Introduction
Gastric cancer (GC) is one of the most common
malignancies worldwide (1).
Currently, rates of early detection and diagnosis for GC are low,
therefore, chemotherapy remains one of the major treatment methods
for GC. However, the effectiveness of chemotherapy is often limited
by the development of drug resistance (2). Cytological mechanisms, which have
contributed to the chemoresistance include enhanced DNA repair
activity, alterations in cell cycle and proliferation, and
defective apoptosis (3). In
addition, molecular mechanisms, including increased rates of drug
efflux, alterations in drug metabolism and mutation of drug targets
may be important in drug resistance (4,5).
Previous studies have found that the activation of certain
signaling pathways, including the, phosphatase and tensin
homolog/phosphoinositide 3-kinase/AKT, nuclear factor
(NF)κB/inhibitor of NFκB, Ras/Raf/mitogen-activated protein kinase
kinase and P53/murine double minute-2 (6–9), are
also associated with drug resistance.
MicroRNAs (miRNAs), a class of short, non-coding
RNAs of 19–22 nucleotides in length, function as
post-transcriptional regulators by directly cleaving target
messenger RNA or inducing translational repression (10). Previously, aberrant miRNAs have
been found to be involved in drug resistance in different types of
tumor, acting as oncogenes or tumor suppressors (11–14).
Accumulating evidence has revealed that miRNA regulates NFκB
signaling (15), and activation of
the NFκB pathway enhances cell resistance to chemotherapeutic drugs
(16). Therefore, the present
study hypothesized that miRNA may induce chemoresistance through
activation of the NFκB pathway.
In the present study, the potential function of
miR-20a in GC chemoresistance was investigated. It was found that
miR-20a was significantly upregulated in samples from patients with
GC, particularly in those with chemoresistance. miR-20a was also
found to be upregulated in a cisplatin (DPP)-resistant GC cell line
(SGC7901/DDP.) miR-20a contributed to DDP resistance when
overexpressed in the SGC7901 GC cell line. Furthermore, the present
study demonstrated that miR-20a may activate the NFκB pathway and
upregulate the downstream targets, survivin and livin, by
repressing the expression of cylindromatosis (CYLD), leading to the
resistance to DDP. The results of the study may aid the development
of personalized treatment for patients with GC that exhibit
abnormal levels of miR-20a.
Materials and methods
Clinical sample collection
A total of 121 patients (age range, 46–75; males,
66; females, 45) with histopathologically confirmed GC and 56
healthy donors (age range, 35–71; males, 33; females, 23) were
recruited between 2012 and 2014 at the First Affiliated Hospital of
Nanjing Medical University (Nanjing, China). A total of 28 GC
plasma samples and an additional 28 formalin-fixed paraffin
embedded (FFPE) sections of GC were obtained from preoperative
patients who had not received chemotherapy. In addition, 30 GC
plasma samples and an additional 35 FFPE sections of GC were
collected from patients with advanced disease, who had undergone
two cycles of platinum-based chemotherapy. Response to chemotherapy
was graded by the standards of Evaluation Criteria in Solid Tumors
recommended by the World Health Organization (WHO), defined as
complete remission (CR), partial remission (PR), stable disease
(SD), and progressive disease (PD). All procedures were approved by
the Institutional Review Boards of the First Affiliated Hospital of
Nanjing Medical University. Written informed consent was obtained
from each participant.
Cell culture and transfection
The SGC7901 human gastric cancer cell line was
purchased from the National Institute of Cells (Shanghai, China).
The DPP-resistant variant (SGC7901/DDP) was obtained from KeyGen
Biotechnology Company (Nanjing, China). All cells were maintained
in RPMI-1640 medium supplemented with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
atmosphere with 5% CO2 at 37°C, as previously described
(17,18). To maintain the DPP-resistant
phenotype, DDP, with final concentration of 1 µg/ml, was
added to the culture media of the SGC7901/DDP cells.
miRNA mimics and their appropriate negative control
were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
All transfection experiments were performed using Lipofectamine
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. UCSC Genome Browser
(genome.ucsc.edu/) was used to search the promoter
regions of miR-20a.
Bioinformatics analysis
TargetScan (www.targetscan.org), a bioinformatics tool for miRNA
target screening, was used to predict the target genes of
miR-20a.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Following tissue homogenization, total RNA from the
cells was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. For
the plasma samples, total RNA was extracted using the mirVana Paris
kit (Ambion, Austin, TX, USA), according to the manufacturer's
protocol. Total RNA from the FFPE tissues was isolated using the
High Pure FFPE RNA Micro kit (Ambion), according to the
manufacturer's protocol. The amplification of miRNA was performed
using the specific primers for the RT-qPCR analysis in the
Bulge-Loop™ miRNA qRT-PCR primer set (RiboBio Co., Ltd.), as
previously described (19,20). RT-qPCR was performed, according to
the manufacturer's protocol. The expression levels of the PCR
products were determined by the level of fluorescence emitted by
SYBR Green (SYBR® Premix Ex Taq™ II, Takara Bio, Inc.,
Otsu, Japan). The relative expression levels of the target miRNAs
from the cells and tissue samples were calculated using the
comparative 2−ΔΔCq method, relative to U6 snRNA, as
previously described (17,18). The levels of miRNA in the plasma
samples were determined based on a standard curve constructed with
using synthetic miRNAs (micrON miRNA mimic; RiboBio Co., Ltd.), as
previously described (19,20).
In vitro drug sensitivity assay
The SGC7901 cells and SGC7901/DDP cells were seeded
into 6-well plates (6×105 cells/well). Either the
miR-20a mimic (100 nM) or mimic control (100 nM) were transfected
into the SGC7901 cells, whereas the SGC7901/DDP cells were
transfected with either miR-20a inhibitor (100 nM) or inhibitor
control (100 nM). The miR-20a mimic, inhibitor and relative
controls were purchased from Gene Pharma (Shanghai, China).
