Introduction
Plasminogen activator inhibitor type-1 (PAI-1) is
the major physiological inhibitor of fibrinolysis and is implicated
in diverse disorders, such as thrombosis, fibrosis and the
vasculopathy associated with diabetes (1,2).
PAI-1 is expressed in vascular endothelial cells, vascular smooth
muscle cells, myocytes, hepatocytes and adipocytes. Its expression
can be modulated by tissue specific factors (3). PAI-1 has been found in plasma and
platelets, as well as in conditioned media of endothelial cells and
hepatocytes (4).
Our previous study demonstrated that in the human
HepG2 liver cell line, sphingosine 1-phosphate (S1P) can promptly
increase the transcription of PAI-1 (5). S1P, synthesized by sphingosine kinase
(SPHK) acting on sphingosine, is a bioactive signaling molecule
that regulates cell movement, differentiation, inflammation,
angiogenesis and immunity through S1P receptors (6–9). S1P
can be released from red blood cells, platelets and endothelial
cells. Receptors of S1P are G protein-coupled receptors consisting
of 5 sub types (6). In the PAI-1
3′-untranslated region (UTR), S1P contributes to RNA decay, thus,
increased PAI-1 transcription due to S1P likely involves the
promoter region (5).
It was recently reported that plasma S1P levels in
patients were correlated with their body mass index and PAI-1
levels. When plasma S1P levels were corrected with hemoglobin or
hematocrit, this correlation was no longer observed (10). In addition, in mouse 3T3-L1
adipocytes, CoCl2 was shown to promote S1P production
and extracellular transport, and increase hypoxia inducible factor
(HIF)-1α and PAI-1 (10).
In HepG2 cells, hypoxia can increase transcription
of PAI-1 by acting on the hypoxia responsive element (HRE) 2 of the
PAI-1 promoter. Transcriptional factor HIF-1α can complex with the
aryl hydrocarbon receptor nuclear translocator and bind to HRE2
(11). HIF-1α is a 'master
regulator' of gene expression. Under conditions of hypoxia the
activity of the oxygen-dependent enzyme, prolyl hydroxylase, is
attenuated. Consequently, degradation of HIF-1 by the
ubiquitin-proteasome pathway is inhibited (12).
SPHK1, the major enzyme involved in S1P synthesis,
is a modulator of HIF-1α during hypoxia in human cancer cells
(13). Stimulation with 1%
O2 elevates S1P in cultured human colon cancer cells
(14,15). Accordingly, S1P is likely to be
pivotal in increasing PAI-1 induced by hypoxia. The results
obtained in the present study suggest that in HepG2 cells, S1P
induced by hypoxia is an essential component in the pathway leading
to induction of HIF-1α and hence increased transcription of
PAI-1.
Materials and methods
Cell culture and reagents
Human hepatocarcinoma-derived HepG2 cells (American
Type Culture Collection, Manassas, VA, USA) were grown in
Dulbecco's modified Eagle's medium (DMEM, Wako Pure Chemical,
Osaka, Japan) containing 4.5 mg/ml glucose and supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in 5% CO2. Cells were grown to 80%
confluency, washed with phosphate-buffered saline (PBS) and serum
starved by incubating in serum-free DMEM containing 0.2% bovine
serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) for 16 h.
S1P (Enzo Life Sciences, Farmingdale, NY, USA) was added to the
media to assess its effects on expression of mRNA and protein. In
certain experiments, cells were cultured under hypoxic condition
(1% O2 and 5% CO2) in a low oxygen incubator
(CO2/multi-gas incubator, Astec, Fukuoka, Japan).
JTE-013 (S1P2 inhibitor; Cayman Chemical, Ann Arbor, MI,
USA) and VPC-23019 (S1P1/3 inhibitor; Avanti Polar
Lipids, Alabaster, AL, USA) were dissolved in dimethyl sulfoxide
(DMSO). They were added to the medium 30 min prior to exposure of
the cells to hypoxia or stimulation by S1P at concentrations of 10
µM. An SPHK inhibitor, SKI
[2-(p-Hydroxyanilino)-4-(p-chlorophenyl) thiazole
HCl; Merck Bioscience, Darmstadt, Germany] was also dissolved in
DMSO and added to the medium 30 min before induction of hypoxia at
a concentration of 10 µM.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was isolated with the use of the Tripure
Isolation Reagent (Roche Diagnostics, Basel, Switzerland) and
converted to cDNA with the use of PrimeScript RT reagent kits (cat.
no. RR037A; Takara Bio Inc., Otsu, Japan). qPCR was performed with
FastStart Universal SYBR Green Master (ROX) (Roche Diagnostics) on
a Thermal Cycler Dice Real Time system (cat. no. TP900; Takara Bio
Inc.). When the reaction had proceeded and the amplification and
melting curves had been confirmed, mRNA was quantified with the use
of the ΔΔCq method as described previously (16). The reactions were performed as
follows: 95°C for 10 min, then 95°C for 15 sec and 60°C for 1 min
for 40 cycles. The primers used for qPCR were as follows: Forward:
5′-TGATGGCTCAGACCAACAAG-3′ and reverse: 5′-CAGCAATGAACATGCTGAGG-3′
for human PAI-1; and forward: 5′-TCATCCAAGAAGCCCTAACG-3′ and
reverse: 5′-CGCTTTCTCTGAGCATTCTG-3′ for human HIF-1α. The human
β-actin primer set (cat. no. HA067803) was purchased from Takara
Bio Inc. and used as a control for normalization.
