Introduction
Slit is a chemorepellent in axon guidance and
neuronal migration, and an inhibitor of leukocyte chemotaxis via
the Roundabout (Robo) receptor. In mammals, the Slit family
consists of three members: Slit homologue 1, 2 and 3. Slit2 is
widely expressed in various types of cancer in humans (1–3), and
the interaction of Slit2 with Robo1 induces tumor angiogenesis
(1). In addition, elevated
expression levels of Slit2 in RIP1-Tag2 transgenic mice enhances
tumor lymphangiogenesis and increases regional lymph node
metastasis, indicating that Slit2 is also a regulator of adult
lymphangiogenesis, and is a causal in Slit2-mediated
lymphangiogenesis in the dissemination of tumor cells (4). In our previous study, R5, an
anti-Robo1 monoclonal antibody, specifically inhibited the
Slit2-Robo1 pathway to inhibit tumor growth and angiogenesis in
human malignant melanoma A375 cells in mice and spontaneous tumors
in a hamster cheek pouch carcinoma model (1,5).
Oral tongue squamous cell carcinoma is the most
common type of malignancy diagnosed in the oral and maxillofacial
regions, and is characterized by a high degree of local
invasiveness and a high rate of metastasis to cervical lymph nodes
(6). A key step in the
infiltration and metastasis of oral tongue carcinoma is the
degradation of the basement membrane between the epithelium and
lamina propria, around cancer nests and the surrounding vascular
structures (7). High levels of
proteases extending, even to the basement membrane, is a key stage
in cancer invasion (8), as high
levels of proteases facilitate degradation of the basement membrane
and extracellular matrix (ECM), providing channels, which allow
tumor cells to migrate and metastasize into the vascular and
lymphatic systems (9).
Furthermore, the invasiveness is associated with the ability of
these proteases to degrade the basement membrane.
Matrix metalloproteinase 2 (MMP2) and MMP9 are
gelatinases, which primarily degrade collagen IV (COL IV), the
predominant component of the basement membrane and ECM, and that
are also involved in neovascularization (10). As COL IV is widely distributed in
tongue tissues, its physiological and pathological significance in
oral tongue carcinoma has attracted increasing attention. Another
molecule, E-cadherin, functions as a cell adhesion molecule in
adherent junctions (11). The loss
of E-cadherin leads to cell dissociation and the acquisition of a
migratory phenotype during development, tissue remodeling or
carcinogenesis (12,13).
However, whether Slit2 inhibits or promotes tumor
cell migration remains controversial, and the role of Slit2-Robo1
signaling in oral cancer remains to be fully elucidated. The aim of
the present study was to investigate the role of Slit2-Robo1
signaling in the adhesion, invasion and migration of tongue
carcinoma cells, and to determine the mechanism by which
Slit2-Robo1 signaling inhibits or promotes tumor cell migration.
The results suggested that Slit-Robo signaling is involved in the
development of tongue carcinoma. The effectiveness of Slit-Robo
signaling blockade in tongue carcinoma cells demonstrates a novel
target for tongue cancer therapy.
Materials and methods
Reagents, antibodies and cell
culture
Gelatin was purchased from Shanghai Shenggong
Biological Co., Ltd. (Shanghai, China). Fibronectin (FN) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The mouse
anti-human Robo1 antibody, R5, (a Slit-Robo signaling-specific
inhibitor), immunoglobuln (Ig)G2b control antibody and rabbit
anti-human Slit2 and Robo1 antibodies were all donated by Dr. Geng
Jianguo (University of Michigan, Ann Arbor, MI, USA). The Tca8113
cells (donated by Professor Wu Junzheng affiliated to The Forth
Military Medical University, Xi'an, Shanxi, China) were cultured in
RPMI 1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in 5%
CO2, and cells at the logarithmic growth phase were used
in the subsequent experiments.
Flow cytometry
Flow cytometry was performed to detect the protein
expression levels of Slit2 and Robo1 in the Tca8113 cells. The
cells (1×106) were harvested and centrifuged at 188 × g
at 25°C for 5 min. Following centrifugation, the cells were
resuspended in RPMI 1640. The cell suspensions (40 µl) were
incubated with 10 µg/ml mouse anti-human Slit2 antibody,
rabbit anti-human Robo1 R5 antibody or IgG2b, and 10 µl
normal mouse or rabbit serum at room temperature for 20 min. The
cells were centrifuged at 1,000 rpm for 5 min and washed and
resuspended in phosphate-buffered saline (PBS) containing 0.1%
NaN3 and 5% FBS. The resuspended cells were incubated
with fluorescein isothiocyanate (FITC)-goat anti-mouse IgG (cat.
no. BA101) or FITC-goat anti-rabbit IgG secondary antibodies (cat.
no. A1105) (1:100; Boster Biological Engineering Co., Ltd., Wuhan,
China) at room temperature for 1 h, centrifuged at 188 × g for 5
min, and washed and resuspended in PBS twice. Subsequently, 5,000
stained cells were analyzed using an ELITE ESP flow cytometer
(Beckman Coulter, Carlsbad, CA, USA).
