Introduction
The World Health Organization has reported that
coronary heart disease (CHD), which is primarily caused by
atherosclerosis, remains the leading cause of mortality worldwide,
resulting in 7.4 million deaths in 2012 (1). Current strategies, including risk
factor control and percutaneous coronary intervention (PCI) are
somewhat effective (2–4). However, lumen restenosis often occurs
following PCI. Therefore, novel treatment strategies are required.
Atherosclerosis, which is critical for the development of CHD, is
often the result of vascular endothelial injury (5). Therefore, maintaining the integrity
of the functional endothelial monolayer is crucial for the
prevention of atherosclerosis initiation and the treatment of CHD
(5). Previous studies have
demonstrated that endothelial progenitor cells (EPCs), from origins
including the spleen and bone marrow, homed to sites of vascular
injury for re-endothelialization upon introduction into the
circulation, suggesting a vascular restoration role for EPCs
(6–9). However, EPCs proliferate slowly and
the number of EPCs in peripheral blood is limited, thus restricting
their clinical application (10).
A previous study has demonstrated that inhibitor of DNA binding 1
(Id1) flips the angiogenic switch via the regulation of EPC
migration from bone marrow (11).
Furthermore, our previous research revealed that Id1 markedly
promoted EPC proliferation (12).
However, the mechanisms underlying Id1-mediated EPC proliferation
remain to be elucidated.
Id1 is a member of the helix-loop-helix (HLH)
family, which is involved in numerous developmental processes,
including cellular proliferation, migration and differentiation,
and functions as a heterodimer with other HLH proteins (13). Previous studies have demonstrated
that Id1 is critical in the regulation of cell cycle progression of
tumor cells by increasing cyclin D1 expression and promoting
G1/S transition (14–17).
Therefore, Id1 may be involved in regulation of the cell cycle of
EPCs.
Cyclin D1, a well-known positive cell regulator from
G1 to S phase, is also a classic target gene of the
canonical wingless-type mouse mammary tumor virus integration site
(Wnt)/β-catenin signaling pathway (17,18).
Among the Wnt family, Wnt2 has been demonstrated to contribute to
vascularization, and acts as a positive signal in the
differentiation of stem cells into vascular endothelial cells
(19–21). Furthermore, a previous study has
revealed that Wnt2 was diminished in liver sinusoidal endothelial
cells of Id1-deficient mice, and administration of exogenous Wnt2
restored hepatovascular regeneration in Id1-deficient mice
(22). However, the association
between Id1 and Wnt2, and the effect of this on cell cycle
regulation in EPCs remains to be elucidated.
In the present study, it was hypothesized that Id1
acts on the cell cycle progression of EPCs via the regulation of
Wnt2 expression. Id1-overexpressed EPCs and Id1-knockdown EPCs were
therefore used as models to investigate the role of Id1 on cell
cycle regulation in EPCs and its possible underlying mechanisms.
The in vitro data generated by the present study indicated
that Id1 promoted cell cycle progression of EPCs from G1
to S phase via a Wnt2-dependent mechanism.
Materials and methods
Culture and characterization of bone
marrow-derived EPCs
All procedures were approved by the Care of Animal
Experiment Committee of Third Military Medical University
(Chongqing, China). A total of 150 C57BL/6J male mice (age, 6–8
week; weight, 22–30 g) were obtained from the Experimental Animal
Center of Third Military Medical University. Mice were housed at
20–26°C with 40–70% humitidy, under a 12-h light/dark cycle and
with ad libitum access to food and water. The culture and
characterization of bone marrow-derived EPCs were performed as
described previously (23,24). Briefly, bone marrow-derived
mononuclear cells were isolated from the tibia and femur of
C57BL/6J mice by density gradient centrifugation using
Histopaque®-1083 (Sigma-Aldrich, St. Louis, MO, USA).
The mononuclear cells were cultured at 37°C in Dulbecco's modified
Eagle's medium (DMEM)/F-12 (GibcThermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 20% fetal bovine serum (FBS;
HyClonGE Healthcare Life Sciences, Logan, UT, USA) in cell culture
flasks coated with gelatin. After 24 h, non-adherent cells were
separated and seeded into a new flask. Following an additional 48
h, non-adherent cells were removed and adherent cells were cultured
continuously for in vitro experiments.
For the characterization assay, cells were incubated
at 37°C with acetylated low density lipoprotein, labeled with
1,1′diocta-decyl-3,3,3′, 3′-tetramethylindocarbocyanine perchlorate
(DiI-Ac-LDL; Biomedical Technologies, Inc., Stoughton, MA, USA) for
4 h, fixed with 4% paraformaldehyde and incubated at 37°C with
fluorescein isothiocyanate-Ulex europaeus agglutinin-1
(FITC-UEA-l; Sigma-Aldrich) for 1 h. Cells were then incubated with
DAPI for 5 min and observed under an immunofluorescence laser
scanning confocal microscope (Leica TCS; Leica Microsystems GmbH,
Wetzlar, Germany). DiI-Ac-LDL and FITC-UEA-l dual-stained cells
were identified as EPCs. Additionally, flow cytometric analysis
(FCM) was performed as described previously (12) with the following FITC-conjugated
antibodies: Rat anti-mouse stem cell antigen-1 (Sca-1; 1
µg/105 cellcatalog no. ab25031; Abcam, Cambridge,
UK), rabbit anti-mouse vascular endothelial growth factor receptor
2 (VEGFR-2; 2 µg/105 cell catalog no. ab11939;
Abcam) and the corresponding rat IgG2a and rabbit IgG isotype
control antibodies (2 µg/105 cell catalog nos.
ab18446 and ab171870, respectively; Abcam).
Infection of recombinant adenoviral
vectors expressing Id1
Recombinant adenoviruses expressing mouse Id1
(Ad-Id1) and negative control adenoviruses (Ad-vector) were
synthe-sized by Hanbio Technology (Shanghai) Co., Ltd. (Shanghai,
China). Infection of adenoviruses was performed according to the
manufacturer's instructions. Following seven days of culture, EPCs
were seeded onto gelatin-coated 6-well plates at a density of
7×105 cells/well and incubated at 37°C for 24 h. Prior
to infection, cells were starved of serum overnight by incubation
in DMEM/F-12 medium supplemented with 0.5% FBS. The culture medium
was subsequently replaced with medium containing adenoviruses (at a
multiplicity of infection of 300) for 2 h, followed by fresh medium
for an additional 72-h culture at 37°C. Infected EPCs were then
harvested for subsequent experiments.