Immunobloting
PAI-1 protein in conditioned media was quantified as
described previously (5). β-actin
and HIF-1α in cell lysates were assayed as follows: HepG2 cells
were washed with PBS and lysed with lysis buffer [62.5 mmol/l
Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS) and 10%
glycerol]. The lysates were centrifuged at 13,000 × g for 5 min at
4°C to remove the cell debris. Samples of supernatant fractions
were subjected to SDS-polyacrylamide gel electrophoresis (PAGE)
with the use of 8 or 10% running gels. After quantification of
concentrations of protein with bicinchoninic acid protein assay
kits (Thermo Fisher Scientific, Inc.), samples were diluted with 1X
SDS sample buffer [62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol,
5% 2-mercaptoethanol and a trace amount of bromophenol blue] and
incubated at 37°C for 5 min. Equal amounts of protein were
separated by SDS-PAGE and transferred to Immobilon-P polyvinylidene
difluoride membranes (Millipore, Billerica, MA, USA). Membranes
were blocked in 3% skim milk in Tris-buffered saline. Membranes
were incubated with mouse anti-HIF-1α IgG (1:5,000 dilution; cat.
no. NB100-105; Novus Biologicals LLC, Littleton, CO, USA), or mouse
anti-β-actin IgG (1;20,000 dilution; cat. no. A5441 Sigma-Aldrich)
for 18 h, and then incubated with peroxidase-conjugated goat
anti-mouse IgG (1:5,000 dilution for HIF-1 and 1:10,000 dilution
for actin detection; cat. no. NA9310; GE Healthcare,
Buckinghamshire, England) for 1 h. Labeling was detected with
enhanced chemiluminescence-Plus reagents (GE Healthcare) or the
SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher
Scientific, Inc.) and the use of a photoimager LAS-3000 mini
(Fujifilm, Tokyo, Japan). Images were analyzed with Image Gauge
software (version 4.0; Fujifilm).
Plasmid constructs
The plasmids used were gifts from Dr Imagawa
(Department of Cardiovascular Medicine, Hokkaido University,
Sapporo, Japan). pGL3-basic vector (Promega Corporation, Madison,
WI, USA) containing the PAI-1 gene promoter region (−825 to +42)
(designated as P-1) was used as previously described (5). PAI-1 gene promoter area fragments
(P-2, −659 to +42; P-3, −536 to +42; P-4, −360 to +42; P-5, −302 to
+42; P-6, −205 to +42; P-7, −165 to +42; P-8, −115 to +42 and P-9,
−60 to +42) were constructed in pGL3-control vectors (Promega
Corporation) (17,18). All the promoters were characterized
by sequencing.
Transfection and luciferase assays
For measuring activity of the promoter region of the
PAI-1 gene, DNA transfection and luciferase assays were performed
as previously described (4). HepG2
cells were inoculated on 12-well plates at 50% confluence and
preincubated in serum free DMEM containing 0.2% BSA. Transient
transfection was performed with the lipofection method with the use
of Lipofectamine LTX (Thermo Fisher Scientific, Inc.).
Co-transfections were performed with 1 mg each of the PAI-1
promoter firefly luciferase fusion DNA reporter constructs in
pGL3-basic vector (Promega Corporation) and 5 ng control pRL-TK
vectors (Promega Corporation). After 5 h, the cells were cultured
in serum-starved media for 18 h, stimulated with S1P or hypoxia for
24 h and harvested. Luciferase activity was detected in cell
extracts with the Passive Lysis Buffer with the use of the
Dual-Luciferase Reporter Assay system (Promega Corporation) and a
luminometer (GloMax 20/20n, Promega Corporation). Normalized
luciferase activity was calculated as the ratio of firefly
luciferase activity to control Renilla luciferase activity.
Results for each reporter construct were expressed as the fold
induction compared with results in transfected, unstimulated
cells.
RNA interference
Control small interfering (si)RNA (MISSON siRNA
Universal Negative Control) and human HIF1A (HIF-1α) siRNA
(SASI_Hs02_00332063) were purchased from Sigma-Aldrich. siRNA (75
pmol) was transfected to HepG2 cells by reverse transfection with
the use of Lipofectamine RNAiMAX (Thermo Fisher Scientific, Inc.).
After 48 h, cells were serum-starved for 16 h and then stimulated
under hypoxic conditions and by S1P for 6 h and 4 h, respectively.
Cells and media were collected and subjected to RT-qPCR and
immunoblotting, respectively.
Measurement of SPHK activity
In vitro sphingosine kinase assays were
performed as described previously (19), with minor modifications. Cells were
lysed by sonication in assay buffer containing 20 mM Tris-HCl (pH
7.5), 0.25 mM EDTA, 12 mM b-glycerophosphate, 1 mM sodium
pyrophosphate, 5 mM sodium fluoride, 5% glycerol, 1X protease
inhibitor cocktail (Complete™ EDTA free; Roche Diagnostics), 1 mM
phenylmethylsulfonyl fluoride, 5 mM sodium orthovanadate, 2 mM
dithiothreitol and 0.5 mM 4-deoxypyridoxine. After removal of cell
debris by centrifugation at 1,000 × g for 3 min at 4°C, the total
lysates were subjected to an in vitro sphingosine kinase
assay. Volumes of samples (50 µg protein) were adjusted to
190 µl with assay buffer and mixed with 10 µM
D-erythro-sphingosine (dissolved in 5% Triton X-100). The reactions
were initiated by adding 2 µCi of [γ-32P] ATP
(200 nmol, Institute of Isotopes Co., Ltd., Budapest, Hungary), in
200 mM MgCl2, followed by incubation at 37°C for 15 min.
Reactions were terminated by addition of 750 µl
chloroform/methanol/HCl (100:200:1, v/v). The organic phase was
separated from the aqueous phase by adding 250 µl chloroform
and 250 µl of 1% KCl. The labeled lipids in the organic
phase were recovered by centrifugation (3,500 rpm, 4°C, 3 min),
dried and suspended in chloroform/methanol (2:1, v/v). Lipids were
separated on Silica Gel G60 high performance TLC plates (Merck
Bioscience) with 1-butanol/acetic acid/water (3:1:1, v/v).
Radioactivity associated with S1P were quantified using a
Bio-Imaging Analyzer, BAS1800II (Fujifilm).
Statistical analysis
Data are expressed as the mean ± standard deviation.
After verification that the data were normally distributed,
differences were assessed with Welch's t-tests. Multiple
comparisons between groups were made by Tukey-Kramer test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Hypoxia and S1P increase PAI-1 promoter
activity in relation to HRE2 and increase transcription of
PAI-1
To determine the association of S1P with
transcription of PAI-1, the effects of S1P on PAI-1 promoter
activity were determined using a luciferase assay and a PAI-1
promoter construct. Hypoxia and S1P increased the luciferase
activity of PAI-1 promoter constructs (P<0.05; P-1 to P-6). No
effects were observed in P-7 to P-9 (Fig. 1A). S1P increased the luciferase
activity of PAI-1 promoter constructs (P-1) in a dose-dependent
manner (Fig. 1B). With the P-1 and
P-6 constructs, S1P had additive effects with hypoxia in inducing
luciferase activity (P<0.05; Fig.
