Introduction
An increased understanding of hepatocellular
carcinoma (HCC), which ranks globally as the third or fourth
leading cause of cancer-associated mortality (1–3), is
key for improving early diagnosis, subsequent patient treatment and
prognosis. HCC has a poor survival rate as it frequently exhibits
local invasion and metastasis. A number of clinicopathological
factors are important in the treatment of HCC, however, it is also
important to develop improved biological indicators for determining
therapeutic strategy.
Lysyl oxidase-like 2 (LOXL2) is a member of the
lysyl oxidase (LOX) gene family, which includes prototypic LOX and
LOX-like (LOXL) proteins, LOXL1, LOXL2, LOXL3 and LOXL4, encoded in
mammalian genomes (4,5). The LOX gene family promotes invasion
and metastatic niche formation in the skin, aorta, heart, lung,
cartilage, kidney, stomach, small intestine, colon, ovaries,
testis, and brain of mice (6,7).
Furthermore, in previous LOXL2 studies, it was demonstrated that
high LOXL2 expression was associated with poor prognosis in colon,
esophageal and squamous cell cancers and that LOXL2 was closely
associated with tumor invasion and metastasis (8–14). A
recent study has reported that LOXL2 is key in tumor
microenvironment and metastatic niche formation in HCC with
hypoxia-inducible factor 1α, transforming growth factor-β, and
microRNAs controlling the expression of LOXL2 (15), however, its effect on proliferation
and clinical features require further elucidation.
The present study aimed to directly detect the
effects of LOXL2 on the proliferation of HCC cell lines via
evaluating the cell apoptosis rate, cell cycle and cell numbers,
and verify the association between LOXL2 expression and clinical
features in 80 HCC patients. These results lay foundation for
future research into HCC.
Materials and methods
Cell lines
SMMC-7721 and HepG2 human HCC and LO2 human
hepatocyte cell lines, were obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were grown as previously described (16). The cells were maintained as
monolayer cultures at 37.8°C in a humidified atmosphere of 5%
CO2. All cell lines were cultured in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 0.25 µg/ml
streptomycin (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA).
Construction of an RNA interference gene
with a lentiviral vector
Cells transfected with LOXL2-small interfering RNA
(LOXL2-siRNA; 5′-ATTACTCCAACAACATCAT-3′) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) were used for
silencing LOXL2. Cells expressing scrambled short hairpin RNA
(shRNA; 5′-TTCTCCGAACGTGTCACGT-3′) in a lentiviral vector served as
the control, and a human LOXL2 dsDNA oligonucleotide sequence was
synthesized with targeted siRNA sequences by GeneChem Co., Ltd
(Shanghai, China). A lentiviral vector, pGCSIL-green fluorescent
protein (GFP) plasmid (synthesized by GeneChem Co., Ltd.), was
digested by digested by AgeI and EcoRI (GeneChem Co.,
Ltd.) and connected with the dsDNA sequence and subsequently
transformed into competent E. coli. Lentiviral vector
production and infection were conducted as previously described
(17). Stable cell lines
expressing LOXL2 shRNAs were selected on lysogeny broth (LB) agar
medium after 16 h culturing at 37°C and were identified by
polymerase chain reaction (PCR) using a Taq polymerase kit (Takara
Bio, Inc., Otsu, Japan). The sense and antisense primers used were
as follows: 5′-CCTATTTCCCATGATTCCTTCATA-3′ and
5′-GTAATACGGTTATCCACGCG-3′. PCR was conducted at 94°C for 30 sec,
followed by 30 cycles at 94, 55 and 72°C for 30 sec, and 72°C for 6
min. The positive clones of recombinant plasmids were sequenced and
extracted.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from HCC cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. cDNA was obtained by reverse
transcription using the Moloney-Murine Leukemia Virus Reverse
Transcriptase cDNA Synthesis kit (Promega Corporation, Madison, WI,
USA) according to the manufacturer's protocols. Subsequently, mRNA
expression levels of the target gene, LOXL2, were detected by qPCR
using the SYBR Premix Ex Taq kit (Takara Bio, Inc., Japan). The
primer sequences were as follows: Sense, 5′-GTCTGCGGCATGTTTGG-3′
and anti-sense, 5′-GCTCTGGCTTGTACGCTTT-3′; and sense,
5′-TGACTTCAACAGCGACACCCA-3′ and antisense,
5′-CACCCTGTTGCTGTAGCCAAA-3′ for GAPDH. The thermocycling conditions
were 95°C for 15 sec, followed by 45 cycles at 95°C for 5 sec and
60°C for 30 sec. Melting curve analysis was conducted to check
amplification. Data was calculated using the comparative
2−ΔΔCq method (18).
Cell counts
HepG2 and SMMC-7721 cells infected with lentiviral
vector were seeded at density of 1×103 cells/well in
96-well plates and incubated. A Cellomics™ instrument (ArrayScan
VT1; Thermo Fisher Scientific, Inc.) was used to measure the two
types of cells stained fluorescent green. The control and
LOXL2-siRNA cell numbers were investigated for five days,
calculated and analyzed, and a cell growth curve was
constructed.
Colony formation assay
Following transfection, the two types of infected
cells were seeded at density of 1×103 cells/well in
6-well plates and cultured for 14 days. The culture medium was
replaced every 3 days. The colonies were rinsed with
phosphate-buffered saline (PBS) and fixed with paraformaldehyde for
30–60 min prior to staining with Giemsa for 20 min. Cells were
washed a number of times with ddH2O until the plate background was
clean and allowed to air dry. Colonies were counted under a
microscope (Micropublisher 3.3RTV; Olympus Corporation, Tokyo,
Japan). All experiments were conducted in triplicate and data were
analyzed using CellQuest Pro software, version 5.1.1.
Flow cytometry to detect the cell
cycle
The two types of infected cells were harvested
following reaching ~85% confluence in a 6-well dish. The cells were
centrifuged at 120 × g for 5 min at room temperature, the
supernatant was discarded, and cells were washed with cold PBS at
4°C, and centrifuged again under the same conditions prior to
collection. The cells were fixed in 70% ethanol and stored at 4°C
for >1 h. The cells were centrifuged at 120 × g for 5 min at
room temperature and washed again prior to addition of staining
solution (propidium iodide) and the cells were subsequently
resuspended. The cell cycle was analyzed with flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) and
CellQuest Pro software, version 5.1.1. All experiments were
performed in triplicate.
Flow cytometry to detect apoptosis
The cells were harvested, washed with D-Hanks
(Haling Biotechnology Co., Ltd., Shanghai, China), trypsinized,
centrifuged at 150 × g for 5 min at room temperature, and the
supernatant was then discarded. The precipitated cells were washed
once with PBS, centrifuged under the same conditions, and then
collected following washing with binding buffer. The cell
suspension was gathered with a final density of
1×106–1×107 cells/ml and 5 µl Annexin
V Apoptosis Detection kit APC (eBioscience, Inc., San Diego, CA,
USA) was added for 10–15 min in a dark room at room temperature.
Flow cytometry analysis was then conducted using FACSCalibur. All
experiments were carried out in triplicate.
MTT assay and BrdU labeling
Cells from the two infected cell lines were seeded
in 96-well plates at an initial density of 2×104/well.
HepG2 cells were stained every 24 h with 10 µl sterile MTT
(5 mg/ml; Beijing Dingguo Changsheng Biotechnology Co., Ltd.) for 4
h at 37°C. The culture medium was removed, and 100 ml of dimethyl
sulfoxide (Sigma-Aldrich) was added to stop the reaction. SMMC-7721
cells were incubated with BrdU reagent [10 µl/well; Cell
Proliferation ELISA, BrdU (colorimetric); Roche Diagnostics, Basel,
Switzerland] and fixed, and the stationary liquid was discarded.
Substrate solution was added to finish the reaction following
staining with anti-BrdU antibodies for 90 min at room temperature.
HepG2 cells were detected at 5 days, and SMMC-7721 cells were
detected at 24 and 96 h. The absorbance was measured at a
wavelength of 490 nm for the MTT assay on HepG2 cells and a
wavelength of 450 nm for the BrdU assay on SMMC-7721 cells with a
microplate reader. All experiments were conducted in triplicate.
The fold change in proliferation of different groups were
calculated, analyzed, and presented in figures.
HCC patients and tissue specimens
A total of 80 samples were collected from patients
(68 males and 12 females; median age, 51; age range, 26–72) at The
First Affiliated Hospital of Dalian Medical University (Dalian,
China) from 2010 to 2014. Tumor tissue (TT) and adjacent non-tumor
tissue (ANT) were resected by surgical excision.
Clinicopathological data were obtained from archived medical
records. The records included patient gender, age, size of tumor,
vascular invasion, tumor number, liver cirrhosis, Child-Pugh grade,
hepatitis B surface antigen (HBsAg), α-fetoprotein (AFP),
histological grade, and pathological stage. The liver cirrhosis was
diagnosed by pathology, and the Child-Pugh grade was divided into
three grades according to evaluation indexes, including hepatic
encephalopathy, ascites, bilirubin, albumin and prothrombin time.
