Introduction
Osteoporosis is a progressive bone disease
characterized by low bone mass, microarchitectural deterioration of
bone tissue and a high risk of fractures (1,2). In
addition, osteoporosis can easily result in serious bone fragility
and susceptibility to fracture (3). It was estimated that over 200 million
individuals has osteoporosis worldwide in 2013 and ~30% of the
postmenopausal women in the USA and Europe suffer with osteoporosis
(4,5). Currently, estrogen is one of the most
commonly used treatment strategies for treating postmenopausal
osteoporosis (6). However, studies
have demonstrated that estrogen treatment could result in serious
adverse effects, such as endometrial carcinoma, breast cancer and
cardiovascular disease (4,7). It is therefore key to identify novel
strategies with low toxicity for treating osteoporosis.
Polydatin
(3,4′,5-trihydroxystilbene-3-β-D-glucoside), predominantly isolated
from the roots of Polygonum cuspidatum Sieb, is a known
natural stilbenes compound with wide pharmacological activity
(8). Previous studies have
demonstrated that polydatin possesses notable anti-inflammatory,
anti-oxidant, anti-shock, anti-asthmatic and anti-hypertrophic
effects (8–13). Postmenopausal osteoporosis is
associated with ovarian hormone deficiency and is a common reason
for age-related bone loss (14).
The ovariectomized rat model is a commonly used osteoporosis animal
model due to its similarities in etiology and pathology to
postmenopausal osteoporosis. Blood calcium and phosphorus are two
important elements that are key for the integrity and remodeling of
bone; alkaline phosphatase (ALP) is a crucial enzyme for bone
remodeling; and receptor activators of nuclear factor-κB
ligand/osteoprotegerin (RANKL/OPG) is reported to be important for
the formation and differentiation osteoclasts. Thus, these
indicators are commonly used to investigate osteoporosis and were
analyzed in this study (2–4, 14).
The present study was designed to investigate the anti-osteoporotic
activity of polydatin on postmenopausal osteoporosis using the
ovariectomized rat model, and to determine its related molecular
mechanisms.
Materials and methods
Chemicals
Polydatin was purchased from Shanghai Tauto Biotech
Co., Ltd. (Shanghai, China), and its purity was >98%. α-modified
minimum essential medium (α-MEM) and fetal bovine serum (FBS) were
purchased from Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Dimethyl sulfoxide (DMSO) and serum OPG enzyme-linked
immunosorbent assay (ELISA) kits were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Rabbit anti-RANKL (cat. no. BA1323 1:500) and
OPG (cat no. BA1475 1:500) polyclonal antibodies were purchased
from Wuhan Boster Bio-engineering Co., Ltd. (Wuhan, China). Rabbit
anti-β-catenin (cat. no. ab6302; 1:2,000), anti-histone (cat. no.
ab1791; 1:2,000) and anti-β-actin (cat. no. ab5694; 1:2,000)
polyclonal antibodies were purchased from the Abcam (Cambridge, MA,
USA). Bicinchoninic acid (BCA) protein assay reagents and western
blot & IP cell lysis buffer kits were purchased from Beyotime
Co. (Hangzhou, China). All other regents used in this study were of
analytical grade.
Animals
Animal protocols were established according to the
generally accepted international guidelines, and approved by the
Animal Care and Use Committee of the Guangdong Province Corps
Hospital (Chinese People's Armed Police Forces, Guangzhou, China;
approval no. 20141109_01#). Female ICR mice (age, 3 months;
n=10/group; weight, 20±2 g) used in the present study were
purchased form the Shanghai Laboratory Animal Center (Shanghai,
China). All animals were housed at 21±1°C and 50–60% humidity under
a 12 h light/dark cycle and had free access to standard pellet diet
and tap water.
Toxicity tests
The 80 ICR mice were random divided into 8 groups
(n=10). Mice of groups 1–7 were administered 2.5, 5, 10, 20, 40, 80
or 100 mg/kg polydatin by intraperitoneal injection (i.p.),
respectively. Group 8 was administered normal saline (10 ml/kg,
i.p.). The mortality rate of the mice in each group was observed
during a 24 h period. Notably, neither death nor any abnormal
neurobehavior were observed, and thus the lethal dose
(LD)50 of polydatin was not obtained.