At 24 h post-transfection, the cells were seeded
into 96-well plates (5×103 cells/well). Following
cellular adhesion, freshly prepared DDP was added at final
concentrations 10, 1, 0.1 and 0.01 times that of the human peak
plasma concentration for DDP (2.0 µg/ml). After 48 h,
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyl-tetrazolium bromide
(MTT) was added to each well to measure the viability of the cells,
and the plate was incubated for 2 h in a humidified incubator. The
absorbance at 490 nm of each well was measured on a
spectrophotometer. The relative survival curve was used to
calculate the concentration of cisplatin, which caused 50%
inhibition of growth.
Dual-luciferase activity assay
The 3′-untranslated region (3′UTR) sequences of
human CYLD cDNA containing the predicted target site of miR-20a,
were chemically synthesized by RiboBio Co., Ltd. HEK293T human
embryonic kidney cells (Cell Bank of the Chinese Academy of
Sciences, Shanghai, China) were plated in 24-well plates
(1.5×105 cells/well) for 24 h, following which 200 ng of
the pGL3-CYLD-3′-UTR, 60 pmol of the miR-20a mimic or miRNA mimic
control, and 80 ng of pRL-TK (Promega Corporation, Madison, WI,
USA) were co-transfected into the cells. Luciferase activity was
measured using the Dual Luciferase Reporter Assay system (Promega
Corporation) consecutively. The firefly luciferase activity was
normalized to the expression of renilla luciferase.
Immunohistochemistry
For immunohistochemical analysis, tissue sections
(4-µm-thick) were formalin-fixed and paraffin-embedded, and
were stained using the avidin-biotin complex method (21). Antigen retrieval was performed
using microwave irradiation in 10 mM citrate buffer (pH 6.0),
followed by incubation with primary antibodies at 4°C overnight.
The antibody for CYLD (cat. no. 8462; dilution 1:200) was purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). The
biotinylated goat anti-rabbit secondary antibody (cat. no. A0227)
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Specimins were incubated in the secondary antibody (1:50)
for 30 min at room temperature. Staining was repeated if the result
was uncertain. The slides were scored independently by two
observers blinded to the clinicopathological characteristics. The
immunostaining of the slides was evaluated under an optical
microscope (magnification ×400). Discordant scores were reevaluated
to reach a consensus. The staining intensity of the expression of
CYLD was scored (scores 0–3 for negative, weak, moderate and strong
expression, respectively), as was the percentage of positive cancer
cells (0 for 0%; 0.1 for 1–9%; 0.5 for 10–49%;and 1.0 for ≥50%).
The staining intensity was multiplied by the proportion score of
the percentage of positive cancer cells.
Western blot analysis
Total protein was prepared from the cells using
radioimmunoprecipitation assay buffer in the presence of proteinase
inhibitor. The nuclear proteins were extracted from the cells using
a nuclear cytoplasmic extraction kit (Beyotime Institute of
Biotechnology). Protein levels were quantified using bicinchoninic
acid assay (Beyotime Institute of Biotechnology). Western blot
analysis was performed, as previously described (17,18).
The primary antibodies for CYLD (cat. no. 8462), P65 (cat. no.
8242), livin (cat. no. 5471), H3 (cat. no. 4499) and survivin (cat.
no. 2808) were obtained from Cell Signaling Technology, Inc. The
blots were incubated with the indicated primary antibodies or GAPDH
antibody (cat. no. AP0063; Bioworld Technology, Inc., St. Louis
Park, MN, USA) overnight at 4°C, then with goat anti-rabbit
horseradish peroxidase (HRP)-conjugated antibody (cat. no. sc-2030;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room
temperature for 1 h. The protein bands were visualized using an
Immobilon Western Chemiluminescent HRP Substrate kit (Beyotime
Institute of Biotechnology,) and a ChemiDoc XRS+ Imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Grey value
analysis of protein bands was performed for quantification of the
protein levels using Image J software (version 1; National
Institutes of Health, Bethesda, MD, USA). The levels of total
protein were normalized to total GAPDH, and nuclear protein to H3.
The fold changes were calculated.
Apoptosis assay
The cells were transfected with miR-20a mimic or
inhibitor, respectively. After 24 h, the cells were treated with
DDP at a final concentration of 10 µg/ml. After 48 h,
apoptosis was assessed via the counting of annexin V-fluorescein
isothiocyanate-positive and propidium iodide-negative cells using
flow cytometry, as described previously (17,18).
Statistical analysis
Data are presented as the mean ± standard deviation
from at least three separate experiments. Two-tailed Student's
t-tests were performed for comparisons using SPSS (version
15.0; SPSS, Inc., Chicago, IL, USA) software. Spearman's
non-parametric correlation test was performed to analyze the
correlation between the expression levels of miR-186 and Twist1 by
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-20a is significantly upregulated in
GC plasma samples and GC tissue samples
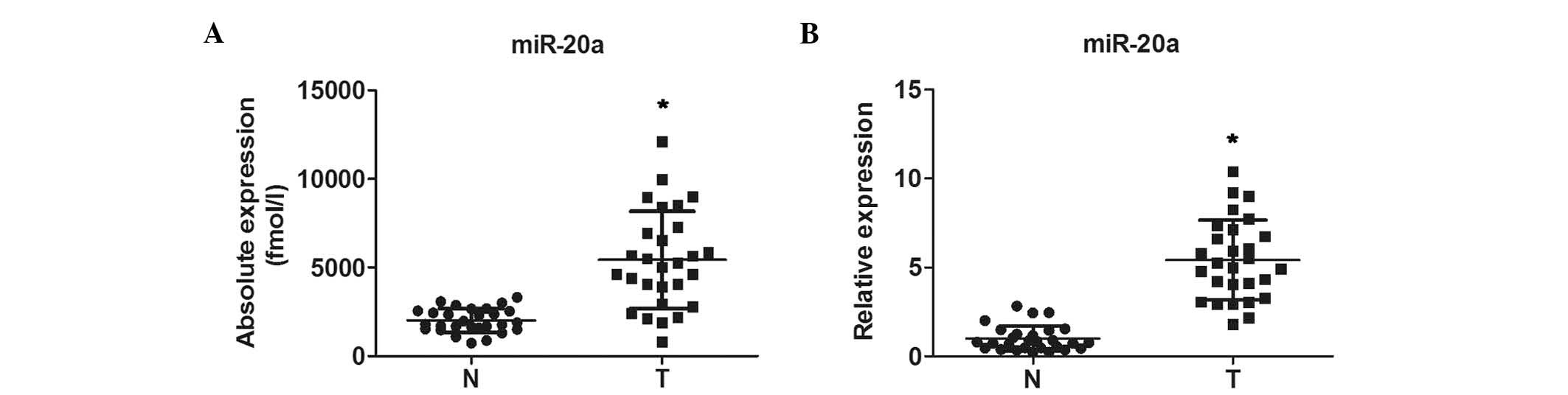
To determine the basal expression of miR-20a in GC,
the present study first performed RT-qPCR analysis to examine the
plasma levels of miR-20a in 28 patients with preoperative GC and 28
healthy subjects. The expression of miR-20a was significantly
increased in the GC group, compared with the normal group (Fig. 1A). Furthermore, it was found that
the expression levels of miR-20a were significantly higher in the
28 GC FFPE tissue samples, compared with those in the 28 samples of
normal gastric tissue (Fig.
