Introduction
Mesenchymal stem cell (MSC)-based therapy has been
widely used in clinical trials and led to exciting developments in
cell therapy, including the prevention of graft versus host disease
(1), reducing liver fibrosis
(2), remodeling of broken bone
(3) and treatment of
cardiovascular diseases (4). An
important characteristic of MSCs is the ability to differentiate
into various cell types (5).
Another feature of MSCs is the lack of co-stimulatory molecules
including CD80, CD86 and HLA-II, which result in MSCs failing to
induce immune responses (6).
Additionally, MSCs also suppress the immune reactions mediated by T
cells, B cells, natural killer (NK) cells, dendritic cells and
complements (7–9). However, recent studies have indicated
that although MSCs exhibit low-immunogenicity and immunosuppressant
abilities, MSCs used as a therapeutic may be rejected or injured by
the host immune system and eventually disappear in vivo
(10–12).
Toll-like receptors (TLRs) are a family of
pathogen-associated molecular patterns (PAMPs) involved in
mediating immune responses induced by invading pathogens (13). There are 10 members in the human
TLRs family, which recognize distinct microbial products from
bacteria, viruses and fungi (14).
The TLRs also important for in MSC functions, including increasing
osteogenic differentiation (TLR3) (15), promoting MSCs migration (TLR5)
(16) and inhibiting MSCs
mediating immunosuppression (TLR4) (17). However, the importance of TLRs in
regulating the immune status of MSC has not been widely
investigated. A previous report demonstrated that TLR7 stimulates
the immunogenicity of MSCs (18),
whereas TLR3 and 4 did not alter the immune status of MSCs.
However, TLR3 and TLR4 agonists enhanced the expression of various
immune-associated molecules (19).
To the best of our knowledge, no previous report has investigated
the importance of TLR1/2 on the immune status of MSCs. The current
study used MSCs isolated from umbilical cord (UC) and activated the
TLR1/2 pathway using a specific agonist, aiming to determine
whether the activation of TLR1/2 changes the immune status of
MSCs.
Materials and methods
Culture and stimulation of MSCs
The MSCs from UC were provided by Sichuan Umbilical
Cord Blood Stem Cell Bank (Chengdu, China). The UCMSCs were
maintained at 37°C with 5% CO2 in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
1×105 cells/well in a 6-well plate.
TLR1/2 agonist, Pam3Csk, was purchased from Novus
Biologicals, Ltd. (Cambridge, UK) and dissolved in sterile water to
0.5 mg/ml as the stock concentration. The final concentration of
Pam3Csk used to stimulate UCMSCs was 100 ng/ml.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of treated and untreated UCMSCs was
extracted using the RNeasy kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's protocol. ReverTra Ace kit (Toyobo
Co., Ltd., Osaka, Japan) was used to perform the synthesis of cDNA
with the following RT conditions: 65°C (5 min), 37°C (15 min) and
98°C (5 min). qPCR was performed using RealMaster Mix SYBR Green
(cat. no. FP202; Tiangen Biotech Co., Ltd., Beijing, China) in an
iCycler iQ (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under
the following conditions: 95°C (30 sec), 58°C (30 sec) and 72°C for
(30 sec), followed by a melt curve from 55–95°C in 0.5°C increments
and 10 sec intervals for 40 cycles. For quantification, GAPDH was
used as the internal control while untreated UCMSC was negative
control. The by 2−ΔΔCq method was used for relative
quantification (20). The primers
used in detection were listed in Table
I. All detections of qPCR were performed three times.
 | Table IPrimers used for reverse
transcription-polymerase chain reaction. |
Table I
Primers used for reverse
transcription-polymerase chain reaction.
| Gene | Forward primer | Reverse primer | Gen bank no. |
|---|
| IFN-β |
CAGCAATTTTCAGTGTCAGAAGCT |
TCATCCTGTCCTTGAGGCAGT | M28622 |
| IL-6 |
GACCCAACCACAAATGCCA |
GTCATGTCCTGCAGCCACTG | M14584 |
| IL-8 |
CTGGCCGTGGCTCTCTTG |
CCTTGGCAAAACTGCACCTT | NM_000584 |
| IL-10 |
GGTGATGCCCCAAGCTGA |
TCCCCCAGGGAGTTCACA | U16720 |
| TNF-α |
GGTGCTTGTTCCTCAGCCTC |
CAGGCAGAAGAGCGTGGTG | M10988 |
| CCL5 |
GACACCACACCCTGCTGCT |
TACTCCTTGATGTGGGCACG | NM_002985 |
| MCP-1 |
AGCAGAGGCTGGAGAGCTACA |
GGGTCAGCACAGATCTCCTTGT | NM_006273 |
| MCP-3 |
CCTCTCCTGCCTCATGCTTATT |
CTCTGTCTCTGCATCATTTGTGAA | U58914 |
| IP10 |
TGAAATTATTCCTGCAAGCCAA |
CAGACATCTCTTCTCACCCTTCTTT | NM_001565 |
| MIP-1 |
GACACCACACCCTGCTGCT |
TACTCCTTGATGTGGGCACG | NM_002985 |
| Nanog |
CCAAAGGCAAACAACCCACTT |
CGGGACCTTGTCTTCCTTTTT | NM_00129769 |
| Sox2 |
CCCCTTTATTTTCCGTAGTTGTATTT |
GATTCTCGGCAGACTGATTCAA | NM_003106.3 |
| Lin28 |
GTCATCAGCGTCAGCAAAGG |
CCCTGCTGCTCAGCACTT | NM_004235.4 |
| Otx2 |
GGTTTCCTCTCCCTCTCCAC |
AATTTGAATTTTTACGTCTGCTG | NM_002448.3 |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC | J04038 |
Antibody array
Supernatants from treated and untreated groups were
collected at 4 h post-stimulation. Supernatants were centrifuged
(800 × g, 10 min, 10°C) to remove the residual cells and then
stored at −80°C. All samples, including TLR1 agonist treated and
untreated, were screened for secreted protein using RayBio Human
Antibody Array C Series 1000 (RayBiotech, Inc., Norcross, GA, USA)
according to the manufacturer's protocol. Blots were analyzed using
ImageJ software, version 1.50 (National Institutes of Health,
Bethesda, MD, USA).