1C and D). However, in the P-7 construct, without HRE2 S1P,
hypoxia failed to induce luciferase activity (Fig. 1E). In addition, S1P and hypoxia
increased PAI-1 mRNA in an additive manner (Fig. 1F). These results suggested that
hypoxia and S1P increased PAI-1 promoter activity in a
HRE2-dependent manner and increased transcription of PAI-1.
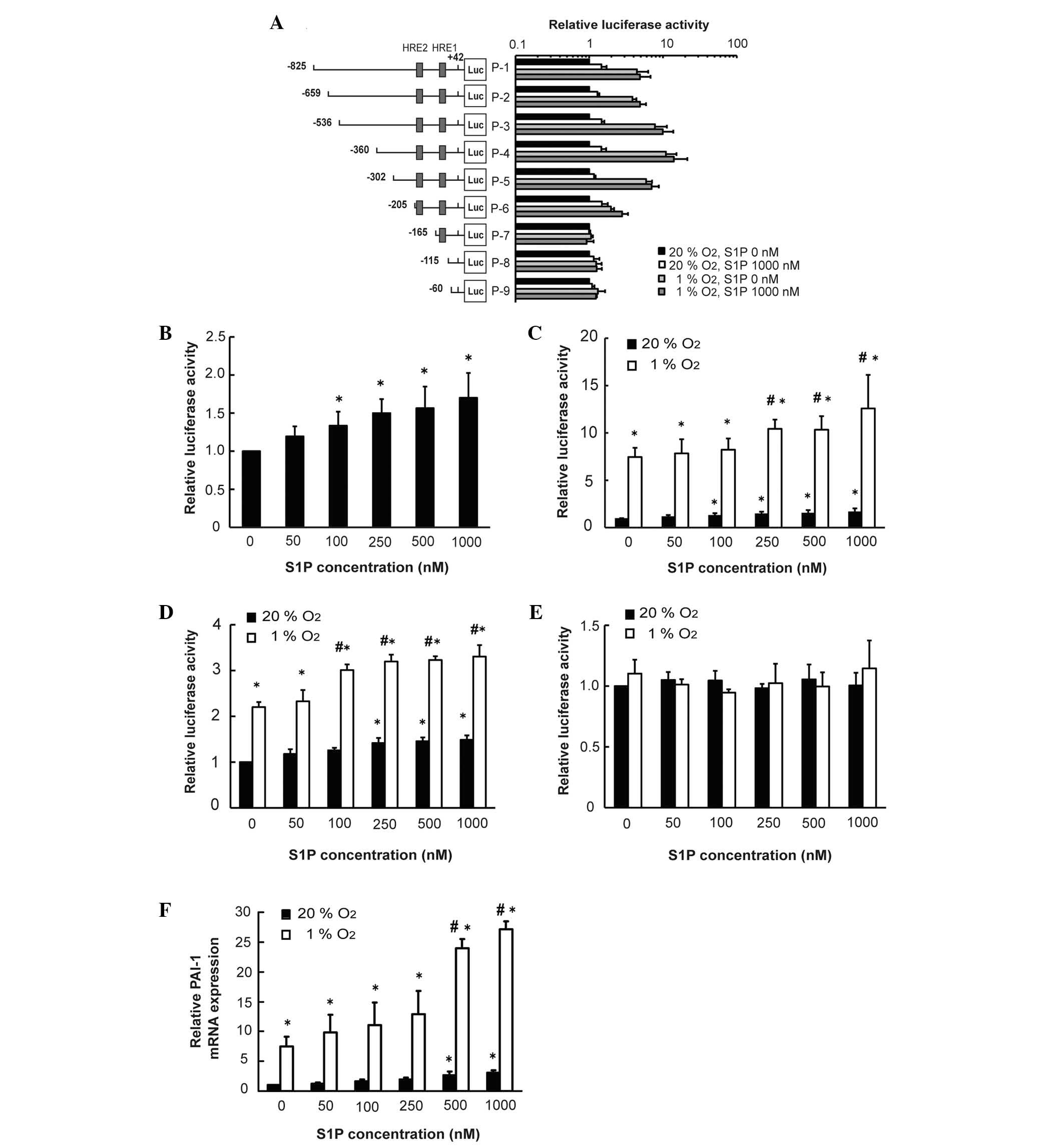 | Figure 1Hypoxia and S1P increase PAI-1
promoter activity through HRE2. HepG2 cells were co-transfected
with 1 µg each of the PAI-1 promoter area firefly luciferase
fusion DNA reporter constructs (P-1 to P-9) in pGL3-basic vector
and 5 ng control pRL-TK vector. After 5 h, the cells were
serum-starved for 18 h, stimulated with S1P (1,000 nM), cultured in
serum-free DMEM under hypoxic conditions for 24 h and harvested.
Luciferase activity was detected in cell extracts. (A) Locations of
HRE1 and HRE2 are shown. (B) Cells were transfected with P-1
reporter construct and treated with S1P. After 24 h, luciferase
activity was detected (n=3, *P<0.05 compared with 0
nM). (C) Cells were transfected with a P-1 reporter construct and
subjected to hypoxia and S1P (at the indicated concentrations).