Transfection of small interfering RNA
(siRNA)
The mRNA sequences of mouse Id1 (NM_010495.3) and
mouse Wnt2 (NM_023653.5) were acquired from the National Center for
Biotechnology Information database (www.ncbi.nlm.nih.gov/). siRNA of mouse Id1 (si-Id1),
mouse Wnt2 (si-Wnt2) and a non-silencing control sequence (si-con)
were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The siRNA sequences are presented in Table I. For siRNA transfection, EPCs were
prepared as per adenovirus infection. The siRNA transfections were
performed with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Following transfection with siRNA, EPCs were cultured
at 37°C for an additional 72 h prior to harvesting for subsequent
experiments.
 | Table IsiRNA sequences. |
Table I
siRNA sequences.
| siRNA | Forward | Reverse |
|---|
| si-Id1 |
5′-CUUGGUCUGUCGGAGCAAATT-3′ |
5′-UUUGCUCCGACAGACCAAGTT-3′ |
| si-Wnt2 |
5′-ACACCCAGAUGUGAUGCGUGCCATT-3′ |
5′-UGGCACGCAUCACAUCUGGGUGUTT-3′ |
| si-con |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
5′-ACGUGACACGUUCGGAGAATT-3′ |
Cell cycle analysis
Cell cycle analyses were performed using FCM to
measure DNA content. Briefly, EPCs were harvested by centrifugation
at 472 × g for 5 min at room temperature and washed with
phosphate-buffered saline (PBS). Cells were fixed with 70% ethanol
overnight at 4°C, washed with PBS and stained using a Cell Cycle
Analysis kit (Beyotime Institute of Biotechnology, Shanghai, China)
at 37°C for 30 min in the dark. Analysis was performed on ~20,000
cells/sample using a MoFlo™ XDP flow cytometer (Beckman Coulter,
Inc., Brea, CA, USA) and ModFit LT™ software version 3.2 (Verity
Software House, Inc. Topsham, ME, USA).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from EPCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse-transcribed to cDNA using a Reverse Transcription
kit (Takara Bio, Inc., Otsu, Japan). RT-PCR was performed using
gene specific primers and 2xEs Taq MasterMix (CWBio, Beijing,
China) according to the manufacturer's instructions. PCR cycling
conditions were as follows: Pre-denaturation at 94°C for 2 min,
followed by 35 cycles of denaturation at 94°C for 30 sec, annealing
at 62°C for 30 sec and extension at 72°C for 30 sec, with a final
incubation at 72°C for 2 min. Bands were quantified with Quantity
One® version 4.6.2 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The gene specific primers are presented in
Table II.
 | Table IIReverse transcription-polymerase
chain reaction primer sequences. |
Table II
Reverse transcription-polymerase
chain reaction primer sequences.
| Gene | Forward | Reverse | Product size,
bp |
|---|
| Id1 |
5′-CGAGGTGGTACTTGGTCTGTC-3′ |
5′-GGTCCCTGATGTAGTCGAT-3′ | 218 |
| Cyclin D1 |
5′-TGACTGCCGAGAAGTTGTGC-3′ |
5′-CTCATCCGCCTCTGGCATT-3′ | 164 |
| Wnt2 |
5′-GTGATGTGTGACAATGTGCCA-3′ |
5′-GTTGCAGTTCCAGCGATGC-3′ | 150 |
| β-actin |
5′-CACTGTGCCCATCTACGA-3′ |
5′-CAGGATTCCATACCCAAG-3′ | 477 |
Western blot analysis
EPCs were lysed in protease inhibitor-containing
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). Cytoplasmic and nuclear proteins were extracted
with a Nuclear and Cytoplasmic Protein Extraction kit (Beyotime
Institute of Biotechnology), used according to the manufacturer's
instructions. Protein samples (20 µg) were separated on 12%
SDS-PAGE gels (concentration, 80 V for 30 min; separation, 120 V
for 70 min) and transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked within 5% non-fat milk for 2 h followed by incubation with
primary antibodies overnight at 4°C. The primary antibodies were as
follows: Rabbit anti-mouse Id1 (1:1,000; catalog no. ab134163;
Abcam), rabbit anti-mouse cyclin D1 (1:2,000; catalog no. ab134175;
Abcam), rabbit anti-mouse Wnt2 (1:500; catalog no. ab27794; Abcam),
rabbit anti-mouse β-catenin (1:1,000; catalog no. ab6302; Abcam),
rat anti-mouse β-actin (1:1,000; catalog no. aa128; Beyotime
Institute of Biotechnology) and rabbit anti-mouse histone (1:1,000;
catalog no. ab1791; Abcam). Membranes were then incubated with the
corresponding secondary antibody conjugated to horseradish
peroxidase, goat anti-rabbit IgG (catalog no. sc-2004; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or goat anti-rat IgG (catalog
no. sc-2006; Santa Cruz Biotechnology, Inc.), diluted 1:5,000 for 1
h at 37°C. The chemiluminescent signal was developed with enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.) and detected by
ImageQuant™ LAS 4000 mini (GE Healthcare Bio-Sciences, Pittsburgh,
PA, USA). The signal was then quantified with ImageQuant TL version
7.0 (GE Healthcare Bio-Sciences).
Statistical analysis
All experiments were performed at least three times
independently. Data are presented as the mean ± standard deviation.
Statistical analysis was performed with with SPSS software version
19.0 (IBM SPSS, Armonk, NY, USA) using one-way analysis of variance
followed by Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of bone marrow-derived
EPCs
Following 4–7 days of culture, the adherent cells
became spindle-shaped and grew in small colonies or linearly, which
were considered to be EPCs (Fig.
1A). Immunofluorescence was performed to confirm the identity
of EPCs. As presented in Fig. 1B,
91.4±2.0% of adherent cells were double-stained with DiI-Ac-LDL and
FITC-UEA-l. The double-stained cells were confirmed as EPCs. In
addition, cells were characterized by FCM to detect the expression
of Sca-1 (a mouse stem cell marker) and VEGFR-2 (an endothelial
cell marker). The percentage of positive cells was 84.5 and 80.2%,
respectively (Fig. 1C).
