Introduction
Prostate cancer is the most common cancer type
diagnosed in men from developed countries and is the leading cause
of cancer mortalities worldwide (1). Despite the various treatments for
prostate cancer having been improved recently, therapy for patients
with advanced prostate cancer still lacks efficacy. This is largely
a result of a lack of knowledge regarding the molecular mechanism
underlying the pathogenesis and progression of prostate cancer.
Therefore, to improve the understanding of the mechanism associated
with prostate cancer progression, it is vital to explore novel
therapeutic strategies for patients with prostate cancer.
Accumulating evidence has demonstrated a significant
role for the Wnt/β-catenin signaling pathway in the development and
progression of human cancer (2–4). The
Dickkopf (DKK) family is one of the Wnt antagonist families, and
inhibits Wnt signaling by binding to the lipoprotein
receptor-related protein 5/6 component of the Wnt receptor complex
(5). DKK consists of four members,
each of which possesses an N-terminal signal peptide and contains
two conserved cysteine-rich domains separated by a linker region
(6). DKK2 is a secretory protein,
and numerous previous studies have shown that it is involved in
tumor cell proliferation, survival, migration and invasion. In
renal carcinoma, the expression of DKK2 is epigenetically
suppressed, and its ectopic expression reduces invasion and induces
apoptosis of cancer cells (7).
DKK2 also increases tumor growth and metastasis through the
transcriptional upregulation of matrix metalloprotease-1 in Ewing's
Sarcoma (8). Additionally, high
expression of DKK2 has also been reported in colorectal cancer
(9). These findings suggested that
DKK2 performs an oncogenic or a tumor-suppressing function
depending on the cell type or context. However, the functions and
mechanism of DKK2 in the development and progression of prostate
cancer remain to be elucidated.
The present study found that DKK2 was upregulated in
prostate cancer tissues and cells, and small interfering (si) RNA
targeting DKK2 inhibited cell growth and invasion. In addition, the
underlying molecular mechanism was also assessed in the present
study.
Materials and methods
Human tissue specimens
Human prostate cancer and adjacent non-tumor liver
tissue samples were collected from patients undergoing resection of
prostate cancer at the Department of Urology, The First People's
Hospital of Yulin City (Yulin, China). All tissues were classified
according to the World Health Organization criteria and staged
according to the tumor-node-metastasis classification. Informed
consent was obtained from each patient and the study was approved
by the Institute Research Ethics Committee of the Department of
Urology, The First People's Hospital of Yulin City.
Cell culture
DU-145 and PC-3 human prostate cancer cell lines and
the RWPE-1 normal prostate epithelial cell line were purchased from
American Type Culture Collection (Manassas, VA, USA) and maintained
in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.,), 100
μg/ml penicillin and 100 μg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified
atmosphere containing 5% CO2.
Stable transfection with siRNA-DKK2
The prostate cancer cells were plated into 12-well
plates (1×105 cells/well) in endothelial cell growth
medium-2 (Invitrogen; Thermo Fisher Scientific, Inc.) without
antibiotics. After incubation for 24 h, the cells were transfected
with siRNA targeting DKK2 (Sangon Biotech Co., Ltd., Shanghai,
China) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from primary tumor
tissues or cells homogenized in TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Superscript First-Strand kit was used to synthesize first strand
cDNA (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was
performed on the Bio-Rad iQ5 Quantitative PCR system (Takara Bio,
Inc., Dalian, China). The primers used were as follows: DKK2,
sense: 5′-AGTACCCGCTGCAATAATGG-3′ and antisense:
5′-GAAATGACGAGCACAGCAAA-3′; β-actin, sense:
5′-GATCATTGCTCCTCCTGAGC-3′ and antisense:
5′-ACTCCTGCTTGCTGATCCAC-3′ (Sangon Biotech Co., Ltd.). A total of
three independent experiments were performed for each reaction in
triplicate.