After 24 h luciferase activity was detected (n=8,
*P<0.05 compared with normoxia and 0 nM,
#P<0.05 compared with hypoxia and 0 nM). (D) Cells
were transfected with P-6 reporter construct and subjected to
hypoxia and S1P (at the indicated concentrations). After 24 h,
luciferase activity was detected (n=3, *P<0.05
compared with normoxia and 0 nM, #P<0.05 compared
with hypoxia and 0 nM). (E) Cells were transfected with P-7
reporter construct and subjected to hypoxia and S1P (at the
indicated concentrations). After 24 h, luciferase activity was
detected. (F) Cells were stimulated with hypoxia and S1P (the
indicated concentration) for 3 h. Total RNA was subjected to
reverse transcription-quantitative polymerase chain reaction for
detection of PAI-1 mRNA (n=3, *P<0.05 compared with
normoxia and 0 nM, #P<0.05 compared with hypoxia and
0 nM). S1P, sphingosine 1-phosphate; PAI-1, plasminogen activator
inhibitor type-1; HRE, hypoxia responsive element. |
Induction of HIF-1α by S1P is transient
compared with that observed with induction by hypoxia
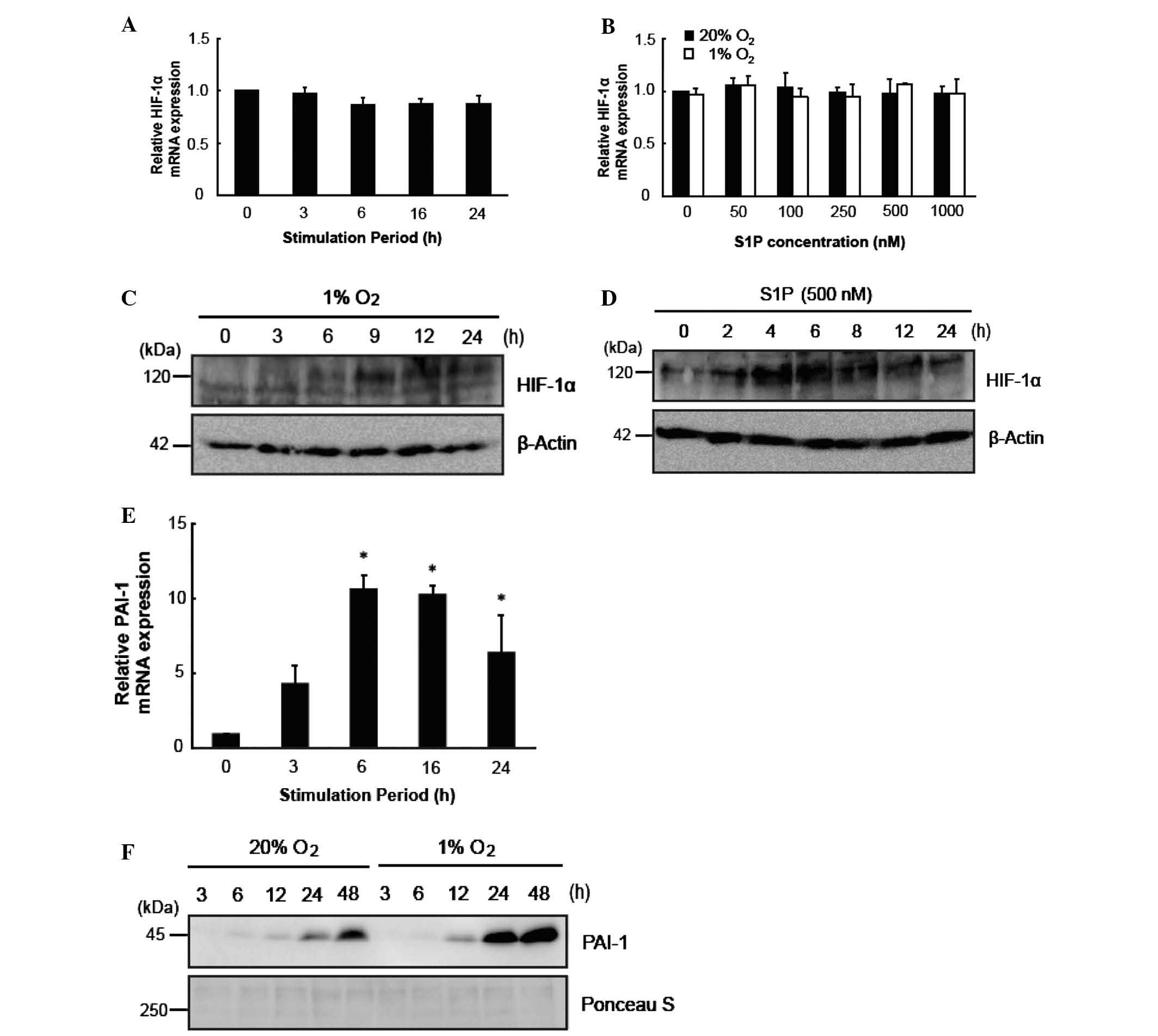
Hypoxia and S1P were not identified to alter the
expression of HIF-1α mRNA (Fig. 2A and
B). Thus, the effects of S1P on HIF-1α protein and PAI-1 mRNA
were elucidated to determine the differences between the effects of
S1P and hypoxia. Hypoxia and S1P increased HIF-1α protein levels
(Fig. 2C and D) and hypoxia was
shown to increase PAI-1 mRNA (P<0.05; Fig. 2E). The increase in PAI-1 protein
with hypoxia was sustained (Fig.
2F). The increase in HIF-1α with S1P was transient (Fig. 2D). Thus, induction of HIF-1α by S1P
was transient compared with that observed with induction by
hypoxia.
Induction of PAI-1 by hypoxia and S1P
involves HIF-1α
It was investigated whether the S1P-induced increase
in PAI-1 transcription involves HIF-1α using siRNA. HIF-1α siRNA
reduced HIF-1α mRNA by 0.5-fold (P<0.01; Fig. 3A). HIF-1α siRNA modestly prevented
the increase in PAI-1 protein induced by hypoxia and S1P (Fig. 3B). HIF-1α siRNA prevented the
increase in PAI-1 mRNA induced by S1P (P<0.01; Fig. 3C). Furthermore, HIF-1α siRNA
prevented the increase in PAI-1 mRNA induced by hypoxia (Fig. 3D). The results suggested that the
induction of PAI-1 by hypoxia and S1P involved HIF-1α.
