Introduction
γ-Herpesviruses, including Epstein-Barr virus (EBV),
are characterized by two distinct life cycles: Latency and lytic
infection, and EBV-associated malignant cells are in the latent
form. In this status, the infected cells are poorly recognized by
the host immune system due to the fact only certain viral gene
products are expressed. Thus, the virus is allowed to persist in
cells for long periods. By contrast, lytic replication is
unfavorable for the virus, as the host cells cannot survive.
There are various factors, both host and viral, that
regulate viral reactivation in vivo. Regarding the viral
factor in EBV infection, latent membrane protein 1 suppresses the
virus reactivation in a nuclear factor (NF)-κB dependent manner
(1). EBV-encoded small RNA also
mediates the NF-κB induction via reactivation of retinoic acid
inducible gene I and its downstream signal pathway, the inhibitor
of κB (IκB) kinase (IKK)α/IKKβ pathway (2). Brown et al (3), demonstrated that NF-κB inhibitors led
to lytic replication in EBV-positive lymphocytes and epithelial
cells, suggesting that NF-κB may be a potential target to disrupt
virus latency. They additionally demonstrated the different
thresholds for reactivation via inhibition of NF-κB among different
cell lines.
Acetylsalicylic acid (aspirin) is a non-steroidal
anti-inflammatory drug commonly used due to its known safety record
and and reasonable price. It has been reported that aspirin
inhibits NF-κB activity by inhibiting IKK activity and thereby
blocking IκBα degradation in the cytoplasm (4,5). Liu
et al (6) demonstrated that
incubation of the EBV-positive malignant cell lines B95-8 and Raji
with aspirin depleted NF-κB (p65) and resulted in EBV lytic
replication, which consequently reduced the viability of
EBV-positive B lymphocytes. Notably, combination treatment with an
anti-herpes agent, ganciclovir, was observed to enhance the
cytotoxic effects only in EBV-positive cells (6). Similar results using other NF-κB
inhibitors, Bay11-7802 and Z-LLF-CHO, were also reported by the
same research group (7).
Commonly used anti-herpes agents can be chemically
classified into three groups: i) Nucleoside analogs, ii) nucleotide
analogs and iii) pyrophosphate analogs (8). Although there are several anti-herpes
agents, ganciclovir and acyclovir, which are nucleoside analogs,
have been considered as standard treatments for herpes simplex
virus (HSV), varicella-zoster virus (VZV) and cytomegalovirus
(CMV). These drugs are monophosphorylated by the viral-encoded
protein kinase (PK) or thymidine kinase (TK), and later converted
into deoxyguanosine-triphosphate (dGTP) by cellular kinases. A
previous study demonstrated that ganciclovir and acyclovir are
monophosphorylated by the EBV-encoded PK (EBV-PK), however not the
EBV-encoded thymidine kinase (EBV-TK) prior to being converted into
dGTP (9). Brivudine, an additional
nucleoside analog, which is an alternative for ganciclovir and
acyclovir, requires the viral-TK for both mono- and
di-phosphorylation (10). For the
treatment of the drug-resistant strain, foscarnet, a pyrophosphate
analog, is also applied in clinical use. Foscarnet directly
inhibits the pyrophosphate binding site on viral DNA polymerases
without requiring activation by viral kinases (11).
EBV has been demonstrated to be a cause of gastric
carcinoma called as EBV-positive gastric cancer (GC) (12–14).
Due to the fact that the episomal EBV genome is detected in almost
all gastric tumor cells, however not in neighboring normal
epithelial cells, the combination treatment of the lytic induction
strategy with cytotoxic drugs such as ganciclovir, which is
converted to its active form by the lytic form of EBV infection, is
expected to selectively destroy tumor cells (15). Ji Jung et al (16) demonstrated the lytic induction by
5-fluorouracil, cisplatin and taxol, and the enhancement of lytic
replication and apoptosis with the combination of ganciclovir in an
EBV-positive GC cell line, SNU-719, which is naturally infected
with EBV. However, these chemotherapeutic agents are also cytotoxic
for normal cells and, thus, safer agents are required for the
induction of lytic replication. Furthermore, it would be beneficial
to examine the effects in combination with other anti-herpes agents
rather than ganciclovir, which cannot be used for the
drug-resistant strain although it is the first-line agent against
HSV, CMV and VZV infections. Liu et al (6,7)
demonstrated the induction of lytic replication by NF-κB inhibitors
including aspirin in EBV-positive lymphocytes, however their
effects on EBV-positive GC cells remain unclear. Thresholds for
reactivation via NF-κB inhibition may vary among different cell
lines, thus it is warranted to confirm the induction of lytic
replication by NF-κB inhibitors using epithelial cell lines
(3).
To determine an effective combination of lytic
inducers and anti-herpes agents leading to selective cytotoxicity
of EBV-positive GC cells, the cytotoxic effects of the NF-κB
inhibitors {aspirin, Bay11-7082 [(E)-3-(4-methylphenylsulf
onyl)-2-propenenenitrile] and Z-LLF-CHO
(benzyloxycarbonyl-t-leucyl-L-leucyl-t-phenylalanilal)} in
combination with the anti-herpes agents (ganciclovir, acyclovir,
brivudine and foscarnet) were examined in EBV-positive and-negative
GC cells.
Materials and methods
Cell lines
The human GC cell lines, SNU-216 (EBV-negative) and
SNU-719 (EBV-positive), which are moderately differentiated
adenocarcinomas, were obtained from the Korean Cell Line Bank
(Seoul, Korea). Cells were cultured in Roswell Park Memorial
Institute-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and antibiotics at
37°C in a humidified 5% CO2 incubator.
Chemical agents
Acetylsalicylic acid (aspirin), Bay11-7082,
Z-LLF-CHO, ganciclovir, acyclovir, foscarnet, brivudine and
12-O-tetradecanoyl-phorbol-13-acetate (TPA) were obtained
from Sigma-Aldrich. TPA was used as a positive control for lytic
replication induction. Stock solutions of aspirin (100 mM),
Bay11-7082 (20 mM), Z-LLF-CHO (20 mM), TPA (1.6 mM), and 10 mg/ml
anti-herpes agents: Ganciclovir (39.2 mM), acyclovir (44.4 mM),
foscarnet (79.4 mM) and brivudine (30.0 mM), were prepared in
dimethyl sulfoxide (DMSO).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was used to test cell viability (MTT Cell Proliferation Assay;
American Type Culture Collection, Manassas, VA, USA).
Cytotoxicity assays
MTT assays were used to assess the effects of NF-κB
inhibitors on cell viability. Briefly, EBV-positive SNU-719 and
EBV-negative SNU-216 cells were plated in 96-well cell culture
plates with serial dilutions of the NF-κB inhibitors for 0, 2, 4, 6
and 8 days. In addition, in vitro cell viability assays were
performed on EBV-positive SNU-719 and EBV-negative SNU-216 cells
using NF-κB inhibitors in combination with the anti-herpes agents.