1B).
miR-20a is involved in GC
chemotherapeutic resistance
To further investigate the potential role of miR-20a
in GC chemo-resistance, 30 plasma samples and 35 FFPE sections were
collected from patients with advanced GC who had undergone two
cycles of platinum-based chemotherapy. The response to chemotherapy
was evaluated using standard WHO criteria, of CR, PR, SD and PD.
According to these criteria, the plasma samples showed that 10
patients had PR, nine patients had SD and 11 patients had PD, with
no patients in CR. In the tissue specimens, 12 had PR, 11 had SD
and 12 had PD, with no cases of CR. The expression of plasma
miR-20a in the PD+SD group were significantly higher, compared with
that in the PR group (Fig. 2A).
The upregulation of miR-20a was also detected in the FFPE tissues
of the PD+SD group, compared with the PR group (Fig. 2B). The results of the RT-qPCR
analysis revealed that the expression of miR-20a was also
significantly increased in the SGC7901/DDP cells, compared with the
parental SGC7901 cell line (Fig.
2C), which was consistent with the results obtained in the
clinical GC samples. These results revealed that the overexpression
of miR-20a was significantly associated with GC
chemoresistance.
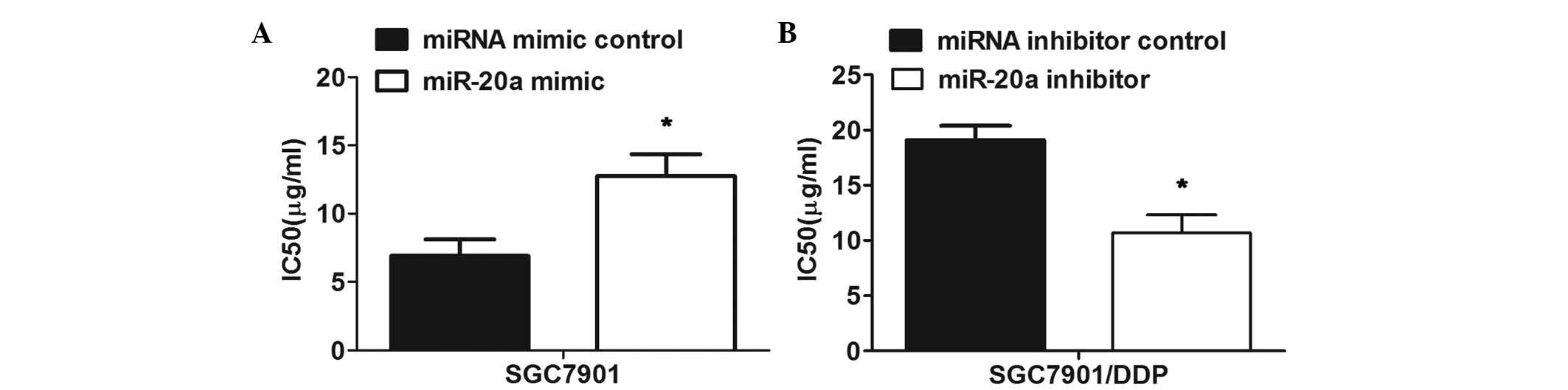
miR-20a regulates resistance to DDP in
the SGC7901/DDP cell line
To examine the functional role of miR-20a in the
chemoresistance of GC, the miR-20a mimic or inhibitor were
transfected into the SGC7901 cells or SGC7901/DDP cells
respectively. Prior to the miRNA transfection, the significant
resistance of the SGC7901/DDP cells to DDP, compared with the
parental SGC7901 cells was verified. The MTT assay revealed reduced
sensitivity of the SGC7901 cells transfected with the miR-20a mimic
to DDP, compared with that of the cells transfected with the mimic
control (Fig. 3A). By contrast,
transfection with the miR-20a inhibitor markedly enhanced the
sensitivity of the SGC7901/DDP cells to DDP, compared with the
control (Fig. 3B). Taken together,
these results indicated that miR-20a modulated the resistance of
the SGC7901/DDP cells to DDP.