Serum HBsAg was detected using ARCHITECT HBsAg (Abbott
Laboratories, Chicago, IL, USA) and AFP was determined using
Elecsys and Cobas analyzers (Roche Diagnostics). The histological
grade was determined according to Edmondson-Steiner modification,
and pathological staging was determined according to the seventh
edition of the tumor node metastasis (TNM) classification of the
International Union Against Cancer (19). Patient consent and approval from
the Institutional Research Ethics Committee were obtained prior to
the use of these clinical materials for research purposes.
Immunohistochemistry analysis
Two tissue microarrays were constructed containing
80 liver TT and ANT samples. Cores of 1.5 mm in diameter were
sampled from each specimen (2 cores/specimen), and were fixed with
10% formalin and embedded in paraffin in the microarray. The
immunohistochemical (IHC) procedure was conducted as previously
described (20). The sections were
treated with the following primary antibody overnight at 4°C:
Monoclonal rabbit anti-human LOXL2 antibody (1:100; cat. no.
ab179810; Abcam, Cambridge, MA, USA). All the sections were
incubated for 30 min at 37°C with peroxidase-labeled goat
anti-rabbit secondary antibody with diaminobenzidine as a chromogen
(1:1,000; cat. no. M080825; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing China). The sections were stained
with hematoxylin. IHC staining was quantitatively analyzed with
Image Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
computerized image analysis system using the automatic measurement
program. The stained sections were analyzed to verify the mean
integral optical density (IOD), which represented the strength of
staining signals as measured per positive pixel.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The intergroup difference was compared using an
independent samples t-test. These analyses were conducted using
SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
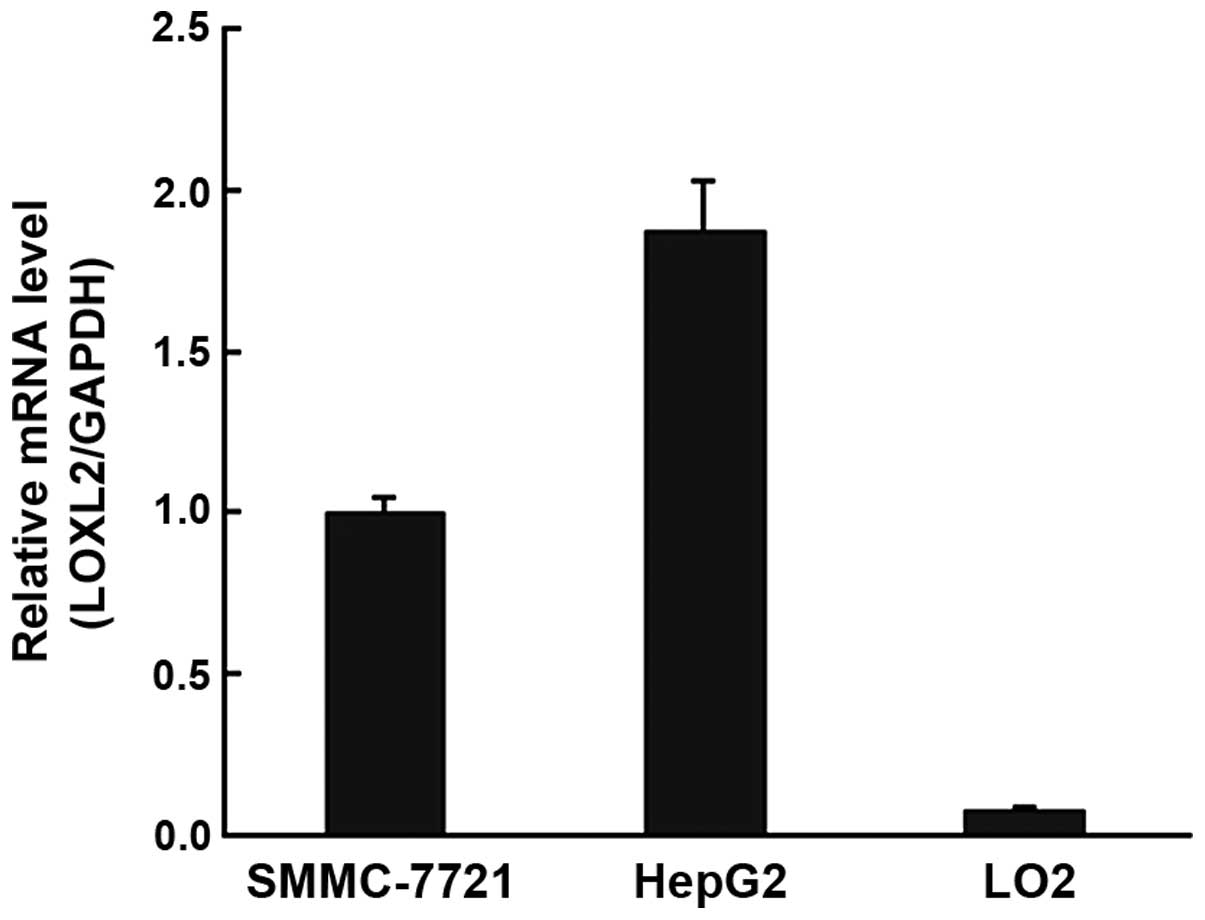
LOXL2 was highly expressed in HCC cell
lines
RT-qPCR demonstrated that LOXL2 mRNA expression
levels were markedly upregulated in HepG2 and SMMC-7721 cells
(Fig. 1). Subsequently, when the
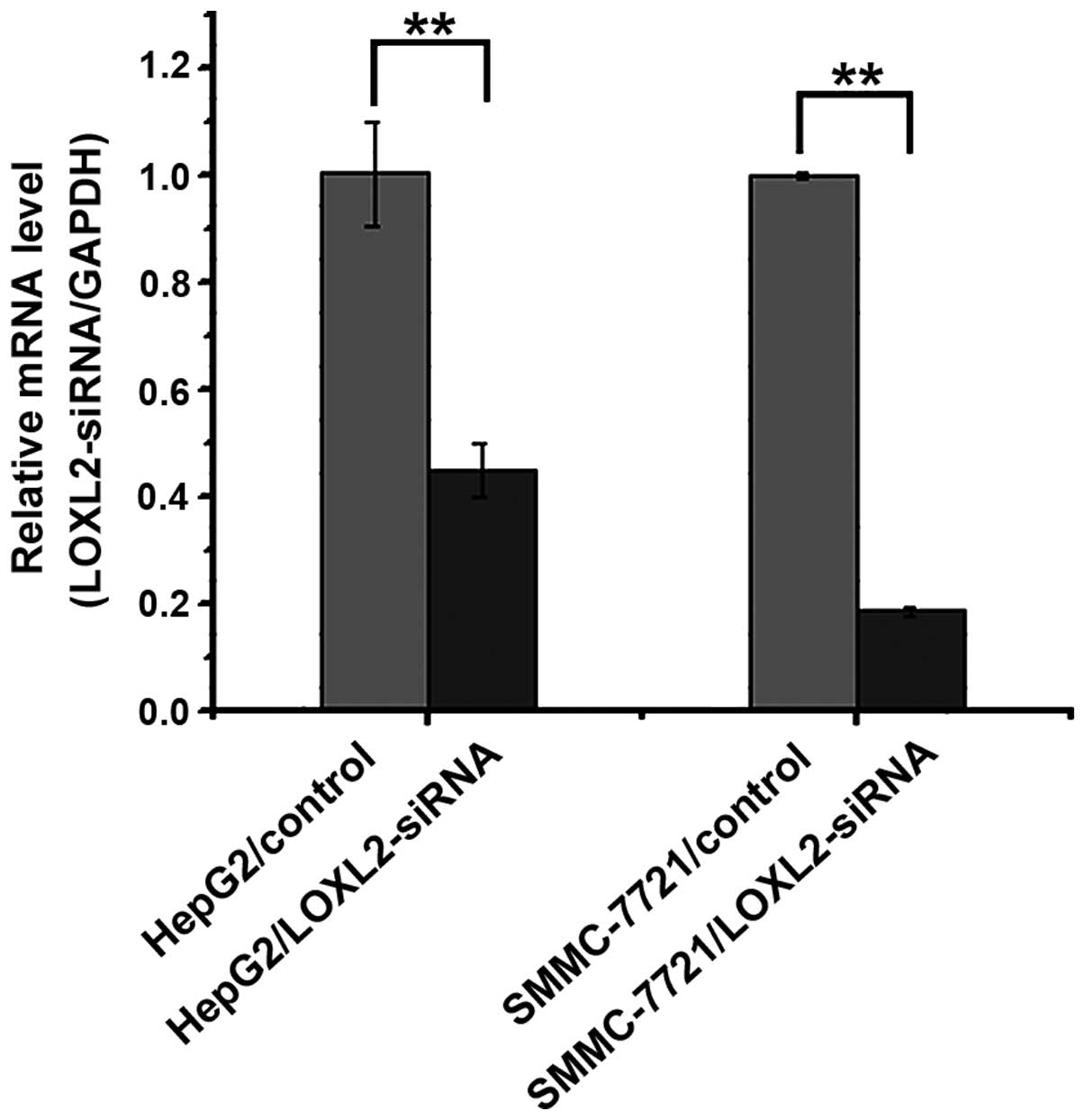
two cell lines were infected with LOXL2-siRNA, the RT-qPCR results
indicated that the expression of LOXL2 mRNA was significantly
decreased in HepG2 (P=0.00310) and SMMC-7721 cell lines
(P<0.0001) (Fig. 2).
