Introduction
Thyroid cancer, derived from the thyroid follicular
cells, is the most common malignancy of the endocrine system
(1). The incidence of thyroid
cancer over the last three decades has increased in the USA,
typically due to improved detection of sub-clinical tumors
(2). There are four major
histological types including papillary thyroid carcinoma (PTC),
follicular thyroid carcinoma (FTC), poorly differentiated carcinoma
and undifferentiated anaplastic carcinoma (ATC) (3). PTC is the most common type of thyroid
cancer and accounts for ~80% of cases (4). The prognosis of PTC is related to
patient age, tumor size and histological parameters including
extra-capsular extension, extra-thyroidal extension, lymph node
invasion, distant metastases and histological variant (5). The majority of PTC cases demonstrate
a good prognosis; however, in a few cases it can recur, transform
or metastasize, which can lead to disease-related mortality
(6). Metastasis of thyroid tumors
often confers a poor prognosis and is the major cause of mortality
(7). Therefore, the identification
of factors associated with metastasis, is important for the
development of novel targeted therapies for thyroid cancer
treatment.
In thyroid cancer, abnormal microRNA (miRNA)
expression has been observed in PTC (8–10),
FTC (11) and ATC (12) tumor subtypes. miRNAs belong to a
group of endogenous, non-protein-coding, single-stranded, small
RNAs that are 20–24 nucleotides in length (13). Previous studies have demonstrated
that miRNAs are involved in major physiological and pathological
cellular pathways including cell growth, differentiation, cell
cycle, apoptosis, migration and invasion (14). In the majority of cases, miRNAs
function to prohibit the expression of specific genes by
interacting preferentially and base-pairing with the
3′-untranslated region (UTR) of targeted mRNA sequences, which
results in mRNA cleavage and repression of translation (15). miRNAs can be divided into
onco-miRNAs and tumor-suppressive miRNAs. Onco-miRNAs are
upregulated in human cancer and function to promote cell
proliferation and inhibit apoptosis, whereas tumor-suppressive
miRNAs are downregulated in human cancer and can prevent cancer
development (16–18). Of particular note, miRNAs have
received widespread attention as a potential targeted therapy
(19). Therefore, the use of miRNA
sequences for the diagnosis, treatment and prognosis of cancer
could be beneficial.
In the present study, the expression and molecular
function of miR-7 in human thyroid cancer was investigated. The
results presented in this study reveal that miR-7 is downregulated
in human thyroid cancer tissues and cells. miR-7 expression levels
were also observed to be associated with thyroid cancer tumor
stage. In addition, ectopic expression of miR-7 suppressed thyroid
cancer cell proliferation, migration and invasion. Moreover,
p21-activated kinase 1 (PAK1) was identified as a novel miR-7
target. Silencing of PAK1 in thyroid tumor cell lines was observed
to confer a similar phenotypic effect on cell growth, migration and
invasion as ectopic expression of miR-7. Ultimately, these results
suggest that miR-7 may function as a tumor suppressor by regulating
cell growth, migration and invasion via direct targeting of PAK1,
and should therefore be investigated as a novel putative target for
the treatment of thyroid cancer.
Materials and methods
Clinical specimens
A total of 32 human PTC tissue pairs, matched normal
adjacent tissues (NATs) and six normal thyroid tissue samples, were
collected from Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China). This study was approved by Tianjin
Medical University Cancer Institute and Hospital's Protection of
Human Subjects Committee and written informed consent was also
obtained from all patients with thyroid cancer. Patients included
in the present study had not received chemotherapy or radiothera py
prior to surgery. Tissue samples were immediately snap frozen in
liquid nitrogen following surgery and stored at −80°C until
required.
Cell culture and transfection
Human PTC cell lines, TPC-1 and HTH83, were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). Cell lines were maintained in Dulbecco's
modified Eagle's medium (DMEM) with 10% (v/v) fetal bovine serum
(FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a
humidified 5% (v/v) CO2 cell incubator at 37°C.
Mature miR-7 mimics, negative controls (NC), PAK1
small interfering RNA (siRNA), NC siRNA and luciferase reporter
plasmids were synthesized and obtained from Shanghai GenePharma
Co., Ltd., (Shanghai, China). Cells were seeded onto a 6-well plate
and cultured in complete DMEM without antibiotics. Transfections
and co-transfections were performed when cells had reached 30–40%
confluence using Lipofectamine 2,000 (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) according to the
manufacturer's instructions.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human PTC tissues,
NATs, normal thyroid tissue samples, TPC-1 cells and HTH83 cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. For detection of
miR-7 expression, reverse transcription was performed using the
M-MLV Reverse Transcription system (Promega Corporation, Madison,
WI, USA). Following, reverse transcription, qPCR was performed
using SYBR green I mix (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's instructions. Each sample
was analyzed in triplicate. U6 small nuclear RNA (snRNA) and
glyceraldehyde 3-phosphate dehydrogenase (GADPH) were used as
internal controls. All primers were obtained from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). Gene expression was quantified using
the 2−ΔΔCq method.
MTT assay
Analysis of cell growth was achieved using the MTT
assay. At 24 h following transfection, cells were seeded on 96-well
plates at 3,000 cells/well. The MTT assay was performed at 24, 48,
72 and 96 h. Briefly, 20 µl MTT (5 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) was added into each sample. The 96-well plates were
then incubated at 37°C for 4 h. Cell culture medium was
subsequently removed and formazan precipitates were dissolved in
200 µl dimethylsulfoxide. A microplate reader (Model 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to read the
absorbance at 490 nm for each sample. Suppression rate was
calculated using the following formula: Suppression rate =
(1-A490miR-7/A490NC) x100%. Experiments were
performed in triplicate.
Cell migration and invasion assay
In vitro cell migration and invasion assays
were performed using Transwell® chambers (Corning, Inc.,
Corning, NY, USA). For the migration assay, 4×104
transfected cells cultured in 200 µl DMEM without FBS, were
added into the top chamber. For the invasion assay,
4×104 transfected cells in 200 µl DMEM without
FBS were placed into the top chamber coated with Matrigel (BD
Biosciences, Franklin Lakes, CA, USA). For both assays, 500
µl DMEM containing 20% FBS was then added to the lower
chamber as a chemo-attractant. Following 24 h incubation, cells
were fixed with 100% methanol (Beyotime Institute of Biotechnology,
Haimen, China) and stained with 0.5% crystal violet (Beyotime
Institute of Biotechnology). Cells that had not migrated or invaded
through the 8-µm pores, were removed carefully with a cotton
swab. For every sample, five fields (magnification, x200) were
imaged at random using an inverted microscope (Olympus Corporation,
Tokyo, Japan). Experiments were performed in triplicate.
Targeting of miR-7
TargetScan (http://www.targetscan.org) software program was used
to search for miR-7 target genes.
