Introduction
Osteoarthritis (OA) is one of the most common causes
of musculoskeletal disability. It is characterized by progressive
degeneration of articular cartilage and synovitis is a significant
contributor, which contributes to the development of OA (1). Diagnosis of OA using magnetic
resonance imaging indicates synovial hyperplasia proximal to
cartilage lesions, particularly in the bursa suprapatellaris and
posterior cruciate ligament of the knee (2). A previous study proposed that the
synovitis is a significant cause of pain and oedema in OA patients
(3). Although interleukin-6
(IL-6), cluster of differentiation 4 (CD4), CD8+ T-cells and
adipocytokines (such as adiponectin and leptin) are vital
inflammatory factors for the process of synovitis (4,5), the
molecular mechanisms of the progressive degeneration of articular
synovial membrane in OA remain to be fully elucidated. Previously,
alterations in the transcriptomes of OA synovial membranes were
investigated using DNA microarray or reverse
transcription-quantitative polymerase chain reaction analysis
(6,7). However, alterations in mRNA
expression levels have been reported to not always correlate well
with the protein levels due to post-transcriptional regulation,
post-translational modification and differential stability of
proteins (8).
The use of proteomics, during which entire proteins
in tissues or cells are identified and quantified directly, has
been identified as a valuable method for elucidating the molecular
basis of disease etiology. Recently, the proteome of human
articular chondrocytes, synovial fluid, serum or urine was
characterized by two-dimensional polyacrylamide gel electrophoresis
(2-DE) and tandem mass spectrometry of cultured chondrocytes
isolated from normal cartilage (9–13).
However, to the best of our knowledge, few proteomic studies
regarding the articular synovial membrane have been conducted
(14), and the present study have
improved knowledge of the proteome of the synovial membrane, and
provided a foundation for further investigation of the pathology of
synovial membrane diseases.
Studies regarding the molecular and cellular
mechanisms of OA have evaluated bilateral or unilateral joint
tissue samples without considering the distinction between
spontaneous and secondary OA (10,15–18).
However, the majority of spontaneous (resulting from the aging
process) and secondary (traumatic) knee OA (KOA) cases occur in
bi/unilateral knee joints, respectively (19), and the pathological process and
treatment for the disease may differ between spontaneous and
secondary KOA (20,21). Furthermore, certain studies
proposed that proteomics may be important in the treatment of OA
(9,22). Thus, the present study hypothesizes
that the mechanisms of proteomic alterations in the progressive
destruction of articular synovial membrane in spontaneous and
secondary KOA are different. The profile of proteins selectively
extracted from rabbit synovial membrane samples of bi/unilateral
KOA were compared by two-dimensional gel electrophoresis (2-DE) and
mass spectral analysis to highlight requirements for establishing
diverse treatments for OA resulting from the aging process and
traumatic OA.
Materials and methods
Animals
New Zealand White (NZW) rabbits were supplied by the
Fujian University of Traditional Chinese Medicine (Fuzhou, China)
animal testing center [batch no. SCXK (Shanghai) 2012-0011]. The
NZW rabbits, 3 male and 3 female (age, 6 months; weight, 2.5–3.0
kg), were provided with a standard laboratory diet with drinking
water and housed in individual cages under a 12-h light/dark cycle
at 20–26°C. The present study complied with national legislation
and with the Ministry of Health of the People's Republic of China
Guide for the Care and Use of Laboratory Animals (23). Local ethical committee approval was
obtained for the current study from the ethics committee of the
Fujian University of Traditional Chinese Medicine. The NZW rabbits
were sacrificed by air embolism 6 weeks following the surgery for
OA model induction.
Animal grouping
All animals were randomly divided into groups A and
B, with 3 rabbits per group, and SPSS 13.0 statistical software was
used (SPSS, Inc., Chicago, IL, USA).
KOA model
The A and B group rabbits were subjected to
bilateral and unilateral anterior cruciate ligament transection
(ACLT), respectively. Briefly, the rabbits were administered with
intraperitoneal injections of 5% chloral hydrate (3 ml/kg; Qingdao
Yulong Algae Co., Ltd., Qingdao, China). in order to sedate and
anesthetize them appropriately. The right knee was shaved,
sterilized, draped under sterile conditions and a medial arthrotomy
was performed. The patella was then dislocated, and the ACL was
isolated and transected. ACLT was confirmed by the surgeon and an
observer using the Lachman test. Following irrigation using sterile
saline solution, the wounds were closed in layers and treated with
antiseptic. Rabbits were provided with the appropriate
postoperative care and allowed to move freely in individual
cages.
Specimen collection for synovial membrane
proteomic detection
The rabbits were sacrificed 6 weeks subsequent to
surgery, and the synovial membrane of the operative right knee
joint was dissected in group A and B rabbits. The samples were
maintained for subsequent evaluation in a nitrogen canister.
Protein extraction of the synovial
membrane
Synovial membrane samples were ground into powdered
tissue using a pestle and mortar, and transferred into a
homogenizer. Lysates (500 µl/100 mg) were added to the
homogenate; RNase (50 µg/ml), DNase (200 µg/ml) and
10 µl/1 ml lysate were added, and maintained at 4°C for 15
min. The tissue was then centrifuged at 12,000 × g for 60 min at
4°C. The supernatant was collected and the protein concentration
was determined using the 2-D Quant kit (GE Healthcare Life
Sciences, Uppsala, Sweden) according to the manufacturer's
protocols. The tissues were refrigerated at −70°C.