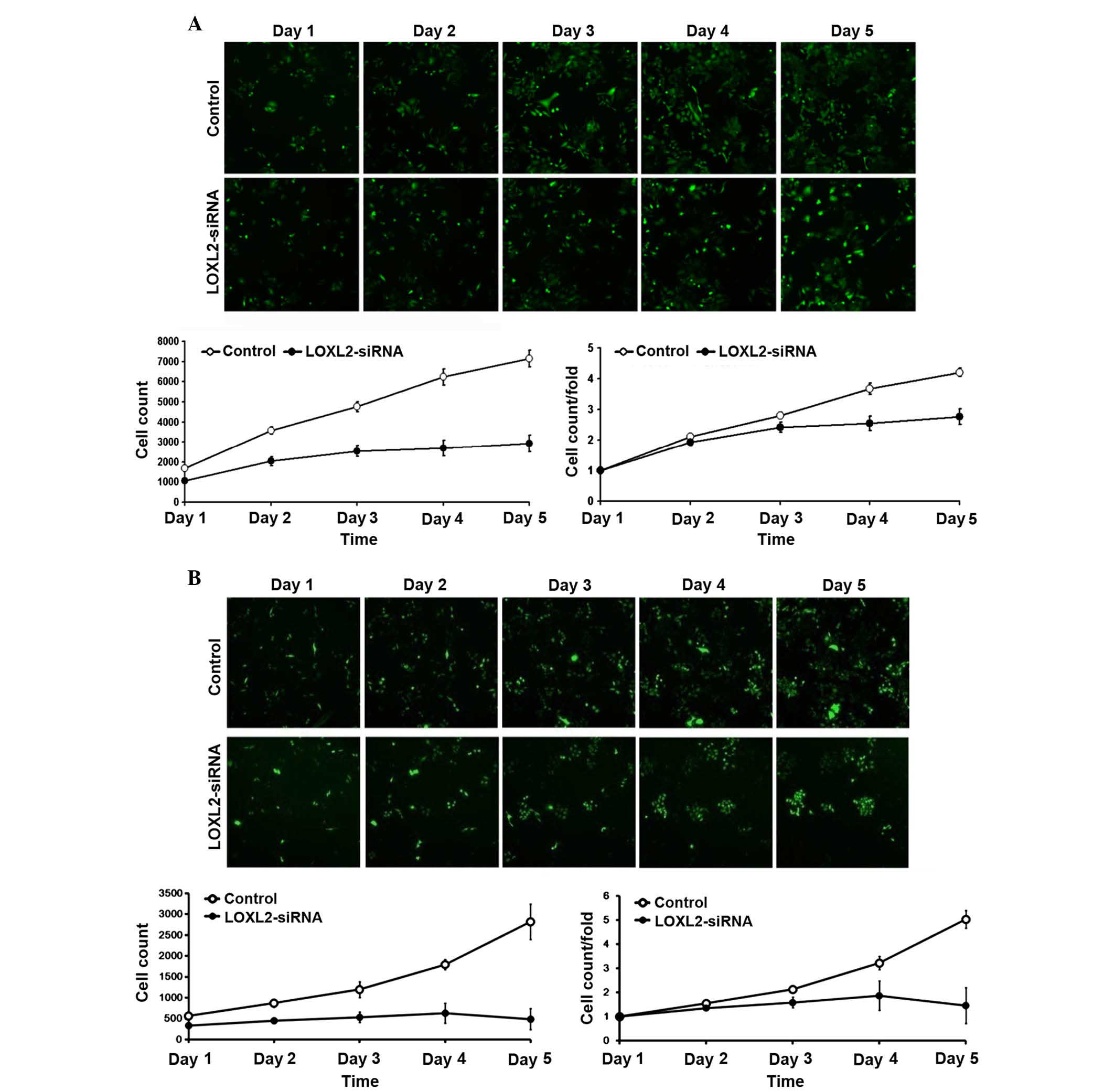
LOXL2 silencing inhibited HepG2 and
SMCC-7721 cell proliferation
As presented in Fig.
3, following LOXL2 gene silencing, the number of cells and the
fold change in proliferation were markedly reduced in the HepG2
(Fig. 3A) and SMMS-7721 (Fig. 3B) cells. These results indicated
that the silencing of LOXL2 was associated with cell
proliferation.
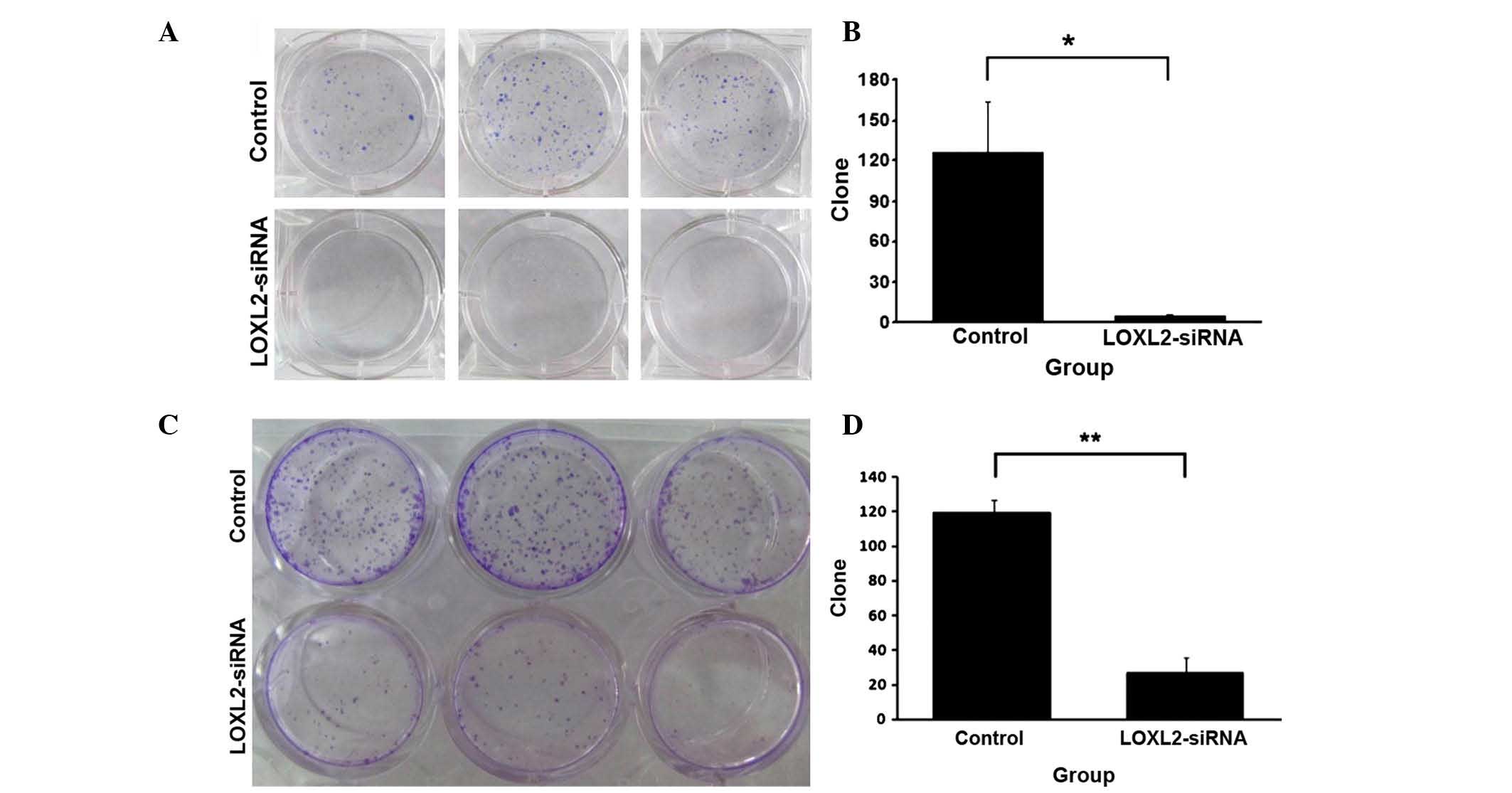
LOXL2 silencing reduced HepG2 and
SMMC-7721 cell colony formation
The effect of LOXL2 silencing on growth and colony
numbers in HCC cell lines is presented in Fig. 4. LOXL2 silencing reduced the
anchorage-independent growth ability of HepG2 (Fig. 4A) and SMMC-7721 cells (Fig. 4C) in soft agar. Cell clone numbers
were significantly decreased in HepG2 (P=0.0287; Fig. 4B) and SMMC-7721 cells (P=0.00022;
Fig. 4D), which were infected with
LOXL2-siRNA.
