Introduction
Pancreatic cancer is the fourth leading cause of
cancer-associated mortality worldwide and has an overall 5-year
survival rate of <5% (1). In
90% of patients, the disease is surgically unresectable at
diagnosis, and the majority of patients who undergo resection for
localized lesions develop recurrent or metastatic disease (2). Therefore, it is important to develop
novel therapeutic strategies in order to combat resistance and
metastasis.
Cancer stem cells (CSCs) are cells that have the
capacity to generate the heterogeneous cancer cell lineages found
in tumors and possess the capacity for self-renewal (3). Other important attributes of CSCs
include active telomerase expression, resistance to harmful
drugs/agents, activation of anti-apoptotic pathways, the ability to
migrate and to metastasize, and increased membrane transporter
activity (3). It has been
demonstrated that solid tumors contain a subpopulation of CSCs
(4), which cannot be eradicated by
current therapeutic strategies and are responsible for tumor
relapse and metastasis (5). In
pancreatic cancer, there are typically 0.2–0.8% CSCs, which are
considered responsible for tumor growth, invasion, metastasis and
recurrence (6). In particular,
CD44, CD24 and epithelial specific antigen (ESA) have been used as
separation markers to identify populations of pancreatic CSCs
(7). However, reports on treatment
targeting CSCs in pancreatic cancer are limited.
Hedgehog (Hh) signaling is key in CSC biology and
may be used a potential therapeutic target in pancreatic cancer
(8,9). A previous study highlighted the
involvement of the Hh signaling pathway in pancreatic
tumorigenesis; inhibition of the pathway and pluripotency
maintaining factors regulated human pancreatic cancer stem cell
characteristics (10).
Furthermore, it has been reported that pharmacological blockade of
aberrant Hh signaling may prove to be an effective therapeutic
strategy for the inhibition of systemic metastases in pancreatic
cancer, likely via targeting CSCs (11).
Bufalin is a toad poison ligand extracted from toad
cake, which is a type of traditional Chinese medicine (12). Bufalin has been shown to be a
potential anticancer agent in various cancer models (13–16).
However, the effects of bufalin on CSCs have not been examined in a
model of pancreatic cancer. The present study aimed to evaluate the
effects of bufalin on pancreatic CSCs in vivo and in
vitro.
Materials and methods
Reagents and antibodies
Bufalin and dimethyl sulfoxide were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Rabbit anti-CD44 polyclonal
antibody (1:1,000; cat. no. BS6825) and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (1:10,000; cat. no. BS13287)
were purchased from Bioworld Technology (Minneapolis, MN, USA).
Rabbit anti-ESA polyclonal antibody (1:1,000; cat. no. 21050-1-AP)
was purchased from Proteintech Group, Inc. (Wuhan, China).
Phycoerythrin-conjugated mouse anti-CD44 monoclonal antibody
(1:100; cat. no. 130-095-180), allophycocyanin-conjugated mouse
anti-CD24 monoclonal antibody (1:100; cat. no. 130-095-954), and
fluorescein isothiocyanate (FITC)-conjugated mouse anti-ESA
monoclonal antibody (1:100; cat. no. 130-080-301) for flow
cytometry were purchased from Miltenyi Biotec GmbH (Bergisch
Glad-bach, Germany). Rabbit anti-Patched (PTCH)1 monoclonal
antibody (1:1,000; cat. no. 2468), PTCH2 polyclonal antibody
(1:1,000; cat. no. 2464), glioma-associated oncogene 1 (Gli1)
monoclonal antibody (1:1,000; cat. no. 3538) and GAPDH monoclonal
antibody (1:1,000; cat. no. 8884) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
Cell culture and serum-free suspension
culture for enrichment of pancreatic CSCs
The human pancreatic cancer gemcitabine-resistant
cell line, MiaPaCa2/GEM, was provided by Professor Yingyi Li from
The Cancer Research Institute, Fudan University Shanghai Cancer
Center (Shanghai, China). The MiaPaCa2/GEM cell line was cultured
in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 5% CO2 at 37°C. For CSCs, the MiaPaCa2/GEM
cells were washed three times for 5 min each with
phosphate-buffered saline (PBS) repeatedly, after which the cells
(2×103) were resuspended in tumor stem medium consisting
of serum-free neural stem cell medium, epidermal growth factor (20
ng/ml), basic fibroblast growth factor (20 ng/ml), leukemia
inhibitory factor (1,000 U/ml), and 15% knockout serum replacement
(Gibco; Thermo Fisher Scientific, Inc.) and cultured in an
ultra-low adhesion dish.
Cell proliferation array and colony
formation assay
Cell proliferation analysis was performed, as
described previously (17).
Briefly, the cells were plated at a density of 5,000 cells per well
in 96-well microtiter plates, and incubated overnight at 37°C in a
humidified incubator containing 5% CO2. On the following
day, various concentrations of bufalin (10, 50, 100, 500 or 1,000
nM) were added to the wells, and the cultures were incubated for an
additional 24, 48 or 72 h at 37°C. Cell viability was determined
using a Cell Counting Kit-8 (Dojindo, Gaithersburg, MD, USA),
according to the manufacturer's protocol. For the colony formation
assays, 2×103 MiaPaCa2/GEM cells or sphere cells were
plated in 6-well plates and cultured with 10% FBS DMEM. The culture
medium was replaced every 3 days. After 2 weeks, the colonies were
fixed with 4% paraformaldehyde and stained with 0.1% crystal violet
(both Sangon Biotech Co., Ltd., Shanghai, China), and images of the
colonies were captured using the Epson Perfection V600 Photo
scanner digital camera (Seiko Epson Corporation, Suwa, Japan).
Flow cytometry
The cell suspension was centrifuged at 300 × g for
10 min at 4°C and the cells (1×107 nucleated cells) were
resuspended in 100 µl PBS. Subsequently, 10 µl
primary antibody was added, followed by mixing and incubation for
10 min in the dark at 4°C. The cells were washed by adding 1–2 ml
buffer and centrifuging at 300 × g for 10 min. The cell pellet was
then resuspended in 500 µl PBS for analysis by flow
cytometry (Cytomics FC 500 Flow Cytometer; Beckman Coulter, Inc.,
Brea, CA, USA).