Preparation of ovariectomy mice and
experimental protocol
Osteoporosis was induced by ovariectomy (OVX)
surgery according to the method described by Kalu (14). After general anesthetic with 50
mg/kg, i.p. sodium pentobarbital, an incision was made through the
back and bilateral ovaries were resected. Mice undergoing sham
surgery mice underwent the same surgical procedures without ovary
resection.
The prepared OVX mice were randomly divided into an
OVX group, 3 polydatin treatment groups (10, 20 and 40 mg/kg) and
sham surgery group. The polydatin treatment lasted 12 weeks;
following treatment body weights of all the mice were recorded.
Subsequently, mice were sacrificed via cervical dislocation under
sodium pentobarbital (50 mg/kg, i.p.) anesthetic. Blood samples (~1
ml) were collected and centrifuged at 4°C at 3,000 × g for 10 min
to obtain serum for further experiments. Furthermore, the uterus
were collected and weighed immediately to calculate the uterine
index, and the thigh-bones of mice were collected to investigate
the dry weights.
Determination of serum ALP, calcium and
phosphorus
The serum ALP, calcium and phosphorus levels were
determined using the automatic biochemistry analyzer (Beckman
Coulter AU5800, Brea, CA, USA).
Determination of OPG in the serum
The serum OPG level was determined using a
commercial ELISA kit, according to the manufacturer's instructions
and was measured using a microplate reader (Multiskan FC, Thermo
Fisher Scientific, Inc.) at 450 nm.
Cell culture
The ST2 mouse bone marrow stromal cell line was
purchased from the American Type Culture Collection (Mannasas, VA,
USA). The cells were cultured in α-MEM medium which was
supplemented with 10% FBS, and the cells were cultured at 37°C in
5% CO2/95% air.
Western blotting
ST2 cells were treated with or without polydatin
(10, 20 and 40 µg/ml) for 48 h, and then were harvested.
Total proteins of cells were extracted using the western blot and
IP cell lysis buffer kit. After quantification of total protein
using the BCA protein assay reagent, 40 µg protein was
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, blotted on polyvinylidene difluoride membranes,
and probed with various primary antibodies, and subsequently with
horseradish-peroxidase-conjugated secondary antibody (Wuhan Boster
Bio-engineering Co., Ltd.) and detected by chemiluminescence with
BeyoECL Plus reagents (Beyotime Institute of Biotechnology,
Jiangsu, China). To measure protein loading, antibodies directed
against β-actin/histone were used.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using one-way analysis of
variance followed by Dunnett's multiple comparisons test using SPSS
software 18.0 (SPSS Inc., SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of polydatin on body weight and
uterine index of ovariectomized mice
As shown in Fig. 1,
after 12 weeks of treatment of polydatin, the ovariectomized mice
had a significantly increased body weight compared with the sham
mice (P<0.01). Notably, the increased weight of ovariectomized
mice was reversed following treatment with polydatin. Treatment of
polydatin at doses of 10, 20 and 40 mg/kg/day significantly
prevented the weight gain observed in ovariectomized mice
(P<0.05, P<0.01 and P<0.01, respectively). Furthermore,
this occurred in a dose-dependent manner.
The uterine index of the mice was decreased
following OVX surgery compared with that in the sham mice
(P<0.01). However, polydatin at doses of 20 and 40 mg/kg/day
increased the uterine index compared with mice in the OVX group
(P<0.01) (Fig. 2).
Effects of polydatin on serum calcium and
phosphorus levels in the ovariectomized mice
As shown in Fig. 3,
following OVX, the serum calcium and phosphorus levels of the mice
in the OVX group were decreased compared with that in the sham mice
(P<0.01). Notably, it was demonstrated that treatment with
polydatin (10, 20 and 40 mg/kg/day) increased calcium (P<0.05)
and phosphorus levels in the serum (P<0.05, P<0.01,
P<0.01, respectively) compared with the OVX group. This occurred
in a dose-dependent manner.
