Introduction
Red blood cell (RBC) excessive hyperplasia and
significant pulmonary hypertension are the main pathophysiological
manifestations of chronic mountain sickness (CMS) in plateau areas
(1). Hypoxemia is a significant
clinical characteristic thereof (2). The neuron is the most oxygen
reaction-sensitive cell. Long-term chronic hypoxemia seriously
affects normal physiological functions, thus cerebrovascular
produces reflective factors to improve the blood supply of neurons
under chronic hypoxia (3).
Cerebrovascular reactivity (CVR) refers to cerebrovascular
pathological factor stimulation by contracting or diastolic change
to achieve stable regional cerebral blood flow (CBF) and cerebral
perfusion (4), and the ability of
reaction is an important index of cerebral reserve capacity. Thus,
CVR is used as an evaluation standard of testing real-time brain
blood supply.
The Transcranial Doppler (TCD) test is becoming a
CVR method and has been extensively applied in clinical research
during recent years (5). In
previous studies, alteration of arterial blood pressure of carbon
dioxide (CO2) from subjects having to hold their breath
were used to detect cerebral arterioles vasomotor responses
(6). As this method is unable to
accurately record the change of the duration of holding breath and
pCO2, the use has been gradually reduced in the clinic.
At present, most scientists use synchronous methods to detect the
CBF velocity and pCO2, including quiet breathing, low
carbonate platform stage and/or hypercapnia platform stage data,
respectively (7). The collected
data are more accurate and straightforward with fewer interference
factors. In the present study, a DWL company Multi-Dop X TCD
ultrasound monitor together with a CO2 monitoring module
were used to accurately determine long-range synchronization
acquisition CO2 partial pressure, breathing graph and
detect changes in middle cerebral artery blood flow and
spectrum.
Endothelin (ET) is a 21 amino acid bioactive peptide
that was separated and purified from pig aortic endothelial cells
by Yanagisawa et al (8).
The peptide is divided subtypes according to the molecular
structure: ET-1, ET-2 and ET-3, respectively. ET-1 is the only one
existing vascular endothelial cell with strong biological effects,
and the most production, while ET-2 and ET-3 are mainly expressed
in the brain, kidney, adrenal gland, and small intestine (9). ET and its receptor have a strong and
lasting vascular contraction effect.
ET receptor (ETR) can be divided into ET B receptor
(ETBR) and ETAR, which are mainly distributed in vascular smooth
muscle, cardiac muscle cells and fibroblasts (9). Strong vasoconstrictor and cell
proliferation occur when it binds to
ET. ETBR is subdivided into ETBRl and ETBR2
subtypes, which are mainly distributed in vascular endothelium,
with a small amount of distribution in vascular smooth muscle. ET
binds ETBR and increases nitric oxide (NO) secretion by endothelial
cells, while prostaglandins play a role in diastolic blood vessels
(10).
NO synthase (NOS) is divided into neuronal NOS
(nNOS), inducable NOS and endothelial NOS (eNOS). The nNOS and eNOS
are mainly expressed in cerebrovascular endothelial cells (11). eNOS can produce NO gas in the
angiogenesis effects of diastolic heart failure (12). eNOS activates hypoxia-induced
factor-1 (HIF-1) and HIF-2, and promotes endothelial cells to
produce more NO gas during hypoxia, which can stimulate the
synthesis of soluble guanylate cyclase acid [cyclic guanosine
monophosphate (cGMP)] in cytoplasm, leading to vasodilation by way
of free diffusion into vascular smooth muscle cells (VSMCs)
(13).
Chronic cerebrovascular diastolic and condensation
reaction under hypoxia are considered controversial issues.
Patients with chronic plateau CVR studies have yet to be reported.
The aim of the present study was to use TCD accompanied with
CO2 (14) to evaluate
CVR and to determine the change in plasma content of ET, ETBR and
eNOS in order to examine the differences in cerebral circulation
reserve capacity of individuals residing at an altitude of 3,600 m
and the healthy controls residing at low attitude. In addition,
scientific data for the prevention and treatment of cerebral
infarction in patients with chronic plateau condition with high
blood clot were provided.