Western blot analysis
The cells were washed with cold PBS and lysed in
culture dishes using PhosphoSafe™ extraction reagent (Merck
Millipore, Darmstadt, Germany) containing 1% protease inhibitor
cocktail (EDTA-free; Thermo Fisher Scientific, Inc.). Protein
concentrations were then determined using Bio-Rad
detergent-compatible protein assays (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A total of 30 µg protein from the
control and treated cell lysates, respectively were loaded onto
8–12% gradient NuPAGE gels (Novex, San Diego, CA, USA), followed by
electrophoresis under reducing conditions and subsequent transfer
onto polyvinylidene difluoride membranes (0.22 µm; Merck
Millipore). Western blot analysis was performed, as described
previously (17). Briefly, the
membranes were incubated overnight at 4°C with anti-CD44, anti-ESA,
anti-PTCH1, anti-PTCH2, anti-Gli1 and anti-GAPDH primary
antibodies, followed by incubation with HRP-conjugated goat
anti-rabbit IgG. Blots were then developed with the SuperSignal
West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific,
Inc.) on a LAS-3000 Imaging system (Fujifilm, Tokyo, Japan). Band
intensities were analyzed using Quantity One 4.6.6 software
(Bio-Rad Laboratories, Inc.)
Animal models and treatment
Female 6-week-old BALBc nu/nu mice were obtained
from the Shanghai Institute of Material Medica, Chinese Academy of
Science (Shanghai, China). All mice were bred in laminar flow
cabinets under pathogen-free conditions. The mice were housed
individually in plastic cages at 25°C and under a 12-h light/dark
cycle, with ad libitum access to food and water. The present
study was performed in accordance with internationally recognized
guidelines on animal welfare (http://www.aaalac.org/resources/theguide.cfm). The
study design was approved by the Animal Ethical Committee of Fudan
University (Shanghai, China).
The MiaPaCa2/GEM cells (2×105) were
subcutaneously inoculated into the right flanks of the 6-week-old
BALBc nu/nu female mice, and treatment commenced the following day.
The mice were randomly separated into two groups, with six mice in
each group. The mice in the experimental group received
intraperitoneal injections of 1.5 mg/kg bufalin (5 days/week),
whereas the control mice were injected with vehicle (20 µl
saline) alone. Treatment continued for 4 weeks, after which the
mice were sacrificed by overdose with anesthesia (4 ml/kg body
weight of 10% chloral hydrate), and tumors were excised from each
mouse, weighed and snap-frozen for further analysis.
In addition, mice were randomly divided into another
two groups, with six mice in each group, in which MiaPaCa2/GEM
cells (2×106/0.2 ml PBS), which were pretreated with
bufalin in one of the groups, were injected via the tail veins of
the female mice. The mice were sacrificed after 6 weeks. The
intestinal tissues and lung tissues were excised from each mouse
for further analysis.
Immunohistochemistry
Immunohistochemical analysis was performed, as
described previously (18).
Briefly, the tumor sections were stained with rabbit anti-ESA at
4°C overnight. Goat anti-rabbit or mouse IgG/HRP was applied as the
secondary antibody, according to the standard protocols provided by
