Introduction
Osterix (OSX) is predominantly expressed in
osteoblasts and cells associated with tooth development during the
human fetal stage, and in craniofacial osteoblasts and chondrocytes
after birth, although there is additionally low-level expression in
the testis, heart, brain, placenta, lung, pancreas, spleen and
myofibroblasts of major arteries. OSX is also highly expressed in
certain tumor cells including those of osteosarcoma cells and giant
cell tumor mesenchymal cells, and is additionally associated with
tumor biological behaviors (1–3).
OSX is an osteoblast-specific transcription factor
with a zinc ion motif structure domain that is required in the
process of osteocyte differentiation and bone formation. It is
located downstream of the runt-related transcription factor-2 gene
as a specific transcription factor that controls osteoblast growth
and differentiation (4,5), which is specifically expressed in
developed bone tissues (6).
Without OSX participation, bone formation within the membrane or
cartilage becomes impossible (4),
and therefore OSX is the key transcription factor in osteoblast
differentiation.
Vascular calcification is a pathological process
universally present in atherosclerosis, hypertension and renal
vasculopathies. The dualistic function of calcified vessels is
reduced and stiffness will be increased, thus facilitating the
development of thrombosis and rupture of atherosclotic plaques.
Approximately 80% patients with vascular injuries and 90% of
patients with coronary artery disease experience vascular
calcification (7). A previous
study (8) demonstrated that
vascular calcification is a cell-controlled and highly regulated
process similar to the active and regulatory process of bone growth
and osteoporosis. Aldosterone (ALD) is the main component of
adrenal mineralocorticoid hormone. ALD results in sodium and water
retention, in addition to promoting collagen deposition and
fibrosis, leading to structural remodeling of the heart and blood
vessels, thus serving an important role in the development and
progression of vascular calcification (9).
The elucidation of a postnatal role of OSX in the
survival of progenitor pools in their interactions with the stem
cell niches (10), together with
evidence associating OSX with vascular function (11), has led to the hypothesis that OSX
may be a key mediator of postnatal phenotypic trans-differentiation
of vascular smooth muscle cells (VSMCs) during calcification. To
the best of our knowledge, this is the first study to investigate
the role of OSX in the process of vascular calcification. The
current in vitro study investigating VSMC calcification
aimed to provide the first fundamental insight into the expression
profiles of OSX during vascular calcification.
In the present study, ALD was used to induce calcium
salt deposition in cultured mouse VSMCs, the gene chip technique
was used to analyze differentially expressed genes of calcified
VSMCs and OSX-specific VSMC small interfering RNA (siRNA) was used
to silence the OSX gene in order to verify the effect of
downregulation of OSX on the calcification of VSMCs.
Materials and methods
Isolation of primary murine VSMCs
Primary VSMCs were isolated from 6-week-old male
C57BL/6 mice (n=6; 20–30 g). All animals were housed in Makrolon
cages, and provided an extruded diet and acidified tap water ad
libitum. Lighting was maintained on a 12:12 dark/artificial
light cycle with lights on from 6.00 a.m. and with a 30-min period
of reduced lighting at transitions. The temperature was maintained
at 22±2°C) with a relative humidity of 55%. The mice were
sacrificed by CO2 inhalation. Following dissection of
the aorta, the adventitia was detached and the blood vessel was
opened to expose the endothelial layer of cells. The aortae
isolated from 16 mice were then digested with 1 mg/ml trypsin for
10 min to remove any remaining endothelium and adventitia. This was
followed by an overnight incubation at 37°C in a humidified
atmosphere of 95% air/5% CO2 in α-minimum essential
medium (α-MEM; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal calf serum (GE Healthcare Life
Sciences) and 1% gentamycin (GE Healthcare Life Sciences). Tissues
were then digested in 425 U/ml collagenase type II for 5 h. Prior
to the experiment, the isolated VSMCs were expanded in α-MEM for
two passages in T25 tissue culture flasks (Greiner Bio-One GmbH,
Frickenhausen, Germany) coated with 0.28 mg/cm2 murine
laminin (Sigma-Aldrich, St. Louis, MO, USA). The current study was
approved by the ethics committee of Shanghai Medical College, Fudan
University (Shanghai, China).
Culture of primary murine VSMCs
VSMCs were plated at
1.5×104/cm2 in a 6-well tissue culture plate
containing α-MEM. To induce calcification of VSMCs, 1.5 mmol/l ALD
(Sigma-Aldrich) was added to confluent cells for 6 days, and cells
were then incubated at 37°C in a humidified atmosphere of 95%
air/5% CO2. The medium was replaced every second
day.
cDNA microarray hybridization
RNA was extracted from cells using the RNeasy Mini
kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's
instructions for microarray analysis. Total RNA content was
assessed by absorbance at 260 nm and purity by A260/A280 ratio for
each sample. The quality of each sample was considered suitable if
it had a ratio >1.9. A l µg-labeled probe was hybridized
at 65°C for the 17-h rolling hybridization. The microarray result
was scanned using an Agilent Microarray Scanner (Agilent
Technologies, Inc., Santa Clara, CA, USA), and the data were read
with Feature Extraction software, version l0.7 (Agilent
Technologies, Inc.) and analyzed using the standard method.
Microarray data analysis
Analysis was conducted as described previously
(12). Normalization across all
arrays was achieved using the robust multi-array average expression
measure which results in expression measures (summarized
intensities) in log base 2 (13).
Comparisons were performed using linear modeling. Following this,
empirical Bayesian analysis was conducted including vertical
(within a given comparison) P-value adjustments for multiple
testing, which control for false discovery rates using the
Bioconductor package limma (14).