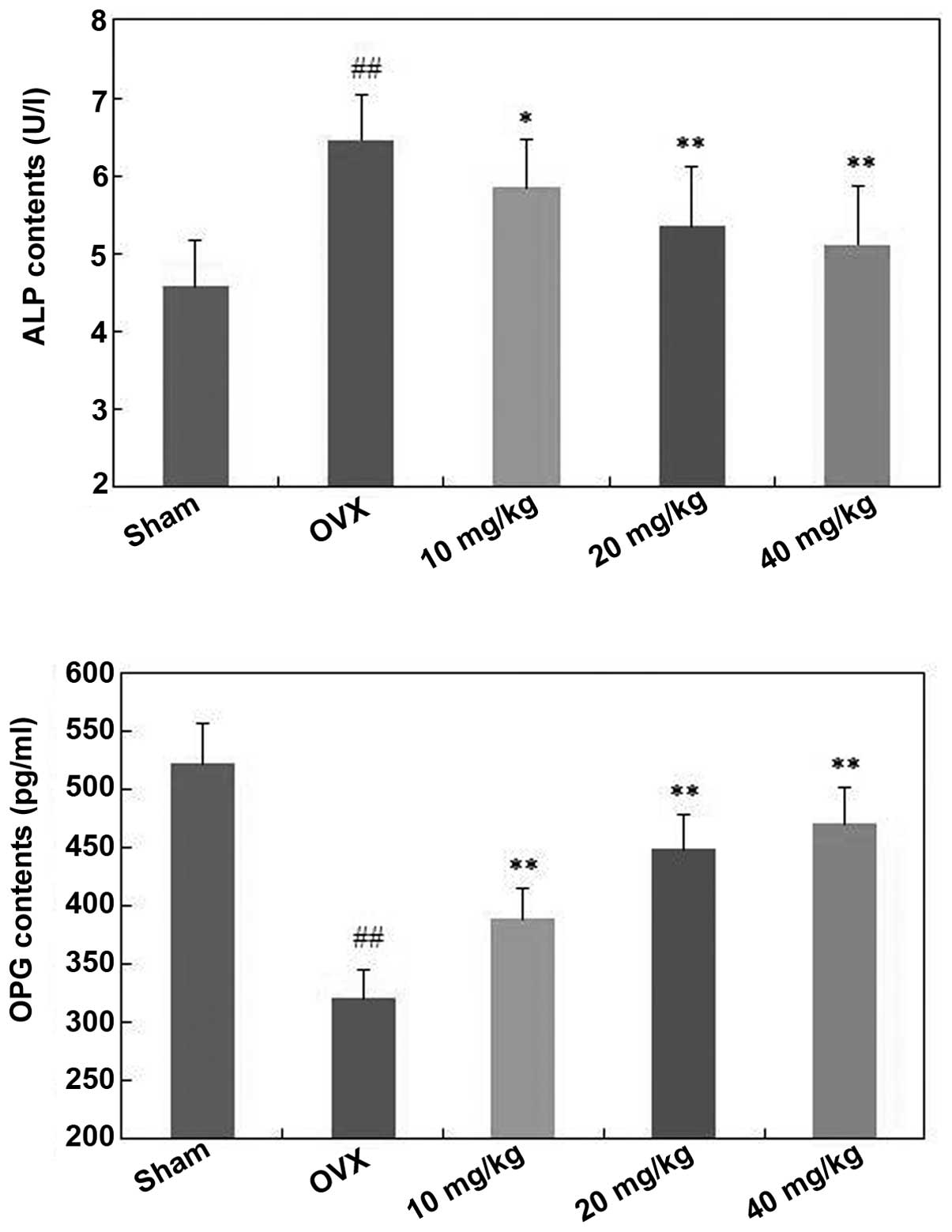
Effects of polydatin on ALP and OPG of
ovariectomized mice
As shown in Fig. 4,
ALP levels of ovariectomized mice were increased following OVX
surgery. Furthermore, polydatin at doses of 10, 20 and 40 mg/kg/day
significantly decreased the ALP level compared with the OVX group
(P<0.05, P<0.01 and P<0.01, respectively) in a
dose-dependent manner.
Conversely, the OPG levels in the mice of the OVX
group were significantly decreased compared with that in the sham
mice (P<0.01) (Fig. 4).
Treatment with polydatin (10, 20 and 40 mg/kg/day) increased the
OPG levels in the serum compared with the OVX group (P<0.01), in
a dose-dependent manner.