the manufacturer. For negative controls, the primary antibodies
were replaced with PBS. Immunopositivity was assessed by two
independent investigators, who were blinded to the model/treatment
type for the series of experiments. The tumor sections were
counterstained for 1 min with hematoxylin.
Immunofluorescence
The tumor sections were stained with rabbit
anti-CD24 (1:50) at 4°C overnight. Following washing with PBS, the
tumor sections were subsequently incubated with
fluorescence-conjugated secondary antibody for 2 h at 37°C. The
sections were then mounted with mounting medium containing DAPI
(Vector Laboratories). For negative controls, primary antibodies
were replaced with PBS. Images were captured using a confocal
fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
All analyses of results were performed using
GraphPad prism version 5.0 (GraphPad Software, San Diego, CA, USA)
and the SPSS 19.0 software package (IBM SPSS, Armonk, NY, USA).
Statistical analyses were performed using χ2 tests,
Student's t-test and one-way analysis of variance models.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Enrichment of pancreatic CSCs using
serum-free suspension culture
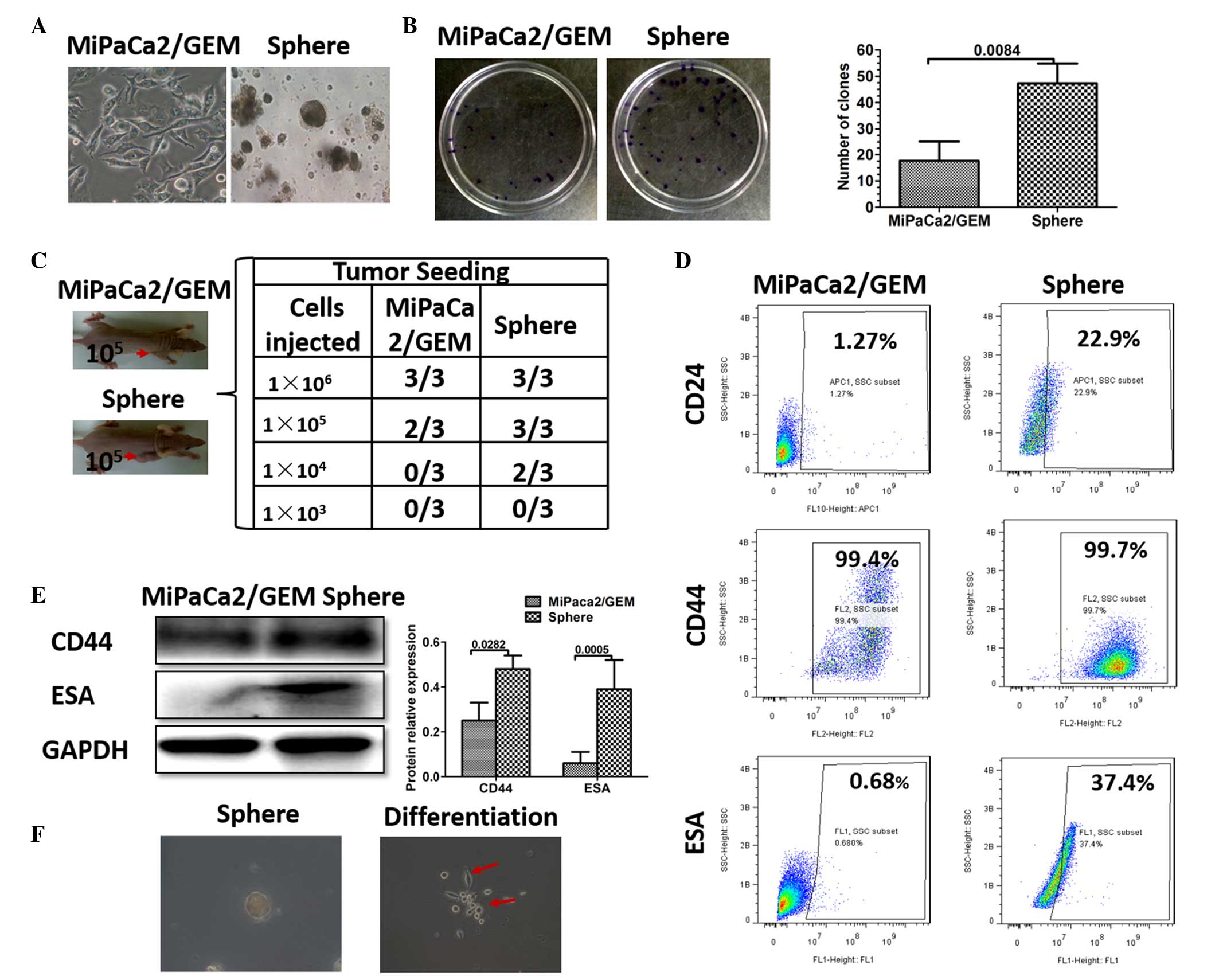
The isolation of CSCs from cancer cells has been
successfully achieved via the use of serum-free suspension culture,
and sphere-forming assays also have been used to identify cancer
stem cells (19). In the present
study, the MiaPaCa2/GEM cell line exhibited a higher proportion of
CSCs, and pancreatic cancer stem cell spheres were successfully
cultured using serum-free suspension culture (Fig. 1A). To determine whether these
sphere cells were CSCs, colony formation and tumor formation assays
were used to identify stem cell properties. The MiaPaCa2/GEM cells
and the sphere cells (1×103/ml) were resuspended and
seeded into a 6 cm dish (2 ml). After 2 weeks, more clones were
detected in the sphere cell group (Fig. 1B). The tumor formation assay
revealed that sphere cell densities of 1×105 and
1×104 were sufficient to induce tumor formation in the
nude mice, and more tumor formation was observed, compared with the
same density of control cells. In particular, no tumor formation
was observed in the control mice implanted with 1×104
MiaPaCa2/GEM cells (Fig. 1C).
Studies have demonstrated that CD44, CD24 and ESA have been used as
separation markers to identify a population of pancreatic CSCs
(7,20,21).
Therefore, western blotting and flow cytometry were performed,
which revealed that the sphere cells had higher expression levels
of CD24, CD44 and ESA (Fig. 1D and
E). Notably, when the sphere cells were cultured in the
adhesive culture system with normal medium, they differentiated
into MiaPaCa2/GEM cells (Fig. 1F).
Taken together, these results demonstrated the successful
enrichment of pancreatic CSCs using serum-free suspension
culture.
Bufalin inhibits pancreatic CSCs in
vitro
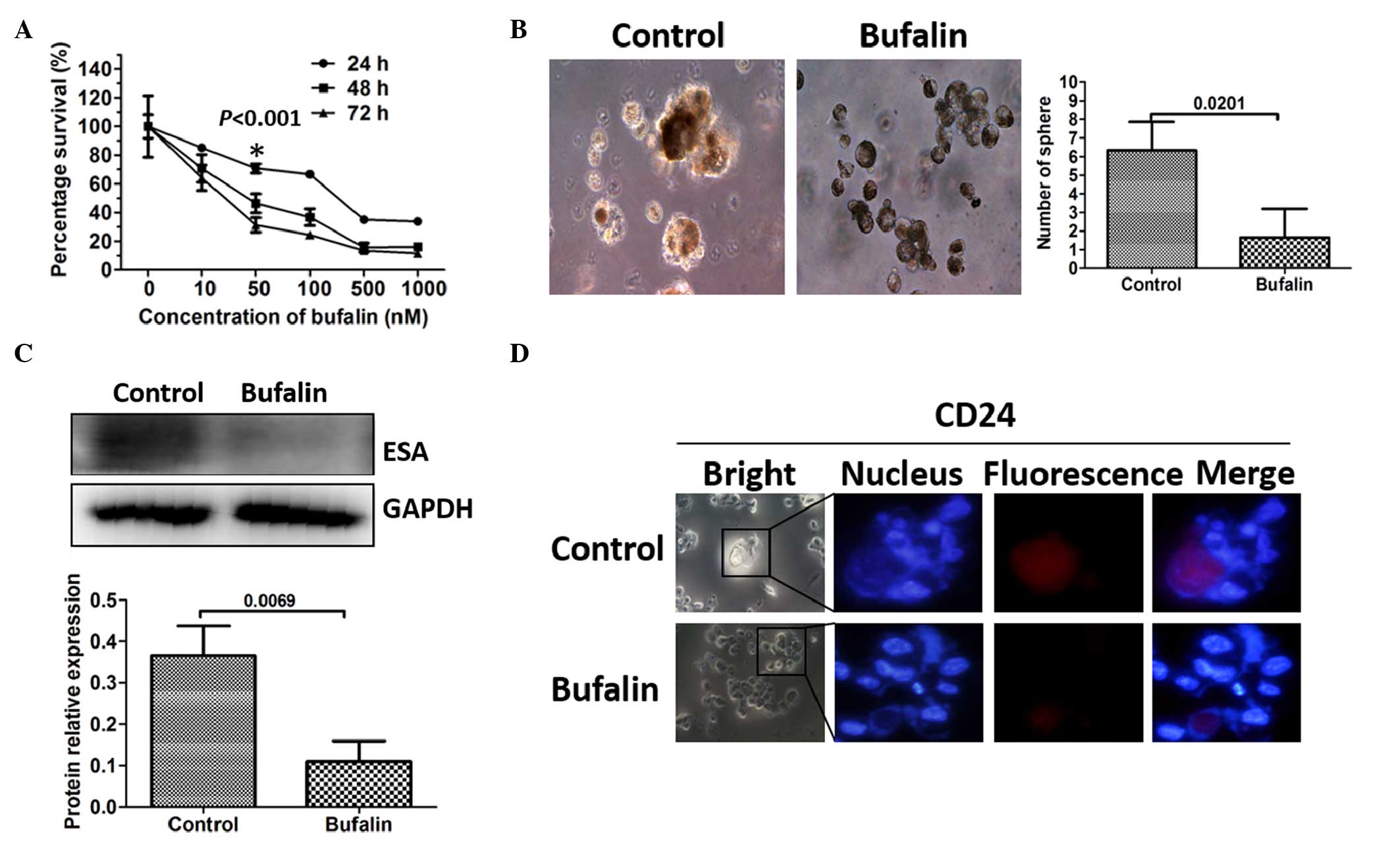
The present study examined whether bufalin had an
effect on the viability of CSCs in vitro. A cell
proliferation assay was used to measure the effects of bufalin on
the proliferative capability of the pancreatic cancer cells.