In the baseline experiments, different concentrations of the
compounds were used. Subsequently, cells were plated in 96-well
cell culture plates with doses selected from baseline experiments
of aspirin (5 mM), Bay11-7082 (20 μM) or Z-LLF-CHO (5
μM). Only the most promising NF-κB inhibitors that blocked
IκB kinase were selected. After 24 h, 100 μg/ml anti-herpes
agents: Ganciclovir (392 μM), acyclovir (444 μM),
foscarnet (794 μM) or brivudine (300 μM), were added,
and cells were incubated for 2, 4, 6 and 8 days at 37°C. In
additional experiments, cells were treated with different
concentrations of NF-κB inhibitors in combination with anti-herpes
agents for 8 days. Cells were also treated with NF-κB inhibitors in
combination with different concentrations of anti-herpes agents
(10, 100 or 200 μg/ml) for 0, 2, 4, 6 and 8 days. After the
indicated times, the plates were incubated with MTT solution for 4
h at 37°C. Detergent reagent was then added, and the absorbance was
measured at 570 nm using a microplate reader. The percentage of
viable cells was set at 100% for untreated controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for BZLF1, BRLF1 and BMRF1
EBV-positive SNU-719 cells were grown to 70%
confluence and treated with aspirin (1, 5 and 10 mM), Bay11-7082
(10, 20 and 30 μM), Z-LLF-CHO (1, 5 and 10 μM) or TPA
(20 ng/ml, 32 nM) for 24 h. Non-treated cells and DMSO-treated
cells were used as negative controls. Total RNA was extracted from
3×106 cells using the the RNeasy Mini kit (Qiagen,
Hombrechtikon, Switzerland), according to the manufacturer's
instructions. DNase I (Roche Diagnostics Japan, Tokyo,
Japan) treatment was performed prior to cDNA synthesis with the
Advantage RT-for-PCR kit (Clontech Laboratories, Inc., Mountain
View, CA, USA). RT-qPCR was performed with validated TaqMan systems
for the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; VIC/MGB Probe, primer limited; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) as an endogenous control and
the lytic EBV genes BZLF1, BRLF1 and BMRF1
using an ABI 7200 Cycling System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer sequences used were as follows:
BZLF1, forward: 5′-AAATTTAAGAGATCCTCGTGTAAAACATC-3′ and
reverse: 5′-CGCCTCCTGTTGAAGCAGAT-3′ and the probe was 5′-(FAM)
ATAATGGAGTCAACATCCAGGCTTGGGC (TAMRA)-3′; BRLF1, forward:
5′-GAGTCCATGACAGAGGATTTGA-3′ and reverse:
5′-GCAGCAGACATTCATCATTTAGA-3′ and the probe was 5′-(FAM)
ATGTATCCAAGATTTCATTAAGTTCG (TAMRA)-3′; BMRF1, forward:
5′-CAACACCGCACTGGAGAG-3′ and reverse: 5′-GCCTGCTTCACTTTCTTGG-3′ and
the probe was5′-(FAM) ATCGTCGGAGGCCAGGCAGAAGCAGAAGC (TAMRA)-3′.
Sequences of primers and probes were as previously described by
Ryan et al (17) and
Hilscher et al (18). Each
TaqMan gene expression assay consisted of a fluorogenic
dye-labelled probe (10 μM; 1.25 μl), two
amplification primers (forward and reverse of 100 μM) 0.45
μl each, PCR master mix (25 μl) and the TaqMan
endogenous control (2.5 μl). The real-time PCR reactions
were run for 25 cycles using cycling conditions (94°C for 45 sec,
followed by 60°C for 45 sec and 72°C for 2 min, and a final
extension at 72°C for 7 min). TaqMan data were analyzed using SDS
software, version 2.2 (Applied Biosystems; Thermo Fisher
Scientific, Inc.), and mRNA expression was normalized to
GAPDH mRNA. RT-qPCR data for the control vector were set to
1, and the expression of lytic genes was compared to the control.
All experiments were performed in triplicate.
RT-qPCR for RelA and RelB
For RT-qPCR, total RNA was isolated from the control
as well as treated cells of SNU-719 using RNeasy Mini kit (Qiagen).
cDNA was synthesized from total RNA using the QuantiTect Reverse
Transcription kit (Qiagen). Expressed genes were detected
quantitatively using a LightCycler® 2.0 Instrument
(Roche Diagnostics Japan) with LightCycler FastStart DNA Master
PLUS SYBR Green I (Roche Diagnostics Japan) according to
the manufacturer's instructions.
The primers for the genes were purchased from FASMAC
Co., Ltd. (Atsugi-shi, Japan). PCR amplification was performed in a
total volume of 20 μl containing cDNA, each primer (0.5
μM) and master mix supplied by the LightCycler FastStart DNA
MasterPLUS SYBR Green I kit (Roche Diagnostics Japan).
The PCR cycling conditions were as follows: 95°C for 10 sec,
followed by 45 cycles at 95°C for 10 sec and 60°C for 10 sec, and
72°C for 15 sec. The fluorescent product was determined at the end
of the 72°C temperature step. All PCR assays were performed a
minimum of four times. The sequences of the primers used were as
follows: GADPH, forward 5′-GCCTCCTGCACCACCAACTG-3′ and
reverse 5′-GACGCCTGCTTCACCACCTTCT-3′; RelA, forward
5′-CTGCCGGGATGGCTTCTAT-3′ and reverse 5′-CCGCTTCTTCACACACTG GAT-3′;
and RelB, forward 5′-TCCCAACCAGGATGTCTAGC-3′ and reverse
5′-AGCCATGTCCCTTTTCCTCT-3′. The data obtained were analyzed using
the LightCycler analysis software version 4.1 (Roche Diagnostics
Japan). To confirm the amplification specificity, the PCR products
were subjected to melting curve analysis. Threshold cycle values of
the target genes were normalized to those of the internal control
genes. Values are presented as the mean ± standard error of three
experiments.
Statistical analysis
All results are expressed as the mean of triplicate
assays ± standard error. The results were tested for significance
using the Mann-Whitney U test using Stata software, version 9.2.