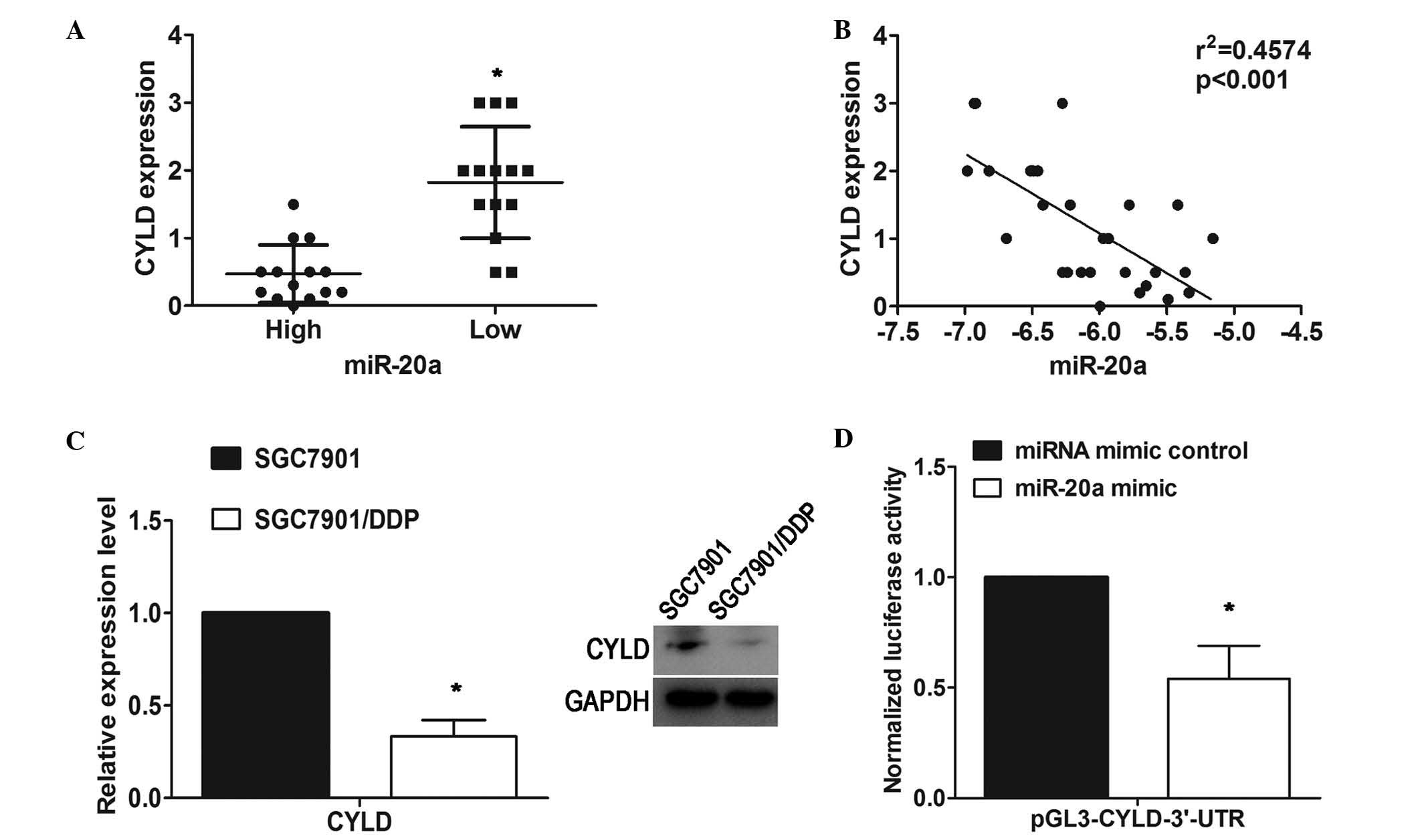
CYLD is a direct target of miR-20a
Using TargetScan software for miRNA target
prediction, the present study found CYLD as a potential direct
target gene of miR-20a. To assess the relevance of miR-20a/CYLD,
immunohistochemistry was performed to examine the expression of
CYLD in 28 GC tissue samples. The expression of miR-20a was also
analyzed uisng RT-qPCR analysis in the same specimens. Among these
28 specimens, using the median expression value of miR-20a as a
cutoff point, the cohort was divided into miR-20a-high or
miR-20a-low tumors. The protein expression levels of CYLD in the
miR-20a-high group were significantly lower, compared with those in
the miR-20a-low group (Fig. 4A).
An inverse correlation (R2=0.4574; P<0.001) between
the expression of miR-20a and the protein levels of CYLD was
observed using Spearman's correlation analysis (Fig. 4B). In addition, western blot
analysis revealed that CYLD was significantly decreased at the
protein level in the SGC7901/DDP cells, compared with the parental
SGC7901 cell line (Fig. 4C).
To further confirm whether CYLD was a target for
miR-20a, luciferase reporter plasmids containing the 3′UTR of CYLD
were constructed and co-transfected with miR-20a mimic in the
HEK293T human embryonic kidney cell line. The results showed that
luciferase activity was significantly decreased in the cells
transfected with the miR-20a mimic, compared with the vector
control (Fig. 4D). These results
showed that miR-20a may negatively regulate the expression of CYLD
by directly targeting its 3′UTR.
miR-20a represses the expression of CYLD,
leading to NFκB activation and upregulation of the downstream
targets, survivin and livin
In the present study, the upregulation of miR-20a in
the SGC7901/DDP cells was concurrent with the downregulation of
CYLD, compared with the parental SGC7901 cells. In addition, the
NFκB pathway-associated proteins, p65, livin and survivin, showed
significantly higher levels of expression in the SGC7901/DDP cells,
compared with the SGC7901 cells (Fig.
5A). The present study verified that CYLD was a direct target
of miR-20a, and also a negative regulator of the NFκB pathway.
Thus, it was hypothesized that miR-20a may activate the NFκB
pathway by repressing the expression of CYLD. To confirm this, the
SGC7901/DDP cells and SGC7901 cells were transfected with miR-20a
inhibitor or mimic, respectively, and western blot analysis was
performed to examine the expression of these proteins. In the
SGC7901/DDP cells, at 72 h post-transfection, the results
demonstrated that the reduced level of miR-20a increased the
expression of CYLD, but decreased the expression of p65, survivin
and livin (Fig. 5B). By contrast,
the expression of CYLD in the SGC7901 cells transfected with the
miR-20a mimic was downregulated, whereas the expression levels of
p65, survivin and livin were upregulated (Fig. 5C). These results suggested that
miR-20a repressed the expression of CYLD, leading to activation of
the NFκB pathway and upregulation of the downstream targets, livin
and survivin.