Western blot analysis
Goat anti-human PAK1 (sc-31685) and mouse anti-human
GADPH primary antibodies (sc-166574) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). At 72 h following
transfection, cells were washed with phosphate-buffered saline
(PBS) and lysed in radioimmunoprecipitation assay (RIPA) lysis
buffer (Beyotime Institute of Biotechnology). Equal quantities of
protein were separated using 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and electroblotted onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked in PBS containing 0.1%
Tween® 20 (Beyotime Institute of Biotechnology) and 5%
non-fat dry milk. The membranes were then probed with PAK1
(1:1,000) or GADPH (1:1,000) primary antibody for an overnight
incubation at 4°C. Corresponding secondary antibodies (sc-2354 for
PAK1; sc-2005 for GADPH; Santa Cruz Biotechnology, Inc.) were then
added followed by incubation at 1:1,000 dilution in Tris-buffered
saline with Tween® 20. Protein bands were detected using
enhanced chemiluminescence solution (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and band intensities were quantified using the
FluorChem imaging system (alpha innotec, GmbH, Kasendorf, DE).
Dual-luciferase reporter assay
Dual-luciferase reporter assays were performed to
determine whether PAK1 was a direct target of miR-7. For miRNA
target validation, cells were transfected with miR-7 mimics or NC,
and co-transfected with PGL3-PAKl-3′-UTR wild-type (wt) or
PGL3-PAKl-3′-UTR mutant (mut) in three independent experiments. At
48 h following transfection, firefly and Renilla luciferase
activities were detected with the Dual-Luciferase Reporter assay
system (Promega Corporation). Renilla luciferase activity
was used as an internal control.
Statistical analysis
Data were presented as the mean ± standard
deviation. and analyzed using SPSS software (version 17.0; SPSS,
Inc., Chicago, IL, USA). Differences were considered significant if
P-values were <0.05.
Results
PTC tissues and cell lines express lower
levels of miR-7 compared with normal thyroid tissues
The expression of miR-7 in PTC tissues, matched
NATs, normal thyroid tissues and PTC cell lines was determined
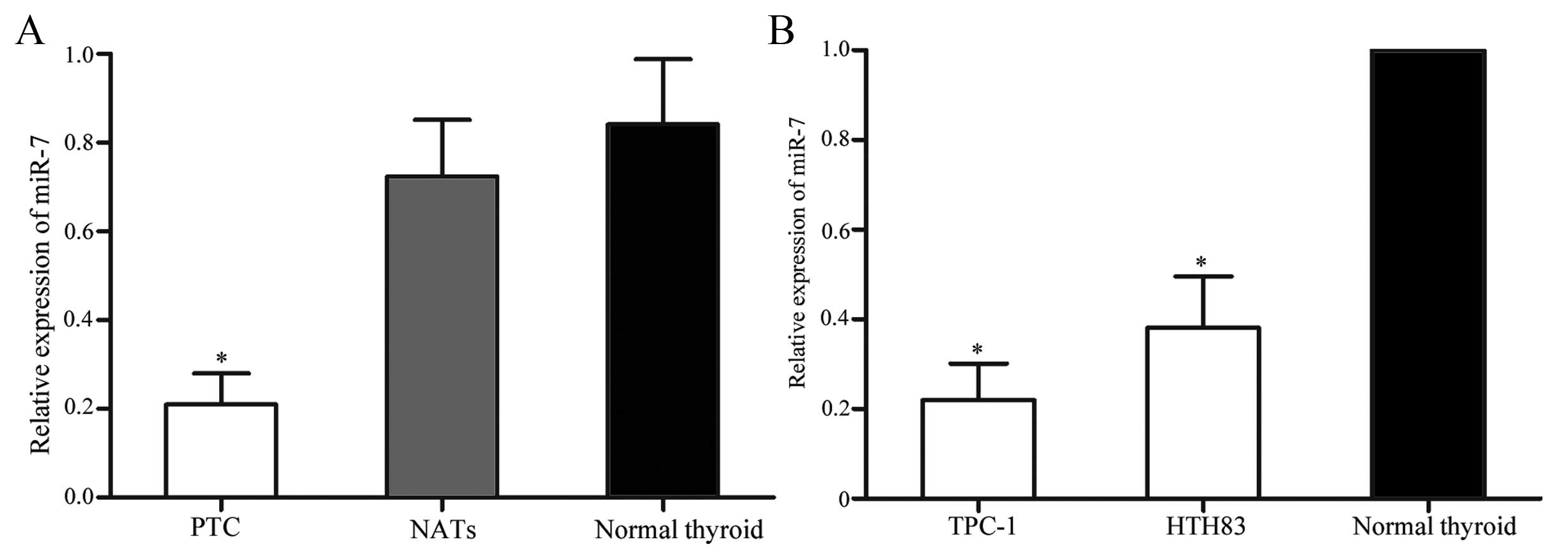
using RT-qPCR. As shown in Fig.
1A, miR-7 was significantly downregulated in PTC tissues when
compared with matched NATs (P=0.015) and normal thyroid tissues
(P=0.007). Downregulation of miR-7 was also observed in TPC-1 and
HTH83 cells compared with normal thyroid tissues ((P=0.010 for
TPC-1; P=0.012 for HTH83). These results suggest that miR-7 may
play tumor-suppressive role in PTC.
