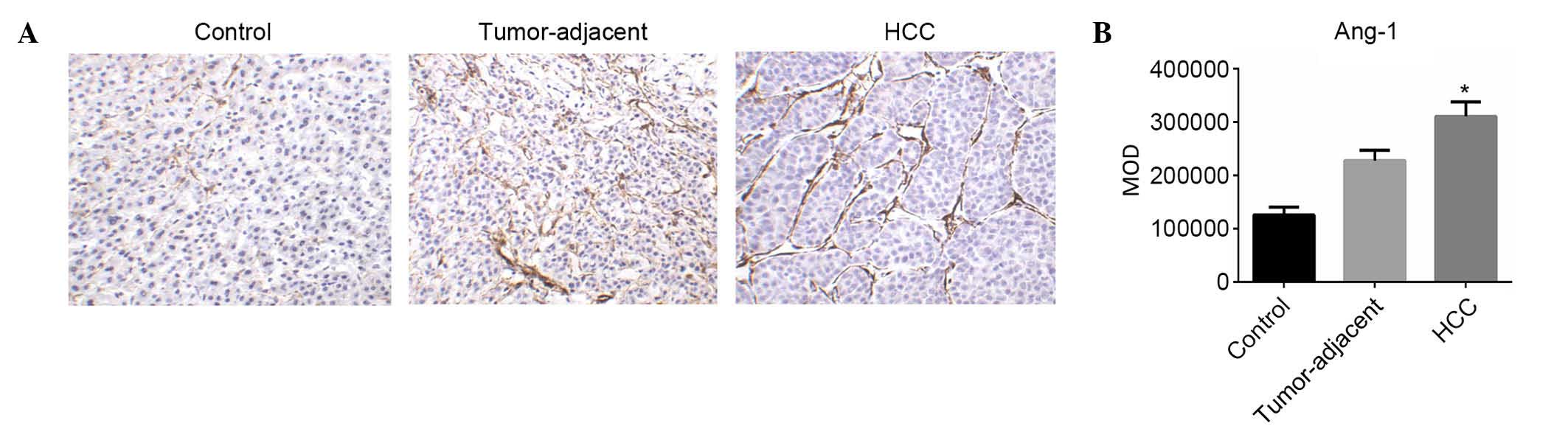
Expression levels of Ang-1 in HCC,
tumor-adjacent and normal liver tissues
The expression of Ang-1 in HSCs in HCC,
tumor-adjacent and normal liver tissues was evaluated using IHC.
Ang-1-positive cells were primarily detected in the cancer cell
nest and hepatic blood sinus (Fig.
2A). The MOD levels of Ang-1 expression in HCC, tumor-adjacent
and normal liver tissues were (3.11±0.27)×105,
(2.28±0.20)×105 and (1.26±0.15)×105,
respectively. The expression of Ang-1 in HCC was significantly
higher compared with tumor-adjacent (F=3.00; P<0.05; Fig. 2B) and normal liver tissues (F=3.14;
P<0.05; Fig. 2B).
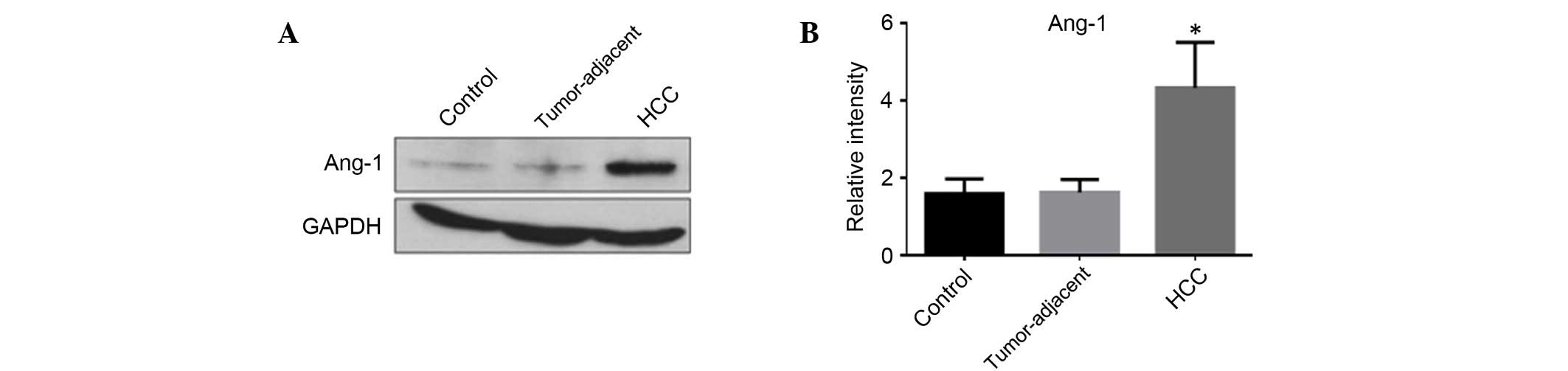
The protein expression levels of Ang-1 in HSCs in
HCC, tumor-adjacent and normal liver tissues were analyzed using
western blot analysis (Fig. 3A).