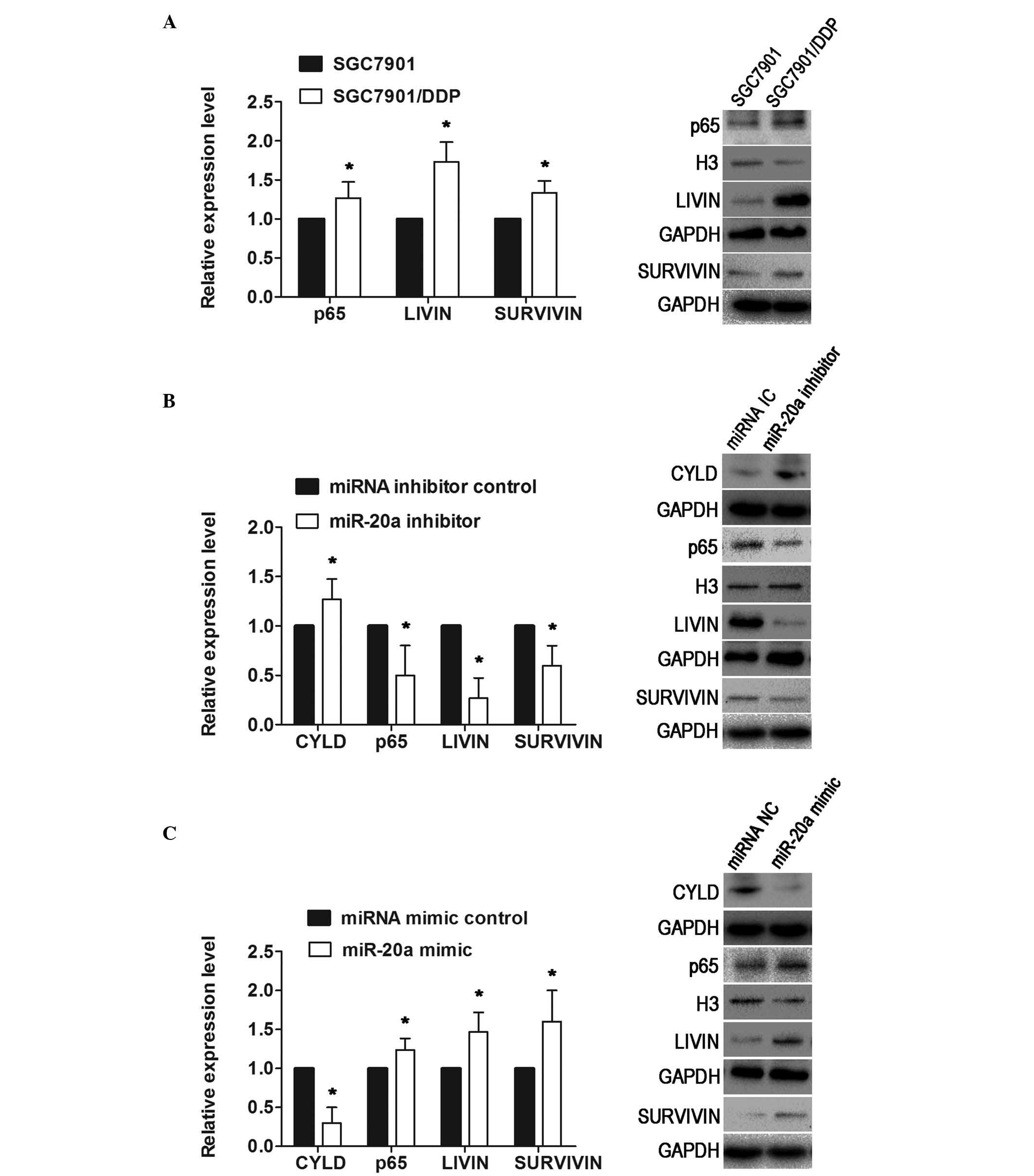 | Figure 5miR-20a represses the expression of
CYLD, leading to NF κB activation and the upregulation of the
downstream targets, survivin and livin. (A) Western blot analysis
showed that p65, livin and survivin were upregulated in the
SGC7901/DDP cells. The levels of p65 in the nuclei were normalized
to H3. (B) In SGC7901/DDP cells transfected with miR-20a inhibitor,
at 72 h post-transfection, the results revealed that low levels of
miR-20a increased the expression of CYLD, and decreased the
expression levels of p65, livin and survivin. (C) Expression of
CYLD in the SGC7901 cells transfected with miR-20a mimic was
downregulated, whereas the expression levels of p65, livin and
survivin were upregulated. Data are presented as the mean ±
standard deviation. *P<0.01. NC, mimic control; IC,
inhibitor control; miRNA/miR, microRNA; CYLD, cylindromatosis; DDP,
cisplatin. |
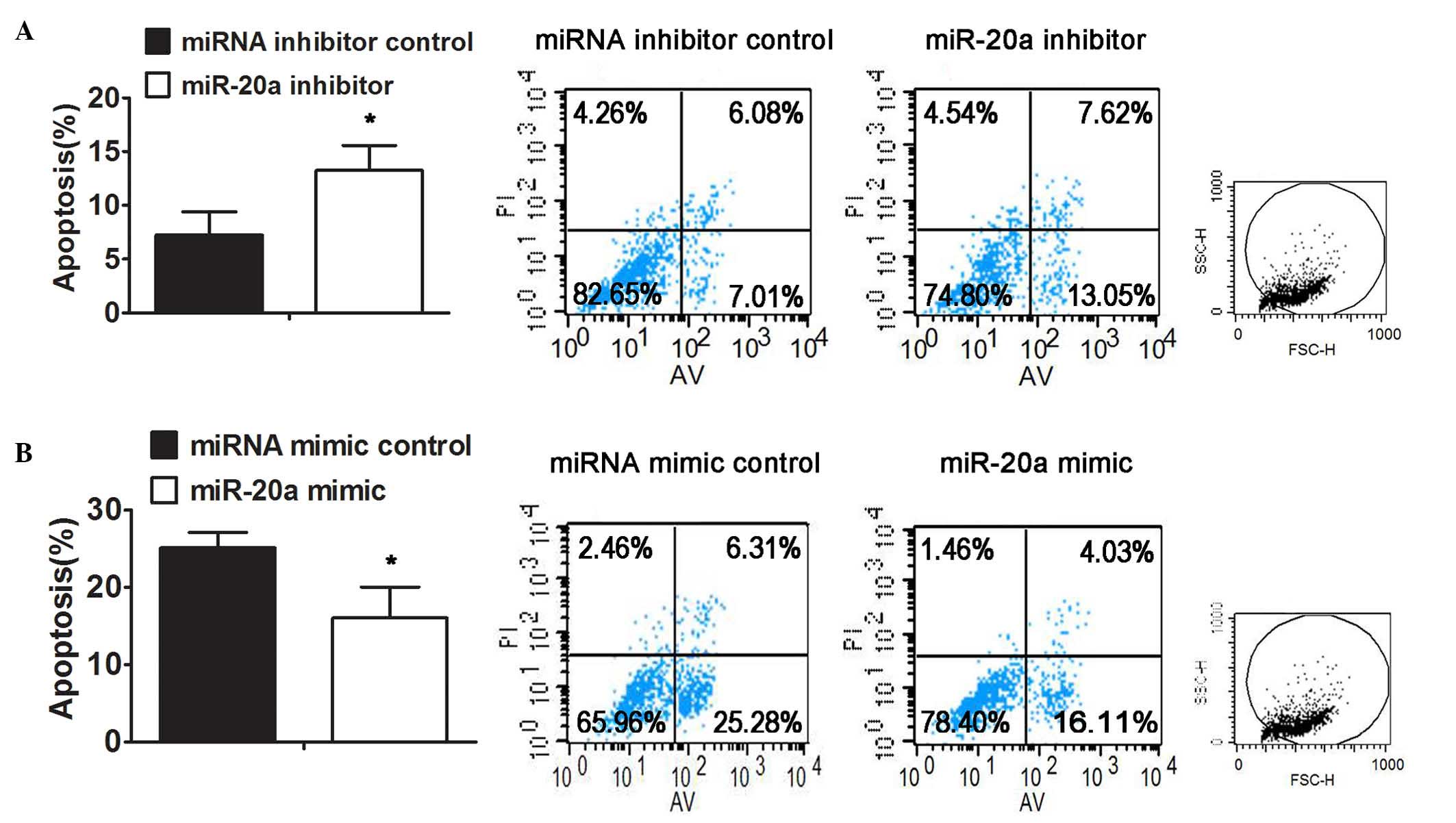
Inhibition of miR-20a sensitizes
SGC7901/DDP cells to DDP-induced apoptosis
The overexpression of anti-apoptotic proteins,
including livin and survivin, has been reported to contribute to
resistance to drug-induced apoptosis in several types of cancer
(17,18,22).
In the present study, it was demonstrated that the overexpression
of miR-20a may enhance the resistance of GC cells to DDP, at least
in part, via the activation of NFκB and its downstream targets,
livin and survivin. Thus, it was hypothesized that miR-20a may also
be associated with DDP resistance by regulating the apoptosis of GC
cells. To further confirm this, the SGC7901/DDP and SGC7901 cells
were transfected with the inhibitor or mimic of miR-20a,
respectively, followed by the analysis of DDP-induced apoptosis.
The results indicated that there was a higher proportion of
apoptotic cells in the SGC7901/DDP cells transfected with miR-20a
inhibitor, compared with those transfected with the inhibitor
control following DDP treatment (Fig.
6A). By contrast, the ectopic expression of miR-20a resulted in
a decrease in the apoptosis induced by DDP in the SGC7901 cells