2-DE
Protein solubility and dry strip
swelling
Lysis buffer [500 µl; 7 mol/l urea, 2 mol/l
thiourea, 4% CHAPS, 1% dithiothreitol (DTT), 0.2% NP-40, 1%
ampholine (pH 4–6) and 1% ampholine (pH 3.5–10)] was added into the
protein solution extraction, which was then vibrated for 5 h.
Dissolution of the protein solution extraction was conducted using
a 200 W ultrasonic instrument for 200 sec. The protein solution
extraction was centrifuged at 20,000 × g for 20 min at 4°C. The
supernatant was collected and the protein concentration was
measured using the Bradford Protein assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The rehydration solution
[800 µl; 8 mol/l urea, 2% CHAPS, 0.5% ampholine (pH 4–7),
0.002% Bromophenol Blue and 800 µg protein solution) was
added into the protein electrophoresis tank and the dry strip was
immersed (pH 4–7; 18 cm), gum down and incubated room temperature
overnight.
Isoelectric focusing
electrophoresis
The expanded 12% gel was placed into the gel strip
slot of the isoelectric focusing electrophoresis apparatus and the
gel was covered with covering oil. The electrophoresis parameters
were as follows: 500 V for 2 h (gradient); 1,000 V for 1 h
(gradient); 8,000 V for 2.5 h (step).
Gel strip equilibration
The gel strip was equilibrated twice following
isoelectric focusing electrophoresis, for 15 min each time, with
gentle agitation. The liquid components for the initial
equilibration were as follows: 50 mmol/l Tris-Hcl (pH 8.8), 6 mol/l
urea, 30% glycerol, 2% sodium dodecyl sulfate (SDS) and 1% DTT. The
second equilibrium liquid components were as follows: 50 mmol/l
Tris-Hcl (pH 8.8), 6 mol/l urea, 30% glycerol, 2% SDS and 2.5%
idoacetamide.
SDS-polyacrylimide gel
electrophoresis
The balance gel was layered on the top of the spacer
gel, taking care to avoid trapped bubbles, and the gel strip was
fixed with 0.5% agarose. The electrode buffer was added following
solidification of the agarose, and electrophoresis was performed
until the Bromophenol Blue indicator reached the bottom of the
separation gel. The concentrations of separation gel and spacer gel
were 15 and 7% respectively, and the current was 30 mA.
Staining
Coomassie Brilliant Blue R-250 was used for
staining. The gel was solidified for 1 h in fixation fluid
comprised of 50% anhydrous ethanol and 10% glacial acetic acid. The
fixation fluid was removed and the gel was stained with 0.1%
Coomassie Brilliant Blue R-250 and vibrated for 10 h. The stain was
removed by rinsing the gel twice with distilled water and adding
destainer (30% anhydrous ethanol and 8% glacial acetic acid) and
vibrating. The destainer was replaced until the background of the
gel was clear.
Silver staining and image
scanning
Following silver staining and coloration the
ImageScanner (GE Healthcare Life Sciences) was used to obtain the
2-DE images. The protein spots were counted using ImageMaster 2D
Platinum software, version 3.0 (GE Healthcare Life Sciences).
Automatic identification of protein spots was conducted with the
software, however, if the boundary between protein spots were
clear, they were segmented into two spots.
Gel image comparison
The distribution of protein isoelectric points of
the two types of protein extraction were compared according to the
2-DE gel image. Bandscan 5.0 (Glyko, Inc., Novato, CA, USA) was
used as comparison software.
In-gel digestion
Samples were spotted onto a MALDI target plate with
an equal volume of matrix solution, containing 5 mg/ml
α-cyano-4-hydroxycinnamic acid in 50% acetonitrile and 0.1%
trifluoroacetic acid. An AutoFlex speed MALDI TOF/TOF MS (Bruker
Corporation, Billerica, MA, USA) was used with a mass accuracy of
50 ppm following external calibration. The samples were analyzed in
MS mode (for generation of peptide mass fingerprints) as well as in
TOF/TOF mode (for fragmentation analysis of the highest intensity
peaks). MS spectra were transformed into peak lists using the
software flexAnalysis version 3.0 (Bruker Corporation). The peak
lists of the MS and MS/MS spectra were merged using BioTools
version 3.0 software (Bruker Corporation).
Protein detection
The amino acid sequence tags obtained from each
peptide fragmentation in MS/MS analyses were used to calculate
match scores and search for protein candidates in three groups
using Mascot software, version 2.3.01 from Matrix Science
(http://www.matrixscience.com). The
retrieval parameters were as follows: Type of search, MS/MS Ion
Search; enzyme, trypsin; fixed modification, Carbamidomethyl (C);
variable modification: Gln->pyro-Glu (N-term Q), Oxidation (M);
mass values, monoisotopic; protein mass, unrestricted; peptide mass
tolerance, ±0.1 Da; and fragment mass tolerance, ±0.1 Da.