Leukocyte proliferation and
leukocyte-mediated cytotoxicity detection
Peripheral blood mononuclear cells (PBMCs) were
isolated from healthy volunteers and labeled by with
carboxyfluorescein diacetate succinimidyl ester (CFSE) at 37°C for
10 min with a final concentration 10 µM. The current study
was approved by the ethics committee of The Central Hospital of
Dazhou (Dazhou, China), and written informed consent was obtained.
The labeling reaction was stopped by adding 5 ml pre-cooled
complete medium. The remaining CFSE was removed by three washes
with cold phosphate-buffered saline (800 × g, 5 min, 4°C). PBMCs
were then co-cultured with UCMSCs with or without TLR1/2 agonist
Pam3Csk. The ratio of PBMCs and UCMSCs in co-culture system was
5:1. PBMCs were collected for proliferation detection by
fluorescence-activated cell sorting (FACS) following 72-h
stimulation.
Supernatants from the PBMC-UCSMSCs were harvested at
24, 48 and 72 h post stimulation. Three centrifugation steps were
performed to remove the remaining cells that may influence the
detection. Release of lactate dehydrogenase (LDH) from injured
cells was detected by a cytotoxicity kit according to the
manufacturer's protocol. Cytotoxicity (% lysis) was calculated
using the following formula: (E − M)/(T − M) × 100; E is the
experimental release, M is the spontaneous release in the presence
of media alone, and T is maximum release in the presence of 5%
Triton X-100.
Detection of surface markers and
co-stimulators detection by FACS
Pam3Csk treated and untreated UCMSCs were collected
for FACS detection by following 72-h stimulation. The UCMSCs were
fixed with 10% formaldehyde for 10 min, then were stained with
different antibodies to detect surface stem cells markers and
co-stimulatory molecules. The assay was performed using CXP flow
cytometry software, version 2.0 (Beckman Coulter, Inc. Brea, CA,
USA). The positive and negative standard was gated according to the
control groups. The antibodies used in detection are listed in
Table II. The dilution of all
antibodies in FACS assay was 1:100 and incubated 30 min at room
temperature. All assays were conducted three times.
 | Table IIMonoclonal antibodies used for
fluorescence-activated cell sorting analysis. |
Table II
Monoclonal antibodies used for
fluorescence-activated cell sorting analysis.
| Name | Company | Cat no. |
|---|
| CD40 | eBioscience, Inc.
(San Diego, CA, USA) | 17-9953 |
| CD80 | eBioscience,
Inc. | 11-0809 |
| CD86 | eBioscience,
Inc. | 12-0869 |
| CD59 | eBioscience,
Inc. | 11-0596 |
| CD74 | eBioscience,
Inc. | 11-0748 |
| CD90 | eBioscience,
Inc. | 45-0909 |
Differentiation detection of UCMSCs
Conditioned medium of chondrocytes (cat. no.
A10071-01), osteocytes (cat. no. A10072-01) and adipocytes (cat.
no. A10070-01) were obtained from Gibco (Thermo Fisher Scientific,
Inc.) and added to UCMSCs (1.5×105 per well) in 6-well
plates in the presence of 100 ng/ml Pam3Csk. Oil-red O for
adipocytes, alizarin red for osteocytes and safranine staining for
chondro-cytes was conducted on day 5, 14 and 20. Prior to staining
[staining solutions diluted at 1:3 with double distilled water
(ddH2O)], cells were fixed with 10% formaldehyde
soulution for 10 min at room temperature, then washed three times
with ddH2O. Subsequently, Mayer's hematoxylin staining
was conducted for 5 min, followed by three further washes with
ddH2O.