Association between miR-7 expression and
thyroid tumor stage
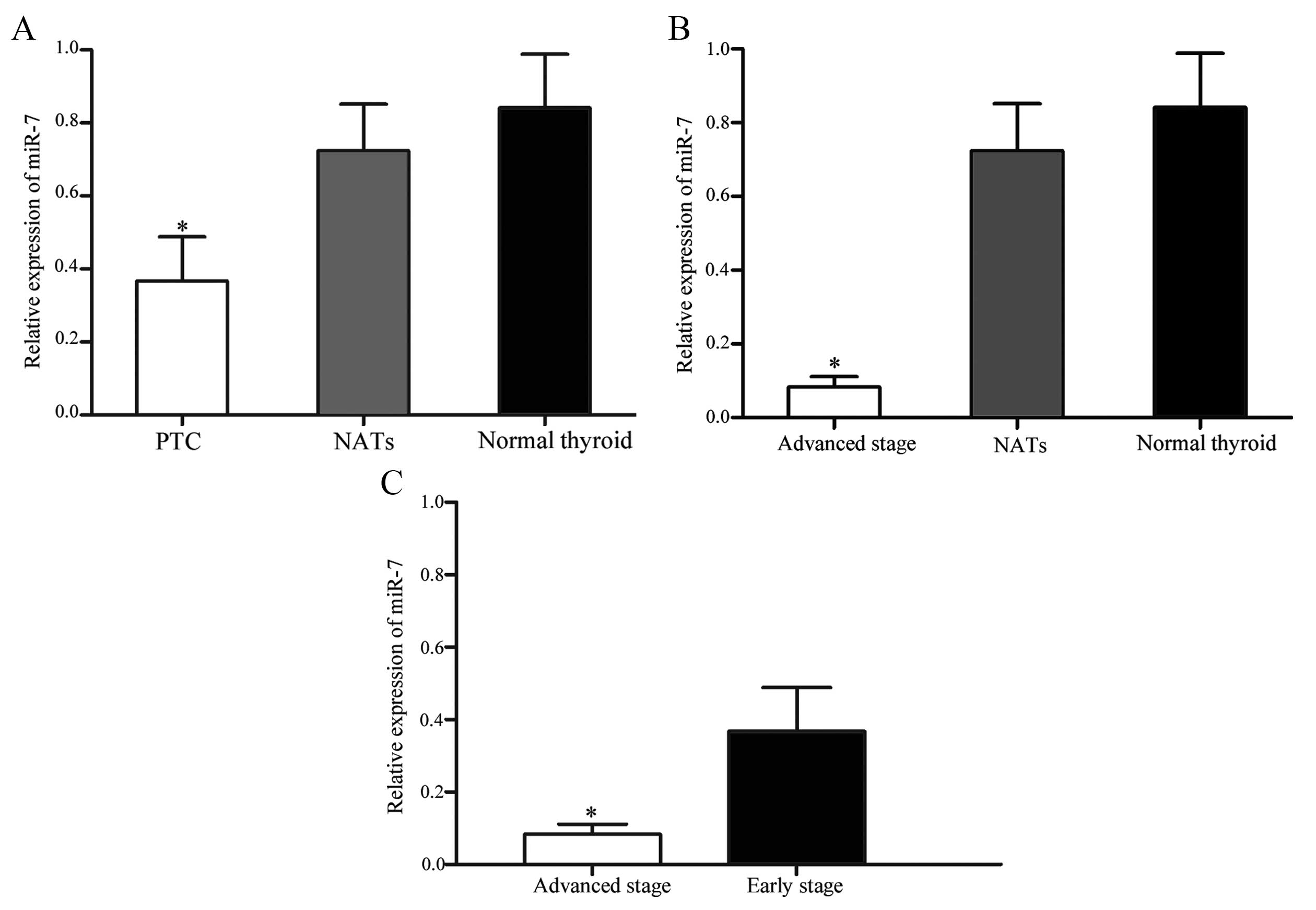
In order to investigate an association between miR-7
expression and thyroid tumor stage, statistical analysis was
performed. Patients with thyroid cancer were divided into
early-stage (T1 and T2) and advanced-stage (T3 and T4) groups. The
expression levels of miR-7 in early-stage (P=0.025; Fig. 2A) and advanced-stage (P=0.005;
Fig. 2B) groups were significantly
lower than NATs and normal thyroid tissues. In addition, miR-7
expression in the advanced-stage group was significantly
downregulated compared with the early-stage group (P=0.034;
Fig. 2C). These results suggest
that lower levels of miR-7 expression are associated with increased
thyroid tumor stage.
miR-7 suppresses TPC-1 and HTH83 cell
proliferation
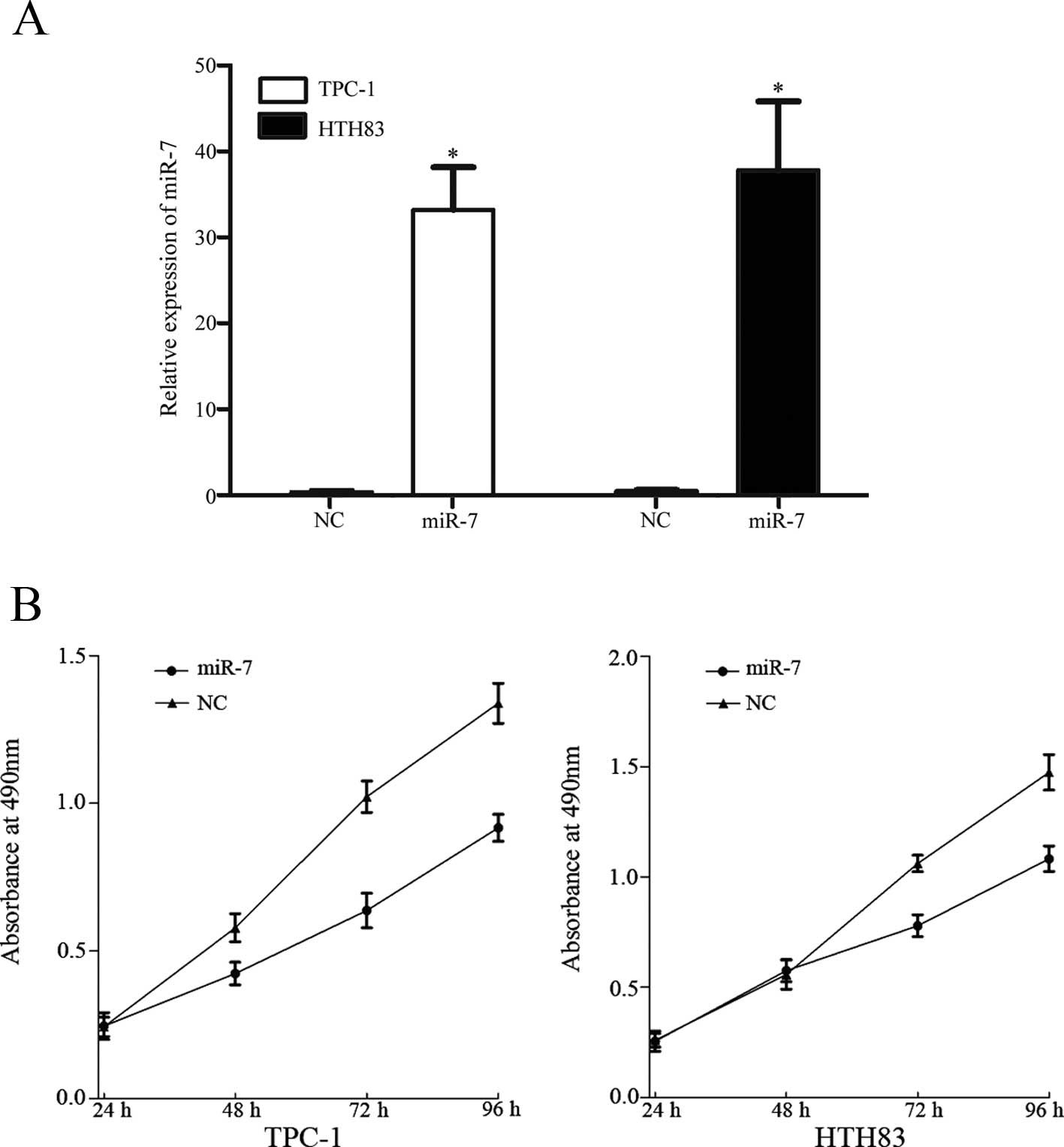
To investigate the role of miR-7 in TPC-1 and HTH83
cells, miR-7 and NC miRNA mimics were transfected into TPC-1 and
HTH83 cells. At 48 h following transfection, total cell RNA was
extracted and RT-qPCR was performed to analyze the expression of
miR-7. As shown in Fig. 3A, miR-7
was significantly upregulated in TPC-1 and HTH83 cells transfected
with miR-7 mimics compared with controls (P=0.001).