In vitro adhesion assay
The wells of 24-well plates were coated with 6.25
mg/l Matrigel (1 ml/well; BD Biosciences, San Jose, CA, USA) and
incubated with RPMI 1640 media containing 1 g/l bovine serum
albumin (BSA; Beyotime Institute of Biotechnology, Shanghai, China)
at 37°C for 1 h to block nonspecific binding sites. The cells were
collected, centrifuged at 188 × g for 5 min, resuspended to
1×105 cells/ml in RPMI 1640 media containing 0.1% BSA,
and incubated at 37°C for 30 min to reconstitute surface proteins.
Aliquots of 2×105 Tca8113 cells were seeded into
individual Matrigel-coated wells and incubated at 37°C in 5%
CO2 for 60 min. The wells were then gently washed three
times with PBS, and the attached cells were harvested and counted
using an Axio Observer A1/Axio Cam, PH (Carl Zeiss AG, Oberkochen,
Germany). The experiment was performed in triplicate.
Cell migration assay
Briefly, 96-well tissue culture plates were coated
with 50 µl of 10 mg/l FN overnight. The Tca8113 cells (100
µl; 1×105/ml) in the logarithmic growth phase
were seeded onto the 96-well plates and grown until confluent. The
confluent cell monolayer was scratched with a probe, and the
original medium was removed following scratching. The scratched
plates were lightly washed with RPMI 1640 serum-free media. Then
samples of media containing 1% FBS, 1% BSA, and 0.1, 1.0 or 10.0
µg/ml R5 or 10 µg/ml IgG2b were added to the 96-well
plates. The widths of the scratches were recorded immediately and
following a 24 h period under an inverted microscope (PH, Axio
Observer A1) at 37°C. The distance of cell migration was determined
as follows: Migration distance = cell-free area at 0 h - cell-free
area at 24 h. The experiments were repeated three times.
Chemotaxis assay
A solution of 1×105/ml Tca8113 cells (200
µl free FBS and RPMI 1640 media) in the logarithmic growth
phase were plated onto each Transwell chamber (BD Biosciences),
following which 10.0 µg/ml R5 or IgG2b was added. The lower
chambers were filled with 400 µl RPMI 1640 media containing
0.1% BSA and 16 µg FN, which was used as a chemoattractant.
After 24 h in continuous culture 37°C, the Transwell chambers were
removed, and the cells in the upper chambers were fixed with 95%
ethanol, stained with Giemsa (Beyotime Institute of Biotechnology,
Shanghai, China), and counted in 10 microscopic fields
(magnification, ×200) per chamber. Each assay was repeated in
triplicate, and the invasion inhibitory rate was calculated as
follows: Inhibitory rate (%) = (1 - cells in R5-treated group /
cells in IgG2b-treated group) × 100%.
Gelatin zymography
The Tca8113 cells (1×105 cells/ml in T-25
flasks) in the logarithmic growth phase were cultured for 24 h. The
medium was removed, and the cells were washed with serum-free RPMI
1640 medium. Subsequently, 3 ml serum-free media containing 0.1,
1.0 or 10.0 µg/ml R5 or 10.0 µg/ml IgG2b was added to
each flask. After 48 h of routine culture, the supernatants were
collected and centrifuged at 3,000 × g at room temperature for 10
min. The clarified supernatants were concentrated using 10-KDa
molecular weight cut off Amicon Ultra Centrifugal Filter Units (EMD
Millipore, Billerica, MA, USA) and quantified using a bicinchoninic
acid protein assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA). A total of 20 µg protein was loaded onto a
gelatin-containing gel (8% acrylamide gel containing 1.5 mg/ml
gelatin; Beyotime Institute of Biotechnology) and separated by
electrophoresis. Subsequently, the gel was washed with 2.5%
Tween-20 solution (Sigma-Aldrich) in PBS (TPBS) and developed at
37°C in zymogram incubation buffer (Beyotime Institute of
Biotechnology), containing 50 mM Tris-HCL and 5 mM CaCl2
(pH 7.6) overnight. Staining was then performed using 0.25%
Coomassie blue R250 solution (Beyotime Institute of Biotechnology),
and destained with 50% methanol and 10% acetic acid (both from
Sigma-Aldrich) until the membrane degraded by MMP2 or MMP9 became
clear.