Materials and methods
Healthy controls
A total of 23 male healthy volunteers, with an
average age of 34.4±15.6 years, residing at an altitude of 2,200 m,
were selected from the Qinghai Xining region between September and
November, 2014, at the Qinghai Provincial People's Hospital Medical
Center. Inclusion criteria for the study were: i) Patients were
18–60 years of age; ii) volunteers did not have any chronic medical
history including hypertension, diabetes, hyperlipidemia and heart,
or cerebrovascular disease; iii) lack of lifestyle habits such as
drinking or smoking; iv) the blood flow spectrum was clearly
evident in the temporal area during the process of TCD examination;
and v) evidence of TCD blood flow turbulence, eddy current signal
and rough vascular murmur.
The study was approved by the Qinghai Provincial
People's Hospital Ethics Committee. Investigators were informed of
the related purpose and method of tests. Written informed consent
was provided by the participants.
CMS patients
A total of 26 male patients with chronic altitude
disease, with an average age of 32.8±16.2 years, were selected at
the Physical Examination Center of Yushu People's Hospital
(Qinghai, China). CMS diagnosis was made according to the 6th
International Plateau Hypoxia Physiological Academic conference
using the chronic plateau disease international diagnostic
criteria, i.e., 'Qinghai Standard' (2004). The main criterion
included erythrocytosis (female hemoglobin 190 g/l, or male
hemoglobin or 210 g/l) (1). The
chronic plateau disease clinical symptoms were alleviated
gradually, when patients descended from a high altitude hypoxic
environment, and while the clinical symptoms appeared again when
the patients became exposed to the plateau environment anew. The
inclusion criteria were: i) The 'standard' in Qinghai score >6;
ii) 18–60 years of age; iii) exclusion of patients with
hypertension, diabetes, hyperlipidemia, cerebrovascular disease,
acute and chronic disease and heart disease; iv) no long-term
drinking, smoking or other unhealthy lifestyle habit; and v)
presenting with TCD blood flow turbulence, vortex signal and rough
vascular murmur. Exclusion criteria were: i) TCD examination did
not clearly show the blood flow spectrum; and ii) the patients were
reluctant to cooperate.
The scoring method of the chronic altitude sickness
'Qinghai Standard' was used to score symptoms as follows: Asthma
and palpitations (0, without asthma/palpitations; 1, mild
asthma/palpitations; 2, moderate asthma/palpitations; and 3, severe
asthma/palpitations); insomnia (0, normal; 1, cannot normally
sleep; 2, lack of sleep; and 3, could not follow sleep); cyanosis
(0, normal; 1, light; 2, moderate; and 3, severe); vasodilation (0,
normal; 1, mild; 2, moderate; and 3, severe); paraesthesia (0,
normal; 1, mild; 2, moderate; and 3, severe) headache (0, no; 1,
mild; 2, mild; and 3, severe) tinnitus (0, without; 1, mild; 2,
mild; and 3, severe) hemoglobin concentration (men: 180
g/l<Hb<210 g/l 0, Hb 210 g/l or >3; women: 160
g/l<Hb<190 g/l 0, Hb 210 g/l or >3). According to the
above symptoms and the level of hemoglobin concentration, chronic
disease of plateau was divided into no chronic plateau sickness,
0–5; mild chronic plateau sickness, 6–10; moderate chronic disease
of plateau, 11–14; and severe chronic plateau sickness, >15.
Blood serum analysis and enzymelinked
immunosorbent assay (ELISA)
Peripheral blood (4 ml) was obtained for detection
(HF-3800 automatic blood cell analyzer) of the RBC count,
hemoglobin concentration, RBC deposit, and to determine blood
oxygen saturation using a portable oxygen saturation device. The
remaining supernatant was collected after centrifugation at 2,800 ×
g for 10 min, and was preserved at −80°C freezer prior to
subsequent analysis. ELISA was performed according to kit
instructions (ETBR: Anti-EDNRB ELISA kit; OriGene Technologies,
Inc., Rockville, MD, USA. eNOS: eNOS ELISA kit; Cell Signaling
Technology, Boston, MA, USA. ET: ET-1 ELISA kit; R&D Systems,
Inc., Minneapolis, MN, USA).