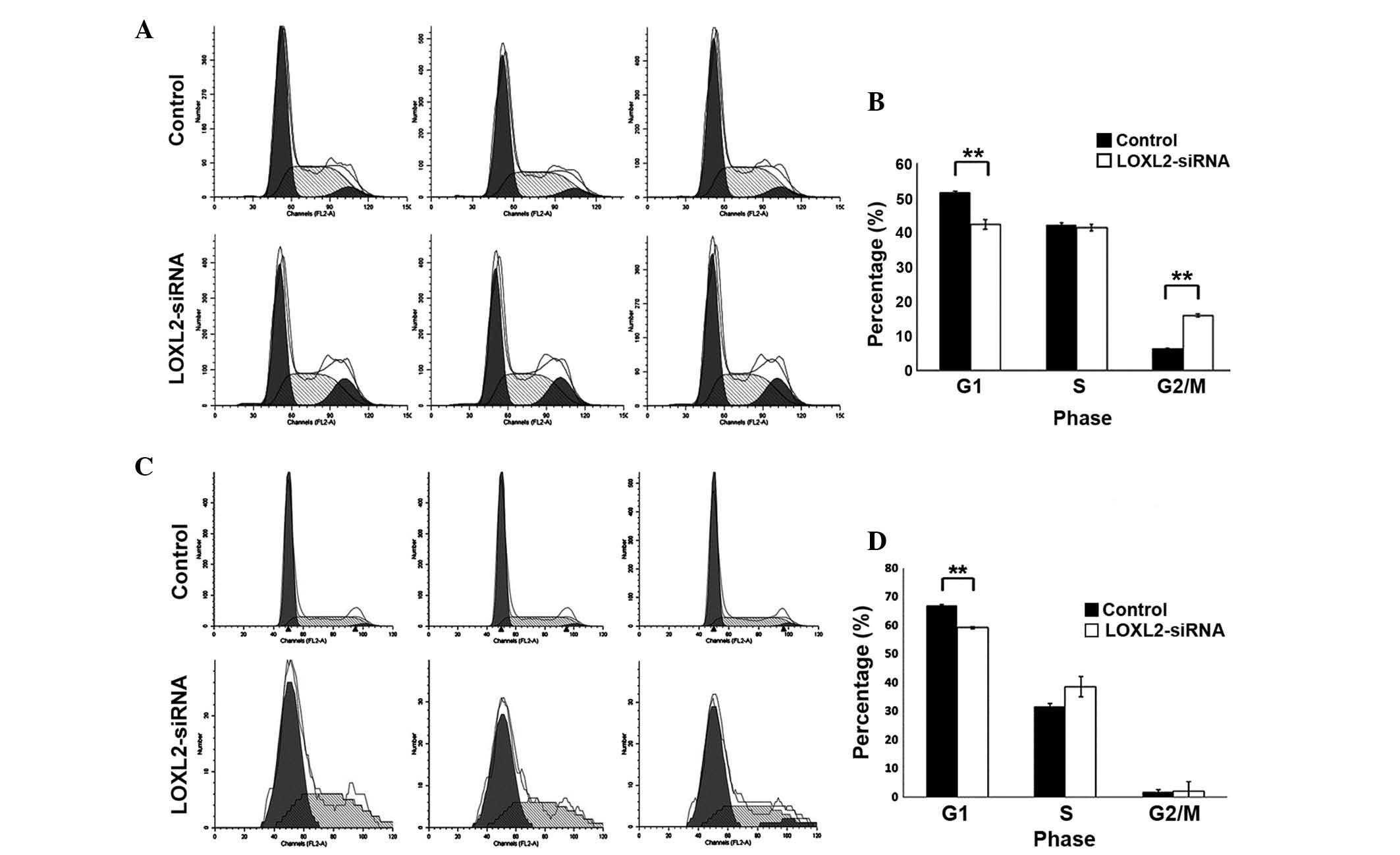
LOXL2 silencing induced cycle arrest in
HepG2 and SMMC-7721 cells
LOXL2 silencing significantly decreased the fraction
of G1 phase cells in HepG2 (P=0.002; Fig. 5A and B) and SMMC-7721 cells
(P=<0.0001; Fig. 5C and D)
compared with the controls. In addition, LOXL2 silencing
significantly increased the percentage of G2/M phase
cells in HepG2 (P<0.0001; Fig. 5A
and B) and markedly decreased the number of cells in S-phase in
SMMC-7721 cells (P=0.06; Fig. 5C and
D). These results suggested that LOXL2 contributed to the cell
phase transition of HCC cells, particularly the G1
phase.
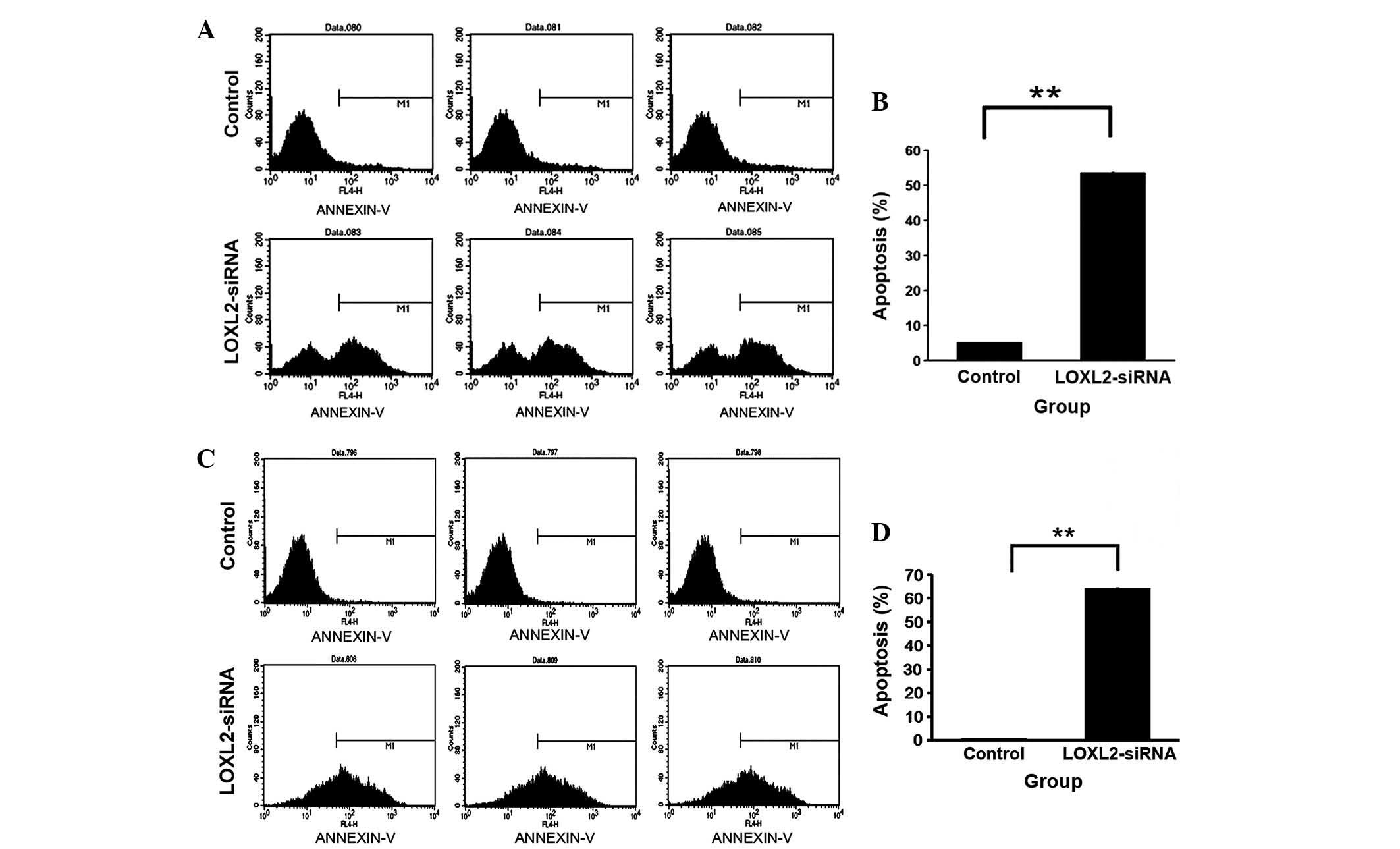
LOXL2 silencing accelerated apoptosis in
HepG2 and SMMC-7721 cells
Following LOXL2 silencing, the percentage of
apoptotic cells was increased in HepG2 (P<0.0001; Fig. 6A and B) and SMMC-7721 cells
(P<0.0001; Fig. 6C and D)
compared with the controls. The results indicated that LOXL2
silencing increased apoptosis in HCC cells.