Western blotting
The Tca8113 cells were treated with 0.1, 1.0 or 10.0
µg/ml R5, or were mock-treated with 10.0 µg/ml IgG2b,
and routinely cultured for 48 h. Subsequently, the cells were lysed
with ice-cold lysis buffer (Cell Signaling Technology, Inc.,
Danvers, MA, USA), containing 50 mM Tris-HCl (pH 7.6), 150 mM NaCL,
1 mM DTT, 10 mM NaF and 2 mM Na3VO4,
containing 0.1% sodium dodecyl sulfate (SDS) and 1X complete
protease inhibitor cocktail (Sigma-Aldrich) for 30 min. This was
followed by centrifugation at 4°C and 13,800 × g for 15 min. The
total protein in the supernatant was determined using Bradford
protein assay reagent (Bio-Rad Laboratories, Inc., Hercules, USA).
Finally, 20 µg of the cell extracts were separated by 12%
SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto
nitrocellulose membranes (GE Healthcare Life Sciences), which were
blocked with 5% skim milk/TPBS (0.1% Tween-20) for 3 h and
incubated with rabbit anti-human E-cadherin (1:1,000; cat. no.
BA0474) or mouse anti-rabbit actin (cat. no. BM0627) (1:200; Boster
Biological Engineering Co., Ltd.) at 25°C for 1 h. The membrane was