The MOD levels of Ang-1 expression in HCC, tumor-adjacent and
normal liver tissues were 4.33±1.17, 1.62±0.33 and 1.60±0.38,
respectively. The expression of Ang-1 in HCC was significantly
higher compared with tumor-adjacent (F=2.71; P<0.05; Fig. 3B) and normal liver tissues (F=2.74;
P<0.05; Fig. 3B).
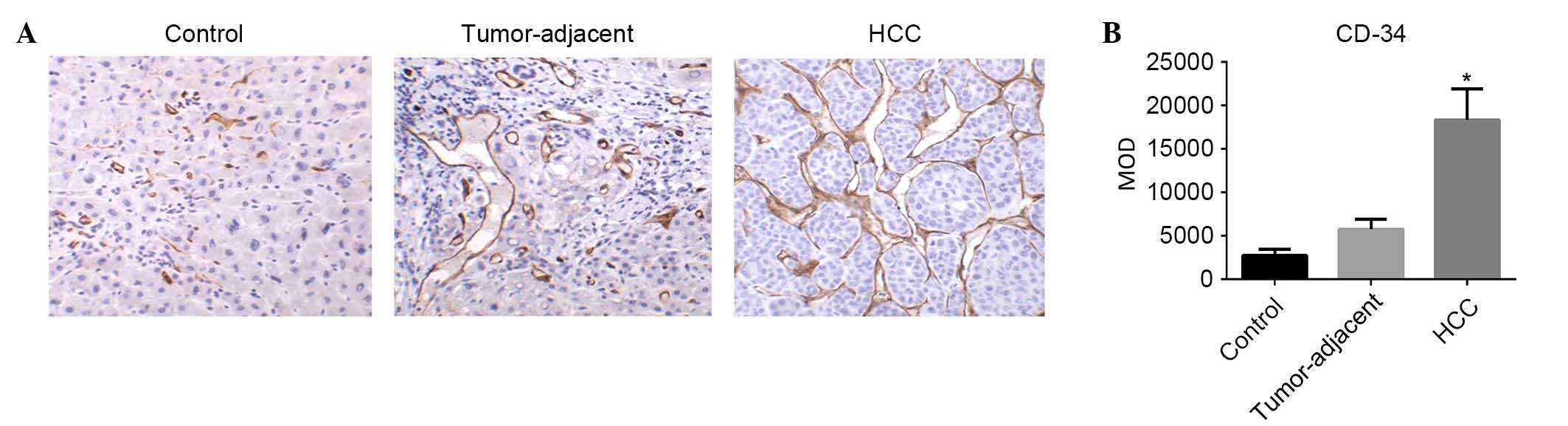
MVD in HCC, tumor-adjacent and normal
liver tissues
The MVD (CD34) in HCC, tumor-adjacent and normal
liver tissues was determined using IHC analysis of CD34 as
previously described (18–21). CD34-positive cells were
predominantly detected in the cancer cell nest and hepatic blood
sinus (Fig. 4A). The MOD levels of
CD34 expression in HCC, tumor-adjacent and normal liver tissues
were (18.3±0.36)×103, (5.75±1.17)×103 and (2.75±0.72)×103,
respectively. The expression levels of CD34 in HCC were
significantly higher compared with tumor-adjacent (F=3.21;
P<0.05; Fig. 4B) and normal
liver tissues (F=3.36; P<0.05; Fig.
4B).
Associations between the expression
levels of Ang-1, α-SMA and MVD (CD34)
Spearman's rank correlation coefficient analysis was
used to evaluate the association among the expression levels of
Ang-1, α-SMA and MVD. As presented in Table I, a positive correlation was
identified between the expression of Ang-1 and CD34 (r=0.610;
P<0.05), Ang-1 and α-SMA (r= 0.576; P<0.05), and α-SMA and
CD34 (r=0.537; P<0.05).
 | Table ISpearman rank analysis to determine
the association between α-SMA, Ang-1 and CD34 expression levels in
hepatocellular carcinoma tissues, tumor-adjacent tissues and normal
liver tissues. |
Table I
Spearman rank analysis to determine
the association between α-SMA, Ang-1 and CD34 expression levels in
hepatocellular carcinoma tissues, tumor-adjacent tissues and normal
liver tissues.