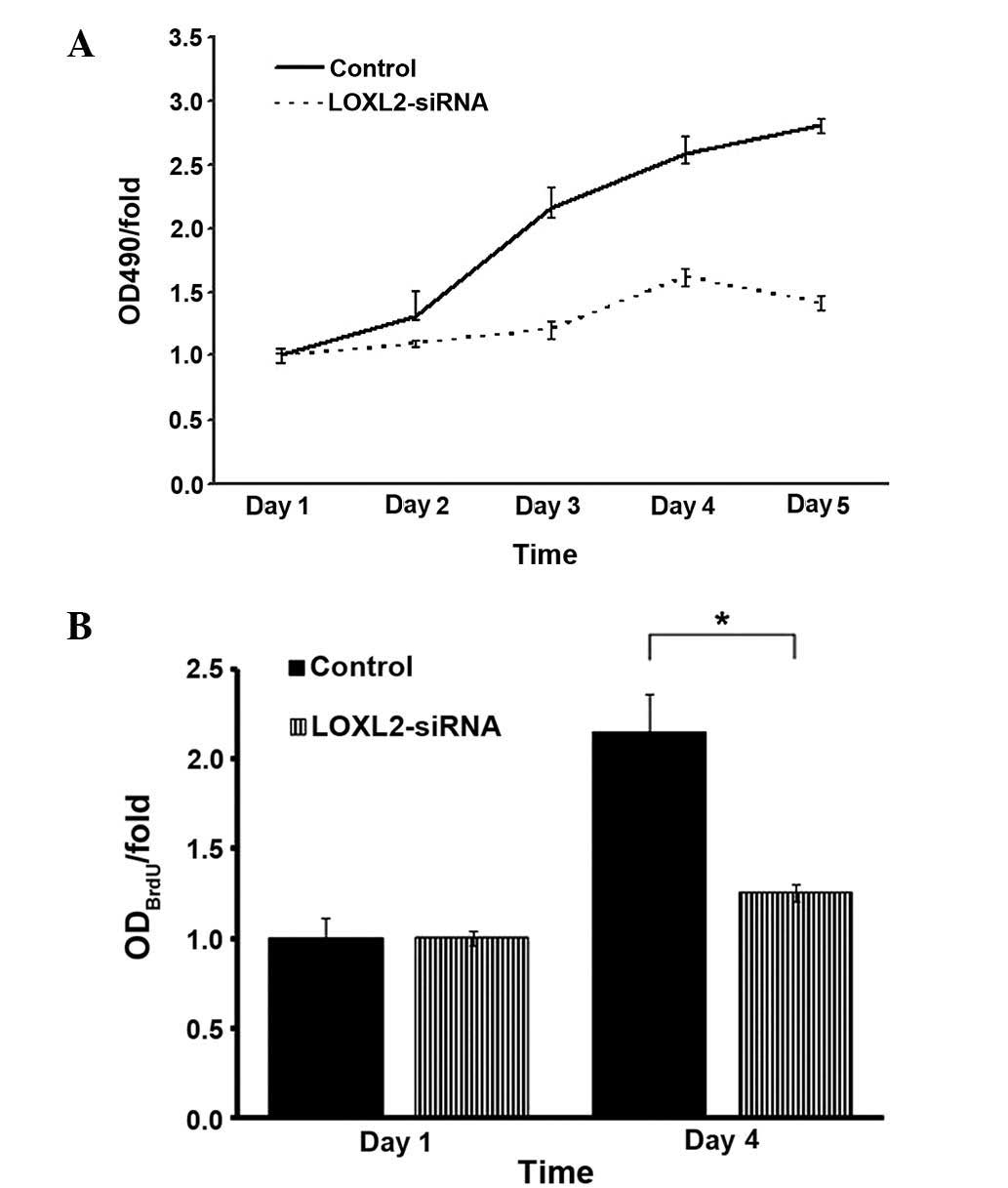
LOXL2 silencing suppressed HepG2 and
SMMC-7721 cell growth as analyzed by MTT and BrdU
MTT assays were used to detect the absorbance of
infected HepG2 cells. LOXL2 silencing decreased the growth of HepG2
cells over five days culture (Fig.
7A). Similarly, BrdU labeling was used in SMMC-7721 cells due
to thymine replacement competition in the S phase, and it was
observed that LOXL2 silencing also decreased the growth of
SMMC-7721 cells on day 4 (P=0.0151; Fig. 7B). These results demonstrated that
LOXL2 is important in suppressing the growth of HCC cells in
vitro.
Characteristics of the HCC patients
Tumor size of the 80 HCC patients ranged from
2.2–12.5 cm (mean, 7.35 cm). The characteristics of all the tissue
samples are summarized in Table
I.
 | Table IClinical information regarding
hepatocellular carcinoma samples. |
Table I
Clinical information regarding
hepatocellular carcinoma samples.
| Characteristic | Number of cases
(%) |
|---|
| Total | 80 (100) |
| Gender |
| Male | 68 (85) |
| Female | 12 (15) |
| Age (years) |
| <50 | 34 (42.5) |
| ≥50 | 46 (57.5) |
| Size (cm) |
| ≤2, no
invasion | 0 (0) |
| ≤5, or 3 nodules,
≤3; no invasion | 11 (13.75) |
| >5, or multiple
nodules | 15 (18.75) |
| Vascular
invasion | 54 (67.5) |
| Vascular
invasion |
| Negative | 26 (32.5) |
| Position | 54 (67.5) |
| Number of
tumors |
| 1 | 62 (77.5) |
| ≥2 | 18 (22.5) |
| Liver
cirrhosis |
| Negative | 6 (7.5) |
| Positive | 74 (92.5) |
| Child-Pugh
grade |
| A | 74 (92.5) |
| B | 6 (7.5) |
| C | 0 (0) |
| HBsAg |
| Negative | 21 (26.25) |
| Positive | 59 (73.75) |
| AFP (ng/ml) |
| ≤20 | 29 (36.25) |
| >20 | 51 (63.75) |
| Tumor histological
grade |
| I | 3 (3.75) |
| I–II | 8 (10) |
| II | 44 (55) |
| II–III | 7 (8.75) |
| III | 18 (22.5) |
| TNM
classification |
| 1 (TIN0M0) | 25 (31.25) |
| 2 (T2N0M0) | 34 (42.5) |
| 3 (T2N1M0 or
T3N0M0) | 21 (25) |
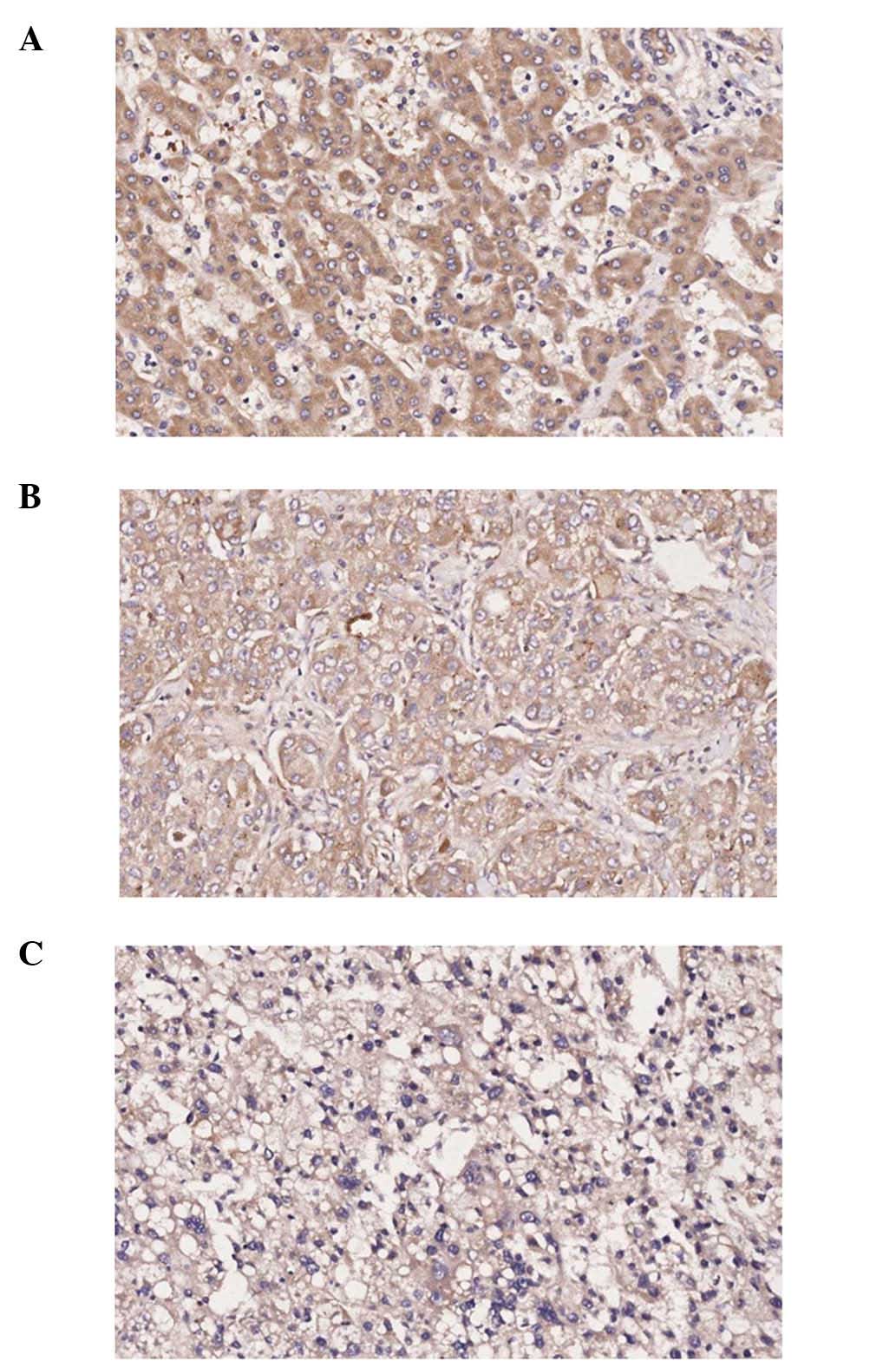
Protein location of LOXL2 in HCC tissue
samples
Expression of LOXL2 was detected in matched ANT and
TT samples by IHC staining, LOXL2 staining was detected in the
cytoplasm, using normal liver tissue as a control (Fig. 8).