(Fig. 6B).
Discussion
miR-20a is a member of the miR-17–92 cluster and
acts as an oncogene. It has been demonstrated that miR-20a is
upregulated, and promotes tumorigenesis and cancer progression in
diverse cancer subtypes, including cervical cancer (23), ovarian cancer (24), osteosarcoma (25), anaplastic thyroid cancer (26) and nasopharyngeal carcinoma
(27). A study by Li et al
(28) revealed that miR-20a was
markedly increased in GC tissues and cell lines, and that the
ectopic expression of miR-20a promoted the proliferation, migration
and invasion of GC cells. A study by Chai et al (29) suggested that miR-20a targeted BNIP2
and contributed to chemotherapeutic resistance in colorectal
adenocarcinoma cell lines. In the present study, it was confirmed
that miR-20a was significantly upregulated in SGC7901/DDP cells and
was associated with GC chemoresistance. In addition, miR-20a
regulated the resistance of the SGC7901/DDP cell line to DDP.
Several studies have shown that constitutively
activated NFĸB signaling and upregulation of drug
resistance-associated proteins, including B cell lymphoma-2,
myeloid cell leukemia-1, multidrug resistance 1 and X-linked
inhibitor of apoptosis protein, are critical in mediating
resistance in various types of cancer (9,30,31).
CYLD, a deubiquitination enzyme, acts as a tumor suppressor in
different types of cancer. Currently, CYLD is known to function as
a negative regulator of the NFκB signaling pathway, and is closely
involved in regulating the apoptosis of cancer cells. The
ubiquitination of key signaling molecules by E3 ubiquitin ligases
is required in NFκB signaling transmission, whereas NFκB activation
is limited by ubiquitin deconjugation induced by deubiquitinases,
including CYLD (32–35). In addition, previous investigations
have suggested that inhibition of the NFκB pathway sensitized cells
to chemotherapeutic drugs in GC (36,37).
In the present study, it was found that miR-20a directly targeted
CYLD, demonstrating a novel mechanism of CYLD dysregulation in GC.