MiaPaCa2/GEM cells were treated with bufalin (10, 50, 100, 500 or
1,000 nM) for 24, 48 or 72 h. Bufalin was observed to have an
anti-proliferative effect on the MiaPaCa2/GEM cells, which was
dose- and time-dependent at concentrations between 10 and 1,000 nM
(Fig. 2A). The maximum nontoxic
concentration of bufalin was 50 nM and the most pronounced
inhibition of CSCs occurred after 24 h, without toxicity.
Therefore, a bufalin concentration of 50 nM and a treatment
duration of 24 h were applied for the subsequent experiments. The
MiaPaCa2/GEM cells were pretreated with 50 nM bufalin for 24 h, and
the subsequent sphere formation arrays detected fewer sphere cells
in the bufalin-pretreated group (Fig.
2B). The markers of pancreatic CSCs were further examined to
determine the effect of bufalin on the CSCs. As expected, the
expression levels of CD24 and ESA were downregulated, demonstrated
using Western blot analysis and immunofluorescence (Fig. 2C and D).
Bufalin inhibits the growth of pancreatic
tumors following MiaPaCa2/GEM cell implantation
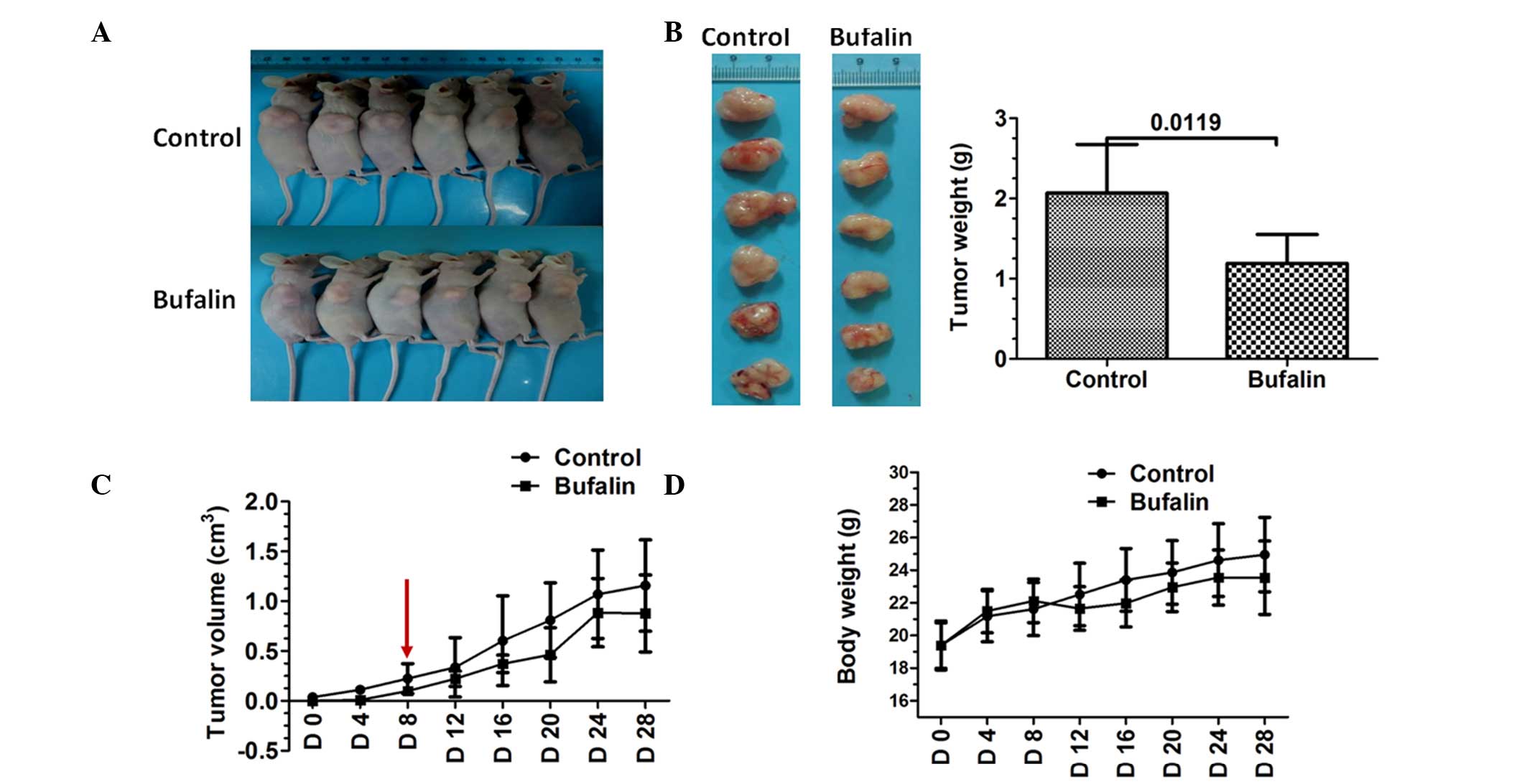
The present study also evaluated the effect of
bufalin on tumor growth in nude mice using MiaPaCa2/GEM cells. The
results showed that bufalin reduced subcutaneous xenograft growth,
compared with the control tumors (Fig.