(StataCorp, College Station, TX, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of NF-κB inhibitors on
EBV-positive and EBV-negative GC cells
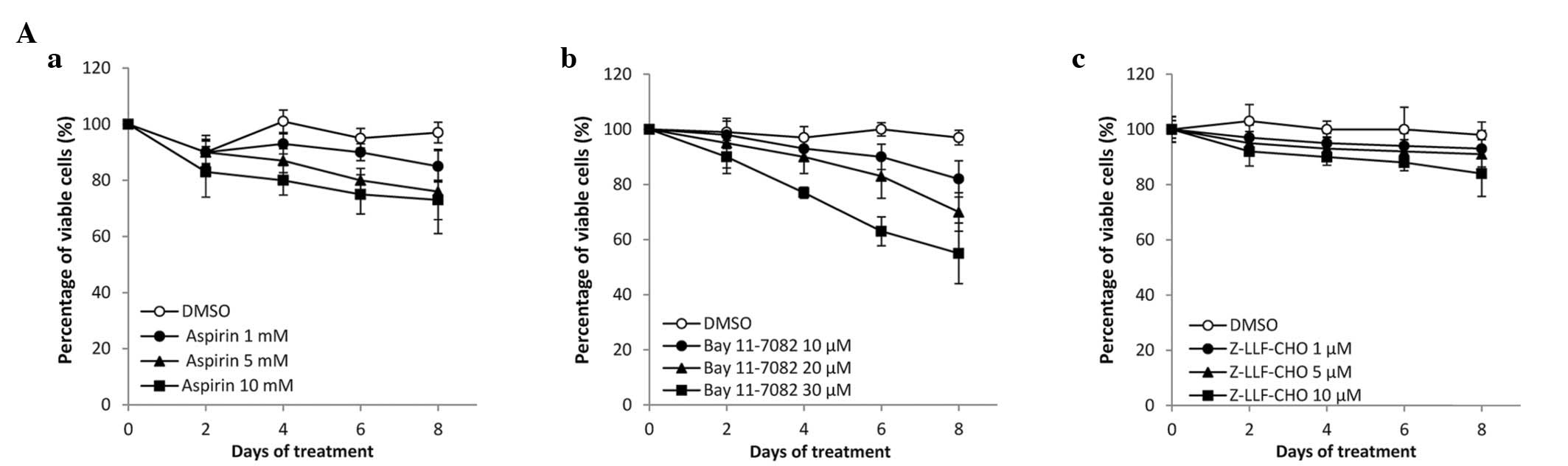
The effects of different concentrations of NF-κB
inhibitors on the cell viability of EBV-positive and EBV-negative
GC cells were examined (Fig. 1).
Aspirin, Bay11-7082 and Z-LLF-CHO reduced the cell viability of the
two GC cell lines in a dose-dependent manner. No significant
difference was observed between the effects observed in
EBV-positive and EBV-negative cells. The cytotoxic effect of
Z-LLF-CHO did not increase significantly over concentrations
ranging from 1 to 10 μM or after 8 days of treatment.
Effect of NF-κB inhibitors in combination
with anti-herpes agents
The combined effects of NF-κB inhibitors and
anti-herpes agents on the cell viability were observed. In the
baseline experiments, treatment with different doses of anti-herpes
agents alone did not influence the cytotoxicity. Thus, presented
are the optimal doses from the combination assays. The cytotoxic
effect of 5 mM of aspirin, 20 μM Bay11-7082 and 5 μM
Z-LLF-CHO on SNU-719 cells was significantly enhanced by the
addition of 100 μg/ml ganciclovir (392 μM), acyclovir
(444 μM), foscarnet (794 μM) or brivudine (300
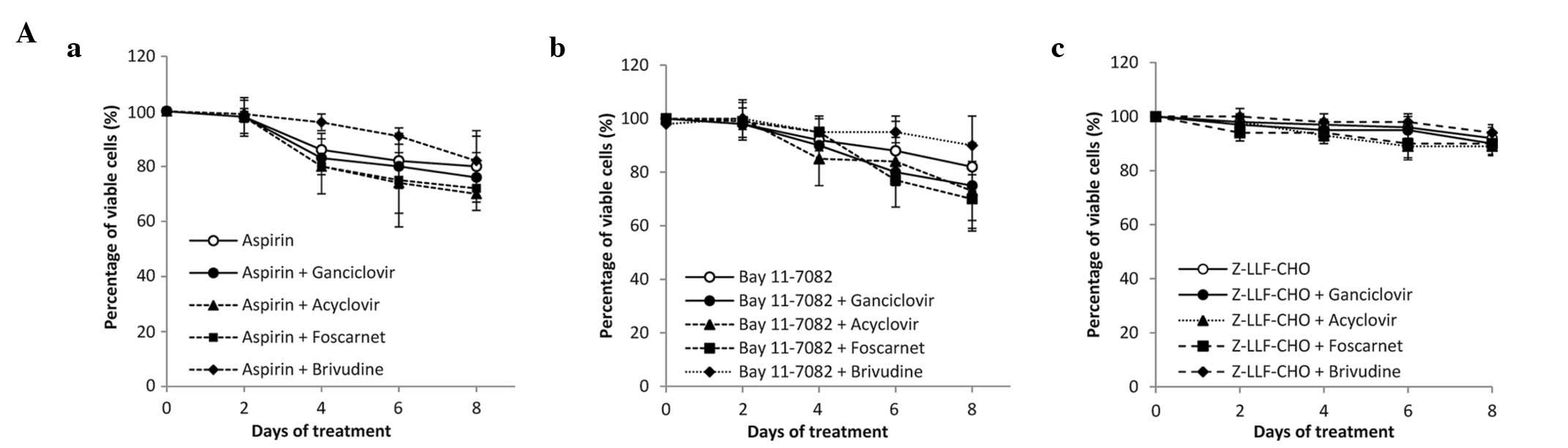
μM) (P<0.05 for all co-treatment; Fig. 2A). To give a specific example, the
combination of ganciclovir and increasing concentrations of NF-κB
inhibitors reduced the cell viability of SNU-719 by 60% in a
dose-dependent manner, while NF-κB inhibitors alone slightly
reduced cell viability (Fig. 2B).
In contrast, NF-κB inhibitors with anti-herpes agents had a
negligible effect on cell viability in SNU-216 cells (Fig. 3A). Additionally, the cytotoxic
effects of increasing concentrations of NF-κB inhibitors on SNU-216
cells was not significantly different with or without ganciclovir
(Fig. 3B). Finally, increasing
concentrations of ganciclovir enhanced the cytotoxic effects of
NF-κB inhibitors in SNU-719 cells in a dose-dependent manner
(Fig. 4A). The same effect was not
observed in SNU-216 cells (Fig.
4B). Thus the cytotoxic effect of ganciclovir was always seen
as EBV-dependent.