Detection of calcification
Calcium deposition was evaluated through
decalcification of the mineralized matrix in 0.6 M HCl for 24 h,
and free calcium was determined colorimetrically using a
spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The protein
content of the cultures was measured and corrected for the
following extraction using 1 mM NaOH in 0.1% sodium dodecyl
sulfate.
Experimental grouping
All parameters obtained in the current study were
classified as i) normal group; ii) ALD (1.5 mmol/l) group; iii)
siRNA transfection (ALD + OSX-siRNA) group, using siRNA-transfected
cells that were effectively inhibited by the above screening and
optimization methods; and iv) negative transfection control (ALD +
negative control siRNA) group, using siRNA-transfected cells that
were ineffectively inhibited using the same methods. Following 3–7
h transfection, the medium was replaced by ALD-containing medium in
all groups except the normal group. Total RNA was extracted at 24
and 48 h subsequent to transfection, and total protein was
extracted at 48 and 72 h subsequent to transfection. The
transfection rate was determined using FAM fluorescence labeled
non-sense siRNA (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The siRNA sequences used were as follows: Sense strand (5-3),
UACAAGUACUGGUUGAACCTT; and anti-sense strand,
GGUUCAACCAGUACUUGCATT. siRNA was synthesized by Shanghai GenePharma
Co., Ltd., Shanghai, China).
siRNA transfection
VSMCs were seeded into a 6-well plate and cultured
in 0.5 ml serum-free Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) without antibiotics at a
density of 75–90% according to the manufacturer's protocol of
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.). siRNA and the
transfectant in each well were diluted with 50 µl serum-free
DMEM, mixed gently and incubated at room temperature for 6 min. The
diluted siRNA and transfectant were then mixed and incubated at
room temperature for 25 min. The 120 µl mixture was directly
added to each well and mixed gently by vibrating the plate, and
then cultured in a CO2 incubator at 37°C for 4–8 h. The
serum-free DMEM was replaced by the DMEM containing 10% fetal
bovine serum to continue with culture for additional 24 h at 37°C.
The DNA (µg)/transfectant (µl) ratio was set at
1.0:3.0 during the process of transfection, and the working
concentration of siRNA was set at 120 nmol/l.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using RNeasy Mini
kit (Qiagen GmbH) according to the manufacturer's instructions. For
each sample, total RNA content was assessed by measuring absorbance
at 260 nm and assessing purity using an A260:A280 ratio. RNA was
reverse transcribed and PCR was conducted as described previously
(15). All genes were analyzed
using the SYBR green detection method with the Stratagene Mx3000P
qPCR system (GE Healthcare Life Sciences, Chalfont, UK). Each
sample was run in triplicate. The reactions were performed as
follows: Initial activation step at 95°C for 2 min; denaturation at
94°C for 10 sec, annealing at 50–68°C for 1 min and extension at
72°C for 1 min/kb, for 40 cycles. All gene expression data were
normalized against β-actin and the control values were expressed as
one to indicate a precise fold change value for each gene of
interest. The primers used were as follows: OSX, forward
5′-GTCCTCTCTGCTTGAGGAA-3′ and reverse 5′-CTTGAGAAGGGAGCTGGGTA-3′;
integrin-binding sialoprotein (IBSP), forward
5′-ATGGAGACGGCGATAGTTCC-3′ and reverse 5′-CTAGCTGTTACACCCGAGAGT-3′;
and β-actin, forward 5′-TCACCCACACTGTGCCCATCTACGA-3′ and reverse
5′-GGATGCCACAGGATTCCATACCCA-3′. The primer sequences were
synthesized by Shanghai GenePharma Co., Ltd.
Western blotting
Cells were lysed in radioimmunoprecipitation assay
buffer (Invitrogen; Thermo Fisher Scientific, Inc.) containing the
cOmplete Protease Inhibitor Cocktail according to the
manufacturer's instructions (Roche Diagnostics, Basel,
Switzerland). Immunoblotting was conducted as described previously
(15). Nitrocellulose membranes
were probed overnight at 4°C with OSX (1:100; cat. no. ab94744;
Abcam, Cambridge, UK) and IBSP (1:100; cat. no. ab84787; Abcam)
antibodies, washed in Tris-buffered saline with Tween-20, and
incubated with anti-rabbit IgG-peroxidase (Abcam) for 1 h (1:1,000;
Abcam). Control membranes were washed in stripping buffer (Pierce
Biotechnology, Inc., Rockford, IL, USA) and re-probed for 1 h for
glyceraldehyde 3-phosphate dehydrogenase expression (1:1,000; cat.
no. ab8245; Abcam). Subsequent to washing 3 times for 5 min with
Tris-buffered saline Tween 20, the membranes were incubated with
the rabbit anti-mouse (cat. no. ab97046) or goat anti-rabbit (cat.
no. ab136636) horseradish peroxidase conjugated IgG for 1 h
(Abcam). The immune complexes were visualized using an enhanced
chemiluminescence reagent (ECL; GE Healthcare Life Sciences).
Alizarin red staining (ARS)
For ARS, cells were washed twice with
phosphate-buffered saline (PBS) and fixed with formalin at room
temperature for 10 min. Formalin was removed and the wells were
washed twice with PBS and once with distilled water. The ARS
solution was then added and incubated at room temperature for 30
min. Finally, the wells were washed with distilled water until the
background staining on the negative wells (wells containing MSCs)
was fully clear. Cells were examined under an inverted optical
microscope (Nanjing Jiangnan Novel Optics Co., Ltd., Nanjing,
China).