Western blotting
The total proteins were extracted from cells using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Inc., Nantong, China). Equal quantities of protein
sample (30 μg) were separated on a 10% sodium dodecyl
sulfate-polyacrylamide gel, transferred onto nitrocellulose
membranes (EMD Millipore, Boston, MA, USA) and blocked with 10%
non-fat milk in phosphate-buffered saline at 4°C overnight. The
membranes were subsequently incubated with the following primary
antibodies: Monoclonal mouse anti-β-catenin (1:3,000; sc-53484),
monoclonal mouse anti-cyclin D1 (1:3,000; sc-20044), mouse
monoclonal anti-c-Myc (1:2,500; sc-47694) and anti-β-actin
(1:1,500; sc-47778; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) overnight at 4°C. After washing with Tris-buffered
saline/0.05% Tween-20 buffer, the membranes were incubated for 1 h
at room temperature with rabbit anti-mouse IgG horseradish
peroxidase-conjugated secondary antibody (1:3,000; sc-358917; Santa
Cruz Biotechnology, Inc.). The membrane was subsequently washed and
visualized by enhanced chemiluminescence detection system (GE
Healthcare Life Sciences, Little Chalfont, UK).
Cell proliferation assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to determine cell proliferation. Briefly, the cells
were seeded into 96-well plates at a density of 1×105
cells/well. Following treatment with siRNA-DKK2 or siRNA-mock, ~20
μl MTT (5 mg/ml) was added to each well and the cells were
incubated for a further 6 h at 37°C. The supernatant was replaced
with 200 μl isopropanol to dissolve the formazan product.
The cell viability was determined by measuring the absorbance at
490 nm using an ELISA microplate reader (Abcam, Cambridge, UK). All
samples were performed in triplicate and the results are presented
as the percentage of growth inhibition.
Cell invasion assay
Cell invasion was measured using a modified Boyden
chamber coated with Matrigel (BD Biosciences, Bedford, MA, USA).
The cells (1×105 cells/well) treated with siRNA-DKK2 or
siRNA-mock were seeded into the upper compartment. The medium,
including 10% fetal bovine serum, was added into the lower
compartment. After 48 h, the cells that passed through the lower
side of the membrane were stained with hematoxylin and eosin
(Sigma-Aldrich), and were counted using a microscope. A total of
five random fields were selected for each membrane, and the results
were expressed as the number of cells migrated per field.
Statistical analysis
All results are presented as the mean ± standard
deviation. Statistical analysis was performed using Student's t