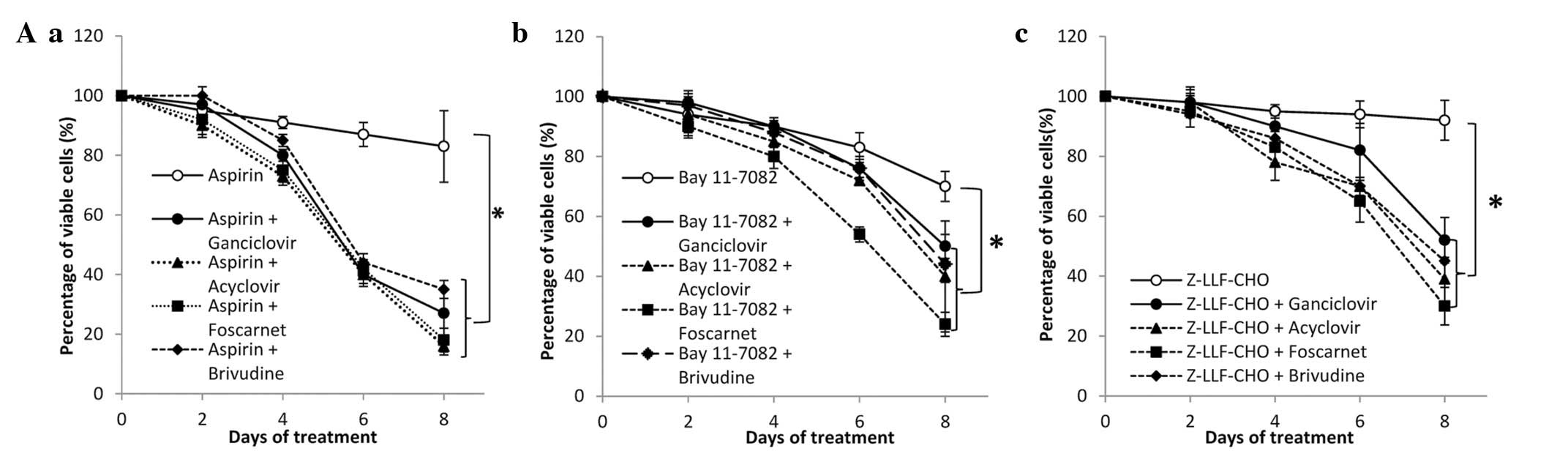 | Figure 2Effect of NF-κB inhibitors in
combination with anti-herpes agents in SNU-719 cells. (A) (a)
Aspirin (5 mM), (b) Bay11-7082 (20 μM) and (c) Z-LLF-CHO (5
μM) were used in combination with GCV, acyclovir, foscarnet
or brivudine (100 μg/ml). An MTT assay was performed
following 0, 2, 4, 6 and 8 days of treatment. (B) Cells were
treated with the indicated concentrations of (a) aspirin, (b)
Bay11-7082 and (c) Z-LLF-CHO in combination with anti-herpes agents
(100 μg/ml). The MTT assay was performed following 8 days of
treatment. The percentage of viable cells was set at 100% for
untreated controls. Values are presented as the mean ± standard
error of three independent experiments (* P<0.05).
NF-κB, nuclear factor κB; GCV, ganciclovir; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; EBV,
Epstein-Barr virus; DMSO, dimethyl sulfoxide. |
 | Figure 3Effects of NF-κB inhibitors in
combination with anti-herpes agents in SNU-216 cells. (A) (a)
Aspirin (5 mM), (b) Bay11-7082 (20 μM) and (c) Z-LLF-CHO (5
μM) were used in combination with GCV, acyclovir, foscarnet
or brivudine (100 μg/ml). The MTT assay was performed
following 0, 2, 4, 6 and 8 days of treatment. (B) Cells were
treated at the indicated concentrations of (a) aspirin, (b)
Bay11-7082 and (c) Z-LLF-CHO in combination with anti-herpes agents
(100 μg/ml). The MTT assay was performed after 8 days of
treatment. The percentage of viable cells was set at 100% for
untreated controls Values are presented as the mean ± standard
error of three independent experiments. NF-κB, nuclear factor κB;
GCV, ganciclovir; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DMSO,
dimethyl sulfoxide. |
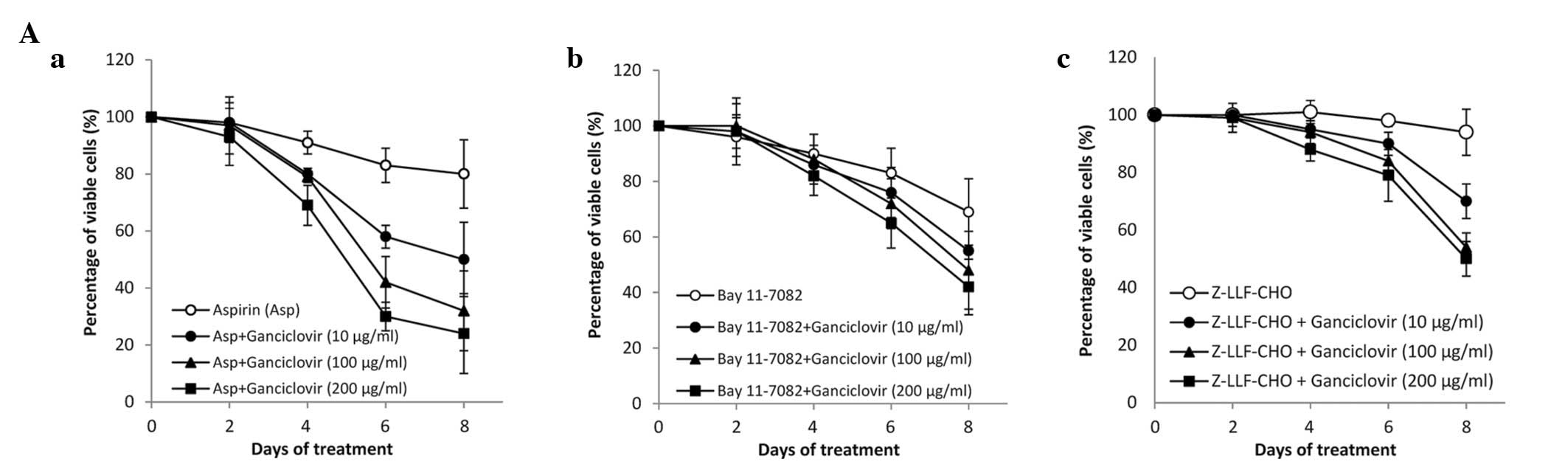 | Figure 4Effect of NF-κB inhibitors in
combination with different concentrations of ganciclovir. (A) In
SNU-719 cells and (B) SNU-216 cells, (a) aspirin (5 mM), (b)
Bay11-7082 (20 μM) and (c) Z-LLF-CHO (5 μM) were used
in combination with ganciclovir (10, 100 or 200 μg/ml
corresponding to 39.2, 392 and 784 μM, respectively). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
was performed after 0, 2, 4, 6 and 8 days of treatment. The
percentage of viable cells was set as 100% for untreated controls.