Statistical analysis
The analysis of RT-qPCR results was performed using
Bio-Rad iQ5 software (Bio-Rad Laboratories, Inc.). Results are
expressed as the mean ± standard error and were analyzed with
Student's t-test using SPSS software (version 16.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. All figures were created
using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
TLR1/2 activation of UCMSCs increases the
proliferation of PBMC and cytotoxicity effect
PBMCs from healthy volunteers were co-cultured with
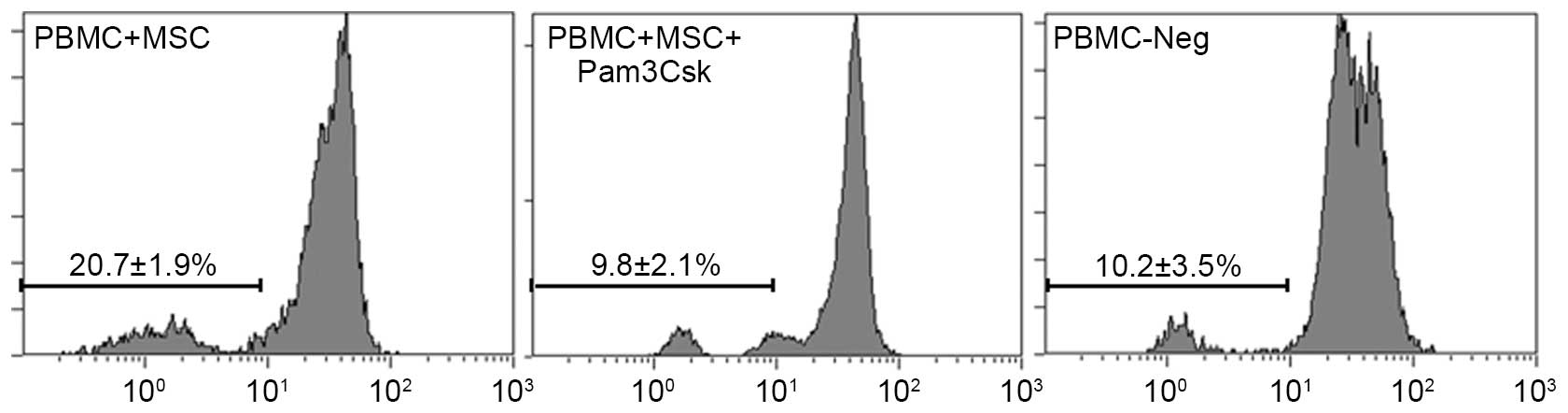
UCMSCs in the presence of 100 ng/ml Pam3Csk. The results indicated
that the proliferation of PBMCs was higher following Pam3Csk
stimulation (20.7%) in PBMC-UCMSCs co-culture system compared with
the untreated group (10.2%; Fig.
1). The results also demonstrated that Pam3Csk only led to
10.2% proliferation rate in PBMC. The results suggested that
activation of TLR1/2 pathway in UCMSCs increases the immune
response.
The immune attack was measured by detecting the LDH
levels in culture supernatants from injured cells, which is a
classical method for measuring leukocyte-mediated cytotoxicity. In
the current study, PBMCs were co-cultured with UCMSCs and Pam3Csk
was added to activate TLR1/2 signaling. The negative was
PBMCs-UCMSCs without Pam3Csk. The results indicated no difference
significant difference between the two groups at 24 h
post-co-culture (10.3% vs. 11.7%: P=0.265), whereas LDH levels were
significantly increased in the Pam3Csk treatment group compared
with the untreated group at 48 h (12.9% vs. 23.9%; P=0.01) and 72 h
(22.3% vs. 32.7%; P=0.037) post-co-culture (Table III).
 | Table IIILactate dehydrogenase levels in the
supernatant of umbilical cord MSC-PBMC co-culture system. |
Table III
Lactate dehydrogenase levels in the
supernatant of umbilical cord MSC-PBMC co-culture system.
| Time | MSC + PBMC | MSC + PBMC +
Pam3Csk | P-value |
|---|
| 24 h | 10.3±2.2% | 11.7±2.8% | 0.265 |