test for the comparison of two groups or one-way analysis of
variance for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
DKK2 expression levels in prostate cancer
tissues and cell lines
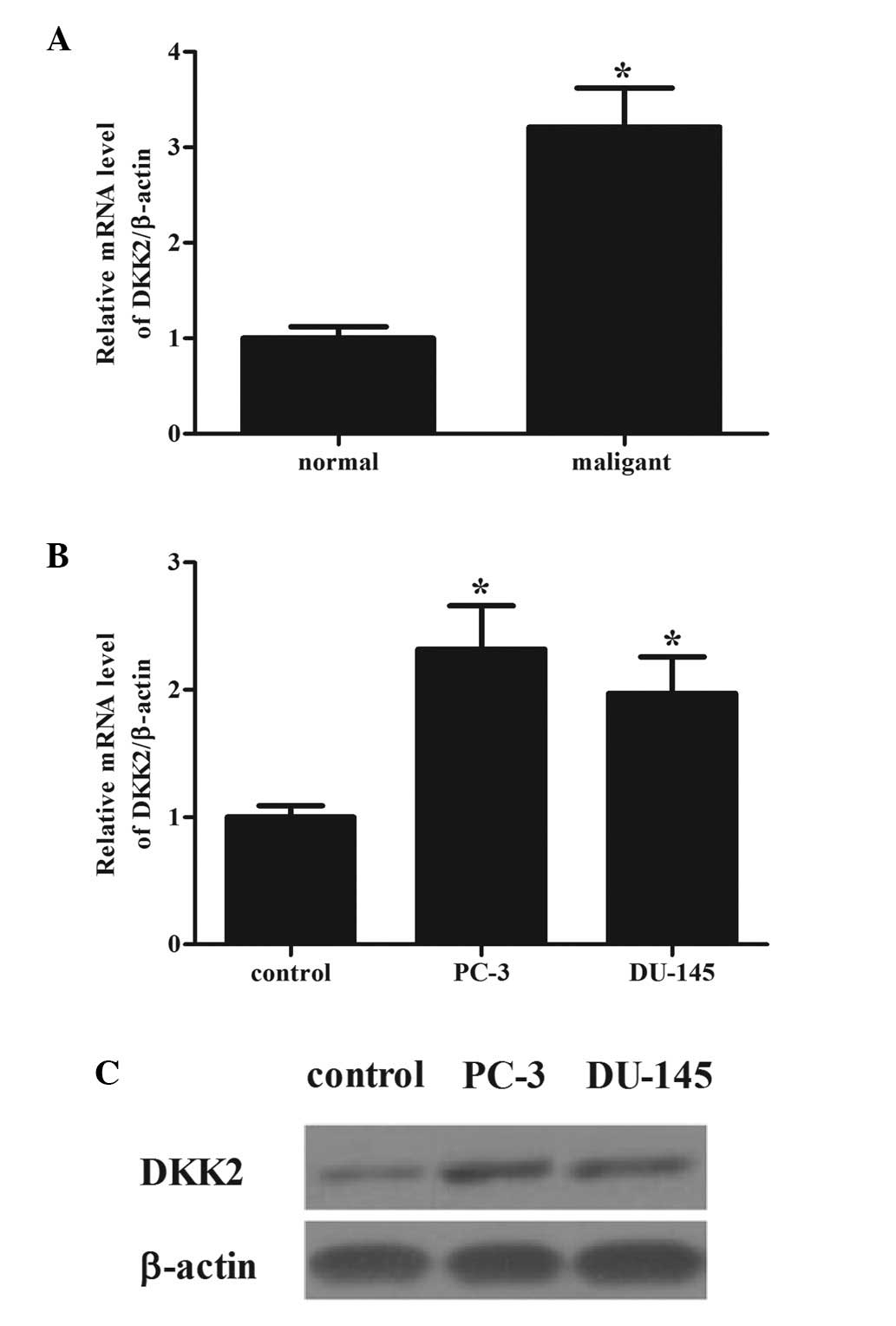
To determine the role of DKK2 in prostate cancer,
the mRNA expression levels of DKK2 were compared between prostate
cancer tissues and adjacent normal prostate tissues. The mRNA
expression of DKK2 was high in prostate cancer tissues compared
with that in normal prostate tissues (Fig. 1A). Consistent with the observations
from tissue samples, it was demonstrated that the mRNA and protein
expression levels of DKK2 were higher in the prostate cancer cells,
PC-3 and DU-145, compared with the normal human prostate cells
(Fig. 1B and C).
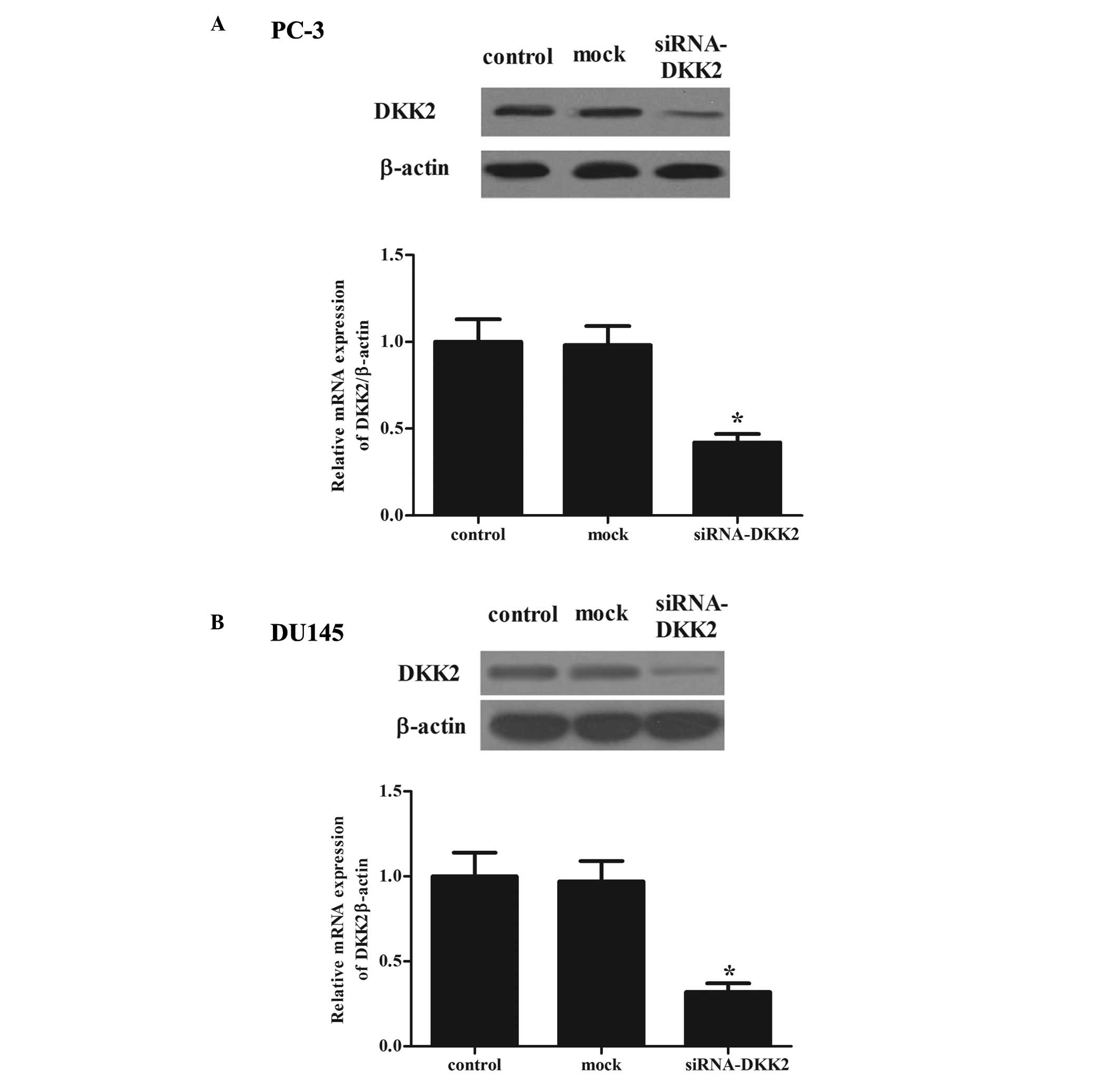
Effects of transfection
It is possible that DKK2 may be important in the
development of prostate cancer. Therefore, the present study
generated the stable knockdown DKK2 in PC-3 and DU-145 cells. To
test the efficiency of DKK2 transfection, RT-qPCR and western
blotting were used to determine the mRNA and protein expression
levels, respectively. As shown in Fig.
2, the siRNA significantly decreased the protein and mRNA
expression levels of DKK2 in PC-3 (Fig. 2A) and DU-145 (Fig. 2B) cells, as compared with the
control group.