The MTT assay was selected to investigate the effect
of miR-7 on cell proliferation. As shown in Fig. 3, upregulation of miR-7
significantly inhibited cell proliferation of TPC-1 (P=0.018) and
HTH83 (P=0.031) cells when compared with controls. This provides
further support to the hypothesis that miR-7 functions as a tumor
suppressor in human thyroid cancer.
miR-7 suppresses TPC-1 and HTH83 cell
migration and invasion
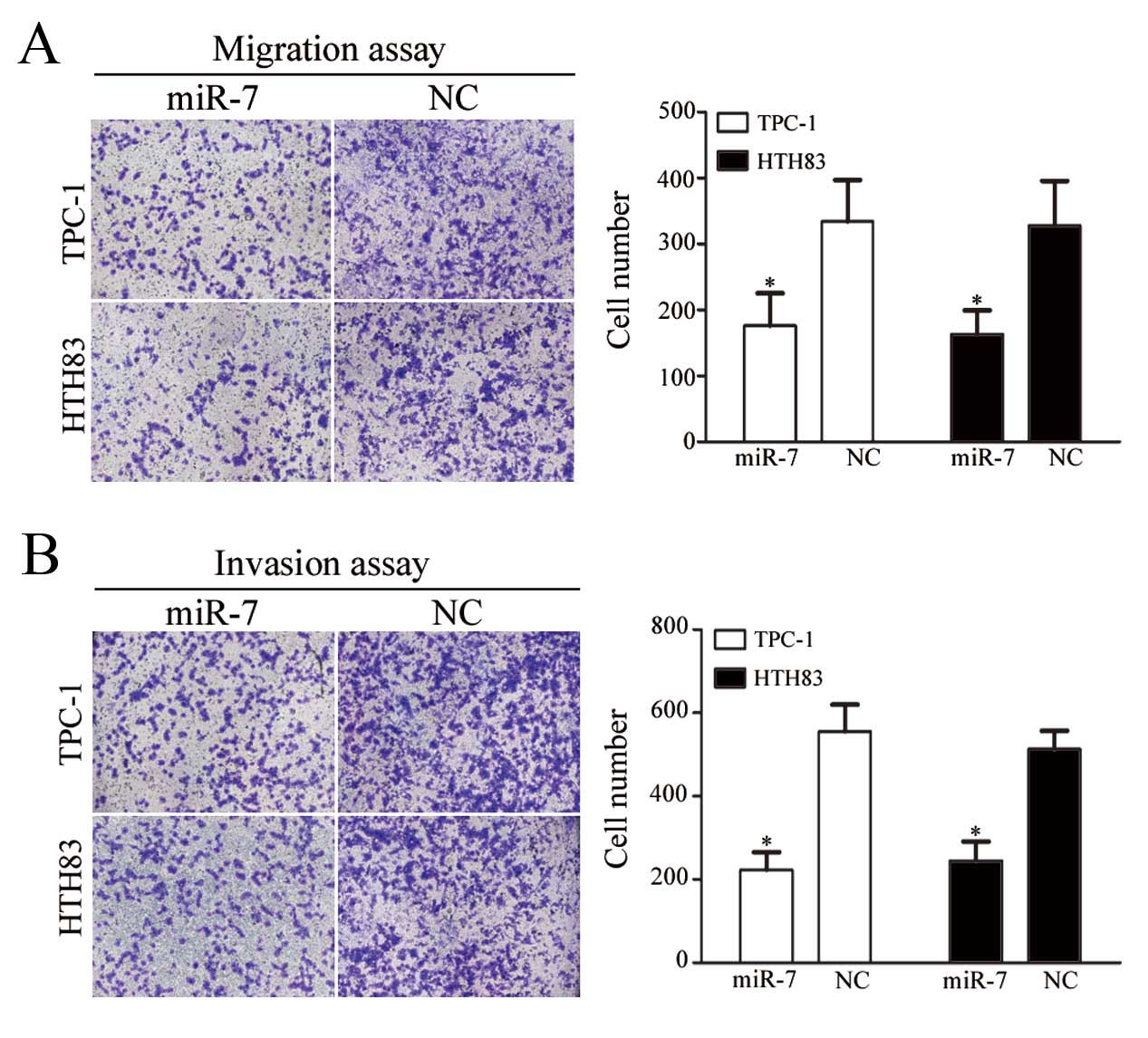
The next aim of the study was to investigate the
role of miR-7 on TPC-1 and HTH83 cell migration and invasion. As
shown in Fig. 4A and B,
miR-7-transfected TPC-1 and HTH83 cells demonstrated a significant
reduction in cell migration (P=0.034 and P=0.029, respectively) and
invasion (P=0.021 and P=0.028, respectively) compared with
NC-transfected cells.
PAK1 is a direct target of miR-7 in
vitro
In order to investigate the potential mechanism by
which miR-7 regulates thyroid cancer cell growth, migration and
invasion the online TargetScan software program was used to search
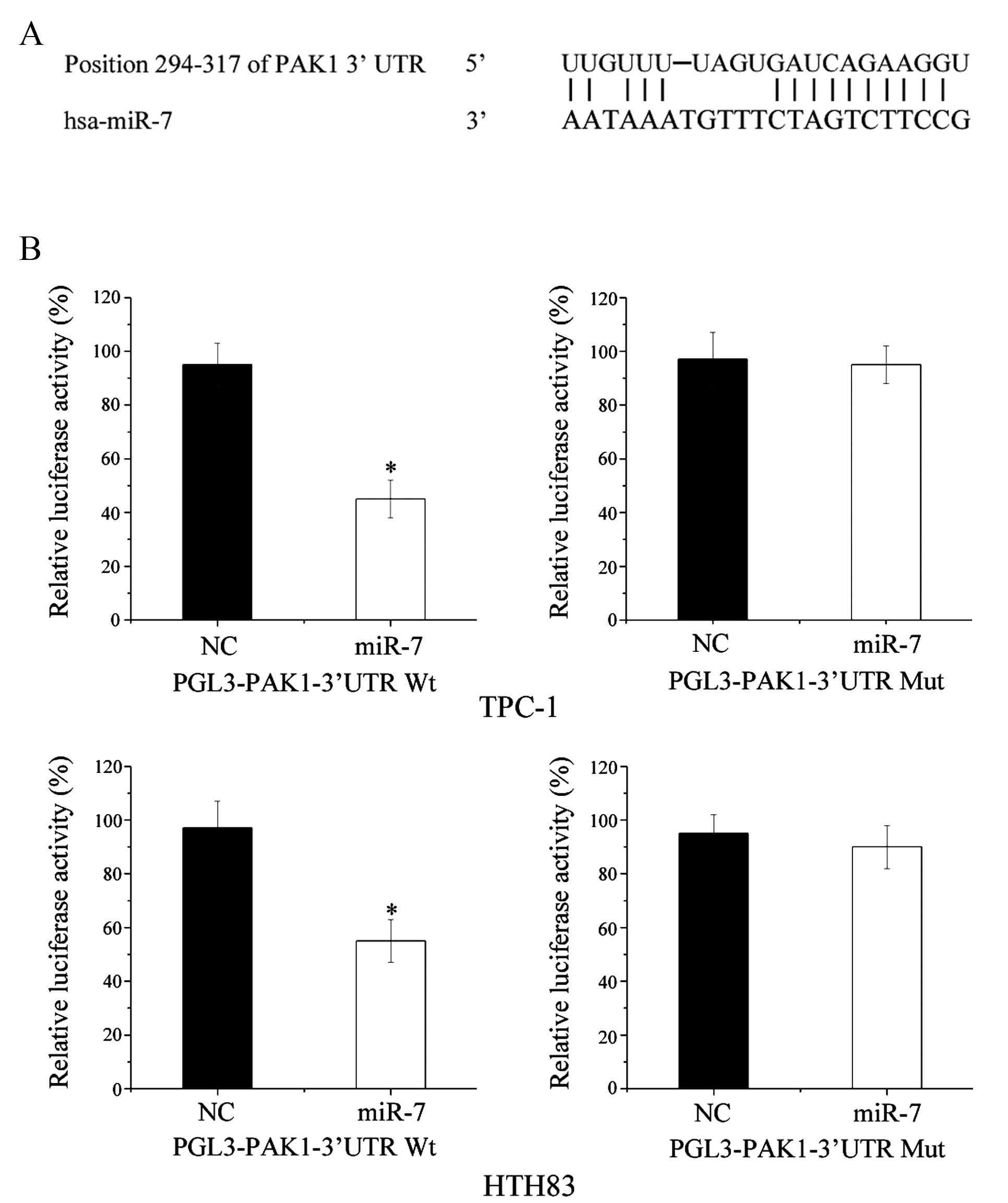
for miR-7 target genes. As shown in Fig. 5A, PAK1 was predicted to be a direct
target of miR-7.