Association of LOXL2 expression with
clinicopathological factors
Characteristics of the 80 HCC patients were
compared. Using horizontal comparative research, LOXL2 expression
was demonstrated to be higher in ANT samples than in TT samples,
and a statistical significance was indicated for the majority of
clinicopathological factors, including gender, age, vascular
invasion, tumor number, Child-Pugh grade A, HbsAg, liver cirrhosis
positive and AFP (P<0.05; Table
II). Vertical comparison in ANT and TT samples indicated no
significant association was observed between LOXL2 expression
levels and all the clinicopathological factors, except histological
grade (P>0.05, data not shown).
 | Table IIExpression of lysyl oxidase-like 2 in
ANT and TT of hepatocellular carcinoma patients. |
Table II
Expression of lysyl oxidase-like 2 in
ANT and TT of hepatocellular carcinoma patients.
| Characteristic | IOD of LOXL2
expression (D/µm2, X±SD)
| P-value (TT vs.
ANT) |
|---|
| ANT | TT |
|---|
| Gender |
| Male |
62079.73±26373.13 |
36455.52±20406.78 | <0.0001b |
| Female |
60380.53±25146.45 |
44101.86±28961.48 | 0.365 |
| Age (years) |
| <50 |
70898.34±26281.21 |
38834.03±22720.20 | 0.003b |
| ≥50 |
55393.36±24145.98 |
36737.76±2146.00 | <0.0001b |
| Vascular
invasion |
| Negative |
58405.79±28093.89 |
37583.48±22365.60 | 0.005b |
| Positive |
63486.61±25120.36 |
37611.61±21813.34 | <0.0001b |
| Tumor size
(cm) |
| Single ≤5, 3
nodules ≤3, and no invasion |
62813.08±30431.63 |
32362.90±18411.26 | 0.117 |
| Single >5, or
multiple nodules |
54942.92±26746.69 |
41411.91±24779.17 | 0.101 |
| Vascular
invasion |
63486.61±25120.36 |
37611.61±21852.23 | 0.110 |
| Number of
tumors |
| 1 |
61491.61±27278.68 |
37091.56±21566.21 | <0.0001b |
| ≥2 |
63055.97±21830.35 |
39362.26±23365.34 | 0.011a |
| Child-Pugh
grade |
| A |
62751.23±24479.83 |
37348.61±21706.92 | <0.0001b |
| B |
51018.5115±42073.69 |
40733.37±25548.37 | 0.412 |
| HBsAg |
| Negative |
60973.84±24476.70 |
38580.76±19154.65 | <0.0001b |
| Positive |
62160.67±26818.31 |
37254.26±22878.54 | <0.0001b |
| Liver
cirrhosis |
| Negative |
57683.43±19908.48 |