Results
2-DE imaging
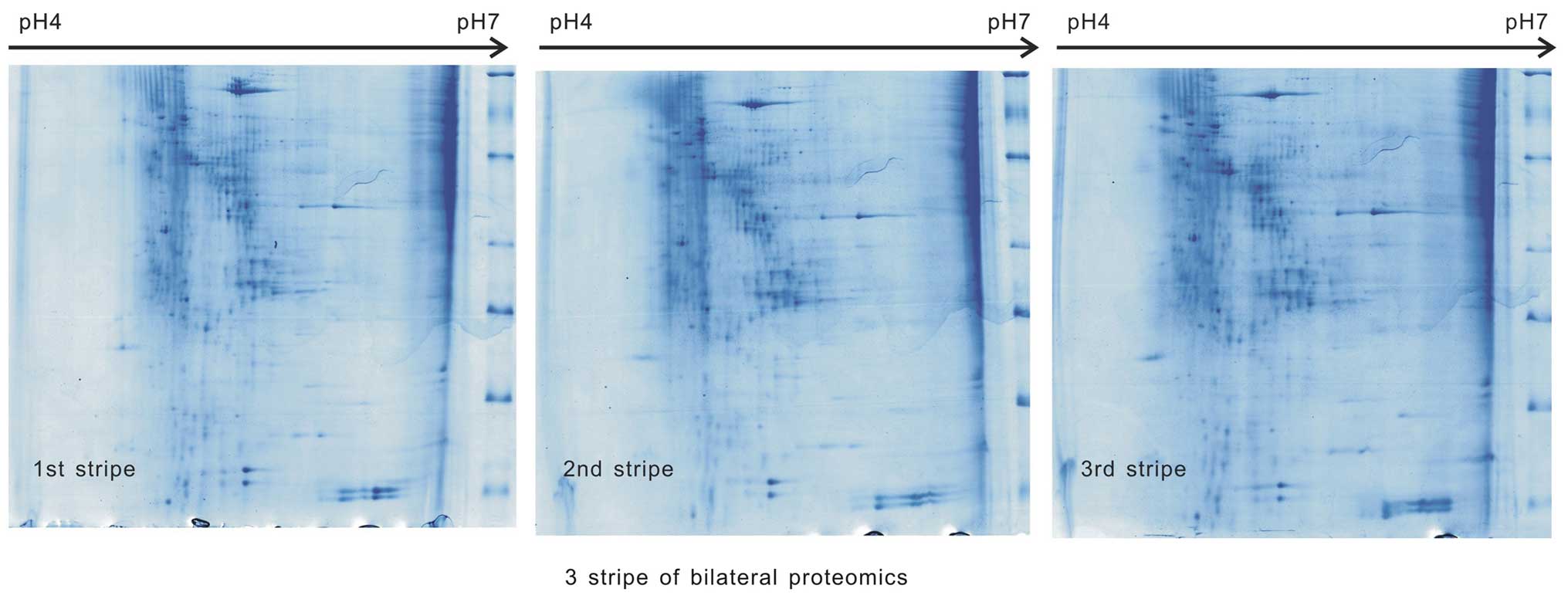
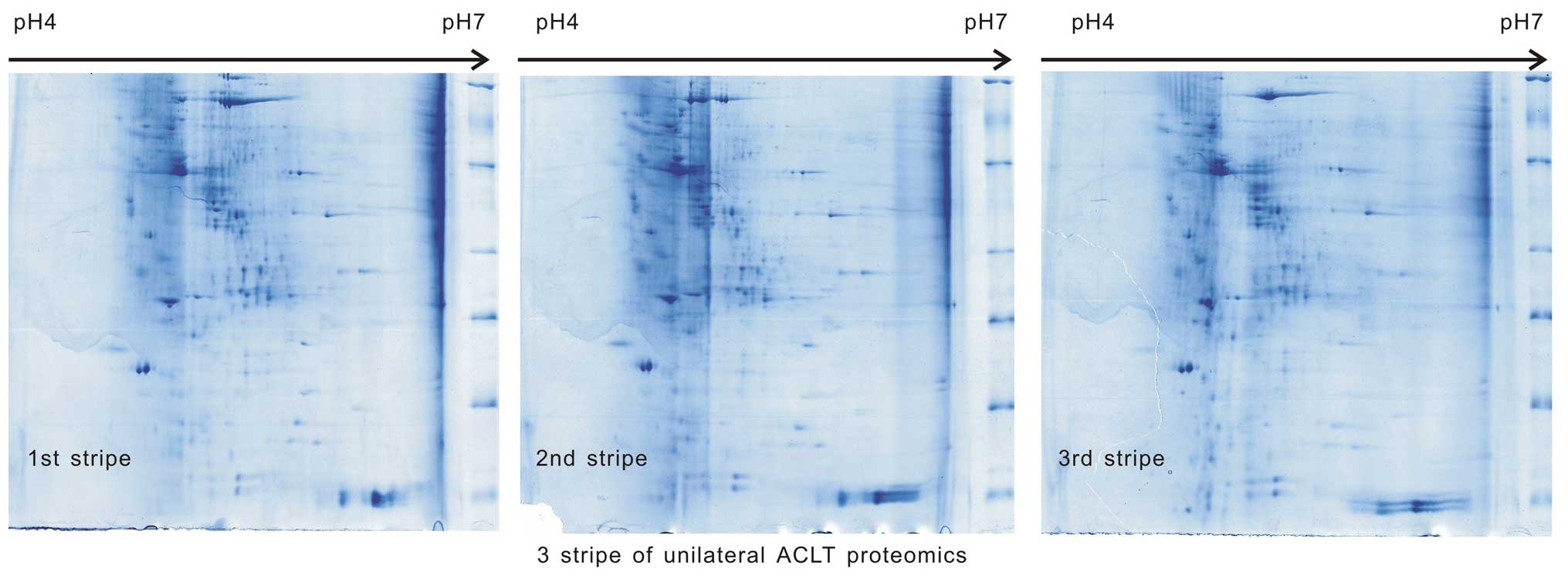
Samples from the two groups underwent 2-DE three
times (500 µg/sample) in the same environment, the images
were scanned and the three images were identified to be comparable
in each group (Figs. 1 and
2). The match scores were 82.1±1%
in group A and 83.2±2% in group B.