Values are presented as the mean ± standard error of three
independent experiments. NF-κB, nuclear factor κB; Asp, aspirin;
DMSO, dimethyl sulfoxide. |
Cytotoxic concentrations of NF-κB
inhibitors and anti-herpes agents
Cytotoxic concentrations required to reduce the
growth of cells by 50% (CC50) were determined for NF-κB
inhibitors and anti-herpes agents in EBV-positive and EBV-negative
GC cells. Aspirin alone exhibited a CC50>10,000
μM in EBV-positive and EBV-negative cells (Table I). However, the addition of 100
μg/ml of an anti-herpes agents increased the cytotoxic
effect, with the combination of aspirin/acyclovir resulting in the
greatest effect (CC50=235 μM). Bay11-7082 and
Z-LLF-CHO resulted in CC50>30 μM and
CC50>10 μM, respectively, and their cytotoxic
effects were enhanced by the addition of an anti-herpes agent
(Table I). The cytotoxic
concentrations of anti-herpes agents were also determined in the
two cell lines (Table II).
Ganciclovir in combination with 5 mM of aspirin resulted in the
greatest cytotoxic effect (CC50=7.2 μg/ml, 28.2
μM).
 | Table ICytotoxic effect of NF-κB inhibitors
in combination with 100 μg/ml anti-herpes agents in
EBV-positive/negative GC cells. |
Table I
Cytotoxic effect of NF-κB inhibitors
in combination with 100 μg/ml anti-herpes agents in
EBV-positive/negative GC cells.
| NF-κB inhibitor +
anti-herpes agent |
CC50a (μM) of NF-κB
inhibitorsb
| Selectivity
indexc |
|---|
| EBV-positive
GC | EBV-negative
GC |
|---|
| Aspirin | >10,000 | >10,000 | >1 |
| Aspirin +
ganciclovir (392 μM) | 675±7.2 | >10,000 | >15 |
| Aspirin + acyclovir
(444 μM) | 235±15.8 | >10,000 | >43 |
| Aspirin + foscarnet
(794 μM) | 338±20.5 | >10,000 | >30 |
| Aspirin + brivudine
(300 μM) | 1,230±13.2 | >10,000 | >8 |
| Bay11-7082 | >30 | >30 | >1 |
| Bay11-7082 +
ganciclovir (392 μM) | 16.5±3.3 | >30 | >2 |
| Bay11-7082 +
acyclovir (444 μM) | 14.5±4.1 | >30 | >2 |
| Bay11-7082 +
foscarnet (794 μM) | 10.6±2.5 | >30 | >3 |
| Bay11-7082 +
brivudin (300 μM) | 13.6±3.2 | >30 | >2 |
| Z-LLF-CHO | >10 | >10 | >1 |
| Z-LLF-CHO +
ganciclovir (392 μM) | 3.5±1.2 | >10 | >3 |
| Z-LLF-CHO +
acyclovir (444 μM) | 2.4±0.5 | >10 | >4 |
| Z-LLF-CHO +
foscarnet (794 μM) | 1.8±0.6 | >10 | >6 |
| Z-LLF-CHO +
brivudin (300 μM) | 2.7±0.6 | >10 | >4 |
 | Table IICytotoxic effect of anti-herpes
agents in combination with NF-κB inhibitors in EBV-positive and
EBV-negative GC cells. |
Table II
Cytotoxic effect of anti-herpes
agents in combination with NF-κB inhibitors in EBV-positive and
EBV-negative GC cells.
| Anti-herpes agent +
NF-κB inhibitor |
CC50a (μM) of anti-herpes