Further investigation was performed to determine whether there was
a correlation between miR-20a and the NFκB pathway, to clarify the
effects of miR-20a on GC chemoresistance. It was found that the
overexpression of miR-20a in the SGC7901/DDP cells was concurrent
with the downregulation of CYLD and the upregulation of p65. The
expression level of CYLD was upregulated and the expression of p65
was downregulated when the SGC7901/DDP cells were transfected with
the miR-20a inhibitor. In the SGC7901 cells, the ectopic expression
of miR-20a decreased the expression of CYLD and increased the
expression of p65. The aforementioned results indicated that
miR-20a was essential, at least in part, for activating the NFκB
pathway in GC. Acting as an upstream regulator of the NFκB pathway,
the downregulation of miR-20a may be vital in NFκB pathway
suppression.
Livin and survivin, members of the inhibitor of
apoptosis protein family, function as anti-apoptotic factors and
are important in inhibiting apoptosis (38). The overexpression of inhibitor of
apoptosis is correlated with cancer chemoresistance. The present
study suggested that miR-20a regulated the expression levels of
livin and survivin via NFκB signaling. The inhibition of miR-20a
rendered the SGC7901/DDP cells more sensitive to DDP-induced
apoptosis. It was hypothesized that miR-20a may also be involved in
the development of DDP resistance by modulating the apoptosis of GC
cells.
However, the mechanism of miR-20a upregulation was
not exact. Using the UCSC database, the present study found that
NFκB binding sites were present in the promoter regions of miR-20a,
which indicated that NFκB may directly regulate the expression of
miR-20a. A study by Zhou et al (39) supported this. Therefore, it was
hypothesized that the positive feedback loop,
miR-20a/CYLD/NFκB/miR-20a, was an important mechanism for the
sustained activation of NFκB in drug-resistant GC cells. However,
further investigation is required to confirm this.
In conclusion, the present study provided the first
evidence, to the best of our knowledge, that miR-20a directly
targeted CYLD, resulting in activation of the NFκB pathway and the
downstream targets, livin and survivin, which potentially
contributed to GC chemoresistance. These results may provide
theoretical information for miR-20a as a key target to reverse
chemotherapy resistance. However, the results of the present study
were obtained from cell lines and do not necessarily reflect the
actual surrogates for clinical tumors. Therefore, further
investigation is required to assess the role of miR-20a in
vivo and clinically.
Acknowledgments
The authors are grateful for the funding support by
the National Natural Science Foundation of China (grant nos.
81201705 and 81201796) and the Natural Science Foundation of
Jiangsu Province (grant no. BK2012442).
Abbreviations:
|
miRNAs
|
microRNAs
|
|
DDP
|
cisplatin
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lippert TH, Ruoff HJ and Volm M: Intrinsic
and acquired drug resistance in malignant tumors. The main reason
for therapeutic failure. Arzneimittelforschung. 58:261–264.
2008.PubMed/NCBI
|
|
4
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakagawa Y, Sedukhina AS, Okamoto N,
Nagasawa S, Suzuki N, Ohta T, Hattori H, Roche-Molina M, Narváez
AJ, Jeyasekharan AD, et al: NF-κB signaling mediates acquired
resistance after PARP inhibition. Oncotarget. 6:3825–3839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halilovic E, She QB, Ye Q, Pagliarini R,
Sellers WR, Solit DB and Rosen N: PIK3CA mutation uncouples tumor
growth and cyclin D1 regulation from MEK/ERK and mutant KRAS
signaling. Cancer Res. 70:6804–6814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaelis M, Rothweiler F, Barth S, Cinatl
J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling
A, Rödel F, et al: Adaptation of cancer cells from different
entities to the MDM2 inhibitor nutlin-3 results in the emergence of
p53-mutated multi-drug-resistant cancer cells. Cell Death Dis.
2:e2432011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin X, Zhang X, Wang Q, Li J, Zhang P,
Zhao M and Li X: Perifosine downregulates MDR1 gene expression and
reverses multidrug-resistant phenotype by inhibiting PI3K/Akt/NF-κB
signaling pathway in a human breast cancer cell line. Neoplasma.
59:248–256. 2012. View Article : Google Scholar
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu
G, Zhao R, Huang H, Wang X, Qiao Y, et al: MiR-152 and miR-185
co-contribute to ovarian cancer cells cisplatin sensitivity by
targeting DNMT1 directly: A novel epigenetic therapy independent of
decitabine. Oncogene. 33:378–386. 2014. View Article : Google Scholar
|
|
12
|
Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li
K, Zhou L, Sun Y, Li M, Zhou J, et al: MiR-508–5p regulates
multidrug resistance of gastric cancer by targeting ABCB1 and
ZNRD1. Oncogene. 33:3267–3276. 2014. View Article : Google Scholar
|
|
13
|
Sui C, Meng F, Li Y and Jiang Y: MiR-148b
reverses cisplatin-resistance in non-small cell cancer cells via
negatively regulating DNA (cytosine-5)-methyltransferase 1 (DNMT1)
expression. J Transl Med. 13:1322015. View Article : Google Scholar
|
|
14
|
Fang L, Li H, Wang L, Hu J, Jin T, Wang J
and Yang BB: MicroRNA-17–5p promotes chemotherapeutic drug
resistance and tumour metastasis of colorectal cancer by repressing
PTEN expression. Oncotarget. 5:2974–2987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Jaarsveld MT, Helleman J, Boersma AW,
van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH,
Berns EM, Verweij J, et al: MiR-141 regulates KEAP1 and modulates
cisplatin sensitivity in ovarian cancer cells. Oncogene.
32:4284–4293. 2013. View Article : Google Scholar
|
|
16
|
Eberle KE, Sansing HA, Szaniszlo P, Resto
VA and Berrier AL: Carcinoma matrix controls resistance to
cisplatin through talin regulation of NF-kB. PLoS One.
6:e214962011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J,
Jiang B, Shu Y and Liu P: MiR-200bc/429 cluster modulates multidrug
resistance of human cancer cell lines by targeting BCL2 and XIAP.