3A and B). Tumors were not detected until day 8 in the
bufalin-treated mice (Fig. 3C).
Additionally, bufalin treatment was well tolerated by the mice, as
no weight loss or signs of acute or delayed toxicity were observed
(Fig. 3D).
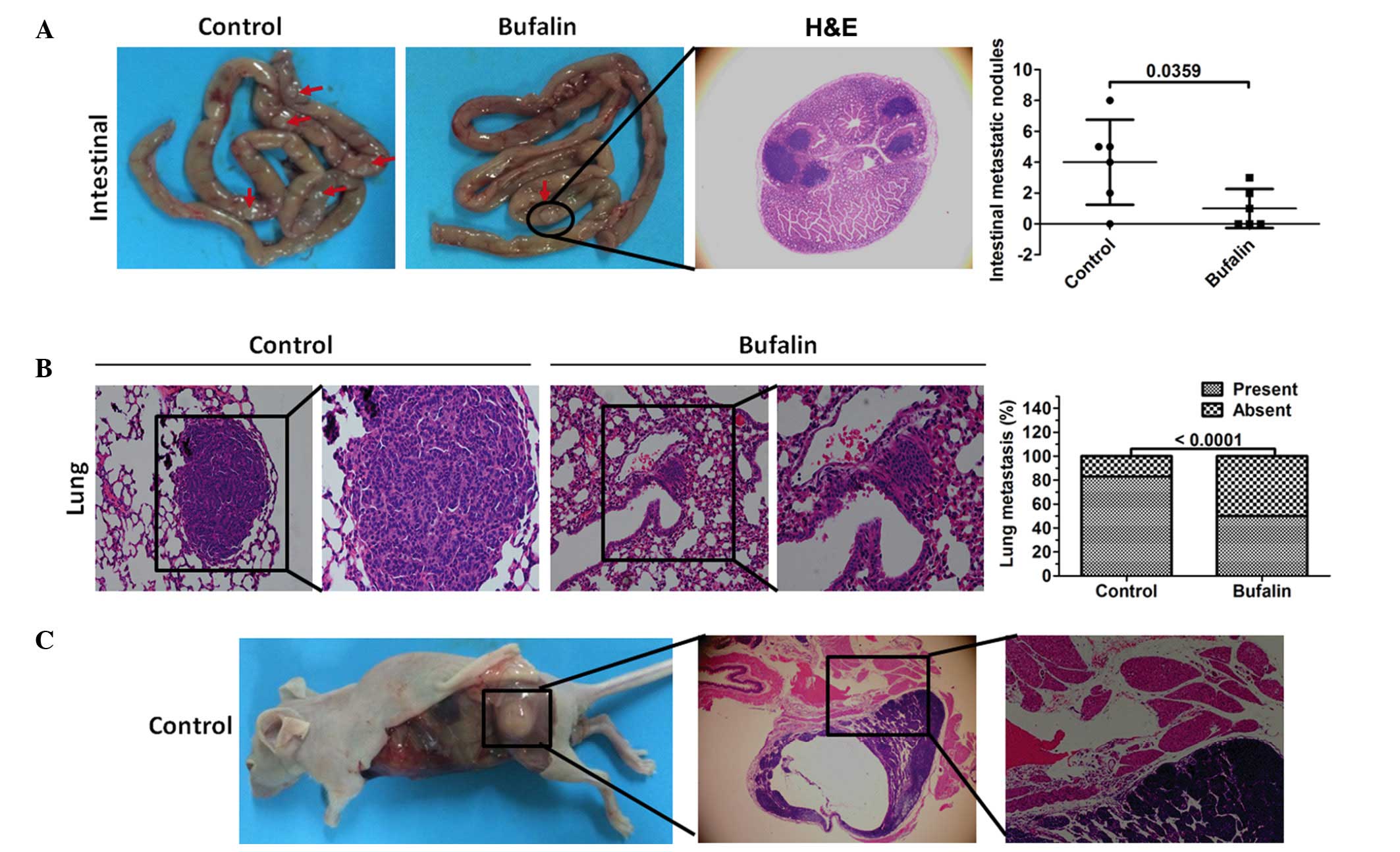
Bufalin inhibits systemic metastasis of
MiaPaCa2/GEM cells in vivo
As is already known, CSCs have the ability to
migrate and to metastasize (3). In
the present study, MiaPaCa2/GEM cells were injected into mice,
which had either been pretreated with 50 nM bufalin for 24 h via
their tail veins or received no pretreatment, and systemic
metastasis was examined. The results showed that fewer intestinal
tumor lesions were detected in the bufalin-pretreated mice
(Fig. 4A). Of note, fewer mice had
metastatic lung lesions in the bufalin-pretreated group (Fig. 4B). A single mouse in the
non-pretreated control group formed metastatic lesions in the
muscle (Fig. 4C).