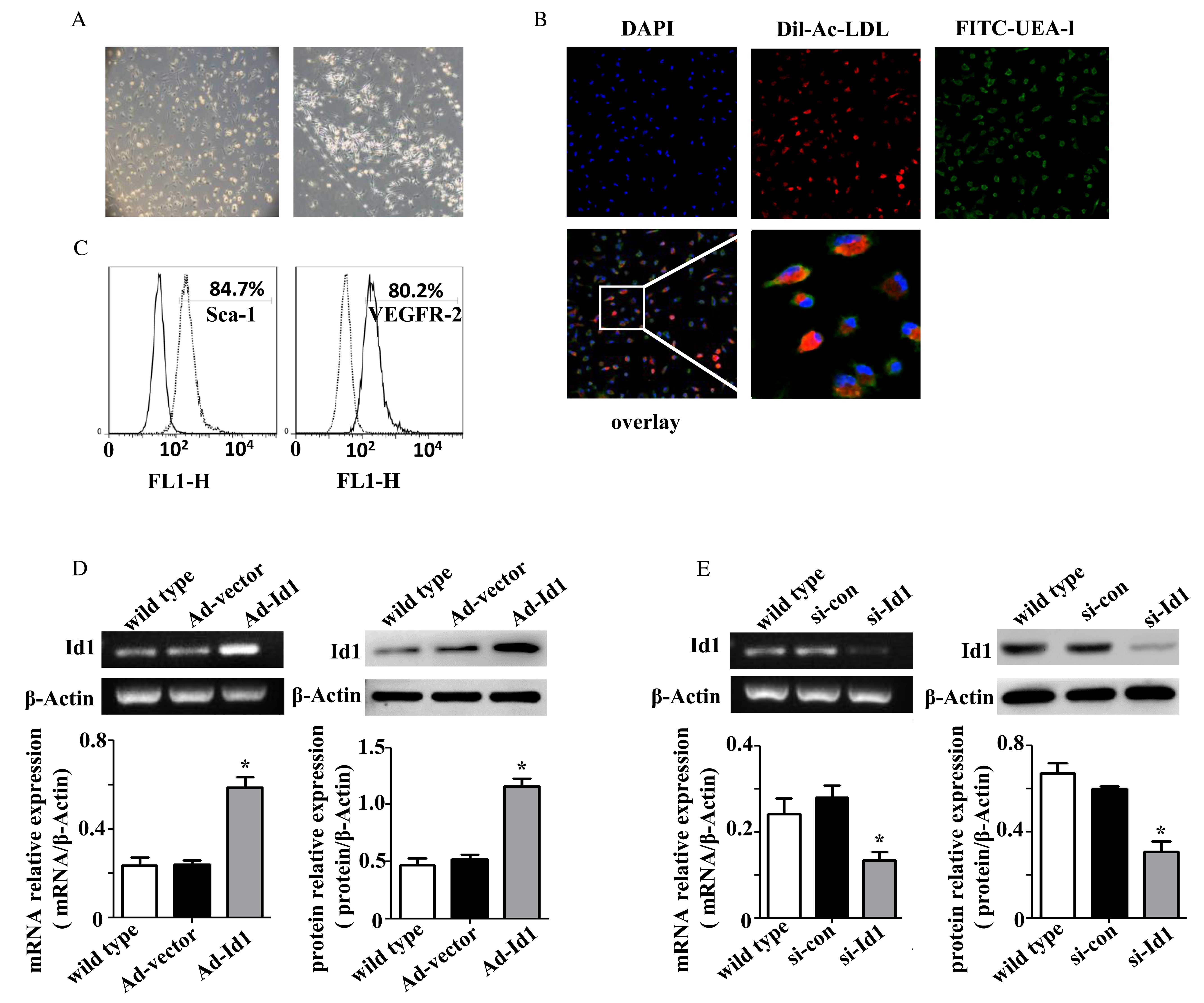 | Figure 1Characterization of bone
marrow-derived EPCs. (A) Following 4–7 days culture, bone
marrow-derived EPCs became spindle-shaped, elliptical or
triangular, and grew in small colonies or linearly (magnification,
×200). (B) EPC uptake of DiI-Ac-LDL (red) and binding of FITC-UEA-l
(green) was determined by fluorescence microscopy (magnification,
×200; magnification of the final image, ×800). The majority of
cells were double stained and therefore confirmed as EPCs. (C)
Cells were incubated with fluorescent antibodies recognizing Sca-1
and VEGFR-2 (right peaks), or the corresponding negative controls
(left peaks). The lines denote the positive gate and the numbers
indicate the percentage of cells within this positive gate. The
expression levels of Id1 mRNA and protein from EPCs treated with
(D) Ad-Id1 or (E) si-Id1 were detected using reverse
transcription-polymerase chain reaction and western blot analysis,
respectively. Overexpression of Id1 using Ad-Id1 increased Id1 mRNA
and protein expression levels, while knockdown of Id1 with si-Id1
decreased Id1 mRNA and protein expression levels. The expression
level was analyzed relative to β-actin (n=3). *P<0.05
vs. wild type. EPCs, endothelial progenitor cellDiI-Ac-LDL,
acetylated low density lipoprotein, labeled with
1,1′dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine
perchloratFITC-UEA-1, fluorescein isothiocyanate-Ulex
europaeus agglutinin-1; Sca-1, stem cell antigen-1; VEGFR-2,
vascular endothelial growth factor receptor 2; Ad, adenoviruId1,
inhibitor of DNA binding 1; si, small interfering; con,
control. |
EPCs were infected with adenoviruses to overexpress
exogenous Id1, or transfected with siRNA to knockdown endogenous
Id1. The efficiency was detected by RT-PCR and western blot
analysis (Fig. 1D and E). The
expression level of Id1 in Ad-Id1 EPCs was upregulated ~3-fold
compared with wild type EPCs (P=0.001; n=3); no difference was
observed between Ad-vector and wild type EPCs (P=0.924; n=3). The
expression level of Id1 in si-Id1 EPCs was downregulated ~70%
compared with wild type EPCs (P=0.039; n=3); no significant
difference was observed between si-con and wild type EPCs (P=0.645;
n=3).