TCD detection
TCD monitor (Multi-DopX DWL Co.) was used to study
CVR (15). The patients were asked
to sit in calm state (breathing rate of 16–20 times/min) in supine
position. Two ultrasonic probes with a head frame (frequency 2 MHz)
were used to make contact with the bilateral temporal of subjects,
with 55–65 mm probing depth, and the speed and direction of
bilateral middle cerebral artery and state of spectrum were
detected. The patients were asked to wear a mask, and were recorded
while breathing quietly for 2 min, followed by hyperventilation for
2 min, calm state breathing for 2 min, autologous CO2
inhalation for 2 min (after 2 m long extension tube) followed by
calm state breathing for 2 min, and holding breath (patients
instructed to inhale and hold breath for as long as possible after
a deep breath) for 2 min to quiet breathing parameters. Each
baseline was selected and its largest middle cerebral artery (MCA),
mean CBF velocity and average ETCO2 were recorded. The
formulae used for the various parameters were: CVR = (V changed−V
baseline)/(ETCO2 change−ETCO2 baseline), and
CVRI = (V change−V baseline)/V baseline × 100%/ETCO2
change-ETCO2 baseline), where V was the baseline for
breathing in a calm state, MCA average, CBF velocity and the
average of ETCO2. V changed after 2 min for autologous
CO2 inhalation, MCA mean, CBF velocity and the average
ETCO2. According to the exhaled gas CO2
partial pressure and blood flow parameters of MCA, CVR and CVRI
were calculated. In the abovementioned formulae, the CVR
calculation constituted the CVR absolute slope (absolute slope),
indicating that alterations in the mm hg CO2 partial
pressure corresponded to the absolute value of blood flow velocity,
whereas under the influence of the baseline flow velocity, the