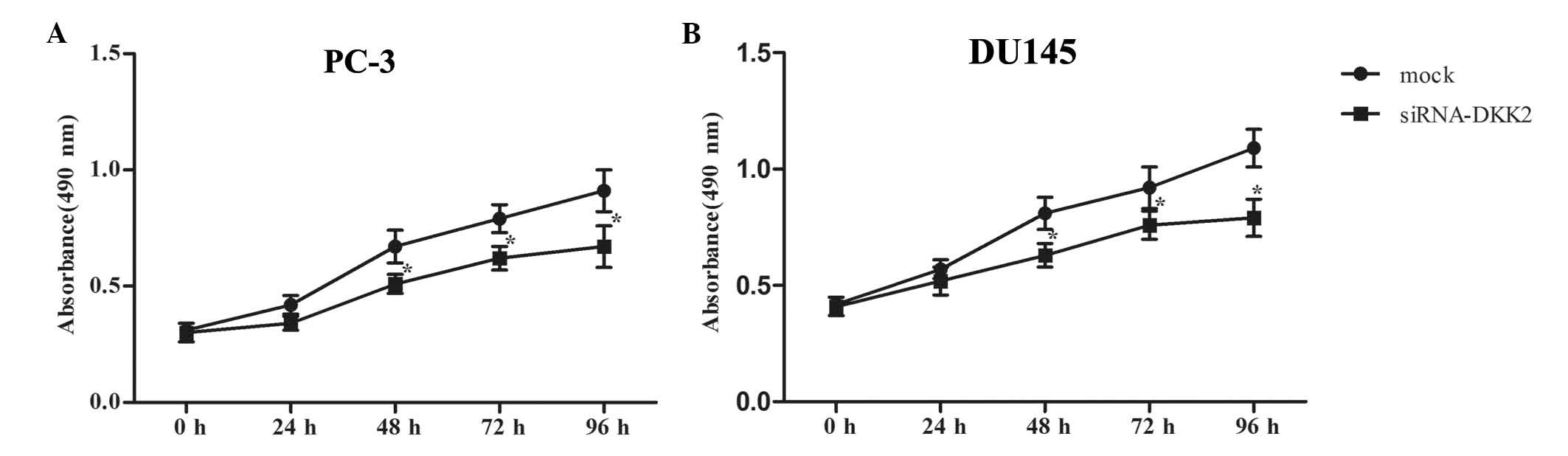
Effects of DKK2 on the proliferation of
prostate cancer cells
To investigate the effects of DKK2 on the
proliferation of human prostate cancer cells, the cells were
transfected with siRNA-DKK2 or siRNA-mock, and cell proliferation
was measured using an MTT assay. As indicated in Fig. 3, compared with the siRNA-mock,
siRNA-DKK2 significantly inhibited the proliferation of PC-3
(Fig. 3A) and DU-145 (Fig. 3B) cells, in a time-dependent
manner. These results indicated that DKK2 promoted the
proliferation of prostate cancer cells.
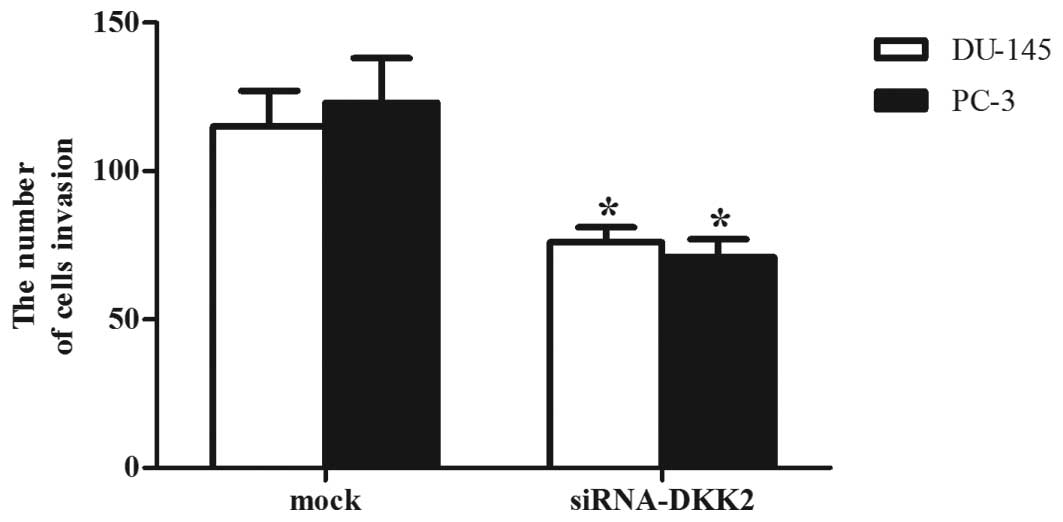
Effects of DKK2 on the invasion of
prostate cancer cells
To assess the effect of DKK2 on prostate cancer cell
invasion, the cells were transfected with siRNA-mock or siRNA-DKK2
and were placed into a Boyden chamber coated with Matrigel. As
shown in Fig. 4, the number of
siRNA-DKK2-transfected PC-3 and DU-145 cells that passed through
the Matrigel was significantly inhibited compared with that derived
from mock or non-transfected cells (P<0.05), suggesting that
DKK2 enhanced the invasion of prostate cancer cells.