Dual-luciferase reporter assays were performed to
confirm whether PAK1 was a direct biological target of miR-7. As
shown in Fig. 5B, miR-7
significantly inhibited PGL3-PAKl-3′-UTR wt but not
PGL3-PAKl-3′-UTR mut luciferase activity in TPC-1 and HTH83 cells
(P=0.022 and P=0.026, respectively). These results indicate that
PAK1 is a direct target of miR-7 in vitro.
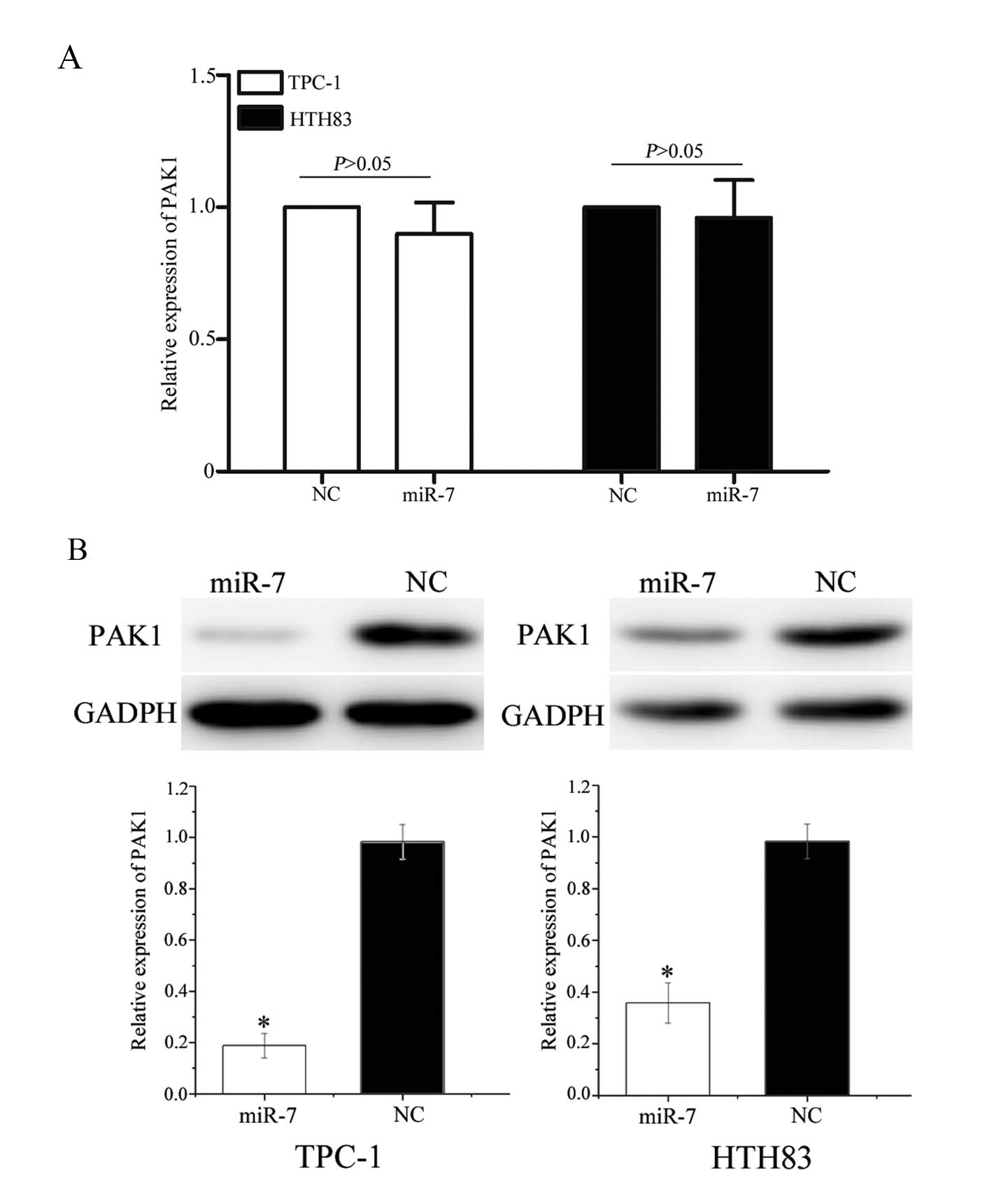
miR-7 negatively regulates PAK1
expression at a post-transcriptional level
To investigate an association between miR-7 and PAK1
at the mRNA and protein levels, miR-7 or NC miRNA mimics were
transfected into TPC-1 and HTH83 cells. PAK1 mRNA levels were
measured using RT-qPCR and protein expression was determined using
western blot analysis. As shown in Fig. 6A, PAK1 mRNA levels were not
significantly altered following transfection with miR-7 mimics
(P=0.715 and P=0.812, respectively). However, western blot analysis
revealed that the expression of PAK1 was significantly
downregulated in TPC-1 and HTH83 cells transfected with miR-7
mimics compared with controls (Fig.
6B, P=0.020 and P=0.028, respectively). These results suggest
that miR-7 regulates PAK1 expression at the post-transcriptional
level.