| 48 h | 13.0±1.6% | 23.9±5.1% | <0.05a |
| 72 h | 22.3±3.3% | 32.7±4.5% | <0.05a |
Activation of TLR1/2 signaling increases
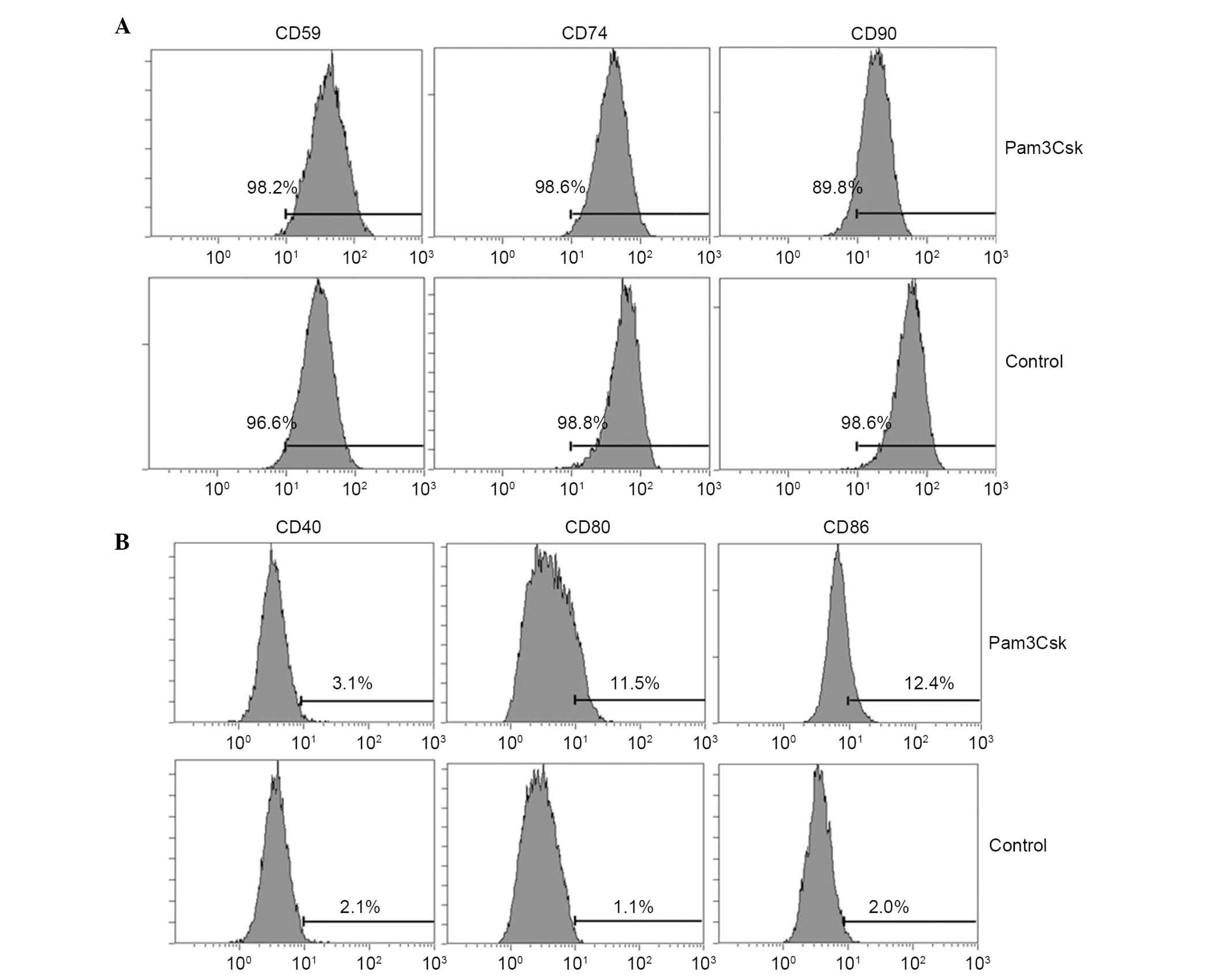
the surface expression of co-stimulators of UCMSCs
The data of the current study indicated that TLR1/2
agonist induces immune attack and causes injury to UCMSCs. Thus,
the study subsequently examined the effect of activation of TLR1/2
signaling on the UCMSCs surface expression of co-stimulators, which
are important for mediating immune responses. The results indicated
that CD80 (11.5 vs. 1.1%) and CD86 (12.4 vs. 2.0%) were
significantly upregulated (P= 0.036 and P= 0.043, respectively) in
UCMSCs treated with Pam3Csk compared with the control group
(Fig. 2A). The expression
variation of specific markers of UCMSCs were also detected and the
results indicated that CD59 (98.2 vs. 96.6%) and CD74 (98.6 vs.
98.8%) and CD90 (89.8 vs. 98.6%) levels were marginally inhibited
following Pam3Csk stimulation compared with untreated cells
(Fig. 2B). The FACS results
indicated that activation of TLR1/2 altered the surface expression
of co-stimulators of UCMSCs, however the effect was not marked.
Immune-modulation molecules were
upregulated in the presence of Pam3Csk
The UCMSCs were stimulated with TLR1/2 agonist,
Pam3Csk, and the expression of pro-inflammatory cytokines (IFN-β,
IL-6, IL-8 and TNF-α), chemokines (CCL-5, MCP-1, IP-10 and MIP-1α)
and stem cell markers (Nanog, Sox2, Lin28 and Otx2) were examined
at 4, 12, 24, 72 and 120 h following agonist treatment. It was
demonstrated that IL-6, CCL-5, IP-10 and MIP-1α were significantly
induced to high expression levels upon Pam3Csk stimulation compared
with the control (Fig. 3A and B).
Additionally, it was observed that although IFN-β, IL-8, TNF-α and
MCP-1 expression levels were significantly induced in the presence
of Pam3Csk compared with the control, the expression levels
decreased markedly at the later time points (Fig. 3A and B).