| CD34
| α-SMA
| Ang-1
|
|---|
| Correlation
coefficient | P-value | Correlation
coefficient | P-value | Correlation
coefficient | P-value |
|---|
| CD34 | 1.000 | – | 0.537 | <0.001 | 0.610 | <0.001 |
| α-SMA | 0.537 | <0.001 | 1.000 | – | 0.576 | <0.001 |
| Ang-1 | 0.610 | <0.001 | 0.576 | <0.001 | 1.000 | – |
Discussion
HCC is a hypervascular cancer; the development of
microvessels is essential for the growth and metastasis of HCC
(7,22). HSCs in normal liver tissues may be
activated by inflammation and mechanical stimulation and become
activated HSCs (23,24). Activated HSCs proliferate and
express α-SMA. A previous study reported that aHSCs contributed to
the growth and metastasis of HCC by expressing hepatocyte growth
factor, interleukin 6 and VEGF (15). However, another previous study has
determined that aHSCs additionally inhibit the proliferation and
metastasis of HCC cells by expressing laminin 5 and other
extracellular matrix components (16). Additionally, aHSCs promote the
development of HCC from cirrhosis, HCC growth and metastasis by
increasing the formation of microvessels in tumor tissues (8,9). The
present study determined that the expression levels of α-SMA in
aHSCs in HCC tissues were significantly higher compared with
tumor-adjacent and normal liver tissues, suggesting that aHSCs may
be involved in microvessel formation and development in HCC.
Previous studies have reported that Ang-1 promoted
the maturation and maintained the stability of vessels (12–14).
In addition, previous studies have determined that Ang-1 regulated
the survival, proliferation and metastasis of endothelial cells and
promoted the formation of microvessels in tumor tissues (12,25).
The expression levels of Ang-1 have been observed to be increased
in glioma and ovarian cancers (26,27).
The present study determined that the expression of Ang-1 in HCC
tissues was significantly higher compared with tumor-adjacent and
normal liver tissues. In addition, the MVD in HCC tissues was
significantly higher compared with tumor-adjacent and normal liver
tissues. These results suggest that Ang-1 is important for
microvessel formation in HCC, which is consistent with the results
of previous studies (18–21).
Spearman's correlation coefficient analysis
identified a positive correlation between the expression levels of
α-SMA, Ang-1 and CD34, suggesting that Ang-1 and aHSCs are involved
in the formation of microvessels in HCC tissues. Ang-1 may also be
involved in the regulatory effects of aHSCs on microvessel
formation. Previous studies have demonstrated that transcription
levels of Ang-1 in aHSCs were increased in response to hypoxia. In
addition, Ang-1 may further promote the proliferation and mobility
of aHSCs, including the deposition of extracellular matrix
(7,16,28–30).
Activated HSCs are also important for the development of cirrhosis
by expressing Ang-1 and promoting angiogenesis (17). The results of the current study
suggest that aHSCs promote the growth and metastasis of HCC by
increasing the expression of Ang-1 and angiogenesis.
The present study initially detected the expression
of α-SMA in HCC tissues using IHC. Positive expression of α-SMA
suggests the existence of aHSCs in HCC tissues. Next, the
expression levels of Ang-1 and CD34 were determined. The results
demonstrated that the expression levels of Ang-1 and CD34 in HCC
were significantly higher compared with tumor-adjacent and normal
liver tissues. The expression of α-SMA and CD34 was primarily
detected between cancer cell nests; however, the majority of Ang-1
expression was detected in cancer cell nests. In addition, a
positive correlation was identified among α-SMA, Ang-1 and CD34. In
conclusion, the results of the present study suggest that aHSCs
increased the expression of Ang-1, resulting in angiogenesis in HCC
tissues, thus promoting the growth and metastasis of HCC. However,
the molecular mechanisms underlying the role of aHSCs in the
development of HCC require further investigation.
Acknowledgments
The present study was supported by grants from the
Basic Research Projects and New Disciplines, Cross Disciplinary
Funding Program of Sun Yat-Sen University (grant no. 15YKJC19C),
the Science and Technology Planning Project of Guangdong Province
(grant no. 2016A020212004) and the Natural Science Foundation of
Guangdong Province (grant nos. 2014A030313067 and
2014A030313144).
References
|
1
|
European Association For The Study Of The
Liver1; European Organisation For Research And Treatment Of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao H, Phan H and Yang LX: Improved
chemotherapy for hepatocellular carcinoma. Anticancer Res.
32:1379–1386. 2012.PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roxburgh P and Evans TR: Systemic therapy
of hepatocellular carcinoma: Are we making progress? Adv Ther.