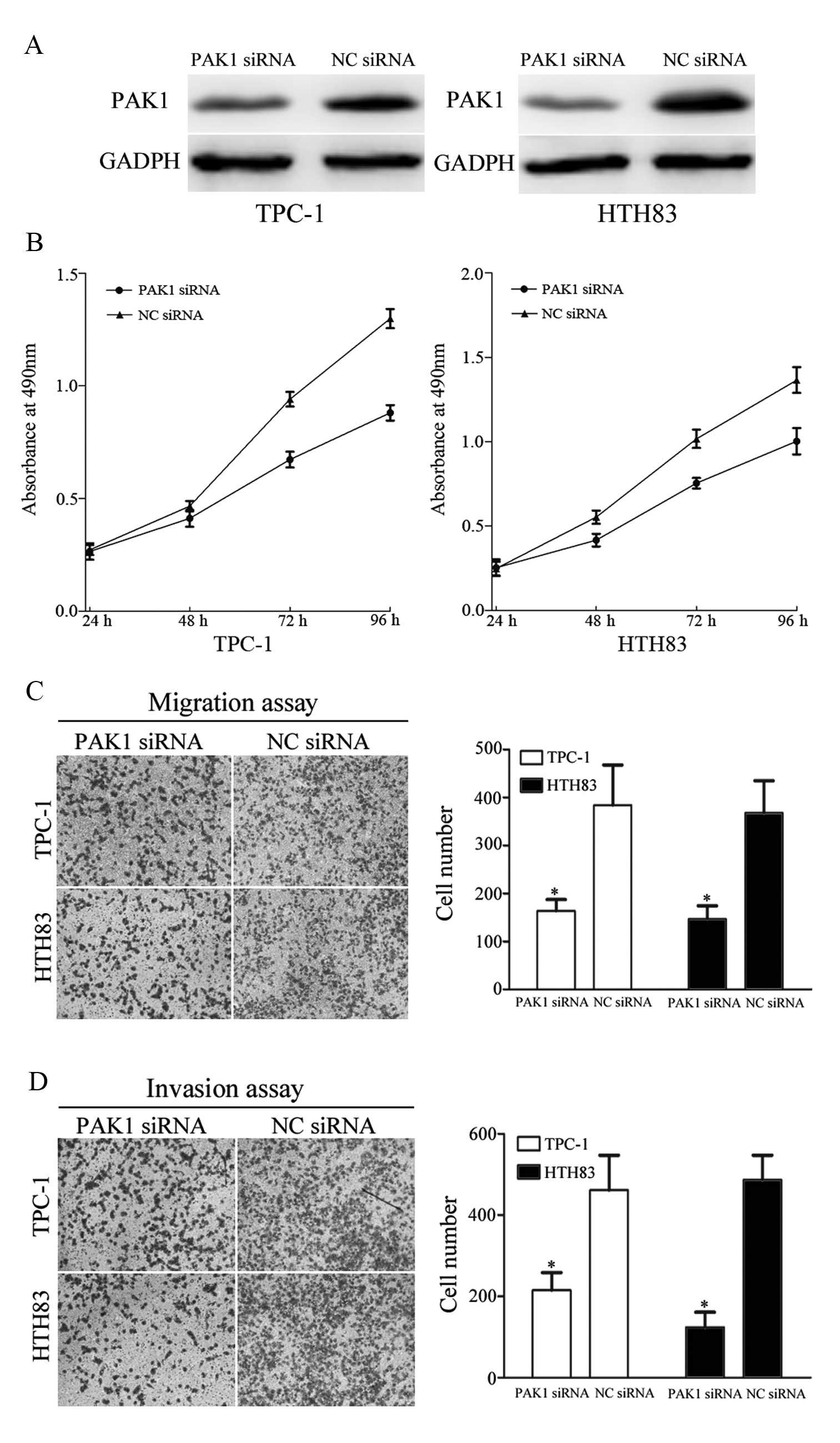
PAK1 is implicated in the effects of
miR-7 on TPC-1 and HTH83 cell growth migration and invasion
To determine whether PAK1 functions as a critical
mediator of miR-7 in TPC-1 and HTH83 cell growth, migration and
invasion, these cells were transfected with PAK1 siRNA and NC siRNA
to silence PAK1 expression. At 72 h following transfection, western
blot analysis demonstrated that PAK1 was down-regulated in TPC-1
and HTH83 cells transfected with PAK1 siRNA compared with controls
(Fig. 7A, P=0.031 and P=0.027,
respectively).
As shown in Fig.
7B, treatment of TPC-1 and HTH83 cells with PAK1 siRNA
significantly inhibited cell growth compared with controls (P=0.014
and P=0.029, respectively). In addition, migration and invasion
assays revealed that PAK1 siRNA markedly suppressed TPC-1 and HTH83
cells migration (P=0.037 and P=0.023, respectively) and invasion
(P=0.039 and P=0.016, respectively) compared with controls. These
results demonstrate that reduced PAK1 expression confers a similar
phenotype as increased miR-7 expression in TPC-1 and HTH83 cells.
Due to the putative interaction between miR-7 and PAK1, it is
possible that miR-7 regulates tumor cell growth, migration and
invasion through direct targeting of PAK1.
Discussion
Over the last decade, accumulating evidence suggests
that aberrant miRNA expression is a common feature of malignancy,
including thyroid cancer (20–22).
miR-7 is an intronic miRNA sequence located in the first intron of
hnRNPK and PGSF1 genes on human chromosomes 9 and 19, respectively
(23). An increasing number of
studies have demonstrated that miR-7 is downregulated in multiple
human tumor types including breast cancer (24), hepatocellular carcinoma (HCC)
(25), cervical cancer (26) and colorectal cancer (27). However, to date, no study has
investigated the expression of miR-7 in thyroid cancer. In the
present study, miR-7 was observed to be significantly downregulated
in human PTC tissues and PTC cell lines. The expression level of
miR-7 was also found to be associated with thyroid tumor stage.
This suggests that miR-7 may play an important role in thyroid
cancer progression.
Previous studies have shown that miR-7 may function
as a tumor suppressor in multiple tumor types (23–26,28,29,31).
miR-7 was found to be significantly downregulated in human cervical
cancer, and ectopic expression of miR-7 was observed to inhibit
cell metastasis and invasion by targeting focal adhesion kinase
(FAK) (26). In human breast
cancer cells, miR-7 decreased cell proliferation, migration,
invasion and induced apoptosis through targeting proteasome
activator subunit 3 and FAK (24,28).
Lee et al (23) also
observed that miR-7 increased the radiosensitivity of breast cancer
cells with activated epidermal growth factor receptor-associated
signaling. In human HCC, miR-7 suppressed cell growth, motility and
caused cell cycle arrest in G1 through targeting the
phosphoinositide 3-kinase (PI3K)/Akt pathway and cyclin E1 (CCNE1)
(25,29). Previous studies also demonstrated
that miR-7 inhibits glioma cells growth, glucose metabolism,
migration, invasion and tumorigenesis by targeting focal adhesion
kinase, insulin-like growth factor 1, eukaryotic translation
initiation factor 5A, annexin A4, PI3K/ATK and Raf/MEK/ERK pathways
(30–33). These results suggest that miR-7
plays an important tumor suppressive role in these cancer types,
and may therefore serve as a potential therapeutic target for the
treatment of these cancers.