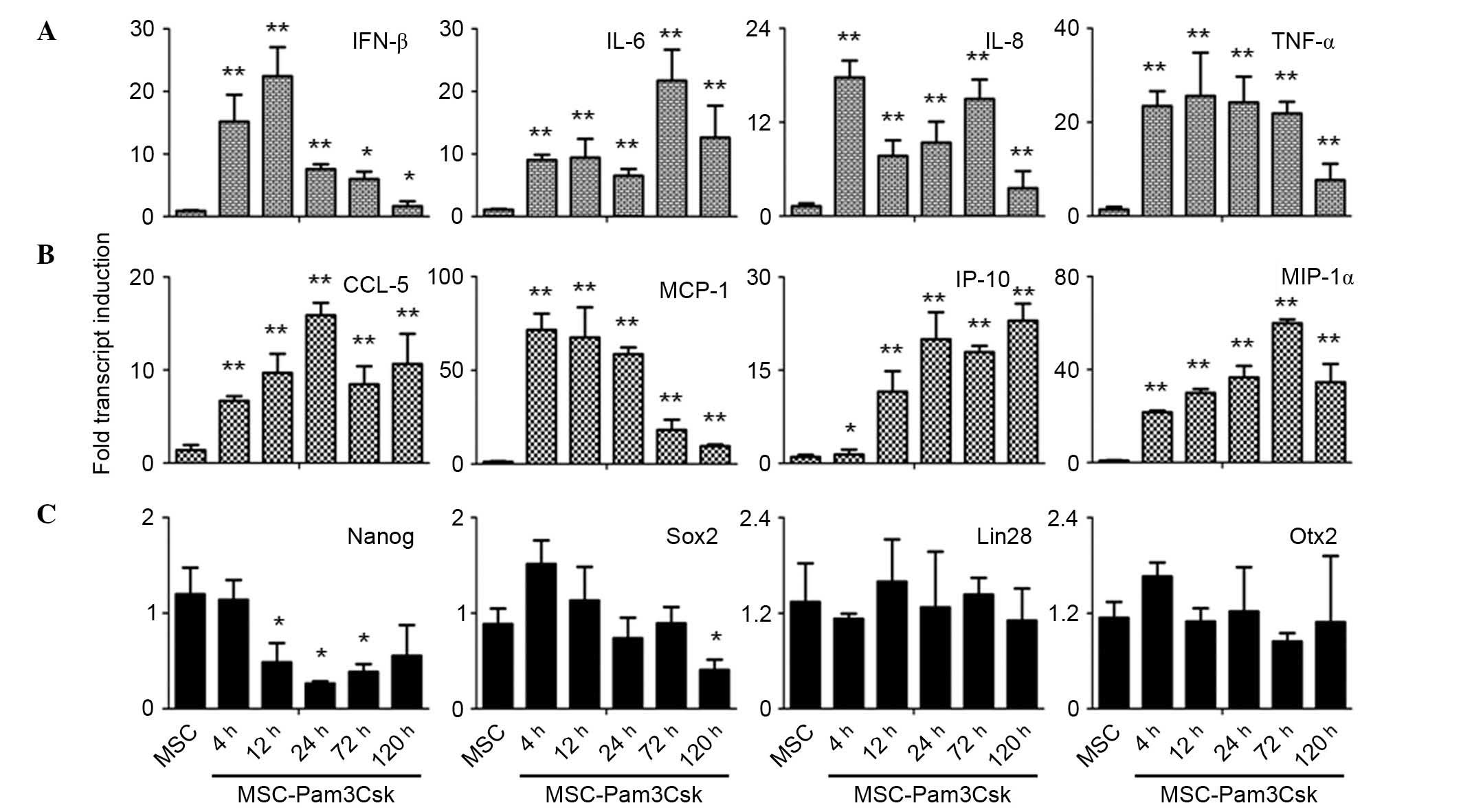 | Figure 3Secretion of pro-inflammatory
molecules was significantly increased in UCMSCs treated with
Pam3Csk. The mRNA levels of (A) cytokines, (B) chemokines and (C)
stem cell markers were measured. *P<0.05,
**P<0.001 vs. control group. Results are expressed as
mean values ± standard error of the mean. IFN-β, interferon-β; IL,
interleukin; TNF-α, tumor necrosis factor-α; CCL5, C-C motif
chemokine ligand 5; MCP, monocyte chemoattractant protein; IP10,
interferon γ-induced protein 10; MIP-1α, macrophage inflammatory
protein-1α; Nanog, Nanog homeobox; SOX2, sex determining region
Y-box 2; Lin28, Lin-28 homolog A; Otx2, orthodenticle homeobox
2. |
Additionally, the expression levels of stem cells
markers were examined to determine whether activation of Pam3Csk
affects the stemness of UCMSCs. The present study demonstrated that
the expression level of Nanog was significantly inhibited following
Pam3Csk stimulation at 12 h compared with control levels (P=0.021),
whereas Sox2 levels were only inhibited compared with the control
at 120 h treatment (P=0.028; Fig.
3C). It was also observed that the expression levels of Lin28
and Otx2 were not altered in the presence of Pam3Csk (Fig. 3C). Thus, the activation of TLR1/2
signaling upregulated the expression of pro-inflammatory molecules
and may inhibit the stemness maintenance of UCMSCs.