agentb
| Selectivity
indexc |
|---|
| EBV-positive
GC | EBV-negative
GC |
|---|
| Ganciclovir | >784 | >784 | >1 |
| Ganciclovir +
Aspirin (5 mM) | 28±9.0 | >784 | >28 |
| Ganciclovir +
Bay11-7082 (20 μM) | 379±9.0 | >784 | >2 |
| Ganciclovir +
Z-LLF-CHO (5 μM) | 543±18.0 | >784 | >1 |
| Acyclovir | >888 | >888 | >1 |
| Acyclovir + Aspirin
(5 mM) | 161±8.9 | >888 | >6 |
| Acyclovir +
Bay11-7082 (20 μM) | 348±32.4 | >888 | >3 |
| Acyclovir +
Z-LLF-CHO (5 μM) | 561±48.0 | >888 | >2 |
| Foscarnet | >1587 | >1587 | >1 |
| Foscarnet + Aspirin
(5 mM) | 189±32.5 | >1587 | >8 |
| Foscarnet +
Bay11-7082 (20 μM) | 519±40.5 | >1587 | >3 |
| Foscarnet +
Z-LLF-CHO (5 μM) | 1351±97.6 | >1587 | >1 |
| Brivudine | >600 | >600 | >1 |
| Brivudine + Aspirin
(5 mM) | 139±10.2 | >600 | >4 |
| Brivudine +
Bay11-7082 (20 μM) | 330±45.6 | >600 | >2 |
| Brivudine +
Z-LLF-CHO (5 μM) | 569±22.5 | >600 | >1 |
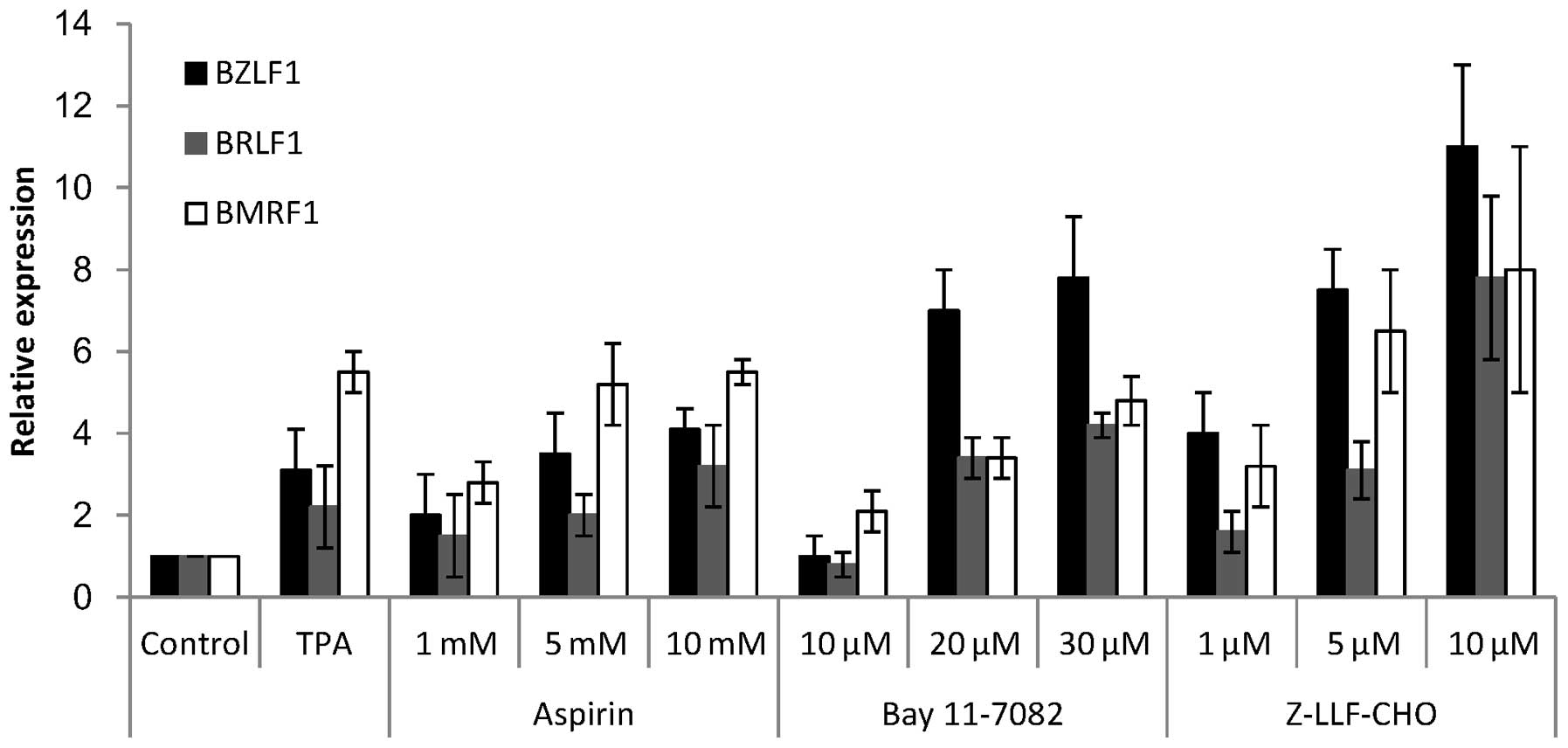
Activation of EBV lytic genes by NF-κB
inhibitors
To explain the cytotoxic effects of NF-κB inhibitors
in combination with anti-herpes agents, the induction of EBV lytic
genes by NF-κB inhibitors was investigated in EBV-positive GC
cells. SNU-719 cells were treated with different concentrations of
NF-κB inhibitors: Aspirin at 1, 5 and 10 mM; Bay11-7082 at 10, 20
and 30 μM; and Z-LLF-CHO at 1, 5 and 10 μM.
Subsequent to 24 h treatment, EBV lytic reactivation was confirmed
by measuring the expression levels of the lytic genes BZLF1,
BRLF1 and BMRF1. All three inhibitors induced the
expression of lytic genes in a dose-dependent manner (Fig. 5). BMRF1 expression levels
subsequent to induction by NF-κB inhibitors were similar to the
level induced by TPA (Fig. 5).
However, induction of the immediate early genes, BZLF1 and
BRLF1, by NF-κB inhibitors was higher than the induction by
TPA, in particular when using 10 μM Z-LLF-CHO (Fig. 5).
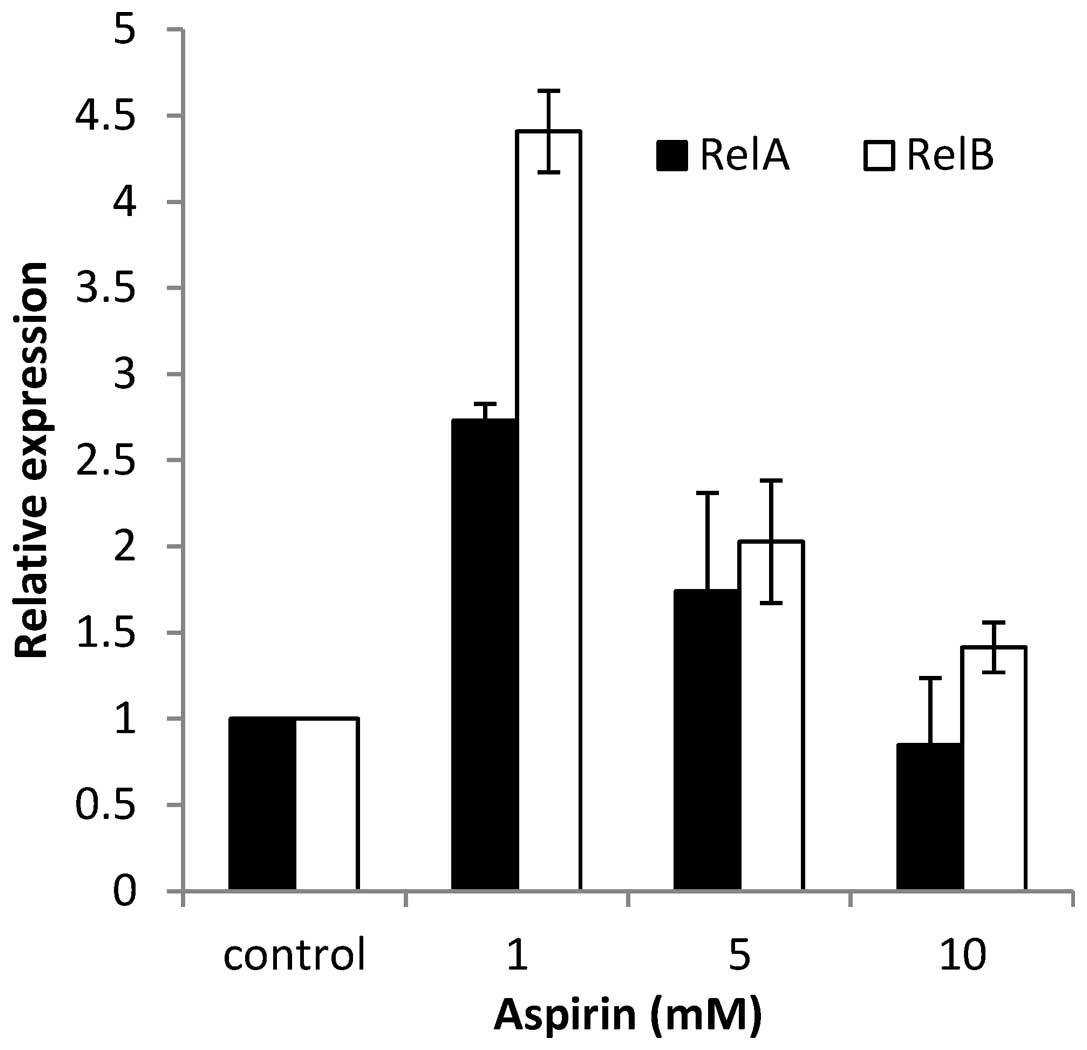
Inhibition of RelA and RelB
Based on the results of the current study, it was
suggested that aspirin was the most promising NF-κB inhibitor in
the SNU-719 cell line. To understand whether EBV reactivation
occurred though NF-κB, the mRNA expression levels of RelA
and RelB, which are the subunits of NF-κB, were examined.
SNU-719 cells were treated with different concentrations of aspirin
(0, 1, 5, 10 mM), and after 24 h the cells were harvested and
examined for the gene expression using RT-qPCR. RelA and
RelB activity was observed to be high prior to the addition
of aspirin, and a dose-dependent reduction was observed following
inhibition (Fig. 6).