washed with Tris-buffered saline (Sigma-Aldrich) twice, each for 15
min, incubated (25°C for 1 h) with horseradish
peroxidase-conjugated goat anti-rabbit and goat anti-mouse
secondary antibodies (cat. nos. BA1055 and BA1051; Boster
Biological Engineering Co., Ltd.), and developed using ECL Plus
chemiluminescent reagents (GE Healtcare Life Sciences, USA). The
relative intensities of the E-cadherin-specific bands of the
R5-treated samples were digitalized and compared with those of the
mock-treated samples using Quantity One software 4.6.2 (Bio-Rad
Laboratories, Inc.), with β-actin used as a loading control.
Statistical analysis
The data were analyzed using Student's t-test and a
χ2 test, using a computer-based SPSS 13.0 software
program (SPSS, Inc., Chicago, IL, USA). The results are expressed
as the mean ± standard deviation. For all analyses, P<0.05 was
considered to indicate a statistically significant difference.
Results
Protein expression levels of Slit2 and
Robo1 in Tca8113 cells
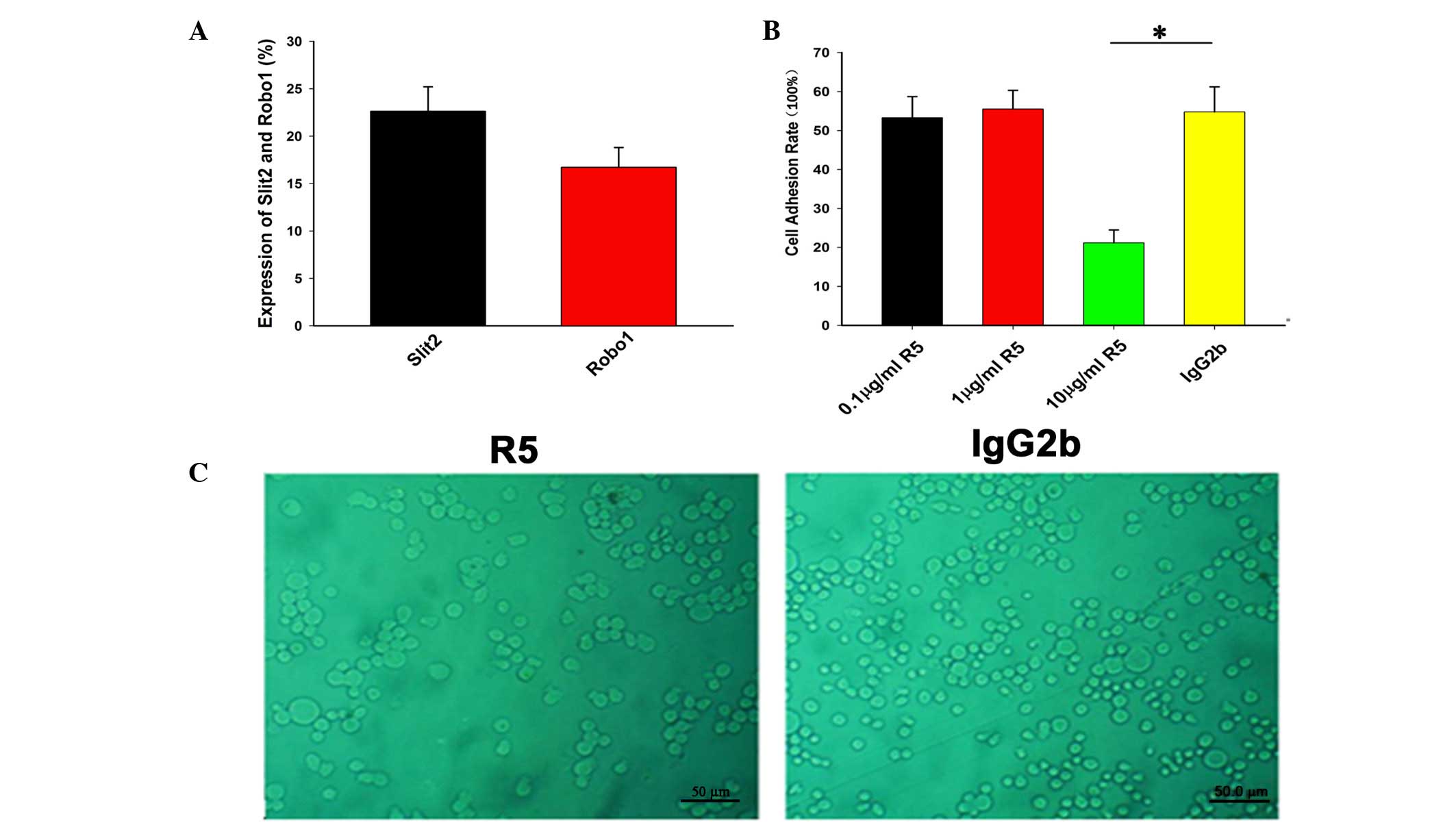
Flow cytometry was used to detect and quantify the
intracellular protein expression levels of Slit2 and Robo1. The