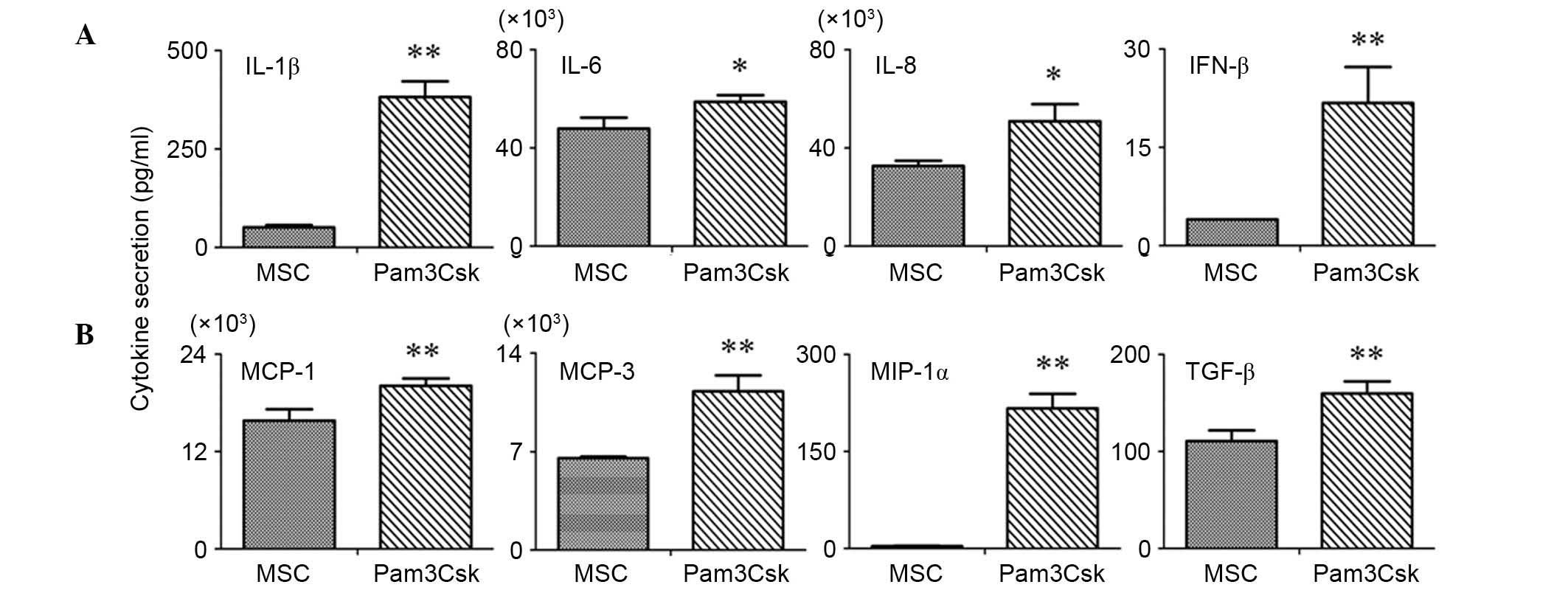
Pam3Csk increases the secretion of
pro-inflammatory cytokines in UCMSCs
The secretion of pro-inflammatory cytokines in the
supernatants of the Pam3Csk-treated and untreated UCMSCs was
measured using a RayBio antibody chip. The results indicated that
IL-1β, IFN-β, MCP-1, MCP-3, MIP-1α and TGF-β were significantly
upregulated in the supernatants of Pam3Csk treated UCMSCs compared
with untreated UCMSCs (P<0.001; Fig. 4). Additionally, IL-6 and IL-8
levels were significantly induced upon TLR1/2 agonist stimulation
compared with controls (P=0.032 and P=0.029, respectively), but not
as markedly as MCP-1, MCP-3, etc (Fig.
4).
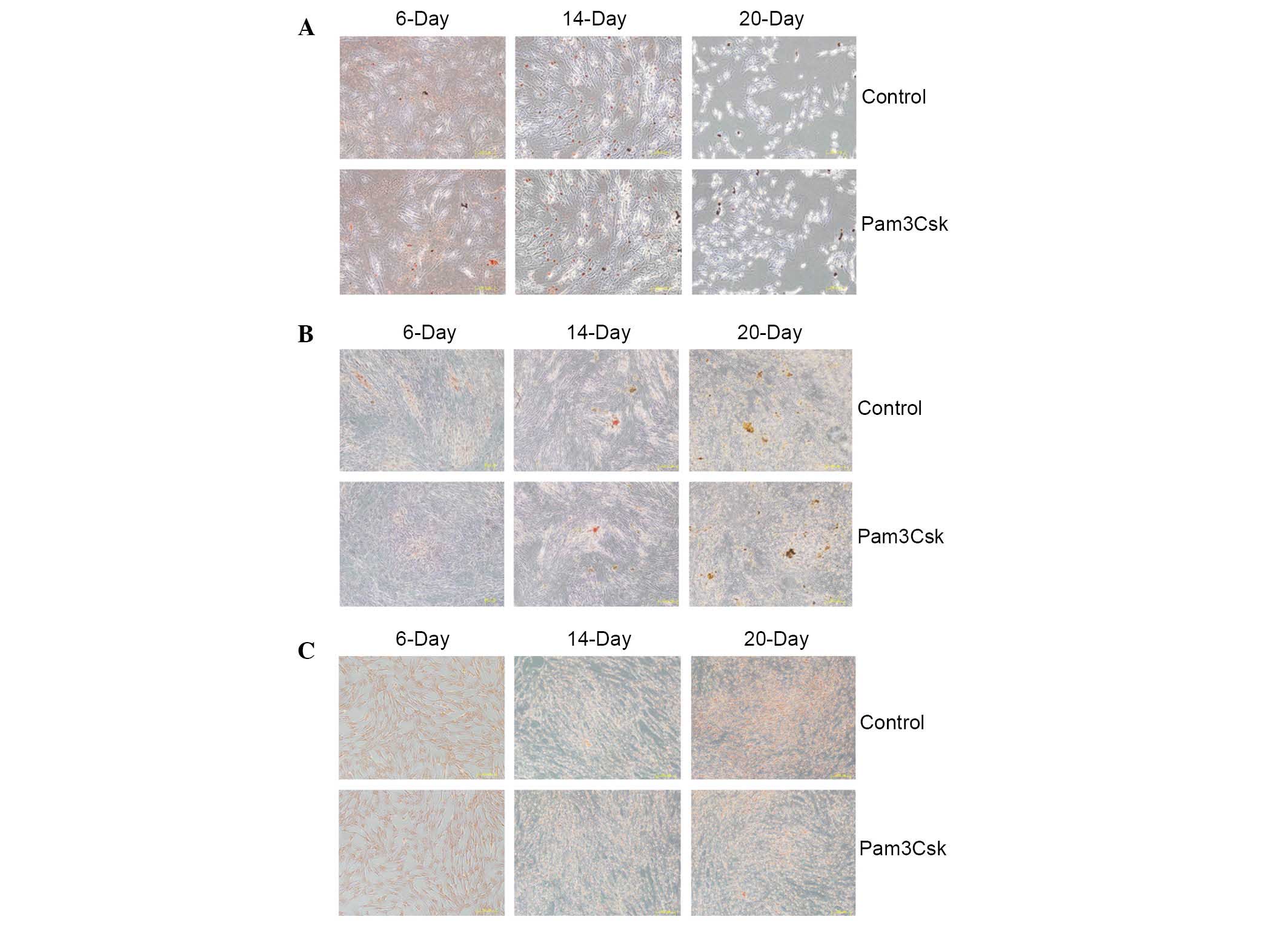
Pam3Csk stimulation had no effect on the
differentiation ability of UCMSCs
A previous study indicated that differentiation of
UCMSCs alters the immune status and increases immune responses
(21). Thus, aimed to determine
whether TLR1/2 activation alters the differentiation of UCMSCs. The
conditioned media for adipocyte, osteoblast and chondrocyte
differentiation were added to UCMSCs following stimulation with
Pam3Csk to detect the importance of TLR1/2 on UCMSCs
differentiation ability. At day 6, 14 and 20 post-stimulation,
alizarin red for osteoblasts, safranine for chondrocytes and
oil-red O staining for adipocytes was conducted to assess the
differentiation of UCMSCs. The results indicated that activation of
TLR1/2 by Pam3Csk stimulation exerted no observable effect on the
differentiation UCMSCs to adipocytes, osteoblasts and chondrocytes
(Fig. 5).
Discussion
Multiple differentiation and self-renewal properties
of MSCs enable its usage in clinical cell-based therapies (21). MSC differentiate into cell types
for various tissues, including bone, cartilage, adipocytes,
connective stomal cells, hepatocytes and muscle (22). In addition to differentiating into
specific cells types, MSCs are also involved in tissue regeneration
due to their trophic effects (23). Thus, knowledge of molecules, and
mechanisms, that regulate the properties and potential
immunogenicity of MSCs is important for the therapeutic use of
MSCs. Immunomodulatory properties enable MSCs to suppress the
activation and proliferation of T and B cell responses, and to
interfere with the maturation of dendritic and NK cells (7–9).
However, previous studies indicated that the in vivo
microenvironment alters the immune status, enhances immune