Effects of polydatin on thigh-bone weight
of OVX mice
As shown in Fig. 5,
thigh-bone weight of ovariectomized mice decreased following
removal of the ovaries compared with that in the sham group
(P<0.01). However, treatment with polydatin (10, 20 and 40
mg/kg) resulted in a significant elevation in thigh-bone weight
compared with that in the OVX group (P<0.05, P<0.01 and
P<0.01, respectively). This occurred in a dose-dependent
manner.
Effect of polydatin on OPG and RANKL
expression in ST2 cells
As shown in Fig. 6,
polydatin (10, 20 and 40 µg/ml) significantly increased the
protein expression of OPG in ST2 cells compared with control
(P<0.01) in a concentration-dependent manner. Conversely, RANKL
protein expression was downregulated by polydatin at a
concentration of 10, 20 and 40 µg/ml compared with the
control (P<0.01), this occurred in a concentration-dependent
manner. Consequently, the ratio of OPG/RANKL was upregulated
following treatment with polydatin.
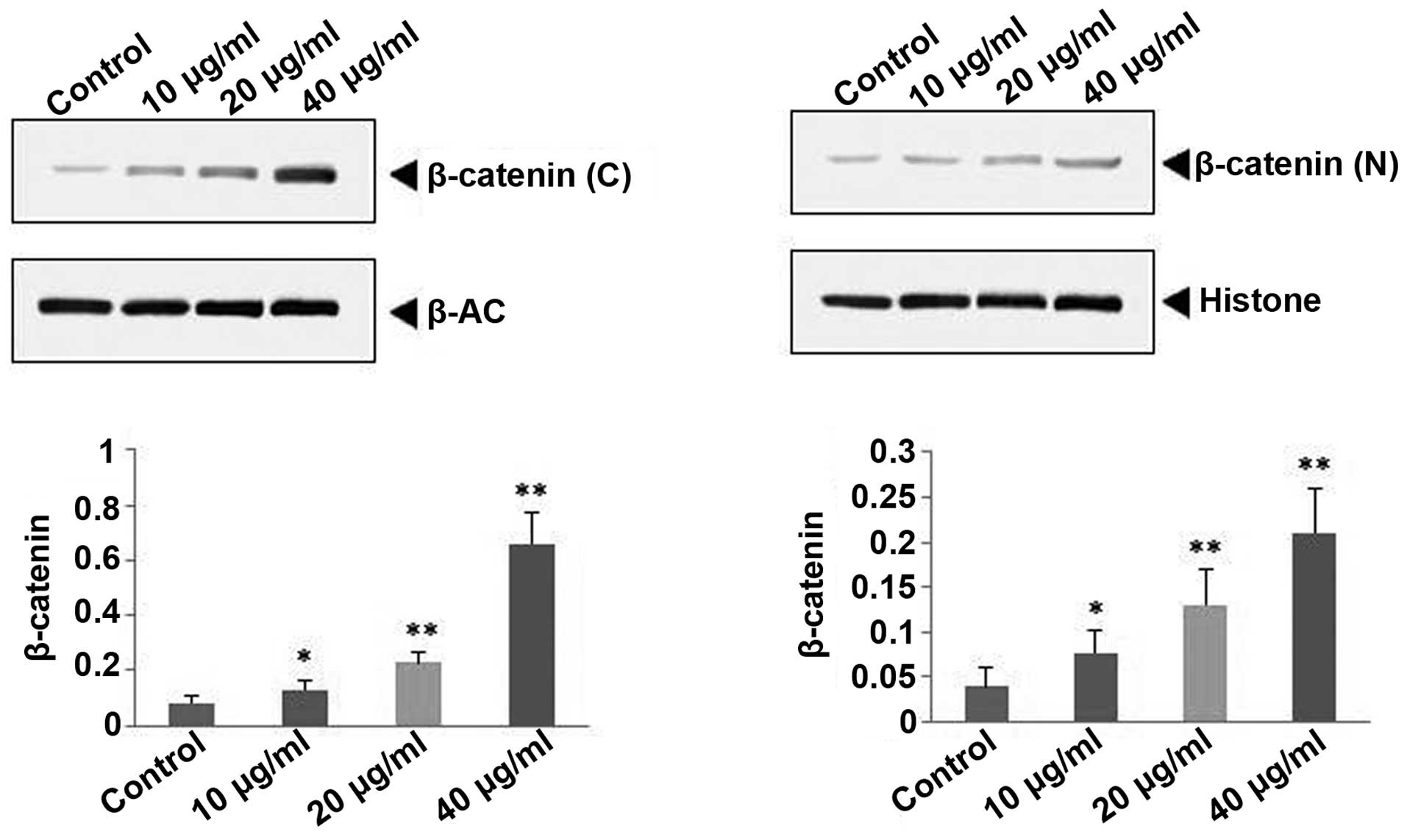
Effect of polydatin on β-catenin
expression in ST2 cells
As shown in Fig. 7,
10, 20 and 40 µg/ml polydatin significantly upregulated the
protein expression of OPG in the cytoplast and nucleus compared
with the control (P<0.05, P<0.01 and P<0.01,
respectively), this occurred in a concentration-dependent
manner.