To date, numerous studies have provided evidence to
suggest that miR-7 functions as a tumor suppressor; however, others
have reported that miR-7 may play an oncogenic role in certain
tumor types (34). miR-7
expression was observed to be upregulated in lung cancer compared
with NATs, and was negatively correlated with disease-free
survival. Moreover, miR-7 promoted cell growth and tumor formation
by targeting the ERF tumor suppressor (35). Consistent with these results, miR-7
was observed to be upregulated in renal cell carcinoma, and
downregulation of miR-7 decreased cell growth, migration and
induced apoptosis (36). These
conflicting observations suggest that miR-7 has a tumor and
tissue-specific expression pattern and function. In the present
study, we demonstrate that miR-7 inhibits thyroid cancer cell
proliferation, migration and invasion, which provides additional
information about the functional role of miR-7 in human cancer.
Identification of miR-7 target genes is important
for understanding its role in tumorigenesis and tumor progression.
It is also important for developing novel targeted therapies. In
the present study, an important functional link between miR-7 and
PAK1 was identified in thyroid cancer. The TargetScan online
software tool predicted that PAK1 was a direct target gene of
miR-7. Using a dual-luciferase reporter assay, we then confirmed
that miR-7 targets the PAK1 3′-UTR directly in vitro.
RT-qPCR and western blot analysis revealed that miR-7 does not
affect PAK1 mRNA stability, but decreases PAK1 expression at the
post-transcriptional level. Knockdown of PAK1 also significantly
inhibited thyroid cancer cell proliferation, migration and
invasion. PAK1 was therefore thought to be involved in
miR-7-induced effects in TPC-1 and HTH83 cells. These results
suggest that miR-7 may play a tumor suppressive role in thyroid
cancer initiation and progression by targeting PAK1 directly.
In 1994, Manser et al (37) discovered PAKs during a screen for
novel proteins that interact with small rho-like G proteins. PAKs
have a common molecular weight of 21 kDa, and are known
collectively as p21 proteins. In humans and other mammals, PAKs are
a class of non-receptor serine/threonine kinases with six known
isoforms (PAK 1-6) (38). Based on
their sequences and functional similarities, PAKs have been
classified into two groups: Group I (PAK1, PAK2 and PAK3) and group
II (PAK4, PAK5 and PAK6) (39).
PAKs have been observed to play important roles in physiological
processes including motility, survival, mitosis, transcription and
translation (40,41). PAK1 has been demonstrated to be a
key regulator of cancer-related signaling pathways (39). An increasing number of studies have
also identified an association between PAK1 expression and tumor
progression, which suggests that PAK1 may be a promising diagnostic
and therapeutic target for cancers (42–46).
In thyroid cancer, PAK1 has been observed to be upregulated in the
invasive fronts of human thyroid cancer tissues. Moreover,
functional studies have revealed that PAK1 is involved in cell
migration and metastasis (47,48).
Therefore, PAK1 may represent a novel anti-cancer target for the
treatment of thyroid cancer and requires further investigation. In
the present study, we identified miR-7 as a negative regulator of
PAK1 expression, which functions to decrease thyroid cancer cell
growth, migration and invasion. Therefore, the use of miR-7 as a
target of PAK1 in thyroid cancer treatment, should be investigated
in future studies.
In conclusion, the results of the present study
indicate that miR-7 is downregulated in thyroid cancer tissue
samples and cell lines compared with normal thyroid tissue samples.
An association between miR-7 and tumor stage was also identified.
The results presented provide evidence to suggest that miR-7
inhibits thyroid cancer cell proliferation, migration and invasion
via the direct targeting of PAK1. miR-7 should therefore be
investigated as a potential target for the treatment of thyroid
cancer. Further studies will be required to determine the efficacy
of miR-7 as an anti-cancer target for thyroid cancer treatment.
Acknowledgments
This study was supported by the Tianjin Cancer
Hospital Research Fund (grant no. 1305).
References
|
1
|
Braun J, Hoang-Vu C, Dralle H and
Hüttelmaier S: Downregulation of microRNAs directs the EMT and
invasive potential of anaplastic thyroid carcinomas. Oncogene.
29:4237–4244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vriens MR, Weng J, Suh I, Huynh N,
Guerrero MA, Shen WT, Duh QY, Clark OH and Kebebew E: MicroRNA
expression profiling is a potential diagnostic tool for thyroid
cancer. Cancer. 118:3426–3432. 2012. View Article : Google Scholar
|
|
3
|
Hartmann C, Mueller W and von Deimling A:
Pathology and molecular genetics of oligodendroglial tumors. J Mol
Med (Berl). 82:638–655. 2004. View Article : Google Scholar
|
|
4
|
Geraldo MV, Fuziwara CS, Friguglieti CU,
Costa RB, Kulcsar MA, Yamashita AS and Kimura ET: MicroRNAs
miR-146-5p and let-7f as prognostic tools for aggressive papillary
thyroid carcinoma: A case report. Arq Bras Endocrinol Metabol.
56:552–557. 2012. View Article : Google Scholar
|
|
5
|
Lv M, Zhang X, Li M, Chen Q, Ye M, Liang
W, Ding L, Cai H, Fu D and Lv Z: miR-26a and its target CKS2
modulate cell growth and tumorigenesis of papillary thyroid
carcinoma. PLoS One. 8:e675912013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng Y, Li C, Luo DC, Ding JW, Zhang W and
Pan G: Expression profile and clinical significance of microRNAs in
papillary thyroid carcinoma. Molecules. 19:11586–11599. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hunt JP, Buchmann LO, Wang L and Abraham
D: An analysis of factors predicting lateral cervical nodal
metastases in papillary carcinoma of the thyroid. Arch Otolaryngol
Head Neck Surg. 137:1141–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pallante P, Visone R, Ferracin M, Ferraro
A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M,
Negrini M, et al: MicroRNA deregulation in human thyroid papillary
carcinomas. Endocr Relat Cancer. 13:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tetzlaff MT, Liu A, Xu X, Master SR,
Baldwin DA, Tobias JW, Livolsi VA and Baloch ZW: Differential
expression of miRNAs in papillary thyroid carcinoma compared to
multinodular goiter using formalin fixed paraffin embedded tissues.
Endocr Pathol. 18:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber F, Teresi RE, Broelsch CE, Frilling
A and Eng C: A limited set of human MicroRNA is deregulated in
follicular thyroid carcinoma. J Clin Endocrinol Metab.
91:3584–3591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Visone R, Pallante P, Vecchione A,
Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V,
Borbone E, et al: Specific microRNAs are downregulated in human
thyroid anaplastic carcinomas. Oncogene. 26:7590–7595. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JC, Gundara JS, Glover A, Serpell J
and Sidhu SB: MicroRNA expression profiles in the management of
papillary thyroid cancer. Oncologist. 19:1141–1147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96(Suppl): R40–R44. 2007.PubMed/NCBI
|
|
15
|
Li X, Abdel-Mageed AB, Mondal D and Kandil
E: MicroRNA expression profiles in differentiated thyroid cancer, a
review. Int J Clin Exp Med. 6:74–80. 2013.
|
|
16
|
Braun J and Hüttelmaier S: Pathogenic
mechanisms of deregulated microRNA expression in thyroid carcinomas
of follicular origin. Thyroid Res. 4(Suppl 1): S12011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
18
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sondermann A, Andreghetto FM, Moulatlet
AC, da Silva Victor E, de Castro MG, Nunes FD, Brandão LG and
Severino P: MiR-9 and miR-21 as prognostic biomarkers for
recurrence in papillary thyroid cancer. Clin Exp Metastasis.