Bufalin downregulates the expression
levels of CD24 and ESA
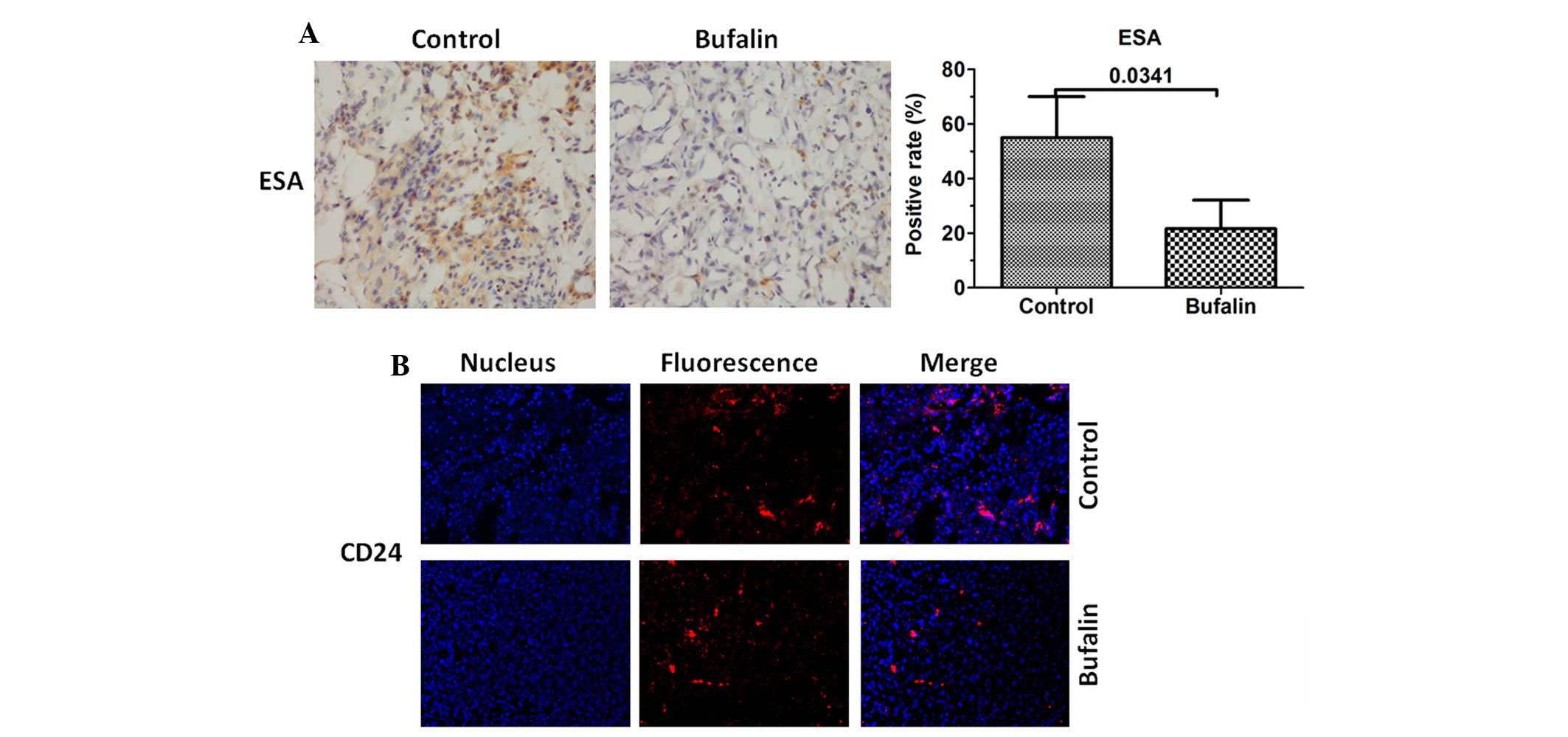
As the expression levels of CD24 and ESA were
downregulated in vitro. Immunohistochemical and
immunofluorescence staining was also used to evaluate the
expression levels of CD24 and ESA in vivo. The results
demonstrated that the expression levels of CD24 and ESA were also
inhibited in the tumor tissues of the bufalin group (Fig. 5).
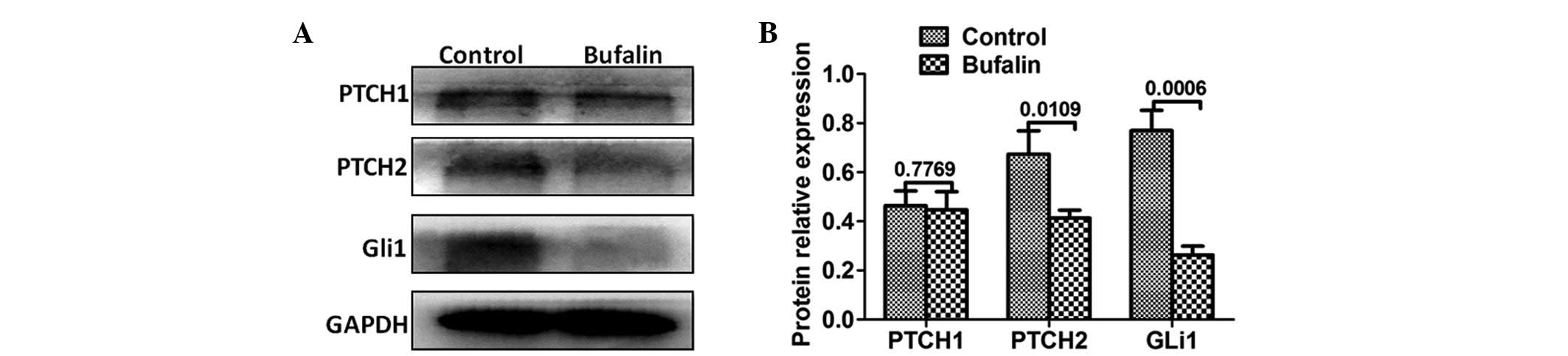
Bufalin inhibits the Hh signaling pathway
in vitro
The Hh signaling pathway is important in pancreatic
CSCs (22). In the present study,
Western blot analysis was used to evaluate the expression levels of
Hh signaling proteins. As expected, the expression levels of PTCH2
and Gli1 were downregulated in the bufalin-pretreated group. No
significant change was observed in the expression of PTCH1
(Fig. 6).
Discussion
In the present study, the effect of bufalin on
pancreatic CSCs was investigated in vivo and in
vitro. Firstly, pancreatic CSCs were successfully enriched by
serum-free suspension culture in a gemcitabine-resistant pancreatic
cancer cell line. Using this CSC model, the results showed that
bufalin inhibited the proportion of pancreatic CSCs, and
downregulated the levels of CD24 and ESA. Notably, bufalin
inhibited tumor growth and extended tumor formation duration, in
addition to downregulating the levels of CD24 and ESA in a
subcutaneous xenograft model established via MiaPaCa2/GEM cell
implantation. In another tumor metastasis model, in which tumor
cells were injected into mice via the tail vein, fewer tumor
metastasis was detected in the bufalin-pretreated group,
demonstrating that pancreatic CSCs may be inhibited in
bufalin-pretreated pancreatic cancer cells.