percentages of Slit2-positive and Robo1-positive cells were
22.6±2.6 and 16.7±2.1%, respectively. These results demonstrated
that Slit2 and Robo1 were expressed in the Tca8113 cells (Fig. 1A).
Effect of R5 on Tca8113 cell attachment
to FN
The present study found that R5 inhibited the
attachment of Tca8113 cells to FN. The attachment rate of the
Tca8113 cells treated with 10.0 µg/ml R5 (21.2±3.3%) was
significantly lower, compared with that of the mock-treated Tca8113
cells (54.8±6.4%; P<0.05; Fig.
1).
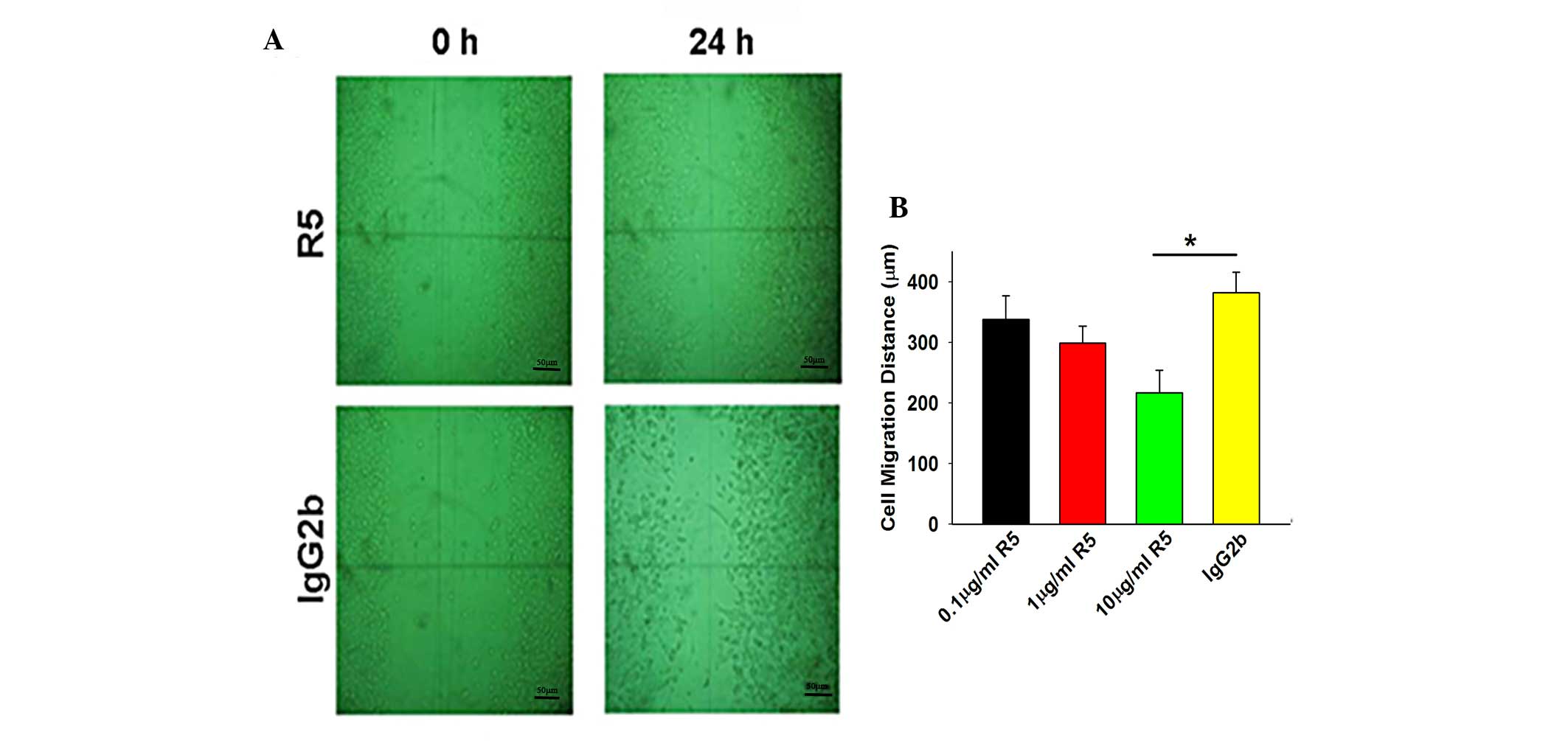
Effect of R5 on Tca8113 cell
migration
An in vitro scratch assay was used to
investigate the migration of the cancer cells on the artificial
basement membrane, Matrigel. The Tca8113 cells were either treated
with R5 at different concentrations or were mock-treated with
IgG2b, and allowed to grow for 24 h under routine conditions,
followed by the introduction of a scratch to the cell monolayer.
The migration distance of the Tca8113 cells treated with 10.0
µg/ml R5 (217±37 µm) was significantly lower,
compared with that of the mock-treated Tca8113 cells (382±34