Differential analysis of protein
spots
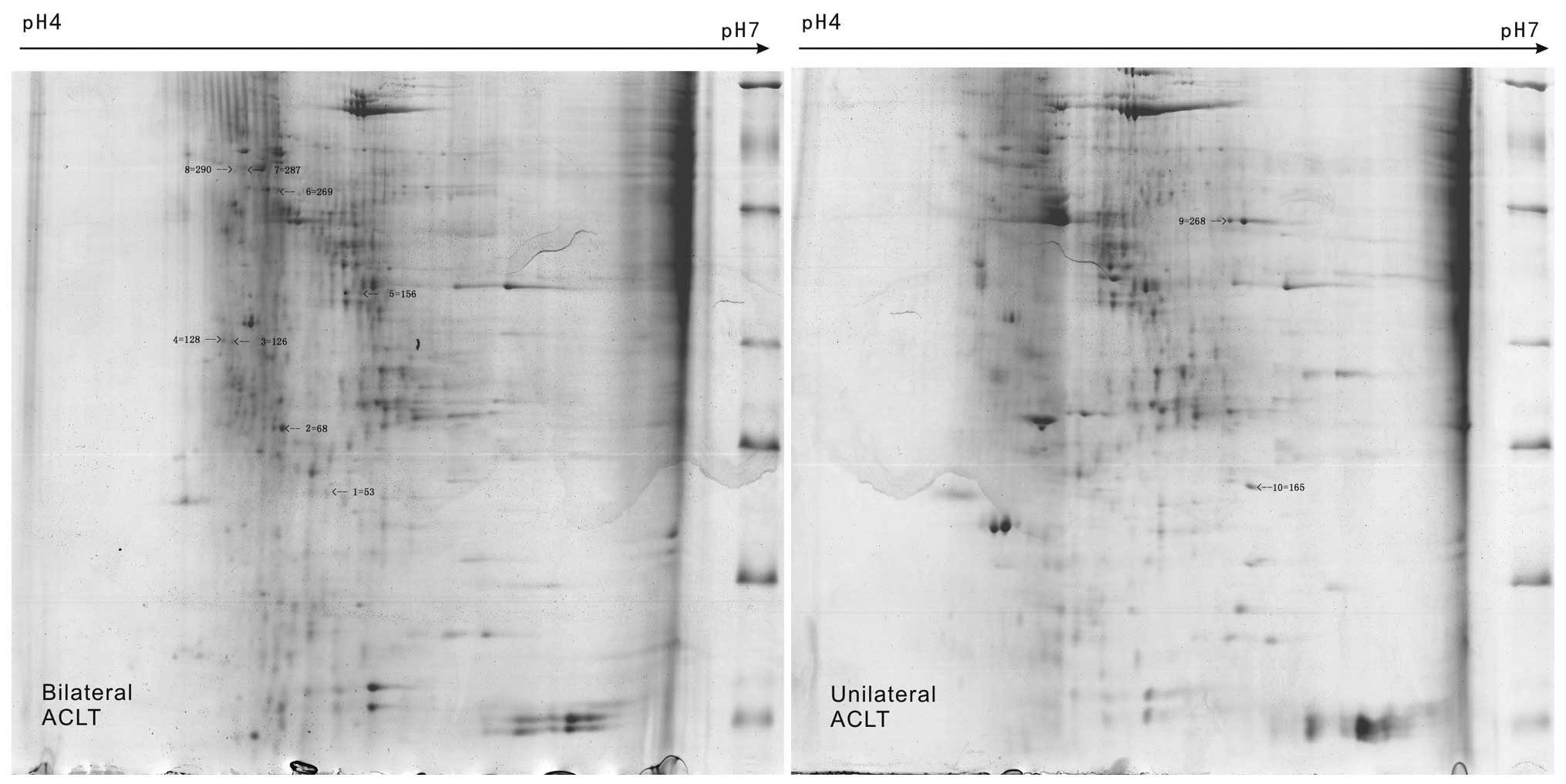
A total of 10 different protein spots were
identified by 2-DE of KOA synovial membrane samples in groups A and
B; the homologous proteins, putative molecular weight and
isoelectric point, and protein scores were determined (Fig. 3). Out of the 10 proteins, certain
protein spots were identified to be the same, such as NO3, NO7 and
NO8 (serum albumin). In samples of unilateral KOA synovial
membrane, NO1 was protein disulfide-isomerase and NO2 was creatine
kinase (CK) M-type. NO6 was identified as lumican, NO10 was
α-2-HS-glycoprotein (AHSG) and NO4, NO5, and NO9 were designated as
uncharacterized proteins from the bilateral KOA synovial membrane
samples (Table I).
 | Table IA total of 10 representative proteins
of knee osteoarthritis rabbits from groups A and B identified by
mass spectrometry of two-dimensional polyacrylamide electrophoresis
gels of the synovium. |
Table I
A total of 10 representative proteins
of knee osteoarthritis rabbits from groups A and B identified by
mass spectrometry of two-dimensional polyacrylamide electrophoresis
gels of the synovium.