Statistical analysis
All values are presented as the mean ± standard
error. Statistical comparisons were conducted by analysis of
variance, followed by Fisher's test using SPSS software (version
17; SPSS, Inc. Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Global transcriptome profiling indicates
upregulation of OSX in the calcification of VSMCs
To identify novel mediators of vascular
calcification, microarray analysis was conducted on VSMCs induced
by ALD culture under calcifying conditions. The result of analysis
using Bioconductor limma software demonstrated that 867 genes were
upregulated and 376 genes were downregulated (n=4, greater than
2-fold change) following normalization, indicating that the
bone-formation-associated gene OSX was differentially expressed at
the highest level in calcifying VSMCs (Table I).
 | Table IGenes exhibiting the greatest
differential expression (log fold change) in vascular smooth muscle
cells induced by aldosterone for 6 days in calcifying
conditions. |
Table I
Genes exhibiting the greatest
differential expression (log fold change) in vascular smooth muscle
cells induced by aldosterone for 6 days in calcifying
conditions.
| Gene ID | Gene name | (Log) fold
change | P-value |
|---|
| OSX | Osterix | 4.11 |
1.23×10−14 |
| IBSP | Integrin binding
sialoprotein | 4.05 |
1.25×10−14 |
| Trib3 | Tribbles homologue
3 | −2.92 |
3.57×10−13 |
| Sept4 | Septin 4 | 2.24 |
4.65×10−13 |
| Art4 |
ADP-ribosyltransferase 4 | 2.05 |
1.19×10−12 |
| Tmem204 | Transmembrane protein
204 | 3.44 |
2.15×10−12 |
| Trib3 | Tribbles homologue
3 | −2.47 |
3.89×10−12 |
| Gpr116 | G protein-coupled
receptor 116 | 2.57 |
1.69×10−11 |
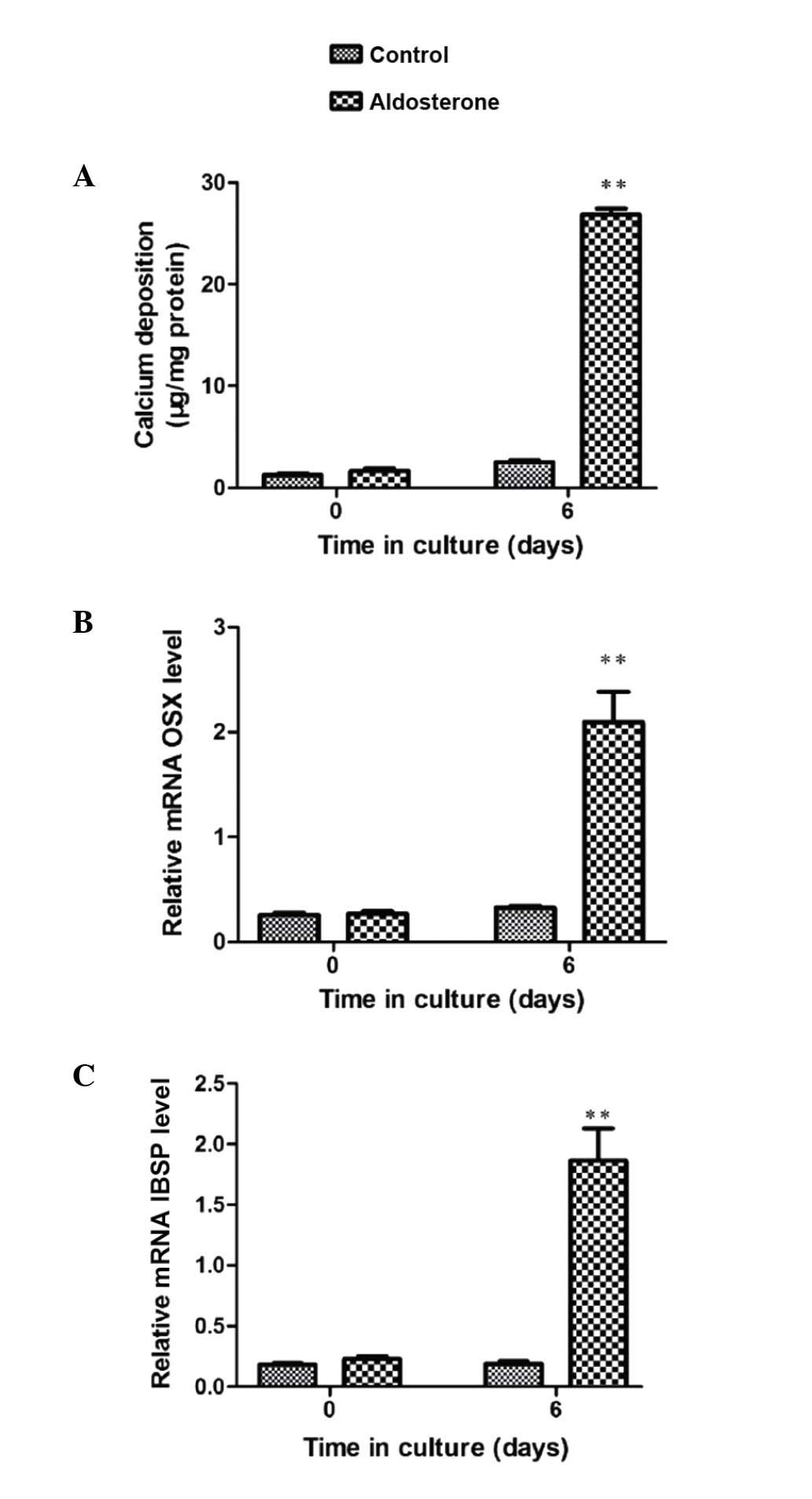
Furthermore, gene expression analysis by RT-qPCR
validated the observation of OSX enhancement. ALD-induced VSMC
calcification was significant subsequent to 6-day culture (Fig. 1A). Associated with this increase in
VSMC mineralization, OSX mRNA expression was significantly
increased at the same time point (P<0.01) (Fig. 1B). A significant increase in mRNA
expression of the osteogenic marker IBSP was observed at 6 days
(P<0.001), indicating that the transition to the osteoblast
phenotype was in progress and thereby validating this in
vitro model to study VSMC calcification (Fig. 1C).