µm; P<0.05; Fig. 2). The
migration distances of the Tca8113 cells treated with 10.0
µg/ml R5 were significantly lower, compared with those of
the IgG2b-treated group.
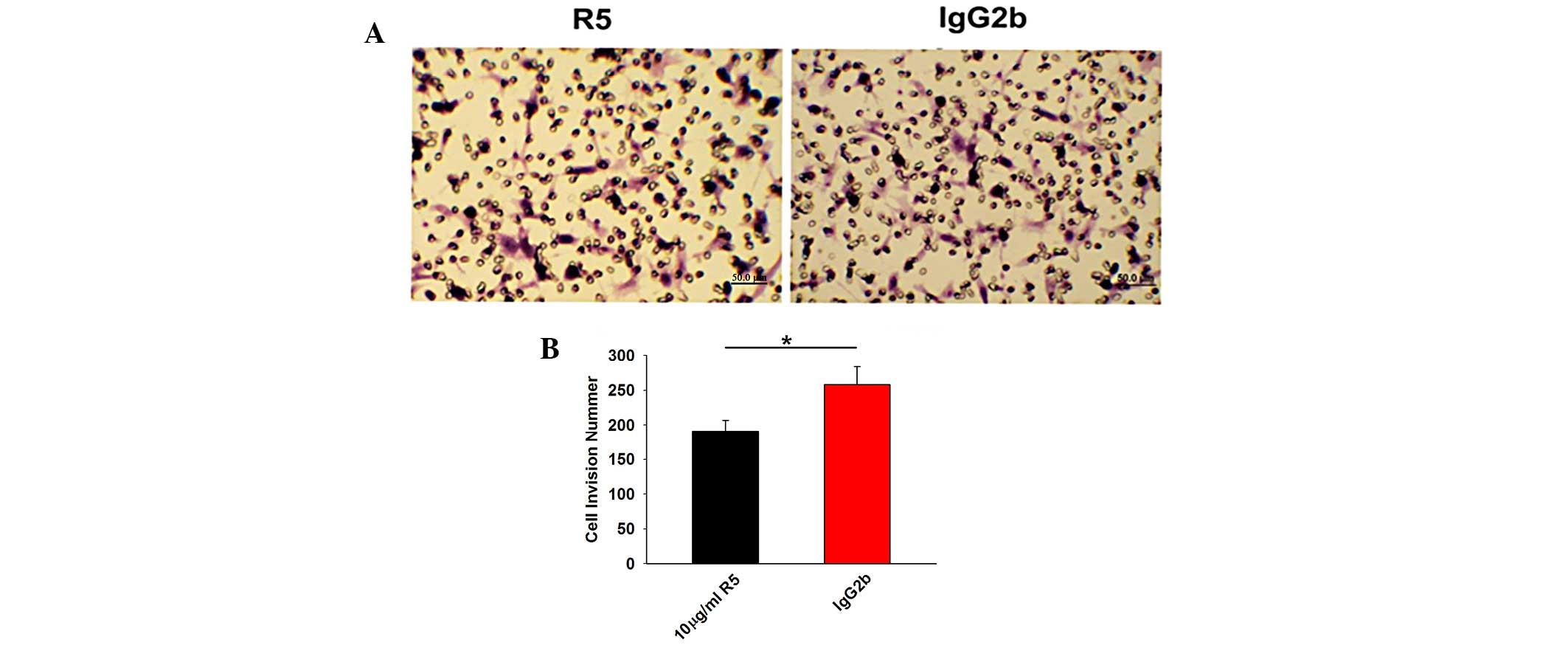
Effect of R5 on the chemotaxis of Tca8113
cells
The recovery of the scratched area in the Transwell
chambers was examined to assess the chemotaxis of the Tca8113 cells
treated with 10.0 µg/ml R5 or IgG2b. The invasion inhibitory
rate of the R5-treated Tca8113 cells (24.67±0.03%) was
significantly lower, compared with that of the mock-treated Tca8113
cells (33.21±0.07%; P<0.05; Fig.
3).
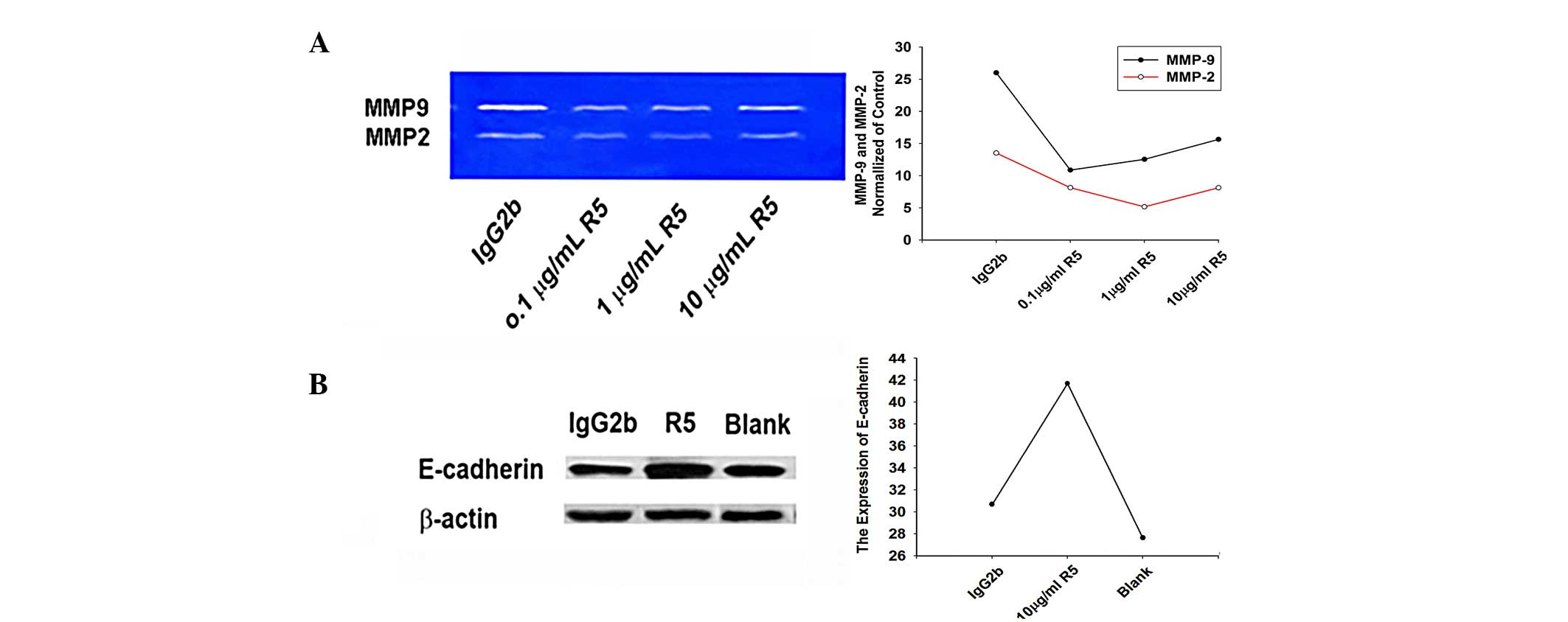
R5 increases the activities of MMP2 and
MMP9 in Tca8113 cells
The supernatants of the Tca8113 cells, following
treatment with 0.1, 1.0 or 10.0 µg/ml R5 or mock treatment
with 10.0 µg/ml IgG2b, were analyzed using
gelatin-incorporated SDS-PAGE to examine the activities of MMP2 and
MMP9 in the cultured tumor cells. The results showed that treatment
with 0.1, 1.0 or 10.0 µg/ml R5 significantly inhibited the
activities of MMP2 (72 KDa) and MMP9 (92 KDa) in the Tca8113 cells
(Fig. 4).