32:521–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong Y, Zhang L, Holloway AK, Wu X, Su L
and Kebebew E: MiR-886-3p regulates cell proliferation and
migration, and is dysregulated in familial non-medullary thyroid
cancer. PLoS One. 6:e247172011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YT, Kitabayashi N, Zhou XK, Fahey TJ
III and Scognamiglio T: MicroRNA analysis as a potential diagnostic
tool for papillary thyroid carcinoma. Mod Pathol. 21:1139–1146.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee KM, Choi EJ and Kim IA: microRNA-7
increases radiosensitivity of human cancer cells with activated
EGFR-associated signaling. Radiother Oncol. 101:171–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong X, Li G, Yuan Y, He Y, Wu X, Zhang W,
Wu Z, Chen T, Wu W, Lobie PE and Zhu T: MicroRNA-7 inhibits
epithelial-to-mesenchymal transition and metastasis of breast
cancer cells via targeting FAK expression. PLoS One. 7:e415232012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao Z, Yang J, Wang C, Li Y, Zhang Y, Dong
X, Zhou L, Liu J, Zhang Y and Qian J: MicroRNA-7 inhibits
metastasis and invasion through targeting focal adhesion kinase in
cervical cancer. Int J Clin Exp Med. 8:480–487. 2015.PubMed/NCBI
|
|
27
|
Suto T, Yokobori T, Yajima R, Morita H,
Fujii T, Yamaguchi S, Altan B, Tsutsumi S, Asao T and Kuwano H:
MicroRNA-7 expression in colorectal cancer is associated with poor
prognosis and regulates cetuximab sensitivity via EGFR regulation.
Carcinogenesis. 36:338–345. 2015. View Article : Google Scholar
|
|
28
|
Shi Y, Luo X, Li P, Tan J, Wang X, Xiang T
and Ren G: miR-7-5p suppresses cell proliferation and induces
apoptosis of breast cancer cells mainly by targeting REGγ. Cancer
Lett. 358:27–36. 2015. View Article : Google Scholar
|
|
29
|
Zhang X, Hu S, Zhang X, Wang L, Zhang X,
Yan B, Zhao J, Yang A and Zhang R: MicroRNA-7 arrests cell cycle in
G1 phase by directly targeting CCNE1 in human hepatocellular
carcinoma cells. Biochem Biophys Res Commun. 443:1078–1084. 2014.
View Article : Google Scholar
|
|
30
|
Wang B, Sun F, Dong N, Sun Z, Diao Y,
Zheng C, Sun J, Yang Y and Jiang D: MicroRNA-7 directly targets
insulin-like growth factor 1 receptor to inhibit cellular growth
and glucose metabolism in gliomas. Diagn Pathol. 9:2112014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu
S, Yu S and Liu X: miR-7 inhibits glioblastoma growth by
simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK
pathways. Int J Oncol. 44:1571–1580. 2014.PubMed/NCBI
|
|
32
|
Lu ZJ, Liu SY, Yao YQ, Zhou YJ, Zhang S,
Dai L, Tian HW, Zhou Y, Deng HX, Yang JL and Luo F: The effect of
miR-7 on behavior and global protein expression in glioma cell
lines. Electrophoresis. 32:3612–3620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun
LH, Wang XF, Zhang JX, Cao L, Wang XR, et al: MicroRNA-7 regulates
glioblastoma cell invasion via targeting focal adhesion kinase
expression. Chin Med J (Engl). 124:2616–2621. 2011.
|
|
34
|
Kalinowski FC, Brown RA, Ganda C, Giles
KM, Epis MR, Horsham J and Leedman PJ: microRNA-7: A tumor
suppressor miRNA with therapeutic potential. Int J Biochem Cell
Biol. 54:312–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chou YT, Lin HH, Lien YC, Wang YH, Hong
CF, Kao YR, Lin SC, Chang YC, Lin SY, Chen SJ, et al: EGFR promotes
lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc
pathway that targets the Ets2 transcriptional repressor ERF. Cancer
Res. 70:8822–8831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y,
Yu W, Wu X, Ye J, Yang S, et al: Identification of miR-7 as an
oncogene in renal cell carcinoma. J Mol Histol. 44:669–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manser E, Leung T, Salihuddin H, Zhao ZS
and Lim L: A brain serine/threonine protein kinase activated by
Cdc42 and Rac1. Nature. 367:40–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jaffer ZM and Chernoff J: p21-activated
kinases: Three more join the Pak. Int J Biochem Cell Biol.
34:713–717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar R, Gururaj AE and Barnes CJ:
p21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
King H, Nicholas NS and Wells CM: Role of
P-21-activated kinases in cancer progression. Int Rev Cell Mol
Biol. 309:347–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dart AE and Wells CM: P21-activated kinase
4-not just one of the PAK. Eur J Cell Biol. 92:129–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shrestha Y, Schafer EJ, Boehm JS, Thomas
SR, He F, Du J, Wang S, Barretina J, Weir BA, Zhao JJ, et al: PAK1
is a breast cancer oncogene that coordinately activates MAPK and
MET signaling. Oncogene. 31:3397–3408. 2012. View Article : Google Scholar :
|
|
43
|
Chow HY, Jubb AM, Koch JN, Jaffer ZM,
Stepanova D, Campbell DA, Duron SG, O'Farrell M, Cai KQ,
Klein-Szanto AJ, et al: p21-Activated kinase 1 is required for
efficient tumor formation and progression in a Ras-mediated skin
cancer model. Cancer Res. 72:5966–5975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Liu F and Li F: PAK as a therapeutic
target in gastric cancer. Expert Opin Ther Targets. 14:419–433.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cai XZ, Wang J, Li XD, Wang GL, Liu FN,
Cheng MS and Li F: Curcumin suppresses proliferation and invasion
in human gastric cancer cells by downregulation of PAK1 activity
and cyclin D1 expression. Cancer Biol Ther. 8:1360–1368. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Singhal R and Kandel ES: The response to
PAK1 inhibitor IPA3 distinguishes between cancer cells with
mutations in BRAF and Ras oncogenes. Oncotarget. 3:700–708. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McCarty SK, Saji M, Zhang X, Jarjoura D,
Fusco A, Vasko VV and Ringel MD: Group I p21-activated kinases
regulate thyroid cancer cell migration and are overexpressed and
activated in thyroid cancer invasion. Endocr Relat Cancer.
17:989–999. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma Y, McCarty SK, Kapuriya NP, Brendel VJ,
Wang C, Zhang X, Jarjoura D, Saji M, Chen CS and Ringel MD:
Development of p21 activated kinase-targeted multikinase inhibitors
that inhibit thyroid cancer cell migration. J Clin Endocrinol
Metab. 98:E1314–E1322. 2013. View Article : Google Scholar : PubMed/NCBI
|