Effects of Id1 on cell cycle progression
and cyclin D1 expression levels in EPCs
Cell cycle progression is closely associated with
proliferation. To investigate whether Id1 is involved in cell cycle
progression of EPCs, FCM was performed to analyze the EPC cell
cycle. The percentage of EPCs in G1 phase decreased from
74.04±2.56 to 59.12±2.87% following Ad-Id1 transfection (P=0.001;
n=3), and the percentage in S/G2M phases increased from
25.96±2.56 to 40.88±2.87% (P=0.001; n=3; Fig. 2A). The percentage of EPCs in
G1 phase increased >10% following transfection with
si-Id1 (P<0.001; n=3) and the percentage in S/G2M
phases decreased significantly compared with wild type EPCs
(P<0.001; n=3; Fig. 2B). These
results demonstrate that Id1 may regulate cell cycle progression of
EPCs.
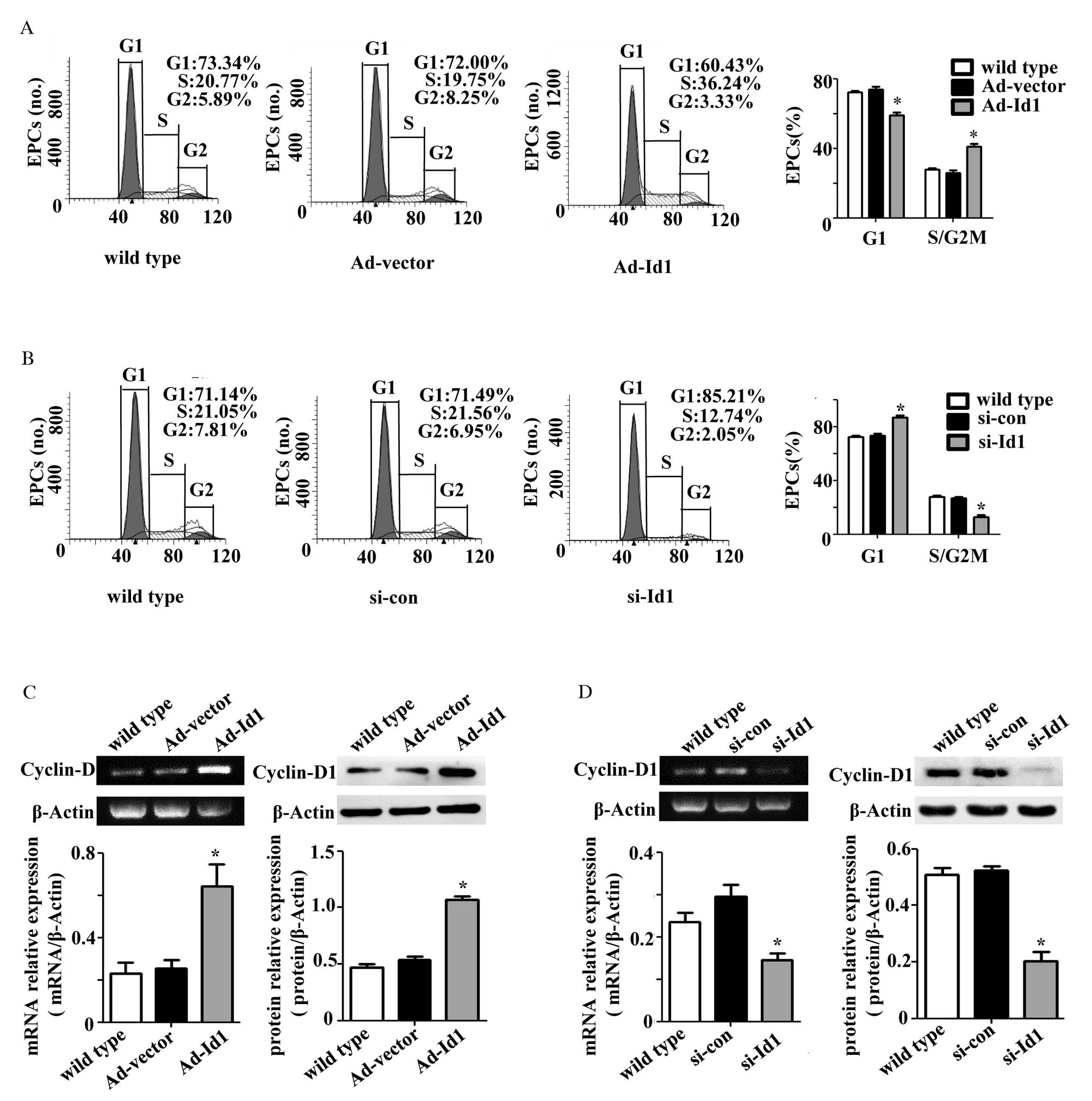 | Figure 2Effects of Id1 on cell cycle
progression of EPCs. The cell cycle distribution of EPCs,
transfected with (A) Ad-Id1 or (B) si-Id1, was analyzed by flow
cytometry. Ad-Id1 transfection decreased the percentage of EPCs in
G1 phase and increased the percentage in
S/G2M phases, while si-Id1 transfection induced the
opposite effect. Cyclin D1 mRNA and protein expression levels from
EPCs treated with (C) Ad-Id1 or (D) si-Id1 were detected using
reverse transcription-polymerase chain reaction and western blot
analysis, respectively. Ad-1d1 transfection increased, and si-Id1
transfection decreased, cyclin D1 mRNA and protein expression
levels. The expression level was analyzed relative to β-actin
(n=3). *P<0.05 vs. wild type. EPCs, endothelial
progenitor cellAd, adenoviruId1, inhibitor of DNA binding 1; si,
small interfering; con, control. |
To further investigate the role of Id1 on cell cycle
regulation of EPCs, the expression level of cyclin D1 was detected
by RT-PCR and western blot analysis. Id1 overexpression
significantly increased cyclin D1 mRNA (P=0.007; n=3) and protein
(P<0.001; n=3) expression levels compared with wild type EPCs
(Fig. 2C). In addition, Id1
knockdown significantly decreased cyclin D1 mRNA (P=0.031; n=3) and
protein (P<0.001; n=3) expression levels (Fig. 2D). These results suggest that Id1
may promote cell cycle progression from G1 to S phase
and regulate cyclin D1 expression levels in EPCs.