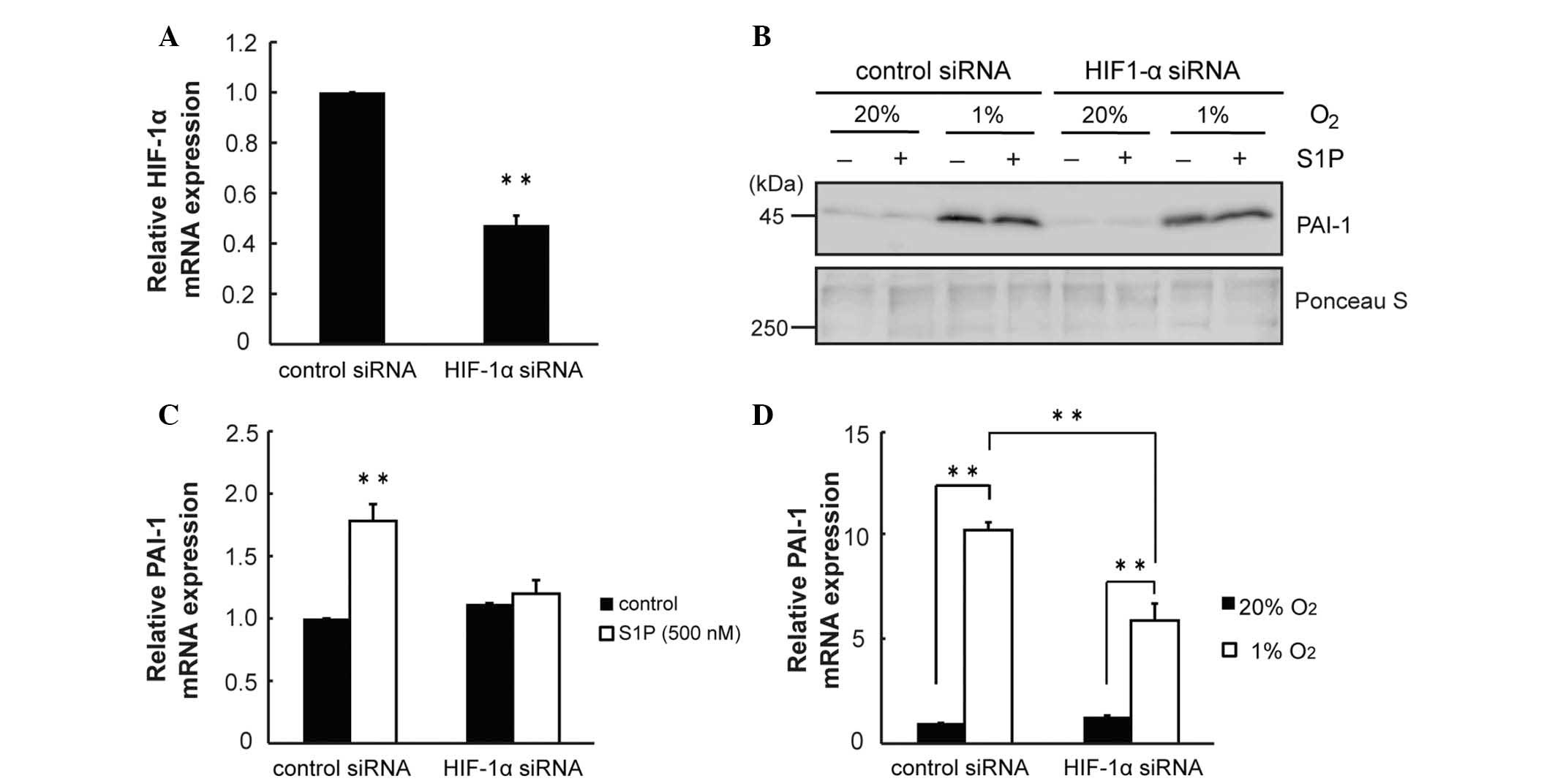 | Figure 3HIF-1α is involved in the increase of
PAI-1 induced by hypoxia and S1P. (A) HepG2 cells were transfected
with HIF-1α siRNA (75 pmol). After 24 h, the expression of HIF-1α
mRNA was determined by RT-qPCR (n=3, **P<0.01
compared with control). (B) After transfection with HIF-1α siRNA,
cells were serum-starved for 16 h. Then, cells were subjected to
hypoxia and S1P (500 nM) for 24 h. Conditioned media were
collected. PAI-1 was quantified by western blot analysis. Ponceau S
staining was used as a loading control. (C) After transfection with
HIF-1α siRNA, cells were serum-starved for 16 h. Then, cells were
stimulated with S1P (500 nM) for 4 h. Total RNA was extracted and
subjected to RT-qPCR for detection of PAI-1 mRNA (n=3,
**P<0.01). (D) After transfection with HIF-1α siRNA,
cells were serum-starved for 16 h. Then, cells were subjected to
hypoxia for 6 h. Total RNA was extracted and subjected to RT-qPCR
for detection of PAI-1 mRNA (n=3, **P<0.01). HIF-1α,
hypoxia inducible factor-1α; PAI-1; plasminogen activator inhibitor
type-1; S1P, sphingosine 1-phosphate; siRNA, small interfering RNA;
RT-qPCR, reverse trancscription-quantitative polymerase chain
reaction. |
Under conditions of hypoxia, S1P
increases HIF-1α in an autocrine and paracrine manner
It was then investigated whether alterations of
HIF-1α induced by hypoxia involves S1P. Hypoxia was observed to
increase the enzymatic activity of SPHK (P<0.05; Fig. 4A). The increase in HIF-1α protein
induced by hypoxia was attenuated by inhibitors of SPHK (Fig. 4B). Increases in HIF-1α induced by
hypoxia and S1P were blunted by inhibitors of S1P1/3
(Fig. 4C and D). Increases in
PAI-1 mRNA induced by S1P and hypoxia were diminished by inhibitors
of S1P1/3 (P<0.01; Fig.
4E and F). Thus, S1P increased HIF-1α in an autocrine and
paracrine manner under conditions of hypoxia.