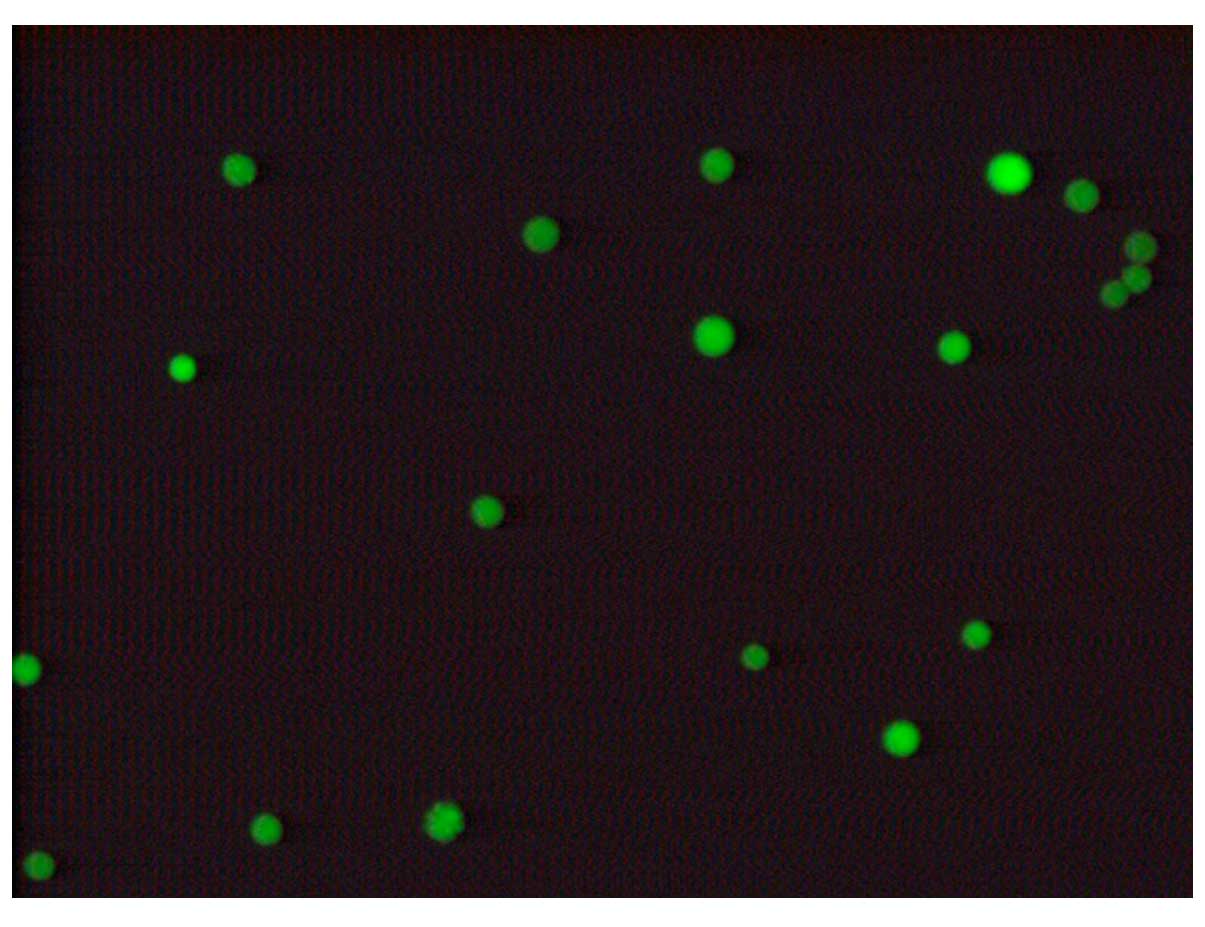
Determination of the siRNA transfection
rate
Subsequent to transfection of VSMCs at the
Lipofectamine transfection dose, green fluorescence was observed
under the fluorescence microscope, indicating positive cell
transfection. Cells were observed under the inverted microscope,
and the cell transfection rate was identified to be greater than
70–75%, which was sufficient for the requirements of the
experiments (Fig. 2).