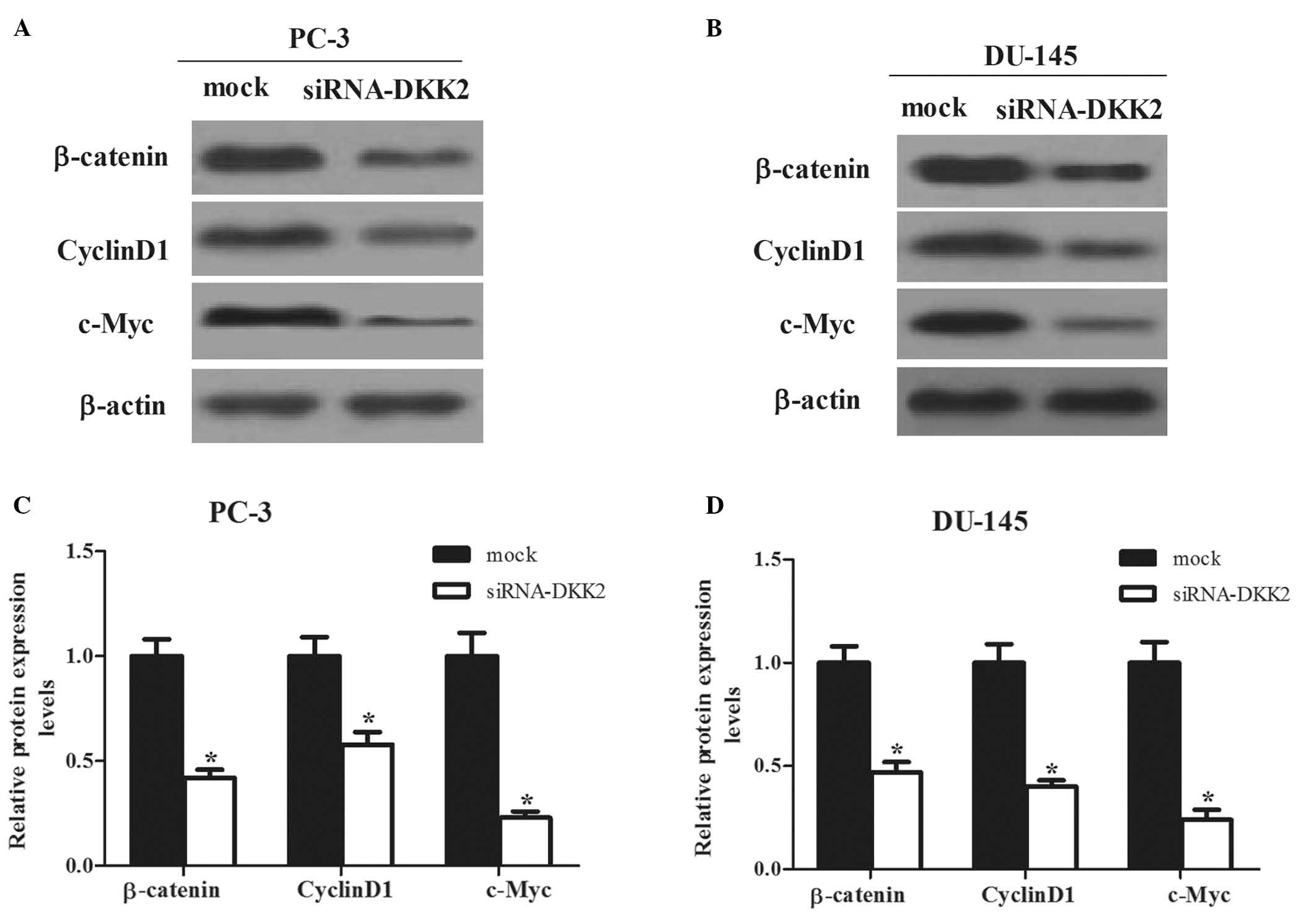
DKK2 exerts its function by promoting
β-catenin signaling
The present study next investigated a potential
mechanism for DKK2-mediated cell migration and invasion. It is well
known that DKK2 is a typical inhibitor of Wnt/β-catenin signaling
pathway, hence the effects of siRNA-DKK2 on the downstream target
genes of Wnt pathway, β-catenin, cyclin D1 and c-Myc, were
investigated. As shown in Fig. 5,
the expression levels of β-catenin, cyclin D1 and c-Myc were
significantly inhibited by siRNA-DKK2 in PC-3 and DU-145 cells.