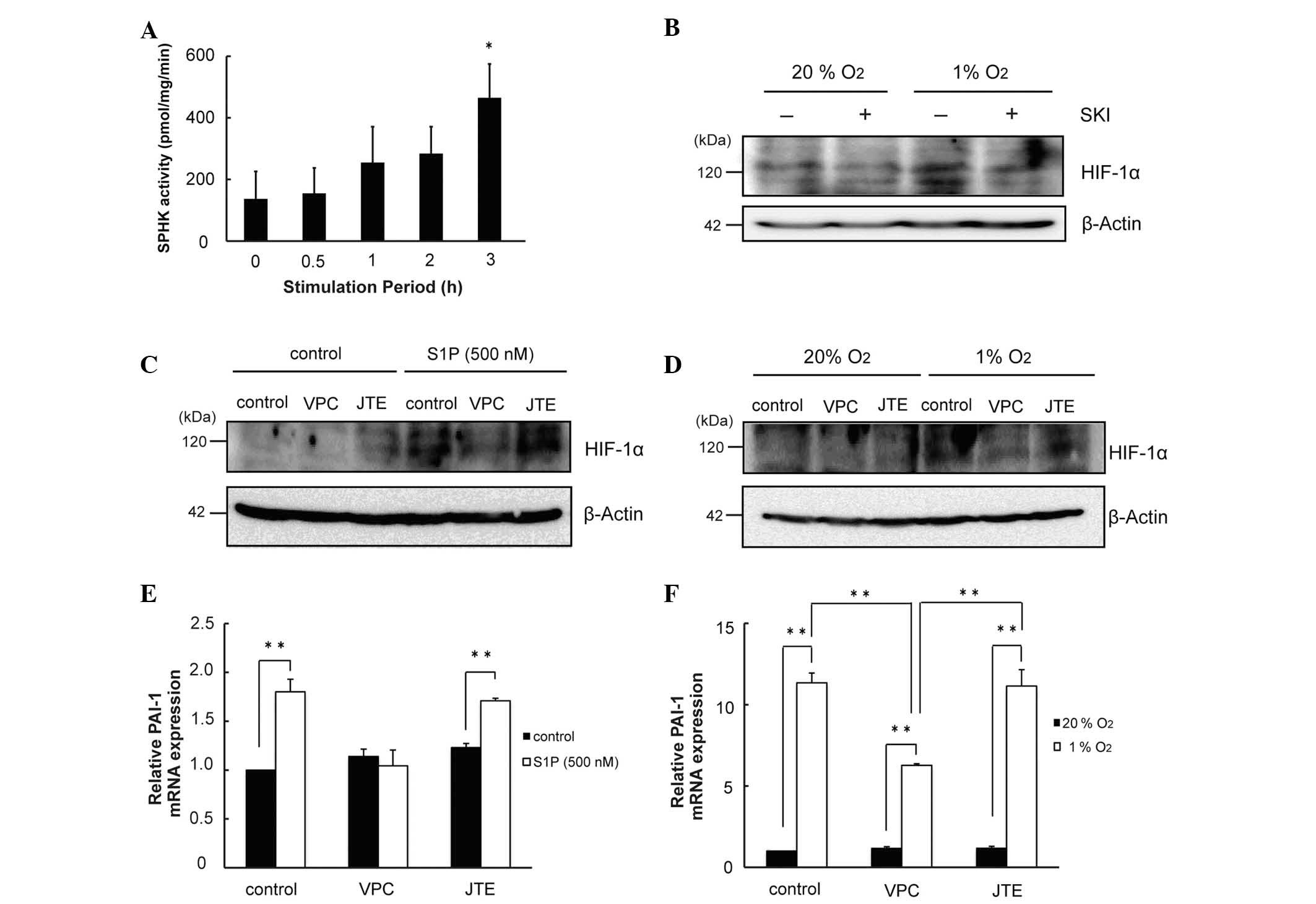 | Figure 4S1P produced by hypoxia contributes
to the increase in HIF-1α protein. (A) Cell lysates were collected
following exposure to hypoxia. Proteins (50 µg) were
incubated with 1 mM [γ-32P]-ATP and 50 µM
sphingosine for 15 min at 37°C. Lipids were extracted and separated
by thin-layer chromatography. Radioactivity associated with S1P was
quantified using a Bio-Imaging Analyzer BAS-1800II (n=3,
*P<0.05 compared with 0 h). (B) Cells were pretreated
with SKI for 30 min, exposed to hypoxia for 6 h, and subjected to
western blot analysis for detection of HIF-1α protein. (C) Cells
were pretreated with S1P receptor antagonists for 30 min, exposed
to S1P (500 nM) for 4 h, and subjected to western blot analysis for
the detection of HIF-1α protein. (D) Cells were pretreated with S1P
receptor antagonists for 30 min, exposed to hypoxia for 6 h, and
subjected to western blot analysis for the detection of HIF-1α
protein. (E) Cells were pretreated with S1P receptor antagonists
for 30 min, exposed to S1P (500 nM) for 4 h, and total RNA
subjected to RT-qPCR for detection of PAI-1 mRNA (n=3,
**P<0.01). (F) Cells were pretreated with S1P
receptor antagonists for 30 min, exposed to hypoxia for 6 h, and
total RNA subjected to RT-qPCR for detection of PAI-1 mRNA (n=3,
**P<0.01). S1P, sphingosine 1-phosphate; HIF-1α,
hypoxia inducible factor-1α; PAI-1; plasminogen activator inhibitor
type-1; RT-qPCR, reverse trancscription-quantitative polymerase
chain reaction; SPHK, sphingosine kinase. |
Discussion
In the present study, hypoxia and S1P acted on the
same region of the PAI-1 promoter. In HepG2 cells, PAI-1 promoter
regions have previously been investigated (11,17,18,20-23).
The region responsive to S1P contained HRE2. As S1P and hypoxia
increased PAI-1 promoter activity in an additive manner, it is
likely that S1P and hypoxia share a common transcription factor and
increase PAI-1. HIF-1α binds to PAI-1 HRE2 (11,22).
In HepG2 cells, increases in HIF-1α protein in response to hypoxia
were persistent. By contrast, the S1P-induced increase in HIF-1α
protein, although rapid, was transient, which was consistent with
the results of a previous study (11). An increase in PAI-1 protein in
conditioned media by hypoxia became apparent at 6 h.
S1P did not significantly increase PAI-1 protein in
the media. Therefore, HIF-1α protein and PAI-1 mRNA were
investigated. Transcription of PAI-1 induced by S1P was strongly
inhibited by knockdown of HIF-1α, suggesting that S1P increases
transcription of PAI-1 via modulation of HIF-1α. Increases in PAI-1
transcription following hypoxia were partially diminished by HIF-1α
knockdown, suggesting that HIF-1α is involved in PAI-1
transcription in a diverse manner under hypoxia and S1P.
In human cancer cells, hypoxia increases the
activity of SPHK. S1P released from such cells can function in an
autocrine or paracrine manner (13,24).
Temporally similar, rapid increases in SPHK activity induced by
hypoxia were observed in HepG2 cells. The increases in SPHK1
protein preceded the increases in HIF-1α protein. Furthermore,
knockdown of SPHK1 prevented the hypoxia-induced increases in
HIF-1α protein (13). Inhibitors
of SPHK also prevented the increases in HIF-1α protein induced by
hypoxia. These results suggest that SPHK or products of SPHK are
central to hypoxia-induced increases in HIF-1α. Hypoxia and/or S1P
did not affect HIF-1α mRNA. However, hypoxia and S1P increased
HIF-1α protein.