37838.50±21361.29 | 0.304 |
| Positive |
62188.00±26583.26 |
34691.35±29525.76 | <0.0001b |
| AFP (ng/ml) |
| ≤20 |
59293.34±30001.59 |
35868.95±17343.50 | 0.005b |
| >20 |
6329.51±23705.50 |
38588.19±24151.63 | <0.0001b |
| Histological
grade |
| I |
26391.16±18678.73 |
25509.08±7018.12 | 0.887 |
| I–II |
46181.98±26941.30 |
30407.17±17293.95 | 0.217 |
| II |
65032.08±24868.29 |
43165.85±24588.47 | <0.0001b |
| II–III |
58476.11±33510.37 |
43891.44±13002.07 | 0.304 |
| III |
67242.60±22814.09 |
27042.97±15674.68 | <0.0001b |
| TNM
classification |
| 1 (TIN0M0) |
58089.55±28652.64 |
36475.76±22086.88 | 0.005b |
| 2 (T2N0M0) |
61571.45±24221.65 |
37021.99±22073.14 | <0.0001b |
| 3 (T2N1M0,
T3N0M0) |
67045.77±26313.12 |
39883.61±22119.33 | 0.001b |
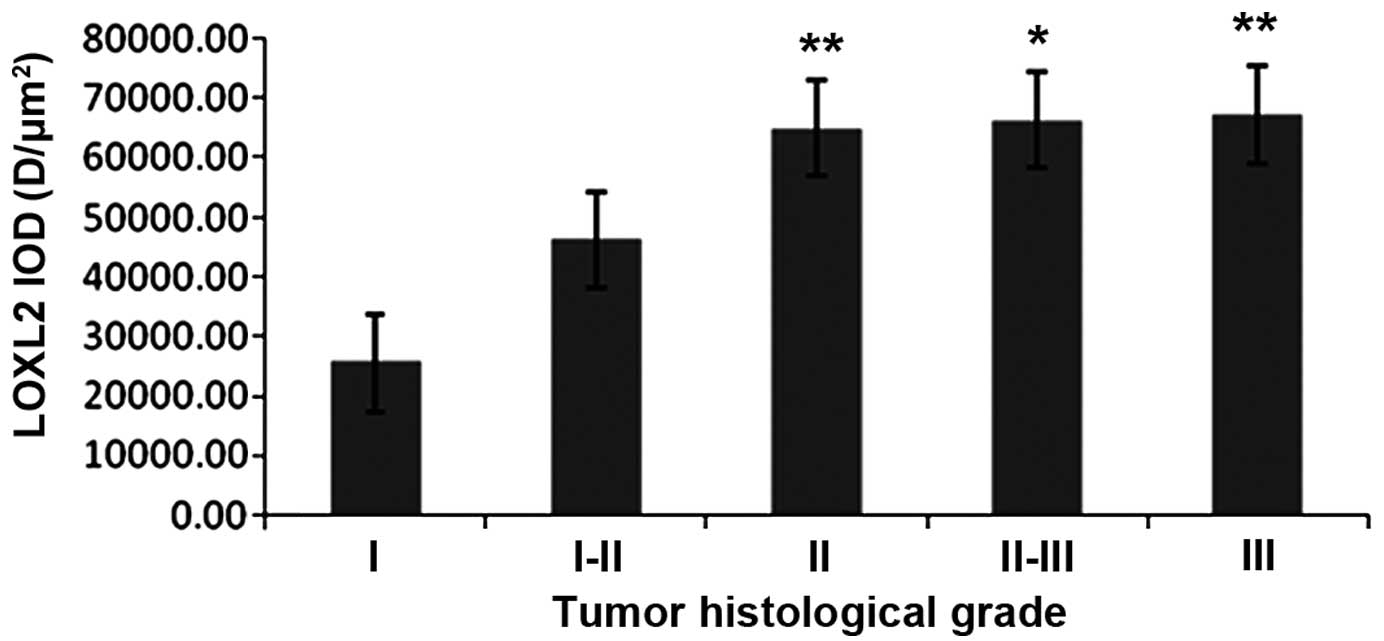
LOXL2 expression levels were altered in
different histological grades
IHC staining of HCC samples indicated that the
protein expression level of LOXL2 was increased in matched ANT
compared with TT in grade II (P=<0.0001) and grade III
(P=<0.0001) subgroup patients (Table II). In matched ANT samples, the
expression levels of LOXL2 was increased with the tumor
histological grade, LOXL2 expression was higher in grade II than in
grade I (P=0.003) and it was gradually increased in grade II–III
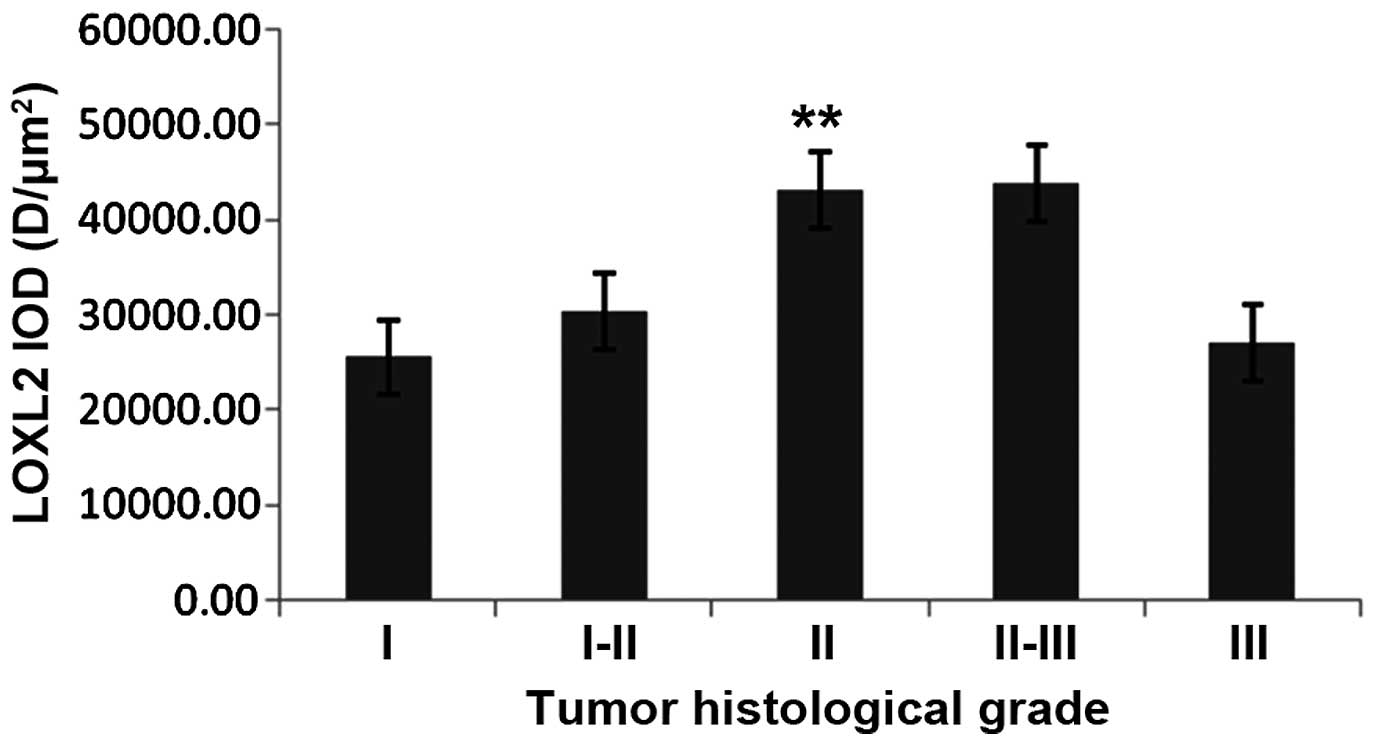
(P<0.05) and grade III (P<0.01) subgroup patients (Fig. 9). In TT samples, the expression
increased to grade II (P<0.01), while it decreased in grade III
with no marked difference between grade II–III and III (P=0.076;
Fig. 10).
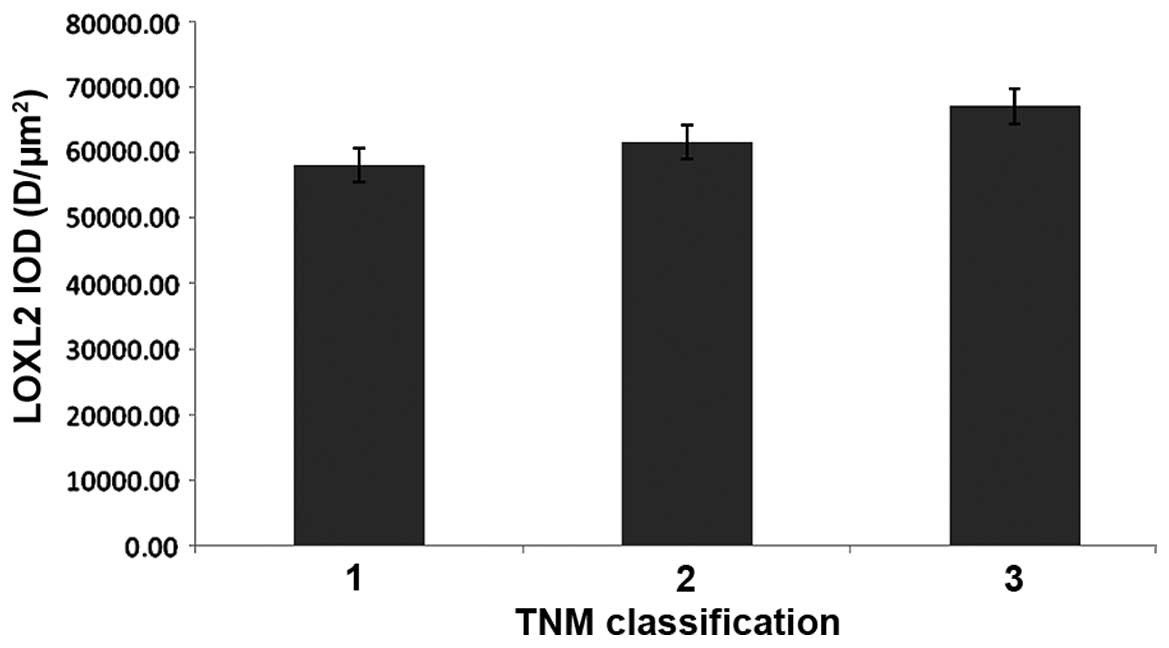
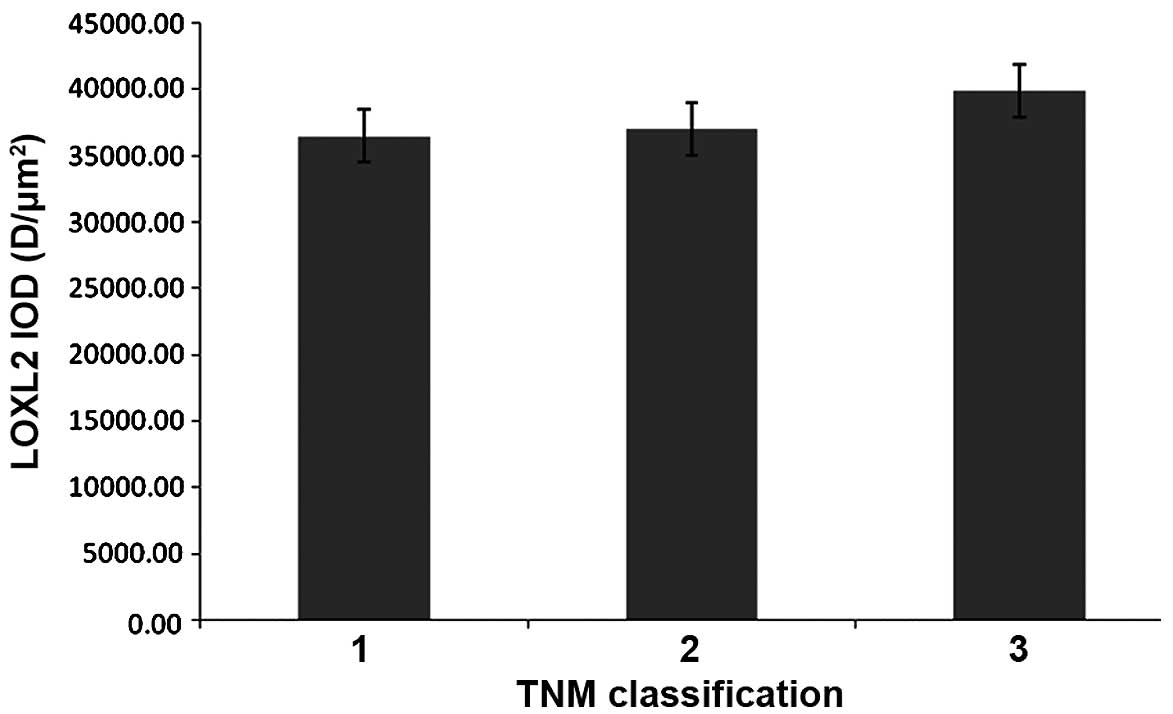
LOXL2 expression differed with TNM
classification
In HCC samples, the protein expression of LOXL2 in
ANT samples was increased compared with TT in TNM classification 1
(P=0.005), classification 2 (P=<0.0001) and classification 3
(P=0.001). In addition, LOXL2 expression was gradually increased
with more advanced TNM classification in matched ANT and TT
(Table II), but there were no
significant differences observed in the ANT or TT samples (Figs. 11 and 12).