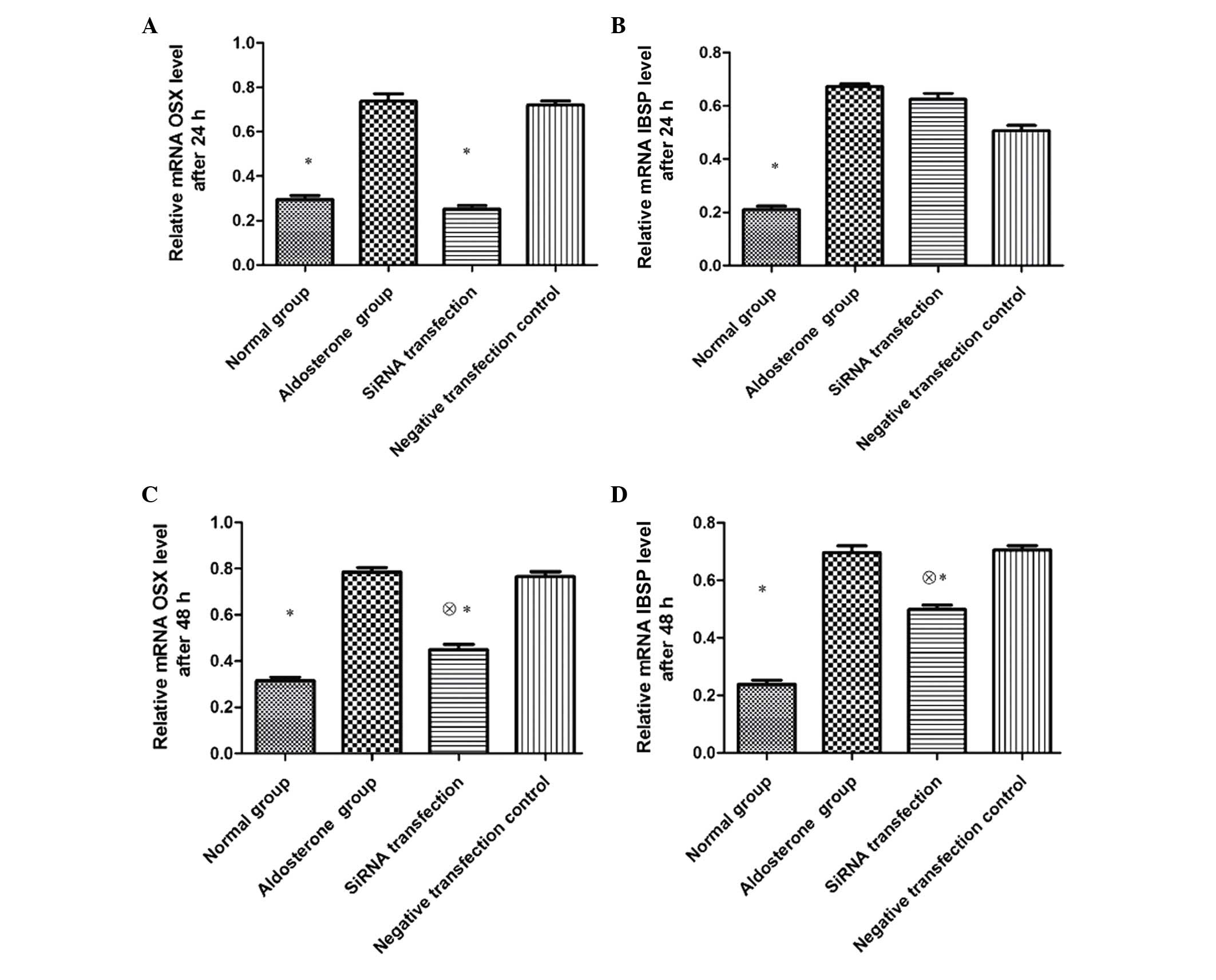
Impact of OSX siRNA on the expression of
VSMC OSX and IBSP mRNA
Following 24-h transfection, the expression of both
OSX and IBSP mRNA in VSMCs of the ALD group was significantly
higher than that of the normal group (P<0.01), and the
expression of OSX mRNA in the siRNA transfection group was
significantly lower than that of the ALD and negative transfection
control groups (P<0.01). No significant differences were
observed in IBSP mRNA expression between the three groups
(P>0.05). Subsequent to 48-h transfection, the expression of OSX
mRNA in the siRNA transfection group remained significantly lower
than that of the ALD and negative transfection control groups
(P<0.05), however was increased compared with the normal group
(P<0.01). The expression of IBSP mRNA in the siRNA transfection
group was significantly lower than that of the ALD and negative
transfection control groups (P<0.05), however was higher than
that of the normal group (P<0.01) (Fig. 3).