Even in the absence of hypoxia, S1P increases HIF-1α
protein via activation of the phosphoinositide 3-kinase pathway
independent of von Hippel-Lindau (VHL) tumor suppressor protein
(pVHL) in mouse endothelial cells (25). By contrast, hypoxia induces
increases in SPHK1 activity that increase HIF-1α protein via pVHL
in human cancer cells (13). In
human follicular thyroid cancer cells, S1P stabilized the HIF-1α
protein independent of pVHL (26).
Conversely, S1P activated translational regulators eIF-4E and
p70S6K, which are known to control HIF-1α synthesis (26). The results obtained in the present
study support the view that S1P and hypoxia increase transcription
of PAI-1 in an additive manner.
S1P1/3 inhibitors prevented the increases
in HIF-1α protein induced by hypoxia and S1P. Downstream
transcription of PAI-1 was attenuated. These results suggested that
S1P produced by SPHK is involved in the early stages of increases
in HIF-1α protein induced by hypoxia. As HepG2 cells express
S1P1, S1P2 and S1P4 subtypes
(5), it is likely that increases
in PAI-1 induced by S1P are mediated by S1P1 (5–6).
However, other S1P receptor subtypes have been demonstrated to also
be involved (10,25,26).
S1P also affects transcriptional regulation with
miRNA and RNA binding protein. S1P acts on PAI-1 mRNA 3′-UTR and
regulates RNA decay (5). At high
concentrations of S1P, PAI-1 is increased. Indeed, in humans,
plasma S1P level and plasma PAI-1 levels are positively correlated
(10).
Sphingolipids are known to be associated with
thrombosis. In Fabry disease, a sphingolipid storage disorder,
plasma S1P level is increased (27) and thrombosis results from cerebral
vasculopathy (28). Npc1 is the
gene responsible for Niemann-Pick disease and, in
ApoE−/−, Npc1−/− mice spontaneous
atherothrombosis and medial degradation are observed (29). Furthermore, S1P is abundantly
present in platelets and red blood cells (30,31).
It is likely that the S1P concentration can be increased with
platelet activation or red blood cell destruction. For instance,
hemoglobin released upon hemolysis can activate platelets, and
thrombosis is the major cause of death in paroxysmal nocturnal
hemoglobinuria, where chronic hemolysis is observed (32). In thrombotic thrombocytopenic
purpura, thrombus formation is facilitated and red blood cells are
mechanically destroyed. In these diseases or animal models PAI-1 is
shown to be increased (29,33–35),
suggesting that excessive S1P can increase PAI-1 and shift the
fibrinolytic balance toward thrombosis.
In normal human plasma, the S1P concentration is
100–300 nM (30) and is maintained
by the release from platelets, red blood cells and endothelium
(31). Thrombus formation, tissue
hypoxia and release of S1P from activated platelets are closely
associated (30,31). Hypoxia can induce the release of
S1P from diverse tissues, leading to a environment characterized by
high levels of S1P. Associations between the increase in PAI-1, and
hypoxia and S1P may underlie the formation of thrombus and create a
vicious cycle. Induction of PAI-1 and diminution of fibrinolysis by
S1P under conditions of hypoxia may be attenuated by pharmacologic
interventions focused on inhibition of S1P receptors.
In conclusion, the present study demonstrated that
S1P induced by hypoxia increases the expression of PAI-1 in HepG2
cells via HIF-1α and that inhibition of S1P receptors may be an
attractive therapeutic target and ameliorate thrombosis following
hypoxia.
Acknowledgments
This study was supported in part by grants-in-aid
for scientific research from the Ministry of Education, Science,
Sport and Culture of Japan. The authors would like to thank
Professor Shin-ichi Hoshino (Department of Biological Chemistry,
Graduate School of Pharmaceutical Sciences, Nagoya City University,
Nagoya, Japan) for useful advice and helpful discussions, and Ms.
Rebecca Aksdal for assistance in preparation of the manuscript.
Abbreviations:
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
PAI-1
|
plasminogen activator inhibitor
type-1
|
|
S1P
|
sphingosine 1-phosphate
|
References
|
1
|
Lucore CL and Sobel BE: Interactions of
tissue type plasminogen activator with plasma inhibitors and their
pharmacologic implications. Circulation. 77:660–669. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sobel BE, Taatjes DJ and Schneider DJ:
Intramural plasminogen activator inhibitor type-1 and coronary
atherosclerosis. Arterioscler Thromb Vasc Biol. 23:1979–1989. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eren M, Painter CA, Gleaves LA, Schoenhard
JA, Atkinson JB, Brown NJ and Vaughan DE: Tissue- and
agonist-specific regulation of human and murine plasminogen
activator inhibitor-1 promoters in transgenic mice. J Thromb
Haemost. 1:2389–2396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asakura T, Iwaki S, Okada H, Sobel BE and
Fujii S: Posttranscriptional regulation of expression of
plasminogen activator inhibitor type-1 by cAMP in HepG2 liver
cells. J Biochem. 150:687–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwaki S, Yamamura S, Asai M, Sobel BE and
Fujii S: Posttranscriptional regulation of expression of
plasminogen activator inhibitor type-1 by sphingosine 1-phosphate
in HepG2 liver cells. Biochim Biophys Acta. 1819:1132–1141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun J, Hla T, Lynch KR, Spiegel S and
Moolenaar WH: International union of basic and clinical
pharmacology. LXXVIII Lysophospholipid receptor nomenclature.
Pharmacol Rev. 62:579–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spiegel S, English D and Milstien S:
Sphingosine 1-phosphate signaling: Providing cells with a sense of
direction. Trend Cell Biol. 12:236–242. 2002. View Article : Google Scholar
|
|
8
|
Spiegel S and Milistein S: Sphingosine
1-phosphate, a key cell signaling molecule. J Biol Chem.
277:25851–25854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takuwa Y, Okamoto Y, Yoshioka K and Takuwa
N: Sphingosine 1-phosphate signaling and biological activities in
the cardiovascular system. Biochem Biophys Acta. 1781:483–488.
2008.
|
|
10
|
Ito S, Iwaki S, Koike K, Yuda Y, Nagasaki
A, Ohkawa R, Yatomi Y, Furumoto T, Tsutsui H, Sobel BE and Fujii S:
Increased plasma Sphingosine 1-phosphate in obese individuals and
its capacity to increase the expression of plasminogen activator
inhibitor-1 in adipocytes. Coron Artery Dis. 24:642–650.