| Spot no. (SSP) | Accession no. (in
IPI_rabbit) | Homologous
protein | Putative Mr (Da) /
pI | Protein score |
|---|
| Group A |
| NO1 | P21195 | Protein
disulfide-isomerase | 57172/0.29 | 207 |
| NO2 | P00563 | Creatine kinase
M-type | 43313/0.27 | 1441 |
| NO3 | G1U9S2 | Serum albumin | 70916/1.04 | 546 |
| NO4 | G1ST52 | Uncharacterized
protein | 182182/0.25 | 497 |
| NO5 | G1SWS9 | Uncharacterized
protein | 53679/2.13 | 511 |
| NO6 | G1SP97 | Lumican | 38736/0.59 | 151 |
| NO7 | G1U9S2 | Serum albumin | 70916/4.96 | 1657 |
| NO8 | G1U9S2 | Serum albumin | 70916/1.50 | 545 |
| Group B |
| NO9 | G1SP97 | Uncharacterized
protein | 50151/3.51 | 728 |
| NO10 | G1SGQ5 |
α-2-HS-glycoprotein | 39539/2.25 | 358 |
Discussion
The synovial membrane is located within joint spaces
and aids in the maintenance of normal joint function. Synovial
membranes produce and secrete hyaluronan to lubricate the tissues
of the joint, and serve an important role in the nutrition of
cartilage, in addition to absorbing inflammation factors. Synovial
fibrosis is a major contributor to joint stiffness in OA, which is
elevated in OA and is key in the onset and persistence of synovial
fibrosis. The process of synovial membrane lesions (from early
inflammation to synovial hyperplasia), and the generation of
inflammatory mediators and cytokines results in cartilage damage.
Therefore, it is hypothesized that investigating and treating the
cartilage alone in OA is not sufficient. Further investigation is
required regarding the prevention of OA, to include the
consideration of diverse pathogenic factors and taking an
interdisciplinary approach, with the synovial membrane becoming a
novel treatment target for OA, which may prevent joint structure
damage and improve the clinical symptoms. In recent years, in order
to further clarify the diagnostic biomarkers and prognostic
indicators in different diseases, increasing numbers of studies are
referring to the use of proteomics. Proteomes of
degenerative/inflamed synovial membranes from rheumatoid arthritis
(RA) and OA and a chronic arthritic condition, spondyloarthropathy
were previously investigated using 2-DE followed by tandem mass
spectrometry (15).
To date, there are few studies regarding the
proteomics of the synovial membrane. Furthermore, to the best of
our knowledge, there are no studies reporting the differences
between proteomics of the synovial membrane in spontaneous and
secondary OA induced by a bilateral and unilateral ACLT model of
KOA. Thus, the present study aimed to elucidate the differences in
the proteomics of the synovial membrane using 2-DE in spontaneous
and secondary KOA rabbit models, in order to establish the diverse
remedies for OA resulting from the aging process and traumatic OA
in the future. The results illustrated that the proteomics of the
synovial membrane in the spontaneous and secondary KOA models were
different. The proteins, disulfide-isomerase and CK M-type, were
identified in the unilateral ACLT synovial membrane tissue, and
serum albumin (three protein spots), lumican and AHSG were observed
in the bilateral ACLT synovial membrane tissue. In addition, three
proteins spots were uncharacterized in the bilateral ACLT synovial
membrane.
Protein disulfide-isomerases (PDIs) have been
reported in different tumors and 19 family members have been
identified. The function of PDI is to catalyze oxidative folding of
novel peptide chains in the endoplasmic reticulum, in addition to
participating in calcium homeostasis and antigen presentation.
Procollagen and thyroglobulin, which are associated with PDI have
been identified in previous studies (24). Li et al (25) investigated mechanical-stress
loading-induced OA of the articulatio mandibularis, and identified
PDI in the mandibular cartilage of rats. However, whether the
synovial membrane of rabbits with KOA contains PDI has not, to the
best of our knowledge, been documented thus far. In the current
study, PDI was identified in the synovial membrane of unilateral
ACLT, while it was not identified in bilateral ACLT. PDI affects
protein metabolism, calcium homeostasis and procollagen synthesis,
which all impact upon the pathological alterations in the tissues
of the KOA joint. Thus, it was hypothesized that the PDI reduces
collagen synthesis, which accelerates the process of KOA due to
increased load in the bilateral ACLT joint.
In addition, the CK M-type protein was identified in
the synovial membrane of unilateral ACLT, while it was not
identified in bilateral ACLT. The components of synovial fluid,
which are secreted by the synovial membrane, can be exchanged, and
enter the circulatory system via synovial membrane capillaries, are
potential biomarkers that can be detected in blood and urine
(26). Therefore, the present
study proposed that the varied expression levels of the CK M-type
protein in the synovial membrane results from the difference in
severity of the synovial membrane lesion between bilateral and
unilateral KOA. As a result of the difference in CK expression
levels between bilateral and unilateral KOA, the density of CK in
the serum is also varied; i.e. the CK density is downregulated in
bilateral KOA. In addition, Chen et al (27) reported that the CK contents
increase then reduce from onset to the later stages of RA. Eimre
et al (28) proposed that
OA was associated with increased sensitivity of mitochondrial
respiration to ADP, causing a reduction in total activities of CK
with marked reductions in the mitochondrial CK fraction. The
authors suggested that due to degenerative remodeling occurring
during the development of OA, these complexes become structurally
and functionally impaired, resulting in increased access of
exogenous ADP to mitochondria and dysfunction of the
CK-phosphotransfer system. Borges et al (29) identified increased plasma
activities of total CK (2.0-fold) in ballet dancers immediately
after class, a finding that is significant in preventing the
development of chronic conditions that are commonly observed in
dancers, such as those with arthritis and synovitis.
Serum albumin is an essential material in cellular
physical activity. Alterations in serum albumin content result in
pathological alterations. Huang et al (30) demonstrated that the serum albumin
status may be important in the utilization and metabolic turnover
of plasma pyridoxal 5-phosphate in the presence of chronic
inflammation and autoimmune disease, such as in patients with RA.