Discussion
The present study found that DKK2 is markedly
overexpressed in both prostate cancer tissues and cells. In
addition, siRNA-DKK2 significantly suppressed the proliferation and
the invasion by inhibiting the Wnt/β-catenin signaling pathway.
DKK1 was directly produced by prostate cancer cells,
whereas normal prostate tissue did not produce this molecule. DKK1
has been reported to be upregulated in the sera of patients with
prostate cancer (10). DKK3 was
also upregulated in the reactive stroma of prostate cancer tissue
(11). Consistent with the above
results, the present study found that DKK2 is overexpressed in both
prostate cancer tissues and cells. All findings indicated that DKK2
is an oncogenic factor in prostate cancer.
Cell proliferation and invasion are two of the most
important features of malignant cell behavior. DKK3 has been
reported to be associated with various cancer types and it is a
tumor suppressor for human cancer growth (12,13).
However, the function and biological role of DKK2 remains to be
elucidated. In Ewing's sarcoma, DKK2 increases the proliferation,
anchorage-independent colony formation, osteolysis and invasion of
the sarcoma cells. In addition, it regulates the expression levels
of different genes important for invasion into bone and osteolysis
(8). By contrast, overexpression
of DKK2 suppressed malignant cell growth and invasion in human
epithelial ovarian cancer cells (14). These findings suggested that DKK2
performs an oncogenic or a tumor-suppressing function dependent on
the cell type or context. The present study found that siRNA-DKK2
markedly inhibited the proliferation and invasion of prostate
cancer cells, suggesting that DKK2 is important for promoting cell
proliferation and invasion of prostate cancer.
Deregulation of the Wnt/β-catenin signaling pathway
is a hallmark of various cancer types. β-catenin is a critical end
component of the Wnt signaling pathway, which regulates cell
growth, apoptosis and migratory behavior in response to
intercellular adhesion molecules (15). It was previously reported that
KIF3a, a subunit of kinesin-II motor protein, functions as an
agonist of the Wnt signaling pathway, and it increases
CK1-dependent DVL2 phosphorylation and β-catenin activation in
prostate cancer cells, leading to transactivation of the
Wnt-signaling target genes, including cyclin D1, HEF1 and matrix
metalloproteinase 9 (16).
Previous studies have shown that the expression levels of Wnt-1 and
β-catenin were increased in invasive prostate cancer cell lines and
in primary prostate cancer specimens (17–20).
In line with these previous reports, the present study demonstrated
that silencing of DKK2 significantly suppressed the expression of
β-catenin, cyclin D1 and c-Myc in prostate cancer cells. β-catenin
forms a cell adhesion complex with E-cadherin raising the
possibility that loss of expression or a change in β-catenin
distribution in the cell can also alter downstream signaling,
decreased inter-cellular adhesion and the promotion of metastasis
(21). A previous study also
demonstrated that a novel small molecule inhibitor of Wnt/β-catenin
signaling, PKF118-310, inhibited proliferation and Wnt/β-catenin
signaling in prostate cancer cells (19). These results suggested that the
inhibitory effect of siRNA-DKK2 on prostate cancer cell
proliferation and invasion may involve the suppression of the
Wnt/β-catenin signaling pathway.
In conclusion, the present findings demonstrated
that DKK2 downregulation suppressed the proliferation and invasion
of prostate cancer cells by inhibiting the Wnt/β-catenin signaling
pathway, which supported the notion that DKK2 may serve as a
diagnostic biomarker for monitoring prostate cancer development and
progression. Therefore, DKK2 is a potential therapeutic strategy
for the treatment of prostate cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
4
|
Maretto S, Cordenonsi M, Dupont S,
Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM and
Piccolo S: Mapping Wnt/beta-catenin signaling during mouse
development and in colorectal tumors. Proc Natl Acad Sci USA.