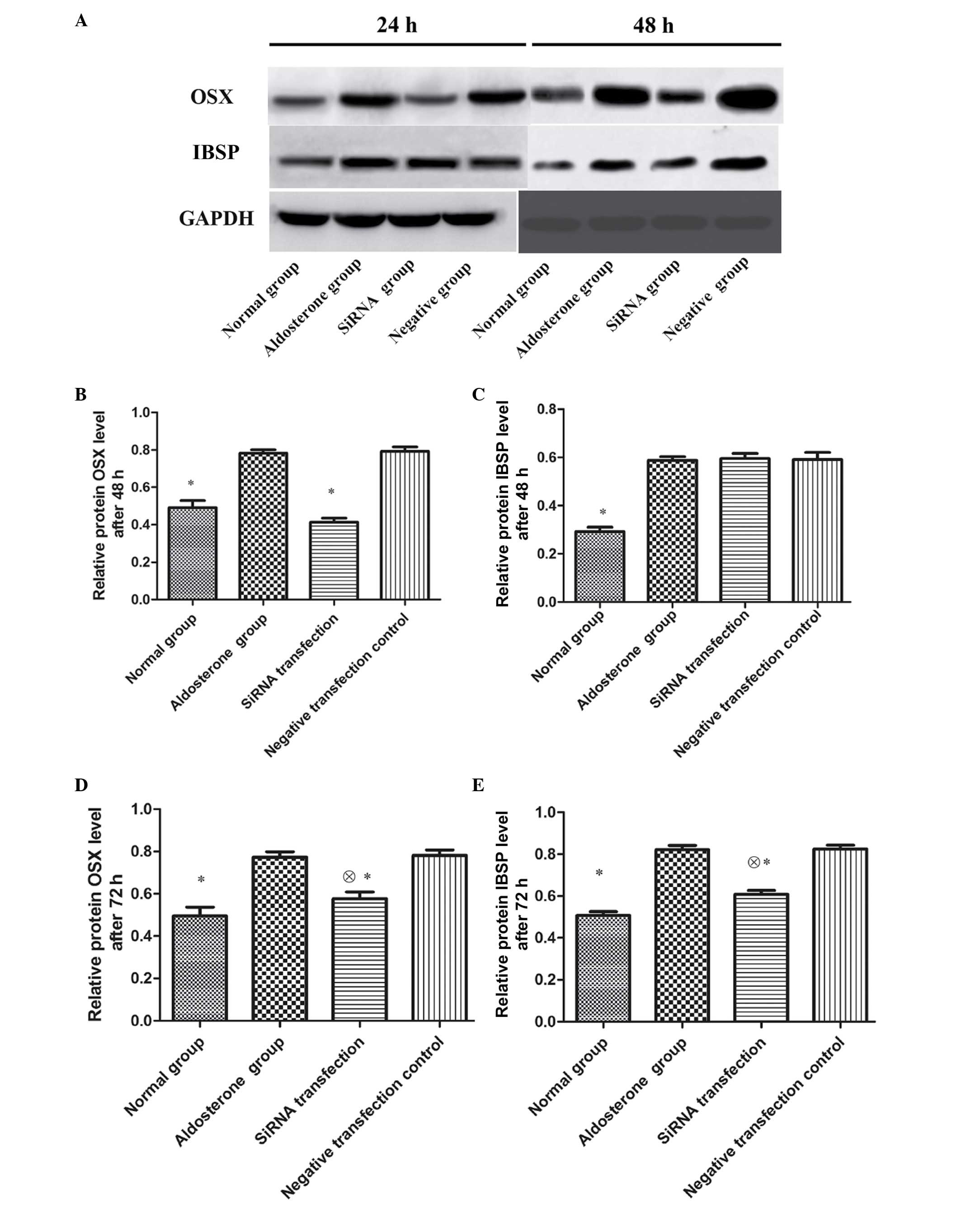
Impact of OSX siRNA on OSX and IBSP
protein expression in VSMCs
Subsequent to 48-h transfection, OSX and IBSP
protein expression in VSMCs of ALD group was significantly
upregulated as compared with that of the normal group (P<0.01);
the OSX protein expression in the siRNA transfection group was
significantly reduced compared with that of the ALD and negative
transfection control groups (P<0.01). No significant differences
were observed in IBSP protein expression between the three groups
(P>0.05). Subsequent to 72-h transfection, the OSX protein
expression in the siRNA transfection group reamained significantly
lower than that of the ADL and negative transfection control groups
(P<0.01), however was higher than that of the normal group
(P<0.01). The IBSP protein expression in the siRNA transfection
group was significantly lower than that of the ADL and negative
transfection control groups (P<0.01), however was higher than
that of the normal group (P<0.01) (Fig. 4).
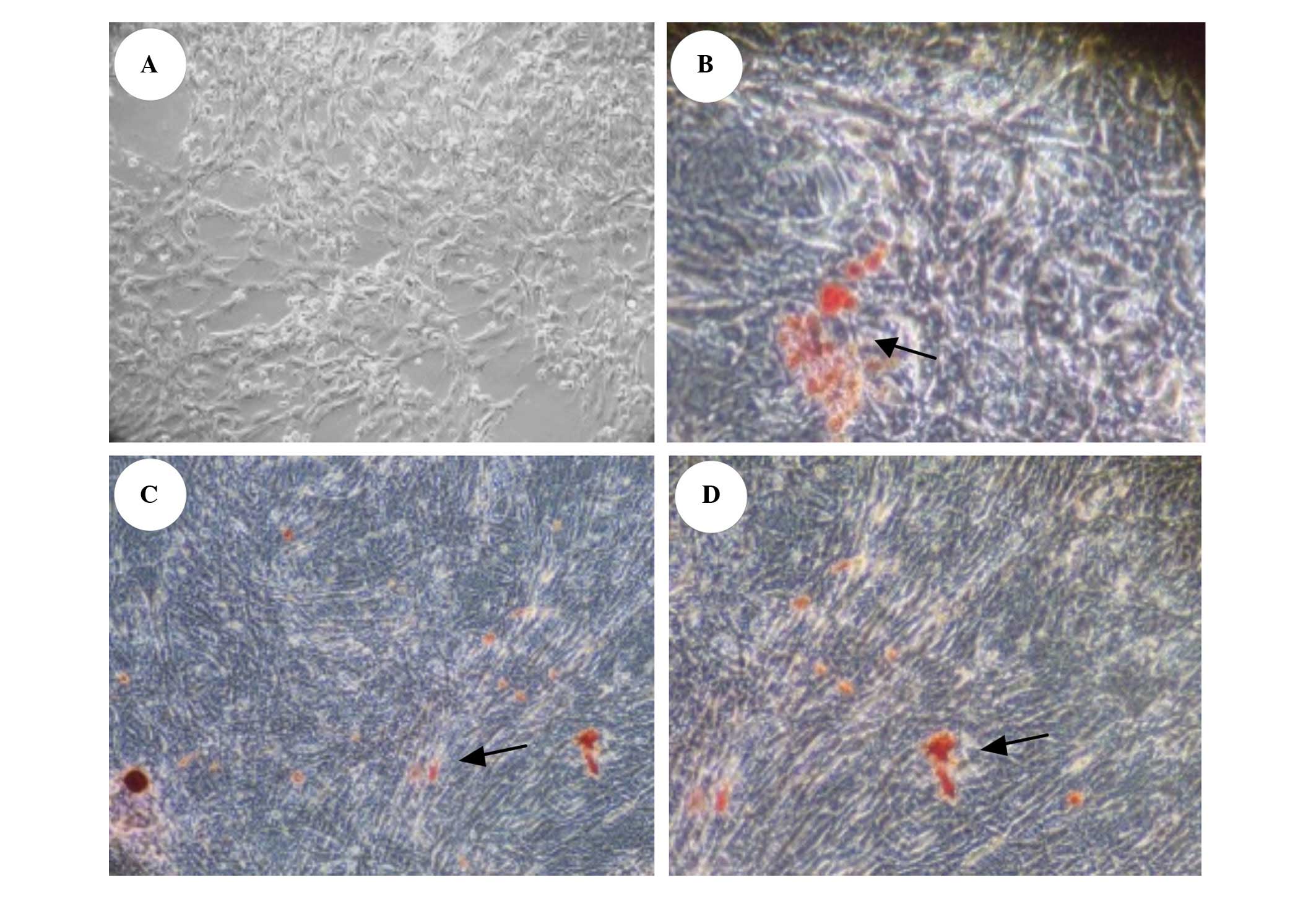
Impact of OSX siRNA on calcium salt
deposition in VSMCs
No calcium salt deposition was observed in VSMCs at
day 6 of transfection in the normal group; large amounts of
orange-red calcium salt deposition was observed in the ALD and
negative transfection control groups. Calcium salt deposition in
the siRNA transfection group was observed to be significantly
reduced (Fig. 5).
Discussion
Stimulation of VSMC phenotype transformation by ALD
is widely accepted. ALD can induce VSMCs to express the osteoblast
phenotype, i.e. promoting the expression of osteoblast-like genes
including OSX and IBSP, and inhibiting the expression of smooth
muscle cell-specific genes such as smooth muscle protein 22-α,
resulting in the induction of calcification of VSMCs. The results
of the present study indicated that the expression of OSX and IBSP
osteoblast marker proteins was increased markedly with the addition
of ALD, indicating that ALD was able to induce differentiation of
VSMCs to osteoblasts, which may be one of the important mechanisms
underlying vascular calcification in chronic kidney disease.
Despite studies that have improved vascular
knowledge, the precise mechanism underlying vascular calcification
remains unclear. The current study demonstrated differential
expression of novel genes in calcifying murine primary aortic VSMC
cultures. Using microarray analysis, functional enrichment analysis
and RT-qPCR, it was identified, for the first time to the best of
our knowledge, that OSX is upregulated during in vitro VSMC
calcification. Mohler et al (16) isolated a group of interstitial
cells from the aortic valve stenosed by calcification, and