Discussion
In the present study, the cytotoxic effects of the
NF-κB inhibitors (aspirin, Bay11-7082 and Z-LLF-CHO), in
combination with four anti-herpes agents; ganciclovir, acyclovir,
brivudine, and foscarnet, using EBV-positive and-negative GC cells.
The cytotoxic effects of NF-κB inhibitors on EBV-positive GC cells
were enhanced by the addition of anti-herpes agents (Fig. 2). However, no significant
alterations in cytotoxicity in EBV-negative GC cells were observed
(Fig. 3). The combination of
aspirin and ganciclovir resulted in the lowest CC50 of
any anti-herpes agent, 7.2 μg/ml (28.2 μM), in
EBV-positive SNU-719 GC cells (Table
I). In contrast, the CC50 of this combination in the
EBV-negative SNU-216 GC cells was greater than 200 μg/ml
(>783.6 μM). These observations are consistent with the
results of a previous study using EBV-positive and -negative
B-lymphocytes (6). Jeong et
al (19) reported that the
inhibition of NF-κB by NF-κB/p65-specific small interfering RNA
induced a near total cessation of cell proliferation in
EBV-positive GC cells, however variably affected cell proliferation
in EBV-negative GC cells, indicating that NF-κB inhibition may be
beneficial in the treatment of EBV-positive GC.
NF-κB inhibitors reduced cell viability of
EBV-positive and EBV-negative GC cells in a dose- and
time-dependent manner (Fig. 1).
However, the cytotoxic effects of Z-LLF-CHO in the two cell lines
were minor compared with the two other NF-κB inhibitors. Z-LLF-CHO,
a reversible proteasome inhibitor, inhibits nuclear translocation
of NF-κB (20). By contrast,
aspirin and Bay11-7082 act on the stabilization of IκBα, resulting
in reduced NF-κB expression. Bay11-7-82 is an irreversible
inhibitor of IκBα phosphorylation (21), and aspirin can inhibit IκB kinase
activity thereby blocking IκBα degradation (5). The weak cytotoxic effects of
Z-LLF-CHO may be partially explained by the difference in the site
of inhibitory function among these NF-κB inhibitors.
The cytotoxic effects of NF-κB inhibitors varied
among the combinations of anti-herpes agents. Due to the fact that
foscarnet, an inhibitor of viral DNA polymerase does not require
activation by viral TK/PK, no increase of the cytotoxicity of the
NF-κB inhibitors was expected. However, marginally increased
enhancement of the cytotoxicity of NF-κB inhibitors was observed in
EBV-positive GC cells with combination treatment with foscarnet
(Fig. 2). Notably, similar
observations have been reported in the study using BCBL-1 cells
(20). The number of viable
HHV-8-positive cells was further reduced by co-treatment of
foscarnet and valoriate, which was more capable of inducing lytic
replication of HHV-8-positive cells than that of treatment with
foscarnet alone (22). Although
the exact mechanism of this phenomenon remains unclear, foscarnet
is suggested to act on virus-infected cells effectively in the
lytic replication stage.
Regarding the cytotoxicity of aspirin, the most
efficient combination with a fixed concentration of anti-herpes
agent was acyclovir/aspirin (235 μM) followed by
foscarnet/aspirin (338 μM), ganciclovir/aspirin (675
μM) and brivudine (1230 μM) (Table I). However, these orders were not
consistent with those when anti-herpes agents were combined with
higher concentrations of aspirin (5 mM) (Table II). Ganciclovir and foscarnet
exhibited the greatest and the least efficient cytotoxity,
respectively. Further investigation into the synergistic effects
between NF-κB inhibitors and anti-herpes agents is required to
explain this discrepancy.
In the present study, the expression levels of the
immediate-early genes BZLF1 and BRLF1, which have
been reported to induce the entire program of lytic EBV gene
expression, were determined (23).
The expression of the early gene BMRF1, which has been
reported to be essential for lytic virus replication, was
additionally confirmed. All NF-κB inhibitors tested, including
aspirin, induced the expression of BZLF1, BRLF1 and
BMRF1 in the EBV-positive GC cell line SNU-719 (Fig. 5). The results of the current study
on EBV-positive gastric cancer cells are in agreement with previous
studies on lytic induction by NF-κB inhibitors in the EBV-positive
B-lymphocyte cell lines B95-8 and Raji (6), indicating that induction of EBV lytic
replication is achievable regardless of cell type.
TPA was used as a positive control of the induction
of lytic replication because of its efficacious induction of EBV
lytic replication in persistently infected lymphoblastoid and
epithelial cells (24). In the
present study, BMRF1 expression levels susbequent to
induction by NF-κB inhibitors were similar to those induced by TPA,
while at the maximum dose (<CC50), the expression
levels of BZLF1 and BRLF1 induced by NF-κB
inhibitors, particularly Z-LLF-CHO, were considerably higher than
those induced by TPA (Fig. 5).
Although the expression levels of these immediate-early/early genes
by aspirin were lower than those of Z-LLF-CHO, the efficiency of
cytotoxicity enhancement induced by anti-herpes agents between
aspirin and Z-LLF-CHO was equivalent or even higher in aspirin
(Fig. 2). Previous reports
demonstrated that RelA expression is involved in the
aspirin-induced EBV lytic replication (6). In addition to RelA, a
dose-dependant reduction in the mRNA levels of RelB was
observed seen in the current study. Thus, it is suggested that EBV
reactivation can be achieved not only through p65 however
additionally through RelB. Bren et al (25) demonstrated that RelB
transcription can be induced by RelA activation, which
indicates that aspirin-induced activation of RelA may have
enhanced the activation of RelB. Further experiments are
required to fully elucidate this effect.
The following limitations were identified in the
current study: i) Only one EBV-positive and one EBV-negative GC
cell line were analyzed, therefore the results of the NF-κB
inhibitor and anti-herpes agent cytotoxicity assays are too limited
to extrapolate and generalize for other epithelial cell lines; ii)
the effects of NF-κB inhibitors on the expression levels of
EBV-TK/-PK and NF-κB activity were not examined. Further studies
are therefore necessary in order to address these issues.
In conclusion, the present study indicated that
NF-κB inhibitors reactivated the EBV lytic genes BZLF1,
BRLF1 and BMRF, in the SNU-719 EBV-positive GC cell
line in a time- and dose-dependent manner. Significant cytotoxicity
of NF-κB inhibitors was enhanced by anti-herpes agents suggesting
that induction of lytic viral transcription using NF-κB inhibitors
in combination with anti-herpes agents may be an effective
therapeutic strategy for treating EBV-associated GC. Further ex
vivo and in vivo studies are warranted to confirm these
results and to evaluate the clinical relevance of the use of NF-κB
inhibitors in combination with anti-herpes agents as a therapeutic
strategy for EBV-positive GC.