Id1 regulates Wnt2 expression levels and
β-catenin nuclear translocation in EPCs
To investigate the underlying mechanism of cell
cycle regulation by Id1, the activation of the Wnt signaling
pathway, which is upstream of cyclin D1 (18), was examined. As Wnt2 is required
for expanding the vascular progenitor population (21), and β-catenin is central to the Wnt
canonical signaling pathway (25),
the Wnt2 expression level and β-catenin nuclear translocation in
EPCs were analyzed. The mRNA (P<0.001; n=3) and protein
(P<0.001; n=3) expression levels of Wnt2 were significantly
increased in Ad-Id1 EPCs compared with wild type EPCs (Fig. 3A and B). In addition, Id1
significantly increased the cytoplasmic (P=0.001; n=3) and nuclear
β-catenin protein (P=0.001; n=3) expression levels (Fig. 3B and C). Id1 knockdown by si-Id1
led to a reduction in Wnt2 mRNA (P<0.001; n=3) and protein
(P=0.001; n=3) expression levels compared with wild type EPCs
(Fig. 3D and E). In addition, Id1
knockdown significantly decreased the β-catenin protein expression
levels in the cytoplasm (P<0.001; n=3) and nucleus (P<0.001;
n=3; Fig. 3E and F). These results
demonstrate that Id1 may upregulate Wnt2 expression levels, and
promote β-catenin accumulation in the cytoplasm and translocation
to the nucleus of EPCs.
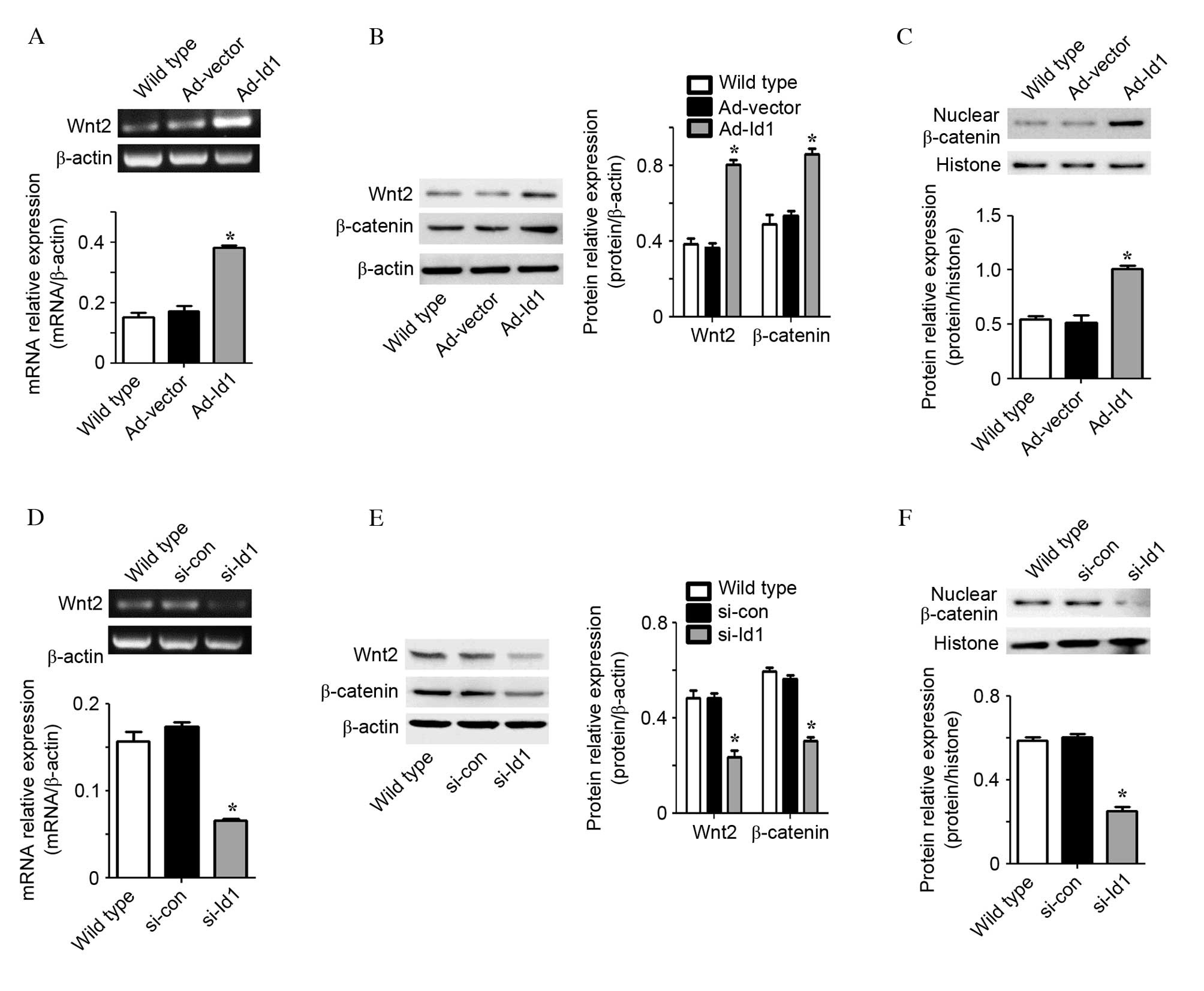 | Figure 3Id1 regulates Wnt2 expression levels
and β-catenin nuclear translocation in EPCs. (A) Wnt2 mRNA
expression levels following treatment of EPCs with Ad-Id1, as
detected by RT-PCR. (B) The protein expression levels of Wnt2 and
cytoplasmic β-catenin in EPCs treated with Ad-Id1 were detected by
western blot analysis. The expression level was analyzed relative
to β-actin. (C) Nuclear β-catenin protein expression levels in
Ad-Id1 EPCs were detected by western blot analysis and the
expression level was analyzed relative to histone. Ad-1d1
transfection increased Wnt2 mRNA and protein expression levels, and
β-catenin protein expression levels. (D) Wnt2 mRNA expression
levels following treatment of EPCs with si-Id1, as detected by
RT-PCR. (E) The protein expression levels of Wnt2 and cytoplasmic
β-catenin in EPCs treated with si-Id1 were detected by western blot
analysis and analyzed relative to β-actin. (F) Nuclear β-catenin
protein expression levels in si-Id1 EPCs were detected by western
blot analysis and the expression level was analyzed relative to
histone (n=3). si-Id1 transfection decreased Wnt2 mRNA and protein
expression levels, and β-catenin protein expression levels.