Effect of R5 on the expression of
E-cadherin in Tca8113 cells
The Tca8113 cells were treated with 0.1, 1, or 10.0
µg/ml R5, or mock-treated with 10.0 µg/ml IgG2b, and
routinely cultured for another 48 h. The results of the western
blotting showed that the expression of E-cadherin in the Tca8113
cells treated with R5 was significantly higher, compared with that
in the mock-treated Tca8113 cells (P<0.05; Fig. 4).
Discussion
The present study aimed to investigate the role of
Slit2-Robo1 signaling in the adhesion, invasion and migration of
tongue carcinoma cells, and examine the mechanism by which
Slit2-Robo1 signaling inhibits or promotes tongue carcinoma cell
migration. The monoclonal anti-Robo1 antibody, R5, was used to
inhibit Slit2-Robo1 signaling, following which changes in cell
invasion and migration, as well as the expression levels of MMP2,
MMP9 and E-cadherin were examined in Tca8113 tongue carcinoma
cells. It was found that R5 inhibited cell adhesion, invasion and
migration and significantly reduced the expression levels of Slit2,
Robo1, MMP2 and MMP9 in the Tca8113 cells, but increased the
expression of E-cadherin.
The present study also found that R5 significantly
inhibited the ability of the Tca8113 cells to attach to FN and
invade the scratched area in vitro, compared with the
mock-treated tongue carcinoma cells, indicating that R5 was capable
of inhibiting the adhesion of the cancerous cells to the ECM. Of
note, adhesion is important in cancer cell invasion and migration,
as adhesion is the initiating step in cancer cell invasion, which
includes a series of adhesion and de-adhesion processes involving
certain ECM and basement membrane components, including FN. Cancer
cells secrete degrading enzymes, including MMP2 and MMP3, to
degrade the matrix following adherence to the matrix, leading to
cancer cell migration into the area via chemotaxis (14).
The present study found that R5 significantly
inhibited Tca8113 cell chemotaxis and migration on the FN-coated
matrix in vitro. In addition to the ability to adhere to
certain matrix components, active cell migration is an important
activity in tumor invasion and metastasis. Cancer cells with high
levels of invasiveness often also exhibit high levels of movement.
Components of the ECM, including FN and certain growth factors in
the ECM exert a chemotactic induction effect on tumor cell
directional migration. For example, Slit2 has been shown to be
associated with angiogenesis, which is important in cancer
metastasis (15).
To delineate the mechanism of Slit2-Robo1-induced
migration in the tongue cancer cells, the enzyme activities of MMP2
and MMP9 were examined using a substrate-specific
gelatin-zymography assay. The resulting data indicated that the
activities of MMP2 and MMP9 were significantly decreased in the
Tca8113 cells treated with R5 for 48 h in vitro, with more
pronounced inhibition of MMP9 activity, suggesting that inhibiting
Slit2-Robo1 signaling upregulated MMP2 and MMP9. MMPs are a class
of proteolytic enzymes, which are closely associated with tumor
invasion and metastasis. Among all MMPs, MMP2 and MMP9 are the most
important types for protease hydrolysis in the degradation of ECM,
particularly COL IV in the cell basement membrane, and are
important in cancer cell invasion and metastasis (14,15).