2013.PubMed/NCBI
|
|
11
|
Fink T, Kazlauskas A, Poellinger L,
Ebbesen P and Zachar V: Identification of a tightly regulated
hypoxia-response element in the promoter of human plasminogen
activator inhibitor-1. Blood. 99:2077–2083. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagorska A and Dulak J: HIF-1: The knowns
and unknowns of hypoxia sensing. Acta Biochim Pol. 51:563–585.
2004.PubMed/NCBI
|
|
13
|
Ader I, Brizuela L, Bouquerel P, Malavaud
B and Cuvillier O: Sphingosine kinase 1: A new modulator of hypoxia
inducible factor 1alpha during hypoxia in human cancer cells.
Cancer Res. 68:8635–8642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leong WI and Saba JD: S1P metabolism in
cancer and other pathological conditions. Biochimie. 92:716–723.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cuvillier O and Ader I: Hypoxia-inducible
factors and sphingosine 1-phosphate signaling. Anticancer Agents
Med Chem. 11:854–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Imagawa S, Fujii S, Dong J, Furumoto T,
Kaneko T, Zaman T, Satoh Y, Tsutsui H and Sobel BE: Hepatocyte
growth factor regulates E box-dependent plasminogen activator
inhibitor type 1 gene expression in HepG2 liver cells. Arterioscler
Thromb Vasc Biol. 26:2407–2413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong J, Fujii S, Li H, Nakabayashi H,
Sakai M, Nishi S, Goto D, Furumoto T, Imagawa S, Zaman TA and
Kitabatake A: Interleukin-6 and mevastatin regulate plasminogen
activator inhibitor-1 through CCAAT/enhancer-binding protein-δ.
Arterioscler Thromb Vasc Biol. 25:1078–1084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olivera A, Kohama T, Tu Z, Milstien S and
Spiegel S: Purification and characterization of rat kidney
sphingosine kinase. J Biol Chem. 273:12576–12583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimova EY, Jakubowska MM and Kietzmann T:
CREB binding to the hypoxia-inducible factor-I responsive elements
in the plasminogen activator inhibitor-I promotor mediates the
glucagon effect. Thromb Heamost. 98:296–303. 2007.
|
|
21
|
Dong J, Fujii S, Imagawa S, Matsumoto S,
Matsushita M, Todo S, Tsutsui H and Sobel BE: IL-1 and IL-6 induce
hepatocyte plasminogen activator inhibitor-1 expression through
independent signaling pathways converging on C/EBPdelta. Am J
Physiol Cell Physiol. 292:209–215. 2007. View Article : Google Scholar
|
|
22
|
Jung SY, Song HS, Park SY, Chung SH and
Kim YJ: Pyruvate promotes tumor angiogenesis through
HIF-1-dependent PAI-1 expression. Int J Oncol. 38:571–576.
2011.
|
|
23
|
Nakayama N, Nakamura T, Okada H, Iwaki S,
Sobel BE and Fujii S: Modulators of induction of plasminogen
activator inhibitor type-1 in HepG2 cells by transforming growth
factor-β. Coron Artery Dis. 22:468–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anelli V, Gault CR, Cheng AB and Obeid LM:
Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma
cells. Role of hypoxia-inducible factors 1 and 2. J Biol Chem.
283:3365–3375. 2008. View Article : Google Scholar
|
|
25
|
Michaud MD, Robitaille GA, Gratton JP and
Richard DE: Sphingosine-1-phosphate: A novel nonhypoxic activator
of hypoxia-inducible factor-1 in vascular cells. Arterioscler
Thromb Vasc Biol. 29:902–908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalhori V, Kemppainen K, Asghar MY,
Bergelin N, Jaakkola P and Törnquist K: Sphigosine-1-phosphate as a
regulator of hypoxia-induced factor-1α in thyroid follicular
carcinoma cells. PLoS One. 8:e661892013. View Article : Google Scholar
|
|
27
|
Brakch N, Dormond O, Bekri S, Golshayan D,
Correvon M, Mazzolai L, Steinmann B and Barbey F: Evidence for a
role of sphingosine-1 phosphate in cardiovascular remodelling in
Fabry disease. Eur Heart J. 31:67–76. 2010. View Article : Google Scholar
|
|
28
|
Moore DF, Kaneski CR, Askari H and
Schiffmann R: The cerebral vasculopathy of Fabry disease. J Neurol
Sci. 257:258–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Welch CL, Sun Y, Arey BJ, Lemaitre V,
Sharma N, Ishibashi M, Sayers S, Li R, Gorelik A, Pleskac N, et al:
Spontaneous atherothrombosis and medial degradation in
Apoe−/−, Npc1−/− mice. Circulation.
116:2444–2452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi
R, Asazuma N, Satoh K, Ozaki Y and Kume S: Sphingosine 1-phosphate,
a bioactive sphingolipid abundantly stored in platelets, is a
normal constituent of human plasma and serum. J Biochem.
121:969–973. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hisano Y, Nishi T and Kawahara A: The
functional roles of S1P in immunity. J Biochem. 152:305–311. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hillmen P, Muus P, Dührsen U, Risitano AM,
Schubert J, Luzzatto L, Schrezenmeier H, Szer J, Brodsky RA, Hill
A, et al: Effect of the complement inhibitor eculizumab on
thromboembolism in patients with paroxysmal nocturnal
hemoglobinuria. Blood. 110:4123–4128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nguyen Dinh Cat A, Escoubet B, Agrapart V,
Griol-Charhbili V, Schoeb T, Feng W, Jaimes E, Warnock DG and
Jaisser F: Cardiomyopathy and response to enzyme replacement
therapy in a male mouse model for Fabry disease. PLoS One.
7:e337432012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Helley D, de Latour RP, Porcher R,
Rodrigues CA, Galy-Fauroux I, Matheron J, Duval A, Schved JF,
Fischer AM and Socié G; French Society of Hematology: Evaluation of
hemostasis and endothelial function in patients with paroxysmal
nocturnal hemoglobinuria receiving eculizumab. Haematologica.
95:574–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anthony MT, Zeigler ZR, Lister J, Raymond
JM, Shadduck RK, Kramer RE, Gryn JF, Rintels PB, Besa EC, George
JN, et al: Plasminogen activator inhibitor (PAI-1) antigen levels
in primary TTP and secondary TTP post-bone marrow transplantation.
Am J Hematol. 59:9–14. 1998. View Article : Google Scholar : PubMed/NCBI
|