CSCs can be isolated by different methods, using
surface markers of CSCs (23–25),
Hoechst dye efflux (26) and
sphere culture using serum-free suspension culture (19). The isolation of CSCs based on
surface markers is the most commonly used method. However, the
percentage of CSCs is only ~0.2–0.8% in pancreatic cancer cell
lines (6), therefore, this method
is difficult to perform and is time-consuming. In the present
study, the MiaPaCa2/GEM gemcitabine-resistant pancreatic cancer
cell line was used to isolate the pancreatic CSCs. Following this,
serum-free suspension culture was used to enrich pancreatic CSC
sphere cells. The colony formation assay and tumor formation assay
demonstrated that these sphere cells exhibited CSC properties. The
cell surface markers were examined further using Western blot
analysis and flow cytometry, and the stem cell-like properties of
the sphere cells were further affirmed.
Pancreatic cancer is typically diagnosed in the late
stages, in which most patients are inoperable, and curable
treatment is not available. Radiotherapy and chemotherapy may
improve prognosis and reduce tumor size, but cannot target all
pancreatic cancer cells (27,28).
Studies have demonstrated that CSCs may be the reason underlying
the inability to eradicate pancreatic cancer using current
therapeutic techniques, and thus are responsible for tumor relapse
and metastasis (6,29–32).
CSCs have been isolated and characterized in pancreas cancer. Li
et al (7) were the first to
identify a population of pancreatic CSCs using CD44, CD24 and ESA
as separation markers. In another study,
CD44+/CD24−cells were isolated from a
pancreatic adenocarcinoma cell line, and these cells exhibited a
markedly higher tumorigenic potential, compared with cellular
subpopulations, which did not express these markers (33). In the present study, the expression
levels of CD24 and ESA were markedly higher in the sphere cells, as
compared with the MiaPaCa2/GEM cells. Conversely, the expression
levels of CD44 were not markedly different between the sphere cells
and the MiaPaCa2/GEM cells.
Bufalin has been shown to be a potential anticancer
agent in various cancer models (34–36).
As with other cardiac glycoside drugs, the anticancer target of
bufalin is predominantly the Na+-K+-ATP
enzyme. Bufalin exerts anticancer effects primarily by inhibiting
tumor cell proliferation, and inducing tumor cell apoptosis,
differentiation and triggering autophagy (37,38).
However, the effect of bufalin on pancreatic CSCs has not been
reported. In the present study, subcutaneous tumor transplantation
was performed in nude mice, which were treated the following day
with bufalin or physiological saline. Tumor formation was not
detected until 8 days in the bufalin-treated mice. This result
suggested that bufalin downregulated the percentage of pancreatic
CSCs exhibiting marked tumorigenicity. In addition, pancreatic
cancer cells were pretreated with bufalin in vitro,
following which pancreatic CSC sphere cells were enriched using
serum-free suspension culture. The results demonstrated the
formation of fewer sphere cells in the bufalin-pretreated cells.
Notably, when these cells were injected into mice via the tail
vein, fewer metastatic lesions were detected, compared with the
non-pretreated mice.
Hh signaling is key in CSC biology, and can be used
a potential therapeutic target in pancreatic cancer (22,39).
Hh signaling comprises effectors (Gli1 and Gli2), receptors (PTCH1,
PTCH2 and Smoothened), and the Sonic hedgehog ligand. Increasing
studies have focussed on targeting Hh signaling to inhibit
pancreatic CSCs. For example, the Gli transcription factor
inhibitor, GANT-61, can inhibit pancreatic CSC growth in
vitro and in a NOD/SCID/IL2R γ null mice xenograft by
downregulating the expression levels of Gli1 and Gli2 (40). GDC-0449 can also inhibit pancreatic
CSC proliferation and survival by inhibiting Hh signaling at the
level of the Gli genes (41). In
the present study, the expression levels of PTCH2 and Gli1 were
suppressed in the bufalin-pretreated cells. Therefore, the Hh
signaling pathway may be involved in the bufalin-induced
suppression of the pancreatic CSC process.
In conclusion, the present study demonstrated that
bufalin inhibited pancreatic tumor growth in vivo and
pancreatic CSC growth in vitro. Furthermore, typical markers
of CSCs, including CD24 and ESA were shown to be downregulated
following treatment with bufalin in vivo. In addition, the
expression levels of two important molecules involved in the Hh
signaling pathway, PTCH2 and Gli1, were altered following bufalin
treatment, which suggested that the Hh signaling pathway may be
involved in bufalin-induced suppression of pancreatic CSCs.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no.81102847).
References
|
1
|
Pandol S, Gukovskaya A, Edderkaoui M,
Dawson D, Eibl G and Lugea A: Epidemiology, risk factors, and the
promotion of pancreatic cancer: Role of the stellate cell. J
Gastroenterol Hepatol. 27(Suppl 2): S127–S134. 2012. View Article : Google Scholar
|
|
2
|
Onkendi EO, Boostrom SY, Sarr MG, Farnell
MB, Nagorney DM, Donohue JH, Kendrick ML, Reid-Lombardo KM, Harmsen
WS and Que FG: 15-year experience with surgical treatment of
duodenal carcinoma: A comparison of periampullary and
extra-ampullary duodenal carcinomas. J Gastrointest Surg.
16:682–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanase CP, Neagu AI, Necula LG, Mambet C,
Enciu AM, Calenic B, Cruceru ML and Albulescu R: Cancer stem cells:
Involvement in pancreatic cancer pathogenesis and perspectives on
cancer therapeutics. World J Gastroenterol. 20:10790–10801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sureban SM, May R, Qu D, Weygant N,
Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG
and Houchen CW: DCLK1 regulates pluripotency and angiogenic factors
via microRNA-dependent mechanisms in pancreatic cancer. PLoS One.
8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Kong D, Ahmad A, Bao B and Sarkar
FH: Pancreatic cancer stem cells: Emerging target for designing
novel therapy. Cancer Lett. 338:94–100. 2013. View Article : Google Scholar
|
|
7
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berman DM, Karhadkar SS, Maitra A, Montes
De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman
JR, Watkins DN and Beachy PA: Widespread requirement for Hedgehog
ligand stimulation in growth of digestive tract tumours. Nature.