responses and causes to failure of MSC-based therapy (10–12).
TLR is the most important (PAMP) family, with an
important role in defending against invading pathogens (15). Among the 11 members of the human
TLR family, TLR1/2, which is located on the cell surface and
recognized by gram-positive bacteria, is involved in the
recognition of a variety of microbial components, including
lipoproteins. Previous research demonstrated that activation of
TLR1/2 exhibited no effect on the immune status of MSC from bone
marrow (17), while no studies
focussing upon the role of TLR1/2 in regulating the immune status
of MSCs from the umbilical cord have been conducted. As UCMSCs
attract attention in cell-based therapy, it is important to analyze
whether activation of TLR1/2 pathway may alter the immunogenicity
of UCMSCs. (24). The current
study demonstrated that activation of TLR1/2 signaling in UCMSCs
promoted immunogenicity by increasing the proliferation of PBMC in
co-culture with UCMSCs and enhancing the release of LDH into the
supernatant of the PBMC-UCMSCs co-culture system. In support of
this observation, the treatment of Pam3Csk also upregulated the
expression of surface co-stimulators, CD80 and CD86, to a certain
extent, however Pam3Csk exhibited no obvious influence on the
levels of stem cell markers, including CD59, CD74 and CD90.
Antibody array chip and RT-qPCR analysis was also performed. The
antibody chip array detecting secretion of pro-inflammatory
molecules indicated the levels of IL-1β, INF-β, MCP-1, MCP-3,
MIP-1α and TGF-β in the supernatants of Pam3Csk-treated UCMSCs were
significantly increased. RT-qPCR for gene expression levels also
indicated that the expression of various pro-inflammatory cytokines
(INF-β, IL-6, IL-8 and TNF-α) and chemokines (CCL-5, MCP-1, IP-10
and MIP-1α) was increased by Pam3Csk. Huang et al (21) suggested that MSCs lost the immune
privilege properties when differentiated into cardiac cells and
finally resulted in the rejection of engrafted MSCs. Thus, the
present study aimed to establish whether the enhanced immune status
was associated with alteration of the differentiation abilities in
UCMSCs. Conditioned media were introduced for adipocyte, osteoblast
and chondrocyte differentiation of UCMSCs upon stimulation with
Pam3Csk, however, no observable change in differentiation ability
was detected following activation of TLR1/2 signaling with
Pam3Csk.