Discussion
Increasing evidence has demonstrated that natural
plant-derived constituents/extracts are promising potential
resources for identifying effective candidate drugs. In addition,
natural compounds are reported to have few side effects and may be
candidates for treating various diseases (15–17).
To the best of our knowledge, this study was the first to
demonstrate that polydatin exhibited significant anti-osteoporotic
activity on OVX-induced osteoporosis in mice.
Osteoporosis is associated with ovarian hormone
deficiency following menopause, and is one of the most common
reasons for bone loss. The ovariectomy-induced osteoporotic mouse
model is a common and reliable animal model, which simulates the
clinical symptoms of postmenopausal osteoporosis in women (18,19).
It is reported that ovariectomy resulted in body weight increases
and uterus weight decreases (20).
In the present study, polydatin was shown to increase the uterine
index and decrease the body weight of mice that had undergone
ovariectomy, suggesting that polydatin could improve the symptoms
of osteoperosis. Bone loss can be reflected by the levels of
calcium and phosphorus in serum (4). ALP is an important enzyme for bone
remodeling, and the increase of ALP is another index for
osteoporosis (21). The results
showed significantly elevated calcium and phosphorus levels in the
serum of ovariectomized mice, and decreased levels of ALP. These
results indicated that polydatin possessed potential
anti-osteoporotic activity for treating ovariectomized mice. ST2
cells are mesenchymal stem cells that have the ability to
differentiate into osteoblast-like cells, and this cell line is
commonly used to explore the mechanism of anti-osteoporotic drugs.
The RANKL/OPG ratio is crucial in osteoclast formation and
differentiation, and bone resorption. RANKL induces osteoclast
differentiation via binding to RANK, and OPG can suppress
osteoclastogenesis and bone resorption via blocking the
communication between RANKL and RANK (4,19).
The present study showed that treatment with polydatin
significantly decreased the expression of RANKL, and increased the
expression of OPG, indicating that polydatin possessed the
potential to inhibit bone loss and resorption. The Wnt/β-catenin
pathway is also important in the generation of osteoblasts, and
bone development and remodeling. In addition, previous studies have
demonstrated that upregulation of the Wnt/β-catenin protein is a
possible strategy for treating osteoporosis (22,23).
The results of the present study also demonstrated that the
polydatin upregulates the expression of β-catenin proteins in the
cytoplast and nucleus of ST2 cells.
In conclusion, the present study suggested that
polydatin could alleviate the osteoporotic symptoms of OVX mice via
upregulating OPG and β-catenin and downregulating RANKL.
Furthermore, the present results may aid the development of
polydatin as an effective drug to treat osteoporosis in the
clinic.
References
|
1
|
Assessment of fracture risk and its
application to screening for postmenopausal osteoporosis. Report of
WHO study group. World Health Organ Tech Rep Ser. 843:1–129.
1994.
|
|
2
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao L, Qin L, Liu L, Wu Y, Han T, Xue L
and Zhang Q: Anthraquinone compounds from Morinda officinalis
inhibit osteoclastic bone resorption in vitro. Chem Biol Interact.
194:97–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Chen B, Pang G, Chen J, Xie J and
Huang H: Anti-osteoporotic activity of puerarin 6′-O-xyloside on
ovariectomized mice and its potential mechanism. Pharm Biol.
54:111–117. 2016. View Article : Google Scholar
|
|
5
|
Zhou ZX and Li LK: Research progress of
traditional Chinese medicine treatment on osteoporosis. Drug
Evaluation. 10:45–47. 2013.
|
|
6
|
Ma XQ, Zheng CJ, Zhang Y, Hu CL, Lin B, Fu
XY, Han LY, Xu LS, Rahman K and Qin LP: Antiosteoporotic flavonoids
from Podocarpium podocarpum. Phytochem Lett. 6:118–122. 2013.