425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abetov D, Mustapova Z, Saliev T and
Bulanin D: Biomarkers and signaling pathways of colorectal cancer
stem cells. Tumour Biol. 36:1339–1353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Ma L, Zhang Z, Liu X, Gao H,
Zhuang Y, Yang P, Kornmann M, Tian X and Yang Y: Hedgehog Signaling
Regulates Epithelial-Mesenchymal Transition in Pancreatic Cancer
Stem-Like. Cells J Cancer. 7:408–417. 2016. View Article : Google Scholar
|
|
12
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, nonsmall-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Y, Zhang Y, Luan J, Duan H, Zhang F,
Yagasaki K and Zhang G: Effects of bufalin on the proliferation of
human lung cancer cells and its molecular mechanisms of action.
Cytotechnology. 62:573–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H,
Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al: Bufalin induces cell
cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour
Biol. 35:10931–10941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu SH, Hsiao YT, Chen JC, Lin JH, Hsu SC,
Hsia TC, Yang ST, Hsu WH and Chung JG: Bufalin alters gene
expressions associated DNA damage, cell cycle, and apoptosis in
human lung cancer NCI-H460 cells in vitro. Molecules. 19:6047–6057.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chueh FS, Chen YY, Huang AC, Ho HC, Liao
CL, Yang JS, Kuo CL and Chung JG: Bufalin-inhibited migration and
invasion in human osteosarcoma U-2 OS cells is carried out by
suppression of the matrix metalloproteinase-2, ERK, and JNK
signaling pathways. Environ Toxicol. 29:21–29. 2014. View Article : Google Scholar
|
|
17
|
Gao Y, Li HX, Xu LT, Wang P, Xu LY, Cohen
L, Yang PY, Gu K and Meng ZQ: Bufalin enhances the
anti-proliferative effect of sorafenib on human hepatocellular
carcinoma cells through downregulation of ERK. Mol Biol Rep.
39:1683–1689. 2012. View Article : Google Scholar
|
|
18
|
Wang P, Chen Z, Meng ZQ, Fan J, Luo JM,
Liang W, Lin JH, Zhou ZH, Chen H, Wang K, et al: Dual role of Ski
in pancreatic cancer cells: Tumor-promoting versus
metastasis-suppressive function. Carcinogenesis. 30:1497–1506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchida S, Yokoo S, Yanagi Y, Usui T,
Yokota C, Mimura T, Araie M, Yamagami S and Amano S: Sphere
formation and expression of neural proteins by human corneal
stromal cells in vitro. Invest Ophthalmol Vis Sci. 46:1620–1625.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Lee CJ and Simeone DM:
Identification of human pancreatic cancer stem cells. Methods Mol
Biol. 568:161–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu J, He J, Liu Y, Simeone DM and Lubman
DM: Identification of glycoprotein markers for pancreatic cancer
CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue
microarray. J Proteome Res. 11:2272–2281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim EJ, Sahai V, Abel EV, Griffith KA,
Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al:
Pilot clinical trial of hedgehog pathway inhibitor GDC-0449
(vismodegib) in combination with gemcitabine in patients with
metastatic pancreatic adenocarcinoma. Clin Cancer Res.
20:5937–5945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24− and
CD133+ cells with cancer stem cell characteristics.
Breast Cancer Res. 10:R102008. View
Article : Google Scholar
|
|
24
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: Let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hadnagy A, Gaboury L, Beaulieu R and
Balicki D: SP analysis may be used to identify cancer stem cell
populations. Exp Cell Res. 312:3701–3710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizuno N, Yatabe Y, Hara K, Hijioka S,
Imaoka H, Shimizu Y, Ko SB and Yamao K: Cytoplasmic expression of
LGR5 in pancreatic adenocarcinoma. Front Physiol. 4:2692013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsuda Y, Kure S and Ishiwata T: Nestin
and other putative cancer stem cell markers in pancreatic cancer.
Med Mol Morphol. 45:59–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chefetz I, Alvero AB, Holmberg JC,
Lebowitz N, Craveiro V, Yang-Hartwich Y, Yin G, Squillace L, Gurrea
Soteras M, Aldo P and Mor G: TLR2 enhances ovarian cancer stem cell
self-renewal and promotes tumor repair and recurrence. Cell Cycle.
12:511–521. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Croker AK and Allan AL: Inhibition of
aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and
radiation resistance of stem-like ALDHhiCD44+
human breast cancer cells. Breast Cancer Res Treat. 133:75–87.
2012. View Article : Google Scholar
|
|
31
|
Rich JN: Cancer stem cells in radiation
resistance. Cancer Res. 67:8980–8984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133(+) cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
View Article : Google Scholar
|
|
33
|
Huang P, Wang CY, Gou SM, Wu HS, Liu T and
Xiong JX: Isolation and biological analysis of tumor stem cells
from pancreatic adenocarcinoma. World J Gastroenterol.
14:3903–3907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H,
Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al: Bufalin induces cell
cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour
Biol. 35:10931–10941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu SH, Wu TY, Hsiao YT, Lin JH, Hsu SC,
Hsia TC, Yang ST, Hsu WH and Chung JG: Bufalin induces cell death
in human lung cancer cells through disruption of DNA damage
response pathways. Am J Chin Med. 42:729–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan S, Qu X, Xu L, Che X, Ma Y, Zhang L,
Teng Y, Zou H and Liu Y: Bufalin enhances TRAIL-induced apoptosis
by redistributing death receptors in lipid rafts in breast cancer
cells. Anticancer Drugs. 25:683–689. 2014.PubMed/NCBI
|
|
37
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar
|
|
38
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar
|
|
39
|
Tang SN, Fu J, Nall D, Rodova M, Shankar S
and Srivastava RK: Inhibition of sonic hedgehog pathway and
pluripotency maintaining factors regulate human pancreatic cancer
stem cell characteristics. Int J Cancer. 131:30–40. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu J, Rodova M, Roy SK, Sharma J, Singh
KP, Srivastava RK and Shankar S: GANT-61 inhibits pancreatic cancer
stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice
xenograft. Cancer Lett. 330:22–32. 2013. View Article : Google Scholar
|
|
41
|
Singh BN, Fu J, Srivastava RK and Shankar
S: Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits
pancreatic cancer stem cell characteristics: Molecular mechanisms.
PLoS One. 6:e273062011. View Article : Google Scholar : PubMed/NCBI
|