100:3299–3304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krupnik VE, Sharp JD, Jiang C, Robison K,
Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et
al: Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Kawakami K, Yamamura S, Ueno K, Majid S, Saini S, et
al: Wnt antagonist gene DKK2 is epigenetically silenced and
inhibits renal cancer progression through apoptotic and cell cycle
pathways. Clin Cancer Res. 15:5678–5687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hauer K, Calzada-Wack J, Steiger K,
Grunewald TG, Baumhoer D, Plehm S, Buch T, Prazeres da Costa O,
Esposito I, Burdach S and Richter GH: DKK2 mediates osteolysis,
invasiveness and metastatic spread in Ewing sarcoma. Cancer Res.
73:967–977. 2013. View Article : Google Scholar
|
|
9
|
Matsui A, Yamaguchi T, Maekawa S, Miyazaki
C, Takano S, Uetake T, Inoue T, Otaka M, Otsuka H, Sato T, et al:
DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer.
Cancer Sci. 100:1923–1930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roato I, D'Amelio P, Gorassini E, Grimaldi
A, Bonello L, Fiori C, Delsedime L, Tizzani A, De Libero A, Isaia G
and Ferracini R: Osteoclasts are active in bone forming metastases
of prostate cancer patients. PloS One. 3:e36272008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zenzmaier C, Untergasser G, Hermann M,
Dirnhofer S, Sampson N and Berger P: Dysregulation of Dkk-3
expression in benign and malignant prostatic tissue. Prostate.
68:540–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawasaki K, Watanabe M, Sakaguchi M,
Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH,
Kumon H and Date H: REIC/Dkk-3 overexpression downregulates
P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces
apoptosis in breast cancer. Cancer Gene Ther. 16:65–72. 2009.
View Article : Google Scholar
|
|
13
|
Yue W, Sun Q, Dacic S, Landreneau RJ,
Siegfried JM, Yu J and Zhang L: Downregulation of Dkk3 activates
beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis.
29:84–92. 2008. View Article : Google Scholar
|
|
14
|
Zhu J, Zhang S, Gu L and Di W: Epigenetic
silencing of DKK2 and Wnt signal pathway components in human
ovarian carcinoma. Carcinogenesis. 33:2334–2343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de la Taille A, Rubin MA, Chen MW,
Vacherot F, de Medina SG, Burchardt M, Buttyan R and Chopin D:
Beta-catenin-related anomalies in apoptosis-resistant and
hormone-refractory prostate cancer cells. Clin Cancer Res.
9:1801–1807. 2003.PubMed/NCBI
|
|
16
|
Liu Z, Rebowe RE, Wang Z, Li Y, Wang Z,
DePaolo JS, Guo J, Qian C and Liu W: KIF3a promotes proliferation
and invasion via Wnt signaling in advanced prostate cancer. Mol
Cancer Res. 12:491–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen G, Shukeir N, Potti A, Sircar K,
Aprikian A, Goltzman D and Rabbani SA: Up-regulation of Wnt-1 and
beta-catenin production in patients with advanced metastatic
prostate carcinoma: Potential pathogenetic and prognostic
implications. Cancer. 101:1345–1356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verras M and Sun Z: Roles and regulation
of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett.
237:22–32. 2006. View Article : Google Scholar
|
|
19
|
Lu W, Tinsley HN, Keeton A, Qu Z, Piazza
GA and Li Y: Suppression of Wnt/beta-catenin signaling inhibits
prostate cancer cell proliferation. Eur J Pharmacol. 602:8–14.
2009. View Article : Google Scholar
|
|
20
|
Davies G, Jiang W and Mason M: Cell-cell
adhesion molecules and signaling intermediates and their role in
the invasive potential of prostate cancer cells. J Urol.
163:985–992. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whitaker HC, Girling J, Warren AY, Leung
H, Mills IG and Neal DE: Alterations in beta. catenin expression
and localization in prostate cancer. Prostate. 68:1196–1205. 2008.
View Article : Google Scholar : PubMed/NCBI
|