The present study found that R5 significantly
increased the protein expression of E-cadherin in the Tca8113
cells, as shown by western blot analysis, indicating that
inhibiting Slit2-Robo1 signaling upregulated the expression of
E-cadherin. E-cadherin is a critical adhesion molecule, and its
downregulation or dysfunction causes cancer cells to lose the
ability to adhere to each other and to migrate to regional lymph
nodes or other remote sites (16).
The knockdown of endogenous Robo1 or the specific inhibition of
Slit2 binding to Robo1 prevents E-cadherin degradation and reverses
the epithelial-mesenchymal transition, resulting in reduced tumor
growth and liver metastasis (17).
In conclusion, the results of the current study have
established that Slit-Robo signaling is important in adhesion,
invasion and migration of tongue carcinoma cells. These results
suggested that Slit2-Robo1 signaling promoted the adhesion,
invasion and migration of tongue carcinoma cells by upregulating
the expression levels of MMP2 and MMP9, and downregulating the
expression of E-cadherin. Slit-Robo signaling may therefore be a
novel target for tongue cancer therapy.
Acknowledgments
The present study was supported by the Fundamental
Research Funds for the Central Universities, China (grant no.
1007RJYA009); the LanZhou University Ji Ben Ke Yan (lzujbky-2015-36
and lzujbky-2015-290); and the National Natural Science Foundation
of China (grant no. 81500835).
References
|
1
|
Wang B, Xiao Y, Ding BB, Zhang N, Yuan Xb,
Gui L, Qian KX, Duan S, Chen Z, Rao Y and Geng JG: Induction of
tumor angiogenesis by Slit-Robo signaling and inhibition of cancer
growth by blocking Robo activity. Cancer Cell. 4:19–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai CF, Jiang YZ, Li Y, Wang K, Liu PS,
Patankar MS and Zheng J: Expression and roles of Slit/Robo in human
ovarian cancer. Histochem Cell Biol. 135:475–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Cheng H, Xu W, Tian X, Li X and Zhu
C: Expression of Robo protein in bladder cancer tissues and its
effect on the growth of cancer cells by blocking Robo protein. Int
J Clin Exp Pathol. 8:9932–9940. 2015.PubMed/NCBI
|
|
4
|
Yang XM, Han HX, Sui F, Dai YM, Chen M and
Geng JG: Slit-Robo signaling mediates lymphangiogenesis and
promotes tumor lymphatic metastasis. Biochem Biophys Res Commun.
396:571–577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang LJ, Zhao Y, Han B, Ma YG, Zhang J,
Yang DM, Mao JW, Tang FT, Li WD, Yang Y, et al: Targeting
Slit-Roundabout signaling inhibits tumor angiogenesis in
chemical-induced squamous cell carcinogenesis. Cancer Sci.
99:510–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byers RM, El-Naggar AK, Lee YY, Rao B,
Fornage B, Terry NH, Sample D, Hankins P, Smith TL and Wolf PJ: Can
we detect or predict the presence of occult nodal metastases in
patients with squamous carcinoma of the oral tongue? Head Neck.
20:138–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garamszegi N, Garamszegi SP,
Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL and
Scully SP: Extracellular matrix-induced transforming growth
factor-beta receptor signaling dynamics. Oncogene. 29:2368–2380.
2012. View Article : Google Scholar
|
|
8
|
Rowe RG and Weiss SJ: Breaching the
basement membrane: Who, when and how? Trends Cell Biol. 18:560–574.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: Changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar
|
|
10
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, Hirsch DS, Sasiela CA and Wu WJ:
Cdc42 regulates E-cadherin ubiquitination and degradation through
an epidermal growth factor receptor to Src-mediated pathway. J Biol
Chem. 283:5127–5137. 2008. View Article : Google Scholar
|
|
13
|
Weng W, Yin J, Zhang Y, Qiu J and Wang X:
Metastasis-associated protein 1 promotes tumor invasion by
downregulation of E-cadherin. Int J Oncol. 44:812–818.
2014.PubMed/NCBI
|
|
14
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
15
|
Guo SW, Zheng Y, Lu Y, Liu X and Geng JG:
Slit2 overexpression results in increased microvessel density and
lesion size in mice with induced endometriosis. Reprod Sci.
20:285–298. 2013. View Article : Google Scholar
|
|
16
|
Masterson J and O'Dea S: Posttranslational
truncation of E-cadherin and significance for tumour progression.
Cells Tissues Organs. 185:175–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF,
Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ and Geng JG: Slit-Robo
signaling induces malignant transformation through Hakai-mediated
E-cadherin degradation during colorectal epithelial cell
carcinogenesis. Cell Res. 21:609–626. 2011. View Article : Google Scholar : PubMed/NCBI
|