25:1089–1104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernández M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia YL, Shi L, Zhou JN, Fu CJ, Chen L,
Yuan HF, Wang YF, Yan XL, Xu YC, Zeng Q, et al: Epimorphin promotes
human hepatocellular carcinoma invasion and metastasis through
activation of focal adhesion kinase/extracellular signal-regulated
kinase/matrix metalloproteinase-9 axis. Hepatology. 54:1808–1818.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sancho Bru P, Juez E, Moreno M, Khurdayan
V, Morales-Ruiz M, Colmenero J, Arroyo V, Brenner DA, Ginés P and
Bataller R: Hepatocarcinoma cells stimulate the growth, migration
and expression of pro-angiogenic genes in human hepatic stellate
cells. Liver Int. 30:31–41. 2010. View Article : Google Scholar
|
|
10
|
Amann T, Bataille F, Spruss T, Mühlbauer
M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff AK and Hellerbrand
C: Activated hepatic stellate cells promote tumorigenicity of
hepatocellular carcinoma. Cancer Sci. 100:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olaso E, Salado C, Egilegor E, Gutierrez
V, Santisteban A, Sancho-Bru P, Friedman SL and Vidal-Vanaclocha F:
Proangiogenic role of tumor-activated hepatic stellate cells in
experimental melanoma metastasis. Hepatology. 37:674–685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holopainen T, Huang H, Chen C, Kim KE,
Zhang L, Zhou F, Han W, Li C, Yu J, Wu J, et al: Angiopoietin-1
overexpression modulates vascular endothelium to facilitate tumor
cell dissemination and metastasis establishment. Cancer Res.
69:4656–4664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji Y, Wang Z, Li Z, Li K, Le X and Zhang
T: Angiotensin II induces angiogenic factors production partly via
AT1/JAK2/STAT3/SOCS3 signaling pathway in MHCC97H cells. Cell
Physiol Biochem. 29:863–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang H, Bhat A, Woodnutt G and Lappe R:
Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer.
10:575–585. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forbes SJ and Parola M: Liver fibrogenic
cells. Best Pract Res Clin Gastroenterol. 25:207–217. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taura K, De Minicis S, Seki E, Hatano E,
Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S
and Brenner DA: Hepatic stellate cells secrete angiopoietin 1 that
induces angiogenesis in liver fibrosis. Gastroenterology.
135:1729–1738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XW, Cai CL, Xu JM, Jin H and Xu ZY:
Increased expression of chitinase 3-like 1 is a prognosis marker
for non-small cell lung cancer correlated with tumor angiogenesis.
Tumour Biol. 36:901–907. 2015. View Article : Google Scholar
|
|
19
|
Ding S, Li C, Lin S, Yang Y, Liu D, Han Y,
Zhang Y, Li L, Zhou L and Kumar S: Comparative evaluation of
microvessel density determined by CD34 or CD105 in benign and
malignant gastric lesions. Hum Pathol. 37:861–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sluimer JC and Daemen MJ: Novel concepts
in atherogenesis: Angiogenesis and hypoxia in atherosclerosis. J
Pathol. 218:7–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong Y, Sun X, Huo L, Wiley EL and Rao MS:
Expression of cell adhesion molecules, CD44s and E-cadherin, and
microvessel density in invasive micropapillary carcinoma of the
breast. Histopathology. 46:24–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
24
|
Sokolović A, Sokolović M, Boers W,
Elferink RP and Bosma PJ: Insulin-like growth factor binding
protein 5 enhances survival of LX2 human hepatic stellate cells.
Fibrogenesis Tissue Repair. 3:32010. View Article : Google Scholar
|
|
25
|
Stoeltzing O, Ahmad SA, Liu W, McCarty MF,
Wey JS, Parikh AA, Fan F, Reinmuth N, Kawaguchi M, Bucana CD and
Ellis LM: Angiopoietin-1 inhibits vascular permeability,
angiogenesis, and growth of hepatic colon cancer tumors. Cancer
Res. 63:3370–3377. 2003.PubMed/NCBI
|
|
26
|
Thomas M and Augustin HG: The role of the
Angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Machein MR, Knedla A, Knoth R, Wagner S,
Neuschl E and Plate KH: Angiopoietin-1 promotes tumor angiogenesis
in a rat glioma model. Am J Pathol. 165:1557–1570. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valfrè di Bonzo L, Novo E, Cannito S,
Busletta C, Paternostro C, Povero D and Parola M: Angiogenesis and
liver fibrogenesis. Histol Histopathol. 24:1323–1341.
2009.PubMed/NCBI
|
|
29
|
Medina J, Arroyo AG, Sánchez-Madrid F and
Moreno-Otero R: Angiogenesis in chronic inflammatory liver disease.
Hepatology. 39:1185–1195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Novo E, Cannito S, Zamara E, Valfrè di
Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S,
Marra F, Pinzani M and Parola M: Proangiogenic cytokines as
hypoxia-dependent factors stimulating migration of human hepatic
stellate cells. Am J Pathol. 170:1942–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|