demonstrated that this cell group exhibited similar behaviors to
that of vascular smooth muscle cells; they were able to
phenotypically transform to osteoblast-like cells and form
calcified nodules. Rajamannan et al (17) reported that the expression of
multiple osteoblast and bone tissue-associated markers was
upregulated in the calcified aortic valves, including OSX,
osteocalcin, osteoprotegerin and IBSP. Alexopoulos et al
(18) identified that the
expression of OSX was increased significantly in the calcified
focus of the human aorta. An in vitro study (19) identified that the expression level
of OSX protein was elevated significantly following VSMC
calcification, and silencing of the OSX gene by siRNA significantly
reduced cell calcium deposition and alkaline phosphatase activity.
At the same time, VSMC calcification was observed to be blocked.
These results suggest that OSX serves an important role in vascular
calcification, and its upregulation is associated with the
transformation and differentiation of VSMCs to osteoblasts. To
further clarify the role of OSX in vascular calcification, the RNAi
technique was used to study cultured murine VSMCs, and it was
identified that the expression of OSX mRNA and protein in the siRNA
transfection group was significantly reduced compared with that of
the ALD and negative transfection control groups, indicating that
OSX siRNA successfully inhibited the ALD-induced expression of VSMC
OSX mRNA and protein in VSMCs at 48 and 72 h.
IBSP is an important non-collagen bone matrix
glycoprotein and is important in the development of the osteoblast
phenotype. There is an OSX binding site on the promoter of the
IBSP-associated bone matrix protein encoding gene. The OSX gene can
induce the expression of the IBSP gene, thus promoting cell
differentiation to osteoblasts (20,21).
It was identified in the current study that when OSX was inhibited
by siRNA in a targeted manner, it could downregulate ALD-induced
IBSP expression and reduce calcium salt deposition in cells,
indicating that OSX serves a role in regulating the downstream
associated matrix protein and preventing the occurrence of VSMC
calcification.
In the present study, gene chip and gene silencing
techniques were used to perform instant transfection using
chemically synthesized siRNA, and it was identified that OSX served
a key role in the development and progression of ALD-induced VSMC
calcification. This observation may aid in the explanation of the
role of OSX in the pathogenesis of vascular calcification, however
further investigation is required in order to more fully elucidate
the mechanism underlying this regulatory effect of OSX.