*P<0.05 vs. wild type. EPCs, endothelial progenitor
cellWnt2, wingless-type mouse mammary tumor virus integration site
family member 2; Ad, adenoviruId1, inhibitor of DNA binding 1; si,
small interfering; con, control; RT-PCR, reverse
transcription-polymerase chain reaction. |
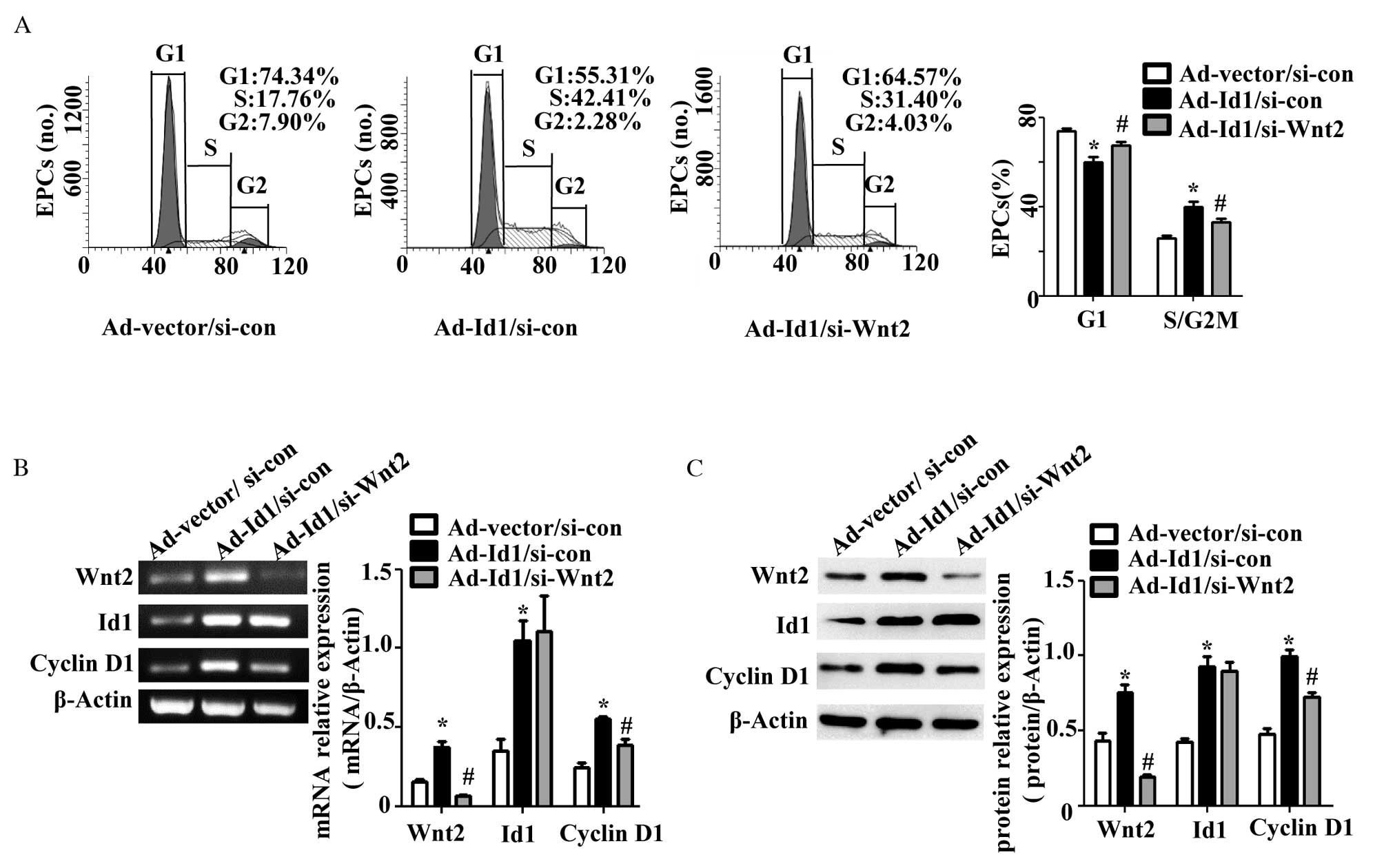
Wnt2 knockdown partially reverses the
effects of Id1 on cell cycle regulation in EPCs
To further investigate the hypothesis that Id1
regulates the cell cycle progression of EPCs via regulation of Wnt2
expression, Ad-Id1 EPCs were transfected with Wnt2 siRNA
(Ad-Id1/si-Wnt2). The G1 phase population of the
Ad-Id1/si-Wnt2 EPCs significantly increased (P=0.025; n=3) and the
S/G2M phase population significantly decreased (P=0.025;
n=3) compared with Ad-Id1/si-con EPCs. However, the percentage of
G1 phase Ad-Id1/si-Wnt2 EPCs was reduced (P=0.038; n=3)
and the percentage of the S/G2M phase population was
increased (P=0.038; n=3) compared with Ad-vector/si-con EPCs
(Fig. 4A). These results were
consistent with the change of cyclin D1 expression levels. The
cyclin D1 mRNA (P=0.008; n=3; Fig.
4B) and protein (P=0.002; n=3; Fig. 4C) expression levels were
significantly reduced in Ad-Id1/si-Wnt2 EPCs compared with those of
the Ad-Id1/si-con EPChowever, the cyclin D1 mRNA (P=0.016; n=3;
Fig. 4B) and protein (P=0.003;
n=3; Fig. 4C) expression levels
were significantly increased compared with the Ad-vector/si-con
EPCs group. Therefore, Wnt2 knockdown partially reversed cell cycle
progression and the increase of cyclin D1 expression in Ad-Id1
EPCs. No significant difference was identified in Id1 mRNA
(P=0.811; n=3) and protein (P=0.707; n=3) expression levels between
Ad-Id1/si-Wnt2 EPCs and Ad-Id1/si-con EPCs (Fig. 4B and C). These results suggest that
Id1 promoted cell cycle progression in EPCs via regulation of Wnt2
expression.
Discussion
EPC proliferation is regarded as one of the crucial
underlying mechanisms of endothelial repair (9). Previous studies have demonstrated
that silencing of Id1 in bone marrow results in a significant
reduction in the EPC population in the peripheral blood of
tumor-bearing mice (26–28). In addition, our previous study
revealed that overexpression of Id1 markedly promoted EPC
proliferation in vitro (12). However, the underlying mechanism
responsible for Id1-induced EPC proliferation remains to be
elucidated.