However, there are few investigations regarding the association
between OA and serum albumin (31). In the present study, the serum
albumin level in the synovial membrane of bilateral ACLT was
observed (it was not identified in unilateral ACLT) with three of
the protein spots identified as serum albumin; thus, it was
inferred that serum albumin levels may increase in the early-middle
stage (6-week ACLT model) in order to increase the elimination of
inflammatory factors. Therefore, regulating serum albumin levels
may present as a novel method for treating OA.
Lumican is a leucine-rich proteoglycan and component
of the extracellular matrix. Lumican and fibromodulin regulate the
assembly of collagens into higher order fibrils in connective
tissues. Jepsen et al (32)
hypothesized that lumican and fibromodulin were candidate genes and
key in the pathogenesis of certain types of Ehlers-Danlos syndrome
and other connective tissue disorders. Previously, numerous lumican
studies were regarding tumors, with few studies focusing on KOA
(31–34). However, the association between
lumican and OA or RA has been reported; Seki et al (33) identified that cultured RA
fibroblastoid synoviocytes contain lumican protein, which encodes
extracellular matrix components. Further investigation of lumican
and fibromodulin may facilitate with the treatment of RA. In the
present results, two protein spots were identified as lumican in
the synovial membrane of bilateral ACLT, however not in unilateral
ACLT (34). Fernández-Puente et
al (34) identified serum
protein biomarkers for moderate and severe OA, and identified six
proteins that were only modulated in moderate OA, 13 proteins that
were only modulated in severe OA and 7 that were modulated in the
two; one of which was lumican. The authors indicated that the
specificity and selectivity of these candidate proteins required
validation prior to the development of novel molecular diagnostic
or prognostic tests for OA. Melrose et al (35) observed that the fragmentation of
small leucine-rich proteoglycans was increased in the degenerate
osteoarthritic articular cartilage and menisci when compared with
the articular cartilage of a normal knee. The authors suggested
that specific decorin and fibromodulin core protein fragments in
degenerate meniscus and/or human articular cartilage may be of
value as biomarkers of disease, and further research may identify
them as therapeutic targets. Clements et al (36) identified that the expression levels
of lumican genes were increased in OA cartilage. Therefore, the
present study proposed that lumican would be upregulated in OA, and
that regulating lumican expression in the synovial membrane may
present as a novel treatment method for OA, consistent with a
previous study (35).
The role of AHSG in the augmentation of neutrophil
phagocytosis by macrophages, thus acting as an anti-inflammatory
molecule, was reported in 1961 (37). Heiss et al (38) suggested that AHSG is a systemic
inhibitor of precipitation of basic calcium phosphate, preventing
unwanted calcification, and that AHSG domain D1 is most efficient
in inhibiting basic calcium phosphate precipitation (38). Liu et al (39) demonstrated that the AHSG gene may
contribute to bone size variation at the hip in a Chinese
population. In addition, Nishio et al (40) observed that AHSG exerted mild
inhibitory effects on calcium oxalate crystallization, and that low
urinary concentrations of prothrombin F1 and osteopontin may
contribute to stone formation. According to further findings,
numerous studies have identified that AHSG is a non-specific
opsonin, with its serum level being demonstrated to vary in
patients who have experienced trauma, or who have diabetes
mellitus. Lebreton et al (41) identified that the serum level of
AHSG was negatively correlated with the acute phase reactants. A
previous study by Mbuyi-Muamba et al (42) reported that treatment of RA did not
appear to modify AHSG plasma levels; thus, the probable biological
role of AHSG in RA is debated. However, Saroha et al
(43) reported that the level of
plasma AHSG was reduced by two-fold in RA patients when compared
with healthy control subjects. To date, the association between OA
and AHSG has required further elucidation. In the present study,
the synovial membrane sample contained AHSG in the bilateral ACLT
group while it was not observed in the unilateral ACLT group. The
present study proposes that during the onset of OA, the synovial
membrane expression of AHSG may be reduced for anti-inflammatory
purposes or to induce the hyperostosis.
The proteomics in the synovial membrane of
spontaneous and secondary KOA models were compared in the present
study. As all 10 proteins have not been reported in the synovial
membrane, their functions in KOA can only be hypothesized. The
present study identified PDI and CK M-type in the unilateral KOA
model, but not in the bilateral KOA model (and more severe
pathological changes than unilateral KOA). PDI may accelerate the
process of KOA, as it regulates protein metabolism, calcium
homeostasis and procollagen synthesis. The levels of CK may reflect
the different disease phases or the degree of pathology of KOA;
with upregulated CK concentrations in the early phase/light KOA and
downregulated CK concentrations at the mid to late phase/severe
KOA.
Serum albumin, lumican, AHSG and three
uncharacterized proteins were observed in the bilateral KOA model,
but not in the unilateral KOA model. It is hypothesized that the
serum albumin levels increase to inhibit the KOA process. The
lumican levels in the synovial membrane may induce cell
proliferation in connective tissues resulting in synovial
hyperplasia, and AHSG proteins in the synovial membrane may be
secreted into the synovial fluid, stimulating bone formation and
resulting in hyperostosis.
In conclusion, the present results demonstrate the
differential proteomic expression and indicate the diverse
pathomechanisms between bilateral and unilateral KOA, highlighting
that spontaneous and secondary KOA require diverse methods of
treatment. Regulation of PDI and CK M-type expression levels may be
necessary in secondary KOA or in the early phase of spontaneous
KOA, and reduced lumican and AHSG levels in spontaneous KOA,
particularly at the mid to late phase, as the majority of patients
are at this stage upon OA diagnosis. However, further
investigations regarding the different mechanisms of synovial
membrane proteomics in spontaneous and secondary KOA are
required.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81273774).