Acknowledgments
The current study was financially supported by the
Shanghai Commission of Science and Technology (grant no.
12ZR1419000).
References
|
1
|
Huang L, Teng XY, Cheng YY, Lee KM and
Kumta SM: Expression of preosteoblast markers and Cbfa-1 and
Osterix gene transcripts in stromal tumour cells of giant cell
tumour of bone. Bone. 34:393–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen MLY and Miao LY: OSX expression
during the development of human teeth. Oral Med Res. 22:372–337.
2006.
|
|
3
|
Cao Y, Zhou Z, de Crombrugghe B, Nakashima
K, Guan H, Duan X, Jia SF and Kleinerman ES: Osterix, a
transcription factor for osteoblast differentiation, mediates
antitumor activity in murine osteosarcoma. Cancer Res.
65:1124–1128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YJ, Kim HN, Park EK, Lee BH, Ryoo HM,
Kim SY, Kim IS, Stein JL, Lian JB, Stein GS, et al: The
bone-related Zn finger transcription factor Osterix promotes
proliferation of mesenchymal cells. Gene. 366:145–151. 2006.
View Article : Google Scholar
|
|
5
|
Zhou YS, Liu YS and Tan JG: Is 1,
25-dihydroxyvitamin D3 an ideal substitute for dexamethasone for
inducing osteogenic differentiation of human adipose tissue-derived
stromal cells in vitro? Chin Med J (Engl). 119:1278–1286. 2006.
|
|
6
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demer LL and Tintut Y: Vascular
calcification: Pathobiology of a multifaceted disease. Circulation.
117:2938–2948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shioi A: Molecular mechanisms of vascular
calcification. Clin Calcium. 20:1611–1619. 2010.In Japanese.
PubMed/NCBI
|
|
9
|
Wu SY, Yu YR, Cai Y, Jia LX, Wang X, Xiao
CS, Tang CS and Qi YF: Endogenous aldosterone is involved in
vascular calcification in rat. Exp Biol Med (Maywood). 237:31–37.
2012. View Article : Google Scholar
|
|
10
|
Radtke CL, Nino-Fong R, Rodriguez-Lecompte
JC, Esparza Gonzalez BP, Stryhn H and McDuffee LA: Osteogenic
potential of sorted equine mesenchymal stem cell subpopulations.
Can J Vet Res. 79:101–108. 2015.PubMed/NCBI
|
|
11
|
Valenzuela CD, Allori AC, Reformat DD,
Sailon AM, Allen RJ Jr, Davidson EH, Alikhani M, Bromage TG, Ricci
JL and Warren SM: Characterization of adipose-derived mesenchymal
stem cell combinations for vascularized bone engineering. Tissue
Eng Part A. 19:1373–1385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Staines KA, Zhu D, Farquharson C and
MacRae VE: Identification of novel regulators of osteoblast matrix
mineralization by time series transcriptional profiling. J Bone
Miner Metab. 32:240–251. 2014. View Article : Google Scholar
|
|
13
|
Irizarry RA, Bolstad BM, Collin F, Cope
LM, Hobbs B and Speed TP: Summaries of Affymetrix GeneChip probe
level data. Nucleic Acids Res. 31:e152003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kauffmann A, Gentleman R and Huber W:
ArrayQuality-Metrics-a bioconductor package for quality assessment
of microarray data. Bioinformatics. 25:415–416. 2009. View Article : Google Scholar
|
|
15
|
Macrae VE, Horvat S, Pells SC, Dale H,
Collinson RS, Pitsillides AA, Ahmed SF and Farquharson C: Increased
bone mass, altered trabecular architecture and modified growth
plate organization in the growing skeleton of SOCS2 deficient mice.
J Cell Physiol. 218:276–284. 2009. View Article : Google Scholar
|
|
16
|
Mohler ER III, Wang H, Medenilla E and
Scott C: Effect of statin treatment on aortic valve and coronary
artery calcification. J Heart Valve Dis. 16:378–386.
2007.PubMed/NCBI
|
|
17
|
Rajamannan NM, Subramaniam M, Rickard D,
Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik
AJ, Bonow RO and Spelsberg T: Human aortic valve calcification is
associated with an osteoblast phenotype. Circulation.
107:2181–2184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alexopoulos A, Bravou V, Peroukides S,
Kaklamanis L, Varakis J, Alexopoulos D and Papadaki H: Bone
regulatory factors NFATc1 and Osterix in human calcific aortic
valves. Int J Cardiol. 139:142–149. 2010. View Article : Google Scholar
|
|
19
|
Taylor J, Butcher M, Zeadin M, Politano A
and Shaughnessy SG: Oxidized low-density lipoprotein promotes
osteoblast differentiation in primary cultures of vascular smooth
muscle cells by up-regulating Osterix expression in an
Msx2-dependent manner. J Cell Biochem. 112:581–588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samee N, Geoffroy V, Marty C, Schiltz C,
Vieux-Rochas M, Levi G and de Vernejoul MC: Dlx5, a positive
regulator of osteoblastogenesis, is essential for
osteoblast-osteoclast coupling. Am J Pathol. 173:773–780. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Igarashi M, Kamiya N, Hasegawa M, Kasuya
T, Takahashi T and Takagi M: Inductive effects of dexamethasone on
the gene expression of Cbfa1, Osterix and bone matrix proteins
during differentiation of cultured primary rat osteoblasts. J Mol
Histol. 35:3–10. 2004. View Article : Google Scholar : PubMed/NCBI
|