Previous studies have suggested that Id1 is
essential for cell cycle regulation, which is closely associated
with proliferation, in various cell lines (14–16).
Thus, the present study investigated whether Id1 regulates
proliferation of EPCs by promoting cell cycle progression. The
results from the present in vitro studies demonstrate that
exogenous Id1 promoted cell cycle progression of EPCs from
G1 to S phase and increased cyclin D1 expression levels.
A decrease in endogenous Id1 expression in EPCs via siRNA prevented
the cell cycle progression of G1 phase EPCs and
inhibited cyclin D1 expression.
Previous studies have reported that Wnt2-deficient
mice exhibit vascular abnormalities (19,20)
and that Wnt2 may expand the vascular progenitor population during
embryonic differentiation (21).
In addition, the Wnt signaling pathway may result in the
accumulation and nuclear translocation of β-catenin, leading to the
transcription of target genes, including cyclin D1; the absence of
Wnts promotes the phosphorylation and, therefore, the degradation
of β-catenin (29). The present
study demonstrated that overexpression of Id1 increased Wnt2
expression levels, and enhanced β-catenin accumulation and nuclear
translocation. Silencing of Id1 reduced Wnt2 expression levels, and
decreased β-catenin expression levels in the cytoplasm and nucleus.
Therefore, the underlying mechanism of Id1 enhancement of cell
cycle progression in EPCs may involve regulation of Wnt2
expression. In addition, Id1 upregulated Wnt2 expression, leading
to enhanced β-catenin stability and nuclear translocation.
β-catenin accumulation in the nucleus may activate cyclin D1
expression to accelerate cell cycle progression of EPCs from
G1 to S phase. To support this hypothesis, Wnt2
expression was knocked down by siRNA in Ad-Id1 EPCs, abrogating the
effects of Id1 on cell cycle regulation. The expression levels of
cyclin D1 and the proportion of EPCs in G2M/S phases
were reduced. These findings suggested that Id1 induced the
expression of Wnt2, leading to cell cycle progression of EPCs from
G1 to S phase. In addition, >50 target genes of the
Wnt/β-catenin signaling pathway have been identified (20), of which certain genes are involved
in the cell cycle of EPCs, including VEGF, connexin 43, c-Myc and
cyclooxygenase-2 (30–33). This suggests that Id1 may
additionally regulate these genes to affect cell cycle progression
of EPCs via Wnt2 expression.
A previous study demonstrated that Id1 inhibited the
expression of p21, a cell cycle associated protein, in EPCs
(34). This may explain why
si-Wnt2 did not fully reverse the effect of Id1 on cell cycle
regulation of EPCs. In addition, Id1 has been reported to regulate
certain other cell cycle-associated factors in numerous cell lines,
including p16, p27 and fibroblast growth factor-2 (34,35).
Therefore, additional mechanisms may underlie Id1 regulation of the
cell cycle of EPCs.
It has been demonstrated by previous studies that
EPCs have functions other than incorporation into injured vascular
endothelium. Paracrine signals derived from EPCs promote the
adhesion and proliferation of neighboring endothelial cells
(36–38). Wnt2 is a secreted extracellular
signaling molecule that binds to Frizzled receptors to transfer
signals (20), and Wnt2 has been
confirmed to promote proliferation of various mature differentiated
endothelial cells (39). These
findings suggest that Id1 regulates the cell cycle to promote EPC
proliferation and induces the release of EPC-derived paracrine
signals to enhance neighboring endothelial cell viability. These
two mechanisms may underlie the repair of endothelial injury by
Id1-overexpressed EPCs.
Although the results of the present study clearly
demonstrate that Id1 regulated the expression of Wnt2 to promote
cell cycle progression of EPCs in vitro, it remains to be
demonstrated whether Id1 has the same function in EPCs in
vivo. Further studies in animal models of vascular injury are
therefore required.
In conclusion, the results of the present study
demonstrate that Id1 acts as a positive regulator of Wnt2 to
activate the expression of cyclin D1 and promote the cell cycle
progression of EPCs. These results suggest that the Wnt2/cyclin D1
signaling pathway may be an important mechanism underlying
Id1-induced EPC proliferation. The present study provides a novel
strategy by which to investigate the proliferation of EPC and
endothelial repair.
Acknowledgments
The present study was supported by grants from the
National Natural and Science Foundation of China (grant no.
81270224). The authors thank Ms. Huali Kang and Ms. Mengyang Deng
for their assistance in cell culture techniques, and Mr. Jie Yang
for his assistance in FCM techniques (Department of Cardiology,
Institute of Cardiovascular Science of PLA, Xinqiao Hospital, Third
Military Medical University).
References
|
1
|
World Health Organization: Health
statistics and information systems. Estimates for 2000–2012.
http://www.who.int/healthinfo/global_burden_disease/estimates/en/.
Accessed June 22, 2016.
|
|
2
|
Wong MC, Zhang de X and Wang HH: Rapid
emergence of atherosclerosis in Asia: A systematic review of
coronary atherosclerotic heart disease epidemiology and
implications for prevention and control strategies. Curr Opin
Lipidol. 26:257–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gillette M, Morneau K, Hoang V, Virani S
and Jneid H: Antiplatelet management for coronary heart disease:
Advances and challenges. Curr Atheroscler Rep. 18:352016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brie D, Penson P, Serban MC, Toth PP,
Simonton C, Serruys PW and Banach M: Bioresorbable scaffold-A magic
bullet for the treatment of coronary artery disease? Int J Cardiol.
215:47–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah P, Bajaj S, Virk H, Bikkina M and
Shamoon F: Rapid progression of coronary atherosclerosis: A review.
Thrombosis. 2015:6349832015. View Article : Google Scholar
|
|
6
|
He T, Smith LA, Harrington S, Nath KA,
Caplice NM and Katusic ZS: Transplantation of circulating
endothelial progenitor cells restores endothelial function of
denuded rabbit carotid arteries. Stroke. 35:2378–2384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Werner N, Junk S, Laufs U, Link A, Walenta
K, Bohm M and Nickenig G: Intravenous transfusion of endothelial
progenitor cells reduces neointima formation after vascular injury.