References
|
1
|
Krasnokutsky S, Attur M, Palmer G, Samuels
J and Abramson SB: Current concepts in the pathogenesis of
osteoarthritis. Osteoarthritis Cartilage. 16(Suppl 3): S1–S3. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi D, Roemer FW, Katur A, Felson DT,
Yang SO, Alomran F and Guermazi A: Imaging of synovitis in
osteoarthritis: Current status and outlook. Semin Arthritis Rheum.
41:116–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pawłowska J, Mikosik A, Soroczynska-Cybula
M, Jóźwik A, Łuczkiewicz P, Mazurkiewicz S, Lorczyński A, Witkowski
JM and Bryl E: Different distribution of CD4 and CD8 T cells in
synovial membrane and peripheral blood of rheumatoid arthritis and
osteoarthritis patients. Folia Histochem Cytobiol. 47:627–632.
2009.
|
|
5
|
Presle N, Pottie P, Dumond H, Guillaume C,
Lapicque F, Pallu S, Mainard D, Netter P and Terlain B:
Differential distribution of adipokines between serum and synovial
fluid in patients with osteoarthritis. Contribution of joint
tissues to their articular production. Osteoarthritis Cartilage.
14:690–695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okabe T, Ohmori Y, Tanigami A, Hishigaki
H, Suzuki Y, Sugano S, Kawaguchi A, Nakaya H and Wakitani S:
Detection of gene expression in synovium of patients with
osteoarthritis using a random sequencing method. Acta Orthop.
78:687–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lambrecht S, Verbruggen G, Elewaut D and
Deforce D: Differential expression of alpha B-crystallin and
evidence of its role as a mediator of matrix gene expression in
osteoarthritis. Arthritis Rheum. 60:179–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anderson NL, Matheson AD and Steiner S:
Proteomics: Applications in basic and applied biology. Curr Opin
Biotechnol. 11:408–412. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiz-Romero C and Blanco FJ: Proteomics
role in the search for improved diagnosis, prognosis and treatment
of osteoarthritis. Osteoarthritis Cartilage. 18:500–509. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo D, Tan W, Wang F, Lv Z, Hu J, Lv T,
Chen Q, Gu X, Wan B and Zhang Z: Proteomic analysis of human
articular cartilage: Identification of differentially expressed
proteins in knee osteoarthritis. Joint Bone Spine. 75:439–444.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mobasheri A: Osteoarthritis year 2012 in
review: Biomarkers. Osteoarthritis Cartilage. 20:1451–1464. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernández-Costa C, Calamia V,
Fernández-Puente P, Capelo-Martinez JL, Ruiz-Romero C and Blanco
FJ: Sequential depletion of human serum for the search of
osteoarthritis biomarkers. Proteome Sci. 10:552012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henrotin Y, Gharbi M, Mazzucchelli G,
Dubuc JE, De Pauw E and Deberg M: Fibulin 3 peptides Fib3-1 and
Fib3-2 are potential biomarkers of osteoarthritis. Arthritis Rheum.
64:2260–2267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruiz-Romero C, Calamia V, Carreira V,
Mateos J, Fernández P and Blanco FJ: Strategies to optimize
two-dimensional gel electrophoresis analysis of the human joint
proteome. Talanta. 80:1552–1560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasegawa A, Nakahara H, Kinoshita M,
Asahara H, Koziol J and Lotz MK: Cellular and extracellular matrix
changes in anterior cruciate ligaments during human knee aging and
osteoarthritis. Arthritis Res Ther. 15:R292013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Revell PA, Mayston V, Lalor P and Mapp P:
The synovial membrane in osteoarthritis: A histological study
including the characterisation of the cellular infiltrate present
in inflammatory osteoarthritis using monoclonal antibodies. Ann
Rheum Dis. 47:300–307. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willett TL, Kandel R, De Croos JN, Avery
NC and Grynpas MD: Enhanced levels of non-enzymatic glycation and
pentosidine crosslinking in spontaneous osteoarthritis progression.
Osteoarthritis Cartilage. 20:736–744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lambrecht S, Verbruggen G, Verdonk PC,
Elewaut D and Deforce D: Differential proteome analysis of normal
and osteoarthritic chondrocytes reveals distortion of vimentin
network in osteoarthritis. Osteoarthritis Cartilage. 16:163–173.
2008. View Article : Google Scholar
|
|
19
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Millington SA, Li B, Tang J, Trattnig S,
Crandall JR, Hurwitz SR and Acton ST: Quantitative and
topographical evaluation of ankle articular cartilage using high
resolution MRI. J Orthop Res. 25:143–151. 2007. View Article : Google Scholar
|
|
21
|
Ratzlaff CR and Liang MH: New developments
in osteoarthritis. Prevention of injury-related knee
osteoarthritis: Opportunities for the primary and secondary
prevention of knee osteoarthritis. Arthritis Res Ther. 12:2152010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mobasheri A: Applications of proteomics to
osteoarthritis, a musculoskeletal disease characterized by aging.
Front Physiol. 2:1082011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
The Ministry of Science and Technology of
the People's Republic of China: Guidance suggestion of caring
laboratory animals. Beijing, P.R. China: 2006
|
|
24
|
Vandenbroeck K, Martens E and Alidza I:
Multichaperone complexes regulate the folding of interferon-gamma
in the endoplasmic reticulum. Cytokine. 33:264–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Zhang XY, Wu TJ, Cheng W, Liu X,
Jiang TT, Wen J, Li J, Ma QL and Hua ZC: Endoplasmic reticulum
stress regulates rat mandibular cartilage thinning under
compressive mechanical stress. J Biol Chem. 288:18172–18183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams A, Smith JR, Allaway D, Harris P,
Liddell S and Mobasheri A: Applications of proteomics in cartilage
biology and osteoarthritis research. Front Biosci (Landmark Ed).