View Article : Google Scholar
|
|
7
|
Du J, Wei YJ, Peng C, Ran X, Zhang H,
Jiang YP, Rahman K and Qin LP: Establishment of a luciferase
assay-based screening system for detecting estrogen receptor
agonists in plant extracts. Bone. 49:572–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng W, Qin R, Li X and Zhou H: Botany,
phytochemistry, pharmacology, and potential application of
Polygonum cuspidatum Sieb.: et Zucc: A review. J Ethnopharmacol.
148:729–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou T, Jiang W, Xu D, Chen T and Fu Y:
Inhibitory effects of polydatin on lipopolysaccharide-stimulated
RAW 264.7 cells. Inflammation. 38:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HL, Gao JP, Han YL, Xu X, Wu R, Gao Y
and Cui XH: Comparative studies of polydatin and resveratrol on
mutual transformation and antioxidative effect in vivo.
Phytomedicine. 22:553–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiyu S, Zhiyu L, Mao Y, Lin B, Lijia W,
Tianbao Z, Jie C and Tingyu L: Polydatin up-regulates Clara cell
secretory protein to suppress phospholipase A2 of lung induced by
LPS in vivo and in vitro. BMC Cell Biol. 12:312011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong M, Ding W, Liao Y, Liu Y, Yan D,
Zhang Y, Wang R, Zheng N, Liu S and Liu J: Polydatin prevents
hypertrophy in phenylephrine induced neonatal mouse cardiomyocytes
and pressure-overload mouse models. Eur J Pharmacol. 746:186–197.
2015. View Article : Google Scholar
|
|
13
|
Wu Y, Xue L, Du W, Huang B, Tang C, Liu C,
Qiu H and Jiang Q: Polydatin restore endothelium-dependent
relaxation in rat aorta rings impaired by high glucose: A novel
insight into the PPARβ-NO signaling pathway. PLoS One.
10:e01262492015. View Article : Google Scholar
|
|
14
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kinghorn AD, Chin YW and Swanson SM:
Discovery of natural product anticancer agents from biodiverse
organisms. Curr Opin Drug Discov Devel. 12:189–196. 2009.PubMed/NCBI
|
|
16
|
Bonifácio BV, dos Santos Ramos MA, da
Silva PB and Bauab TM: Antimicrobial activity of natural products
against Helicobacter pylori: A review. Ann Clin Microbiol
Antimicrob. 13:542014.PubMed/NCBI
|
|
17
|
Cheng YC: Opportunities for traditional
Chinese medicine to address unmet challenges in modern healthcare.
J Tradit Complement Med. 5:2–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riggs BL and Melton LJ III: Involutional
osteoporosis. New Engl J Med. 314:1676–1686. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue L, Jiao L, Wang Y, Nie Y, Han T, Jiang
Y, Rahman K, Zhang Q and Qin L: Effects and interaction of icariin,
curculigoside, and berberine in er-xian decoction, a traditional
Chinese medicinal formula, on osteoclastic bone resorption. Evid
Based Complement Alternat Med. 2012:4908432012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li F, Yang XL, Yang YN, Guo C, Zhang C,
Yang Z and Li P: Antiosteoporotic activity of echinacoside in
ovariectomized rats. Phytomedicine. 20:549–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Chen Q, Wang F and Zhang G:
Antiosteoporotic compounds from seeds of Cuscuta chinensis. J
Ethnopharmacol. 135:553–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong BC, Kim TS, Kim HS, Lee SH and Choi
Y: Transmembrane protein 64 reciprocally regulates osteoblast and
adipocyte differentiation by modulating Wnt/β-catenin signaling.
Bone. 78:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian J, Xu XJ, Shen L, Yang YP, Zhu R,
Shuai B, Zhu XW, Li CG, Ma C and Lv L: Association of serum Dkk-1
levels with β-catenin in patients with postmenopausal osteoporosis.
J Huazhong Univ Sci Technolog Med Sci. 35:212–218. 2015. View Article : Google Scholar : PubMed/NCBI
|