In clinical trials, numerous endogenous ligands,
including heparin sulfate, oxidized low-density lipoprotein, uric
acid and heat shock proteins have been previously demonstrated to
activate TLRs. These endogenous TLR agonists may regulate functions
of UCMSCs by endogenous stimuli during tissue repair. Future
studies are required to study the regulatory mechanisms of the
biological functions of UCMSCs. The current study firstly confirmed
that activation of the TLR1/2 pathway increased the immunogenicity
of UCMSCs. In clinical cell-based therapy, the engrafted MSCs
encountered numerous endogenous ligands which may activate TLR
pathways. Thus, the present study identified the potential risks of
the use of MSCs in clinical therapy
References
|
1
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kharaziha P, Hellström PM, Noorinayer B,
Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost
M, Zali MR and Soleimani M: Improvement of liver function in liver
cirrhosis patients after autologous mesenchymal stem cell
injection: A phase-II clinical trials. Eur J Gastroenterol Hepatol.
21:1199–1205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vojtassák J, Danisovic L, Kubes M, Bakos
D, Jarábek L, Ulicná M and Blasko M: Autologous biograft and
mesenchymal stem cells in treatment of the diabetic foot. Neuro
Endocrinol Lett. 27(Suppl 2): S134–S137. 2006.
|
|
4
|
Guiducci S, Porta F, Saccardi R, Guidi S,
Ibba-Manneschi L, Manetti M, Mazzanti B, Dal Pozzo S, Milia AF,
Bellando-Randone S, et al: Autologus mesenchymal stem cells foster
revascularization of ischemic limbs in systemic sclerosis: A case
report. Ann Intern Med. 153:650–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bordignon C, Carlo-Stella C, Colombo MP,
De Vincentiis A, Lanata L, Lemoli RM, Locatelli F, Olivieri A,
Rondelli D, Zanon P and Tura S: Cell therapy: Achievements and
perspectives. Haematologica. 84:1110–1149. 2011.
|
|
7
|
Bassi EJ, Aita CA and Câmara NO: Immune
regulatory properties of multipotent mesenchymal stromal cells:
Where do we stand? World J Stem Cells. 3:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom
HJ, Kim MG, Oh KH, Ahn C and Yang J: Immunosuppressive mechanisms
of embryonic stem cells and mesenchymal stem cells in alloimmune
response. Transpl Immunol. 25:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y and Lin F: Mesenchymal stem cells are
injured by complement after their contact with serum. Blood.
120:3436–3443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allison M: Genzyme backs Osiris, despite
prochymal flop. Nat Biotechnol. 27:966–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spaggiari GM, Capobianco A, Becchetti S,
Mingari MC and Moretta L: Mesenchymal stem cell-natural killer cell
interactions: Evidence that activated NK cells are capable of
killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell
proliferation. Blood. 107:1484–1490. 2006. View Article : Google Scholar
|
|
13
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Opitz CA, Litzenburger UM, Lutz C, Lanz
TV, Tritschler I, Köppel A, Tolosa E, Hoberg M, Anderl J, Aicher
WK, et al: Toll-like receptor engagement enhances the
immunosuppressive properties of human bone marrow-derived
mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1
via interferon-beta and protein kinase R. Stem Cell. 27:909–919.
2009. View
Article : Google Scholar
|
|
16
|
Pevsner-Fischer M, Morad V, Cohen-Sfady M,
Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR and Zipori D:
Toll-like receptors and their ligands control mesenchymal stem cell
function. Blood. 109:1422–1432. 2007. View Article : Google Scholar
|
|
17
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Liu D, Pu D, Wang Y, Li L, He Y,
Li Y, Li L and Li W: The TLR7 agonist imiquimod promote the
immunogenicity of msenchymal stem cells. Biol Res. 48:62015.
View Article : Google Scholar :
|
|
19
|
Zhang L, Liu D, Pu D, Wang Y, Li L, He Y,
Li Y, Li L, Qiu Z, Zhao S and Li W: The role of toll-like receptor
3 and 4 in regulating the function of mesenchymal stem cells
isolated from umbilical cord. Int J Mol Med. 35:1003–1010.
2015.PubMed/NCBI
|
|
20
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diag Mol Path. 15:56–61. 2006. View Article : Google Scholar
|
|
21
|
Huang XP, Sun Z, Miyagi Y, McDonald
Kinkaid H, Zhang L, Weisel RD and Li RK: Differentiation of
allogeneic mesenchymal stem cells induces immunogenicity and limits
their long-term benefits for myocardial repair. Circulation.
122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Augello A, Kurth TB and De Bari C:
Mesenchymal stem cells: A perspective from in vitro cultures to in
vivo migration and niches. Eur Cell Mater. 20:121–133. 2010.
|
|
24
|
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee
IH, Lin WS, Wu CH, Lin WY and Cheng SM: Mesenchymal stem cells from
human umbilical cord express preferentially secreted factors
related to neuroprotection, neurogenesis, and angiogenesis. PLoS
One. 8:e726042013. View Article : Google Scholar : PubMed/NCBI
|