16:2622–2644. 2011. View
Article : Google Scholar
|
|
27
|
Chen X and Wang F: Variation and clinical
significance of serum cardiac enzymes in patients with rheumatoid
arthritis. China Medical Herald. 10:51–52. 2013.
|
|
28
|
Eimre M, Puhke R, Alev K, Seppet E, Sikkut
A, Peet N, Kadaja L, Lenzner A, Haviko T, Seene T, et al: Altered
mitochondrial apparent affinity for ADP and impaired function of
mitochondrial creatine kinase in gluteus medius of patients with
hip osteoarthritis. Am J Physiol Regul Integr Comp Physiol.
290:R1271–R1275. 2006. View Article : Google Scholar
|
|
29
|
Borges LS, Bortolon JR, Santos VC, de
Moura NR, Dermargos A, Cury-Boaventura MF, Gorjão R, Pithon-Curi TC
and Hatanaka E: Chronic inflammation and neutrophil activation as
possible causes of joint diseases in ballet dancers. Mediators
Inflamm. 2014:8460212014. View Article : Google Scholar :
|
|
30
|
Huang SC, Wei JC, Lin PT, Wu DJ and Huang
YC: Plasma pyridoxal 5-phosphate is not associated with
inflammatory and immune responses after adjusting for serum albumin
in patients with rheumatoid arthritis: A preliminary study. Ann
Nutr Metab. 60:83–89. 2012. View Article : Google Scholar
|
|
31
|
Izai M, Miyazaki S, Murai R, Morioka Y,
Hayashi H, Nishiura M and Miura K: Prorenin-renin axis in synovial
fluid in patients with rheumatoid arthritis and osteoarthritis.
Endocrinol Jpn. 39:259–267. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jepsen KJ, Wu F, Peragallo JH, Paul J,
Roberts L, Ezura Y, Oldberg A, Birk DE and Chakravarti S: A
syndrome of joint laxity and impaired tendon integrity in lumican
and fibromodulin deficient mice. J Biol Chem. 277:35532–35540.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seki T, Selby J, Häupl T and Winchester R:
Use of differential subtraction method to identify genes that
characterize the phenotype of cultured rheumatoid arthritis
synoviocytes. Arthritis Rheum. 41:1356–1364. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernández-Puente P, Mateos J,
Fernández-Costa C, Oreiro N, Fernández-López C, Ruiz-Romero C and
Blanco FJ: Identification of a panel of novel serum osteoarthritis
biomarkers. J Proteome Res. 10:5095–5101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Melrose J, Fuller ES, Roughley PJ, Smith
MM, Kerr B, Hughes CE, Caterson B and Little CB: Fragmentation of
decorin, biglycan, lumican and keratocan is elevated in degenerate
human meniscus, knee and hip articular cartilages compared with age
matched macroscopically normal and control tissues. Arthritis Res
Ther. 10:R792008. View
Article : Google Scholar
|
|
36
|
Clements DN, Fitzpatrick N, Carter SD and
Day PJ: Cartilage gene expression correlates with radiographic
severity of canine elbow osteoarthritis. Vet J. 179:211–218. 2009.
View Article : Google Scholar
|
|
37
|
Schmid K and Burgi W: Preparation and
properties of the human plasma Ba-alpha2-glycoproteins. Biochim
Biophys Acta. 47:440–453. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heiss A, DuChesne A, Denecke B, Grötzinger
J, Yamamoto K, Renné T and Jahnen-Dechent W: Structural basis of
calcification inhibition by alpha 2-HS glycoprotein/Fetuin-A.
Formation of colloidal calciprotein particles. J Biol Chem.
278:13333–13341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu YJ, Liu XH, Lei SF, Li MX and Deng HW:
Alpha2-HS glycoprotein gene is associated with bone size at the hip
in Chinese. Yi Chuan Xue Bao. 32:1128–1135. 2005.PubMed/NCBI
|
|
40
|
Nishio S, Hatanaka M, Takeda H, Iseda T,
Iwata H and Yokoyama M: Analysis of urinary concentrations of
calcium phosphate crystal-associated proteins:
Alpha2-HS-glycoprotein, prothrombin F1, and osteopontin. J Am Soc
Nephrol. 10(Suppl 14): S394–S396. 1999.PubMed/NCBI
|
|
41
|
Lebreton JP, Joisel F, Raoult JP, Lannuzel
B, Rogez JP and Humbert G: Serum concentration of human alpha 2 HS
glycoprotein during the inflammatory process: Evidence that alpha 2
HS glycoprotein is a negative acute-phase reactant. J Clin Invest.
64:1118–1129. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mbuyi-Muamba JM, Dequeker J and Stevens E:
Alpha 2 HS-glycoprotein in rheumatoid arthritis. Its plasma
concentration and possible biological role. Rev Rhum Mal
Osteoartic. 49:515–518. 1982.In French. PubMed/NCBI
|
|
43
|
Saroha A, Kumar S, Chatterjee BP and Das
HR: Jacalin bound plasma O-glycoproteome and reduced sialylation of
alpha 2-HS glycoprotein (A2HSG) in rheumatoid arthritis patients.
PLoS One. 7:e463742012. View Article : Google Scholar : PubMed/NCBI
|