Acknowledgments
The current study was financed by the Grants-in-Aid
for Scientific Research on Priority areas (grant no. 17015037) from
the Ministry of Education, Culture, Sports, Science and Technology,
Japan. The authors would like to thank Professor Kim Woo Ho for his
scientific advice.
References
|
1
|
Adler B, Schaadt E, Kempkes B,
Zimber-Strobl U, Baier B and Bornkamm GW: Control of Epstein-Barr
virus reactivation by activated CD40 and viral latent membrane
protein 1. Proc Natl Acad Sci USA. 99:437–442. 2002. View Article : Google Scholar
|
|
2
|
Kawai T, Takahashi K, Sato S, Coban C,
Kumar H, Kato H, Ishii KJ, Takeuchi O and Akira S: IPS-1, an
adaptor triggering RIG-I- and Mda5-mediated type I interferon
induction. Nat Immunol. 6:981–988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown HJ, Song MJ, Deng H, Wu TT, Cheng G
and Sun R: NF-κB inhibits gammaherpesvirus lytic replication. J
Virol. 77:8532–8540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kopp E and Ghosh S: Inhibition of NF-kappa
B by sodium salicylate and aspirin. Science. 265:956–959. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin MJ, Yamamoto Y and Gaynor RB: The
anti-inflammatory agents aspirin and salicylate inhibit the
activity of I (kappa)B kinase-beta. Nature. 396:77–80. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu SF, Wang H, Li ZJ, Deng XY, Xiang H,
Tao YG, Li W, Tang M and Cao Y: Aspirin induces lytic cytotoxicity
in Epstein-Barr virus-positive cells. Eur J Pharmacol. 589:8–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu SF, Wang H, Lin XC, Xiang H, Deng XY,
Li W, Tang M and Cao Y: NF-κB inhibitors induce lytic cytotoxicity
in Epstein-Barr virus-positive nasopharyngeal carcinoma. Cell Biol
Int. 32:1006–1013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visalli P and van Zeijl M: DNA
encapsidation as a target for anti-herpesvirus drug therapy.
Antiviral Res. 59:73–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng Q, Hagemeier SR, Fingeroth JD,
Gershburg E, Pagano JS and Kenney SC: The Epstein-Barr virus
(EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase
(EBV-TK), is required for ganciclovir and acyclovir inhibition of
lytic viral production. J Virol. 84:4534–4542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris SA, McGuigan C, Andrei G, Snoeck R,
De Clercq E and Balzarini J: Synthesis and antiviral evaluation of
phosphoramidate derivatives of
(E)-5-(2-bromovinyl)-2′-deoxyuridine. Antivir Chem Chemother.
12:293–300. 2001. View Article : Google Scholar
|
|
11
|
Meyer PR, Rutvisuttinunt W, Matsuura SE,
So AG and Scott WA: Stable complexes formed by HIV-1 reverse
transcriptase at distinct positions on the primer-template
controlled by binding deoxynucleoside triphosphates or foscarnet. J
Mol Biol. 369:41–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burke AP, Yen TS, Shekitka KM and Sobin
LH: Lymphoepithelial carcinoma of the stomach with Epstein-Barr
virus demonstrated by polymerase chain reaction. Mod Pathol.
3:377–380. 1990.PubMed/NCBI
|
|
13
|
Shibata D, Tokunaga M, Uemura Y, Sato E,
Tanaka S and Weiss LM: Association of Epstein-Barr virus with
undifferentiated gastric carcinomas with intense lymphoid
infiltration lymphoepithelioma-like carcinoma. Am J Pathol.
139:469–447. 1991.PubMed/NCBI
|
|
14
|
Takada K: Epstein-Barr virus and gastric
carcinoma. Mol Pathol. 53:255–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Israel BF and Kenney SC: Virally targeted
therapies for EBV-associated malignancies. Oncogene. 22:5122–5130.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji Jung E, Mie Lee Y, Lan Lee B, Soo Chang
M and Ho Kim W: Ganciclovir augments the lytic induction and
apoptosis induced by chemotherapeutic agents in an Epstein-Barr
virus-infected gastric carcinoma cell line. Anticancer Drugs.
18:79–85. 2007.
|
|
17
|
Ryan JL, Fan H, Glaser SL, Schichman SA,
Raab-Traub N and Gulley ML: Epstein-Barr virus quantitation by
real-time PCR and targeting multiple gene segments: A novel
approach to screen for the virus in paraffin-embedded tissue and
plasma. J Mol Diagn. 6:378–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hilscher C, Vahrson W and Dittmer DP:
Faster quantitative real-time PCR protocols may lose sensitivity
and show increased variability. Nucleic Acids Res. 33:e1822005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong JY, Woo JH, Kim YS, Choi S, Lee SO,
Kil SR, Kim CW, Lee BL, Kim WH, Nam BH and Chang MS: Nuclear
factor-kappa B inhibition reduces markedly cell proliferation in
Epstein-Barr virus-infected stomach cancer, but affects variably in
Epstein-Barr virus-negative stomach cancer. Cancer Invest.
28:113–119. 2010. View Article : Google Scholar
|
|
20
|
Ding L, Li LL, Yang J, Tao YG, Ye M, Shi
Y, Tang M, Yi W, Li XL, Gong JP and Cao Y: Epstein-Barr virus
encoded latent membrane protein 1 modulates nuclear translocation
of telomerase reverse transcriptase protein by activating nuclear
factor-kappaB p65 in human nasopharyngeal carcinoma cells. Int J
Biochem Cell Biol. 37:1881–1889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pierce JW, Schoenleber R, Jesmok G, Best
J, Moore SA, Collins T and Gerritsen ME: Novel inhibitors of
cytokine-induced IkappaBalpha phosphorylation and endothelial cell
adhesion molecule expression show anti-inflammatory effects in
vivo. J Biol Chem. 272:21096–21103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sergerie Y and Boivin G: Evaluation of
susceptibility of human herpesvirus 8 to antiviral drugs by
quantitative real-time PCR. J Clin Microbiol. 41:3897–3900. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Westphal EM, Mauser A, Swenson J, Davis
MG, Talarico CL and Kenney SC: Induction of lytic Epstein-Barr
virus (EBV) infection in EBV-associated malignancies using
adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485–1491.
1999.PubMed/NCBI
|
|
24
|
Takimoto T, Iwawaki J, Tanaka S and Umeda
R: Interactions between Epstein-Barr virus (EBV) and human cell
lines: Growth and EBV induction. ORL J Otorhinolaryngol Relat Spec.
52:40–46. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bren GD, Solan NJ, Miyoshi H, Pennington
KN, Pobst LJ and Paya CV: Transcription of the RelB gene is
regulated by NF-kappaB. Oncogene. 20:7722–7733. 2001. View Article : Google Scholar : PubMed/NCBI
|