Discussion
In the present study, the mRNA expression levels of
LOXL2 were decreased in the two cell lines infected with siRNA
lentiviral vector, and the cell numbers, proliferation and
anchorage-independent growth ability were significantly decreased.
In addition, the rate of cell apoptosis increased. The results of
the current study provide novel evidence that silencing LOXL2 may
decrease the proliferation of HCC cells in vitro.
Furthermore, in the cell cycle distribution, LOXL2 silencing
notably decreased the fraction of G1 phase cells in
HepG2 and SMMC-7721 cells and increased the fraction of
G2/M phase cells in HepG2 cells and S-phase cells in
SMMC-7721 cells. This indicates that LOXL2 contributes to HCC cell
growth in the G1 phase as when the expression is
reduced, HCC growth may be blocked. This may be beneficial in novel
therapeutic strategies for HCC patients in the future.
Wong et al (15) demonstrated that LOXL2 was
overexpressed in human HCC. The present study of HCC patients
further demonstrated that LOXL2 was positively expressed in the
cytoplasm and that the expression of LOXL2 in matched ANT samples
was markedly increased compared with TT samples. Furthermore, LOXL2
expression increased with histological grade and more advanced TNM
classification. The results of the present study were consistent
with recent reports. The tumor-stroma crosstalk has been
demonstrated to be important in tumor progression. Increased LOXL2
expression resulted in tumor progression and metastasis, likely by
promoting tumor cell invasion and remodeling of the tumor
microenvironment (21–25). It has been demonstrated that LOXL2
mediates induction of epithelial-mesenchymal transition and matrix
remodeling enzymes to modify the tumor microenvironment, thus,
promoting survival and proliferation of the tumor cells (26–29).
These results contribute to improved understanding of the
association between LOXL2 expression levels and clinicopathological
factors in HCC.
In conclusion, LOXL2 promotes HCC proliferation and
is highly expressed in ANT samples compared with TT samples. These
results aid improved understanding of the importance of LOXL2 in
HCC, laying foundation for future research. Furthermore, additional
in vitro and clinical studies are required to fully
understand the role of LOXL2 in HCC.
Acknowledgments
The authors would like to thank GeneChem Co., Ltd.
(Shanghai, China) for construction of the lentiviral vector and
synthesis of the pGCSIL-GFP plasmid. The present study was
supported by the National Natural Science Foundation of China
(grant no. 81273925).
References
|
1
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma-epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
2
|
Abrams P and Marsh JW: Current approach to
hepatocellular carcinoma. Surg Clin North Am. 90:803–816. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin CL and Kao JH: Optimal management of
hepatocellular carcinoma: Challenges and opportunities. J
Gastroenterol Hepatol. 25:1336–1338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cano A, Santamaría PG and Moreno-Bueno G:
LOXL2 in epithelial cell plasticity and tumor progression. Future
Oncol. 8:1095–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akiri G, Sabo E, Dafni H, Vadasz Z,
Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M
and Neufeld G: Lysyl oxidase-related protein-1 promotes tumor
fibrosis and tumor progression in vivo. Cancer Res. 63:1657–1666.
2003.PubMed/NCBI
|
|
6
|
Payne SL, Hendrix MJ and Kirschmann DA:
Paradoxical roles for lysyl oxidases in cancer-a prospect. J Cell
Biochem. 101:1338–1354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayashi K, Fong KS, Mercier F, Boyd CD,
Csiszar K and Hayashi M: Comparative immunocytochemical
localization of lysyl oxidase (LOX) and the lysyl oxidase-like
(LOXL) proteins: Changes in the expression of LOXL during
development and growth of mouse tissues. J Mol Histol. 35:845–855.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peinado H, Moreno-Bueno G, Hardisson D,
Pérez-Gómez E, Santos V, Mendiola M, de Diego JI, Nistal M,
Quintanilla M, Portillo F and Cano A: Lysyl oxidase-like 2 as a new
poor prognosis marker of squamous cell carcinomas. Cancer Res.
68:4541–4550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li TY, Xu LY, Wu ZY, Liao LD, Shen JH, Xu
XE, Du ZP, Zhao Q and Li EM: Reduced nuclear and ectopic
cytoplasmic expression of lysyl oxidase-like 2 is associated with
lymph node metastasis and poor prognosis in esophageal squamous
cell carcinoma. Hum Pathol. 43:1068–1076. 2012. View Article : Google Scholar
|
|
10
|
Macartney-Coxson DP, Hood KA, Shi HJ, Ward
T, Wiles A, O'Connor R, Hall DA, Lea RA, Royds JA, Stubbs RS and
Rooker S: Metastaticsusceptibility locus, an 8p hot-spot for tumour
progression disrupted in colorectal liver metastases: 13 candidate
genes examined at the DNA, mRNA and protein level. BMC Cancer.
8:1872008. View Article : Google Scholar
|
|
11
|
Offenberg H, Brünner N, Mansilla F,
Orntoft Torben F and Birkenkamp-Demtroder K: TIMP-1 expression in
human colorectal cancer is associated with TGF-B1, LOXL2, INHBA1,
TNF-AIP6 and TIMP-2 transcript profiles. Mol Oncol. 2:233–240.
2008. View Article : Google Scholar
|
|
12
|
Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z,
Sun YM, Sun LC, Pan J, Sun LX, et al: Secreted LOXL2 is a novel
therapeutic target that promotes gastric cancer metastasis via the
Src/FAK pathway. Carcinogenesis. 30:1660–1669. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sano M, Aoyagi K, Takahashi H, Kawamura T,
Mabuchi T, Igaki H, Tachimori Y, Kato H, Ochiai A, Honda H, et al:
Forkhead box A1 transcriptional pathway in KRT7-expressing
esophageal squamous cell carcinomas with extensive lymph node
metastasis. Int J Oncol. 36:321–330. 2010.PubMed/NCBI
|
|
14
|
Brekhman V and Neufeld G: A novel
asymmetric 3D in-vitro assay for the study of tumor cell invasion.
BMC Cancer. 9:4152009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong CC, Tse AP, Huang YP, Zhu YT, Chiu
DK, Lai RK, Au SL, Kai AK, Lee JM, Wei LL, et al: Lysyl
oxidase-like 2 is critical to tumor microenvironment and metastatic
niche formation in hepatocellular carcinoma. Hepatology.
60:1645–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrandon S, Saultier P, Carras J,
Battiston-Montagne P, Alphonse G, Beuve M, Malleval C, Honnorat J,
Slatter T, Hung N, et al: Telomere profiling: Toward glioblastoma
personalized medicine. Mol Neurobiol. 47:64–76. 2013. View Article : Google Scholar
|
|
17
|
Hahn WC, Dessain SK, Brooks MW, King JE,
Elenbaas B, Sabatini DM, DeCaprio JA and Weinberg RA: Enumeration
of the simian virus 40 early region elements necessary for human
cell transformation. Mol Cell Biol. 22:2111–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song S, Zhou J, He S, Zhu D, Zhang Z, Zhao
H, Wang Y and Li D: Expression levels of microRNA-375 in pancreatic
cancer. Biomed Rep. 1:393–398. 2013.
|
|
19
|
Zheng Y, Wang DD, Wang W, Pan K, Huang CY,
Li YF, Wang QJ, Yuan SQ, Jiang SS, Qiu HB, et al: Reduced
expression of uroplakin 1A is associated with the poor prognosis of
gastric adenocarcinoma patients. PLoS One. 9:e930732014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li TY, Xu LY, Wu ZY, Liao LD, Shen JH, Xu
XE, Du ZP, Zhao Q and Li EM: Reduced nuclear and ectopic
cytoplasmic expression of lysyl oxidase-like 2 is associated with
lymph node metastasis and poor prognosis in esophageal squamous
cell carcinoma. Hum Pathol. 43:1068–1076. 2012. View Article : Google Scholar
|
|
21
|
Erler JT, Bennewith KL, Nicolau M,
Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barry-Hamilton V, Spangler R, Marshall D,
McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien
H, Wai C, et al: Allosteric inhibition of lysyl oxidase-like-2
impedes the development of a pathologic microenvironment. Nat Med.
16:1009–1017. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barker HE, Chang J, Cox TR, Lang G, Bird
D, Nicolau M, Evans HR, Gartland A and Erler JT: LOXL2-mediated
matrix remodeling in metastasis and mammary gland involution.
Cancer Res. 71:1561–1572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peinado H, Del Carmen Iglesias-de la Cruz
M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A and
Portillo F: A molecular role for lysyl oxidase-like 2 enzyme in
snail regulation and tumor progression. EMBO J. 24:3446–3458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahadevan D and Von Hoff DD: Tumor-stroma
interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther.
6:1186–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radisky D, Hagios C and Bissell MJ: Tumors
are unique organs defined by abnormal signaling and context. Semin
Cancer Biol. 11:87–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tlsty TD and Coussens LM: Tumor stroma and
regulation of cancer development. Annu Rev Pathol. 1:119–150. 2006.
View Article : Google Scholar
|