Cancer Chemother Pharmacol. 69:723–731. 2012. View Article : Google Scholar
|
|
18
|
Zhu W, Shan X, Wang T, Shu Y and Liu P:
MiR-181b modulates multidrug resistance by targeting BCL2 in human
cancer cell lines. Int J Cancer. 127:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao DS, Chen Y, Jiang H, Lu JP, Zhang G,
Geng J, Zhang Q, Shen JH, Zhou X, Zhu W and Shan QJ: Serum miR-210
and miR-30a expressions tend to revert to fetal levels in Chinese
adult patients with chronic heart failure. Cardiovasc Pathol.
22:444–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou X, Zhu W, Li H, Wen W, Cheng W, Wang
F, Wu Y, Qi L, Fan Y, Chen Y, et al: Diagnostic value of a plasma
microRNA signature in gastric cancer: A microRNA expression
analysis. Sci Rep. 5:112512015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bratthauer GL: The avidin-biotin complex
(ABC) method and other avidin-biotin binding methods. Methods Mol
Biol. 588:257–270. 2010. View Article : Google Scholar
|
|
22
|
Zhu DX, Zhu W, Fang C, Fan L, Zou ZJ, Wang
YH, Liu P, Hong M, Miao KR, Liu P, et al: MiR-181a/b significantly
enhances drug sensitivity in chronic lymphocytic leukemia cells via
targeting multiple anti-apoptosis genes. Carcinogenesis.
33:1294–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao S, Yao D, Chen J, Ding N and Ren F:
MiR-20a promotes cervical cancer proliferation and metastasis in
vitro and in vivo. PLoS One. 10:e01209052015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan X, Liu Y, Jiang J, Ma Z, Wu H, Liu T,
Liu M, Li X and Tang H: MiR-20a promotes proliferation and invasion
by targeting APP in human ovarian cancer cells. Acta Biochim
Biophys Sin (Shanghai). 42:318–324. 2010. View Article : Google Scholar
|
|
25
|
Huang G, Nishimoto K, Zhou Z, Hughes D and
Kleinerman ES: MiR-20a encoded by the miR-17–92 cluster increases
the metastatic potential of osteosarcoma cells by regulating Fas
expression. Cancer Res. 72:908–916. 2012. View Article : Google Scholar
|
|
26
|
Xiong Y, Zhang L and Kebebew E: MiR-20a is
upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS
One. 9:e961032014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng X, Xiang J, Wu M, Xiong W, Tang H,
Deng M, Li X, Liao Q, Su B, Luo Z, et al: Circulating miR-17,
miR-20a, miR-29c and miR-223 combined as non-invasive biomarkers in
nasopharyngeal carcinoma. PLoS One. 7:e463672012. View Article : Google Scholar
|
|
28
|
Li X, Zhang Z, Yu M, Li L, Du G, Xiao W
and Yang H: Involvement of miR-20a in promoting gastric cancer
progression by targeting early growth response 2 (EGR2). Int J Mol
Sci. 14:16226–16239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chai H, Liu M, Tian R, Li X and Tang H:
MiR-20a targets BNIP2 and contributes chemotherapeutic resistance
in colorectal adeno-carcinoma SW480 and SW620 cell lines. Acta
Biochim Biophys Sin (Shanghai). 43:217–225. 2011. View Article : Google Scholar
|
|
30
|
Wang ZH, Chen H, Guo HC, Tong HF, Liu JX,
Wei WT, Tan W, Ni ZL, Liu HB and Lin SZ: Enhanced antitumor
efficacy by the combination of emodin and gemcitabine against human
pancreatic cancer cells via downregulation of the expression of
XIAP in vitro and in vivo. Int J Oncol. 39:1123–1131.
2011.PubMed/NCBI
|
|
31
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.PubMed/NCBI
|
|
32
|
Jono H, Lim JH, Chen LF, Xu H, Trompouki
E, Pan ZK, Mosialos G and Li JD: NF-kappaB is essential for
induction of CYLD, the negative regulator of NF-kappaB: Evidence
for a novel inducible autoregulatory feedback pathway. J Biol Chem.
279:36171–36174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Regamey A, Hohl D, Liu JW, Roger T,
Kogerman P, Toftgard R and Huber M: The tumor suppressor CYLD
interacts with TRIP and regulates negatively nuclear factor kappaB
activation by tumor necrosis factor. J Exp Med. 198:1959–1964.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kovalenko A, Chable-Bessia C, Cantarella
G, Israël A, Wallach D and Courtois G: The tumour suppressor CYLD
negatively regulates NF-kappaB signalling by deubiquitination.
Nature. 424:801–805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brummelkamp TR, Nijman SM, Dirac AM and
Bernards R: Loss of the cylindromatosis tumour suppressor inhibits
apoptosis by activating NF-kappaB. Nature. 424:797–801. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia JT, Chen LZ, Jian WH, Wang KB, Yang
YZ, He WL, He YL, Chen D and Li W: MicroRNA-362 induces cell
proliferation and apoptosis resistance in gastric cancer by
activation of NF-κB signaling. J Transl Med. 12:332014. View Article : Google Scholar
|
|
37
|
Manu KA, Shanmugam MK, Ramachandran L, Li
F, Fong CW, Kumar AP, Tan P and Sethi G: First evidence that
γ-tocotrienol inhibits the growth of human gastric cancer and
chemosensitizes it to capecitabine in a xenograft mouse model
through the modulation of NF-κB pathway. Clin Cancer Res.
18:2220–2229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vucic D and Fairbrother WJ: The inhibitor
of apoptosis proteins as therapeutic targets in cancer. Clin Cancer
Res. 13:5995–6000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou R, Hu G, Gong AY and Chen XM: Binding
of NF-kappaB p65 subunit to the promoter elements is involved in
LPS-induced transactivation of miRNA genes in human biliary
epithelial cells. Nucleic Acids Res. 38:3222–3232. 2010. View Article : Google Scholar : PubMed/NCBI
|