Circ Res. 93:e17–e24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin
ZX, Liu J and Huang L: An essential role for the
Id1/PI3K/Akt/NFkB/survivin signalling pathway in promoting the
proliferation of endothelial progenitor cells in vitro. Mol Cell
Biochem. 363:135–145. 2012. View Article : Google Scholar :
|
|
9
|
Kirton JP and Xu Q: Endothelial precursors
in vascular repair. Microvasc Res. 79:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafii S and Lyden D: Cancer. A few to flip
the angiogenic switch. Science. 319:163–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Yu Y, Guo RW, Shi YK, Song MB,
Chen JF, Yu SY, Yin YG, Gao P and Huang L: Inhibitor of DNA
binding-1 promotes the migration and proliferation of endothelial
progenitor cells in vitro. Mol Cell Biochem. 335:19–27. 2010.
View Article : Google Scholar
|
|
13
|
Norton JD: ID helix-loop-helix proteins in
cell growth, differentiation and tumorigenesis. J Cell Sci.
113:3897–3905. 2000.PubMed/NCBI
|
|
14
|
Zebedee Z and Hara E: Id proteins in cell
cycle control and cellular senescence. Oncogene. 20:8317–8325.
2001. View Article : Google Scholar
|
|
15
|
Hasskarl J and Münger K: Id proteins-tumor
markers or oncogenes? Cancer Biol Ther. 1:91–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng Q, Jia Z, Wang W, Li B, Ma K and Zhou
C: Inhibitor of DNA binding 1 (Id1) induces differentiation and
proliferation of mouse embryonic carcinoma P19CL6 cells. Biochem
Biophys Res Commun. 412:253–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komiya Y and Habas R: Wnt signal
transduction pathways. Organogenesis. 4:68–75. 2008. View Article : Google Scholar
|
|
19
|
Monkley SJ, Delaney SJ, Pennisi DJ,
Christiansen JH and Wainwright BJ: Targeted disruption of the Wnt2
gene results in placentation defects. Development. 122:3343–3353.
1996.PubMed/NCBI
|
|
20
|
Goodwin AM and D'Amore PA: Wnt signaling
in the vasculature. Angiogenesis. 5:1–9. 2002. View Article : Google Scholar
|
|
21
|
Wang H, Charles PC, Wu Y, Ren R, Pi X,
Moser M, Barshishat-Kupper M, Rubin JS, Perou C, Bautch V and
Patterson C: Gene expression profile signatures indicate a role for
Wnt signaling in endothelial commitment from embryonic stem cells.
Circ Res. 98:1331–1339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding BS, Nolan DJ, Butler JM, James D,
Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D,
et al: Inductive angiocrine signals from sinusoidal endothelium are
required for liver regeneration. Nature. 468:310–315. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sekiguchi H, Ii M, Jujo K, Yokoyama A,
Hagiwara N and Asahara T: Improved culture-based isolation of
differentiating endothelial progenitor cells from mouse bone marrow
mononuclear cells. PLoS One. 6:e286392011. View Article : Google Scholar
|
|
25
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao D, Nolan DJ, Mellick AS, Bambino K,
McDonnell K and Mittal V: Endothelial progenitor cells control the
angiogenic switch in mouse lung metastasis. Science. 319:195–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et
al: Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumor angiogenesis and growth.
Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaked Y, Ciarrocchi A, Franco M, Lee CR,
Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R and
Kerbel RS: Therapy-induced acute recruitment of circulating
endothelial progenitor cells to tumors. Science. 313:1785–1787.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoeppner LH, Sinha S, Wang Y, Bhattacharya
R, Dutta S, Gong X, Bedell VM, Suresh S, Chun C, Ramchandran R, et
al: RhoC maintains vascular homeostasis by regulating VEGF-induced
signaling in endothelial cells. J Cell Sci. 128:3556–3568. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang HH, Su CH, Wu YJ, Li JY, Tseng YM,
Lin YC, Hsieh CL, Tsai CH and Yeh HI: Reduction of connexin43 in
human endothelial progenitor cells impairs the angiogenic
potential. Angiogenesis. 16:553–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang L, Chen MF, Xiao ZL, Yu GL, Chen XB
and Xie XM: The effect of endothelial progenitor cells on
angiotensin II-induced proliferation of cultured rat vascular
smooth muscle cells. J Cardiovasc Pharmacol. 58:617–625. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colleselli D, Bijuklic K, Mosheimer BA and
Kähler CM: Inhibition of cyclooxygenase (COX)-2 affects endothelial
progenitor cell proliferation. Exp Cell Res. 312:2933–2941. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang G, Zhang Y, Xiong J, Wu J, Yang C,
Huang H and Zhu Z: Downregulation of Id1 by small interfering RNA
in gastric cancer inhibits cell growth via the Akt pathway. Mol Med
Rep. 5:1075–1079. 2012.PubMed/NCBI
|
|
35
|
Passiatore G, Gentilella A, Rom S,
Pacifici M, Bergonzini V and Peruzzi F: Induction of Id-1 by FGF-2
involves activity of EGR-1 and sensitizes neuroblastoma cells to
cell death. J Cell Physiol. 226:1763–1770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Z, von Ballmoos MW, Faessler D,
Voelzmann J, Ortmann J, Diehm N, Kalka-Moll W, Baumgartner I, Di
Santo S and Kalka C: Paracrine factors secreted by endothelial
progenitor cells prevent oxidative stress-induced apoptosis of
mature endothelial cells. Atherosclerosis. 211:103–109. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Santo S, Seiler S, Fuchs AL, Staudigl J
and Widmer HR: The secretome of endothelial progenitor cells
promotes brain endothelial cell activity through PI3-kinase and
MAP-kinase. PLoS One. 9:e957312014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burlacu A, Grigorescu G, Rosca AM, Preda
MB and Simionescu M: Factors secreted by mesenchymal stem cells and
endothelial progenitor cells have complementary effects on
angiogenesis in vitro. Stem Cells Dev. 22:643–653. 2013. View Article : Google Scholar :
|
|
39
|
Klein D, Demory A, Peyre F, Kroll J,
Géraud C, Ohnesorge N, Schledzewski K, Arnold B and Goerdt S: Wnt2
acts as an angiogenic growth factor for non-sinusoidal endothelial
cells and inhibits expression of stanniocalcin-1. Angiogenesis.
12:251–265. 2009. View Article : Google Scholar : PubMed/NCBI
|