difference between the data were more obvious. CVRI referred to the
result of the relative slope (relative slope), indicating changes
in mm hg CO2 partial pressure corresponded to changes in
the percentage of blood flow velocity, providing an objective
description of the degree of change in CBF velocity with
CO2 partial pressure.
Statistical analysis
Data were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Measurement data were
presented as mean ± standard deviation (using two independent
samples t-test, inspection level α= 0.05. P<0.05 was considered
to indicate a statistically significant difference.
Results
Blood and SO2
The CMS group comprised 26 males, with an average
age of 34.4±15.6 years while the healthy controls comprised 23
males, with an average age of 32.8±16.2 years. There was no
statistically difference in average age composition and comparable
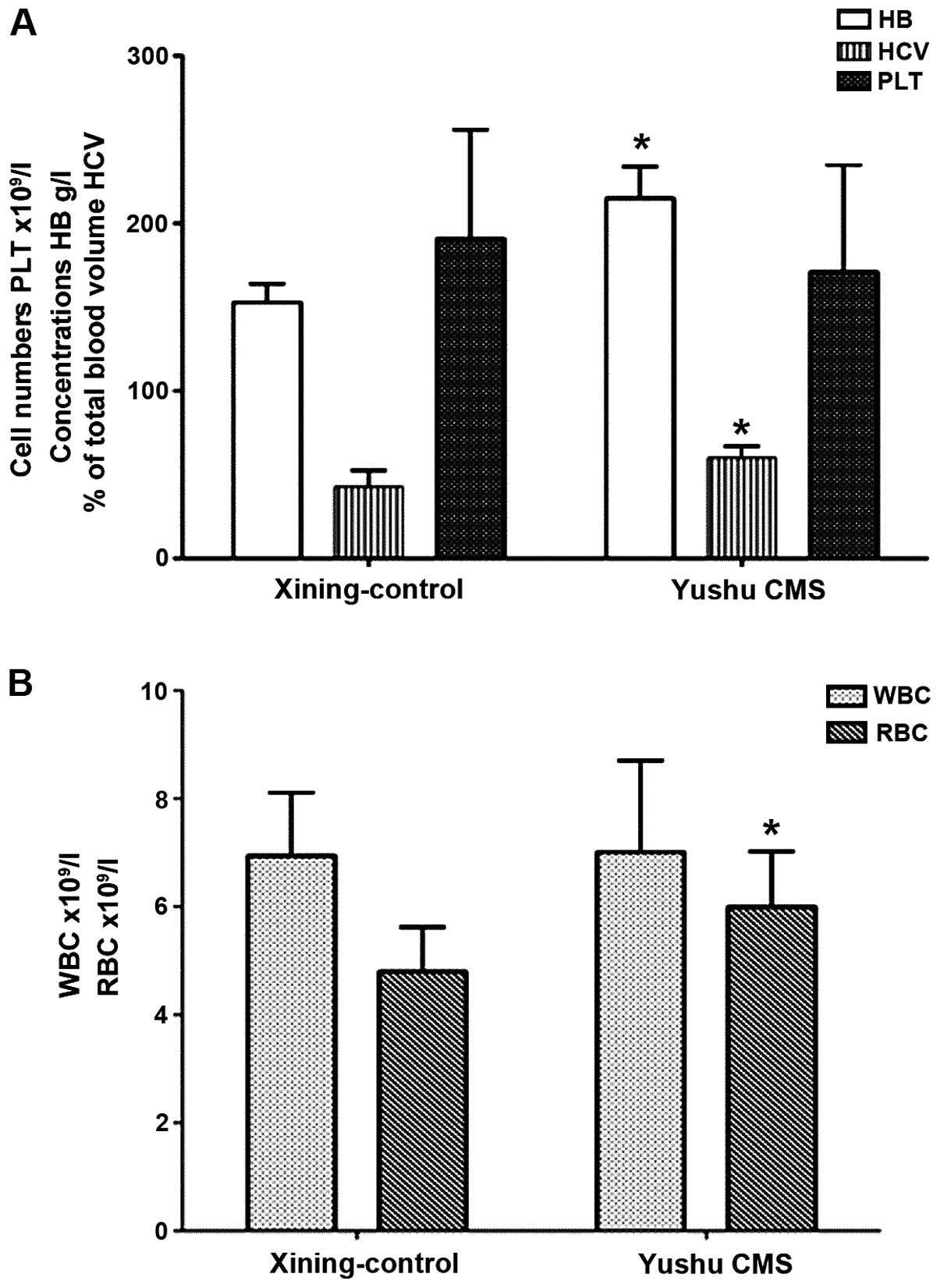
(P>0.05). Patient characteristics are shown in Table I and various cell counts are shown
in Fig. 1A and B.
 | Table IGeneral characteristics of the
research subjects (mean ± standard deviation). |
Table I
General characteristics of the
research subjects (mean ± standard deviation).
| Groups | No. of cases | Hba (g/l) | WBC
(×109/l) | PLT
(×109/l) | HCVb (%) |
SaO2c (%) |
|---|
| Control | 23 | 165.7±8.9 | 5.9±1.6 | 189.6±35.0 | 55.7±6.9 | 87.4±3.5 |
| CMS | 26 | 220.6±15.3 | 6.8±1.5 | 179.1±46.0 | 71.9±5.7 | 77.9±6.9 |
| t-value | | 26.464 | 2.896 | 1.302 | 12.703 | 8.934 |
| P-value | | 0.000 | 0.002 | 0.098 | 0.000 | 0.000 |
Comparison of CVR, CVRI and eNOS, ET-1,
and ETBR
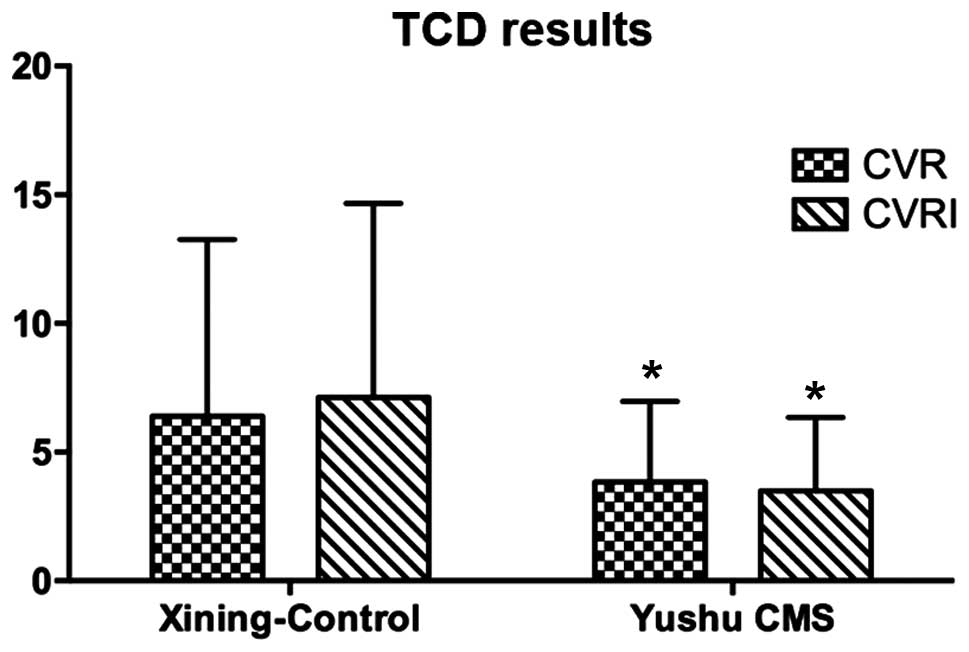
The cerebrovascular response values of CMS patients
were examined, and it was found that CVR and CVRI were
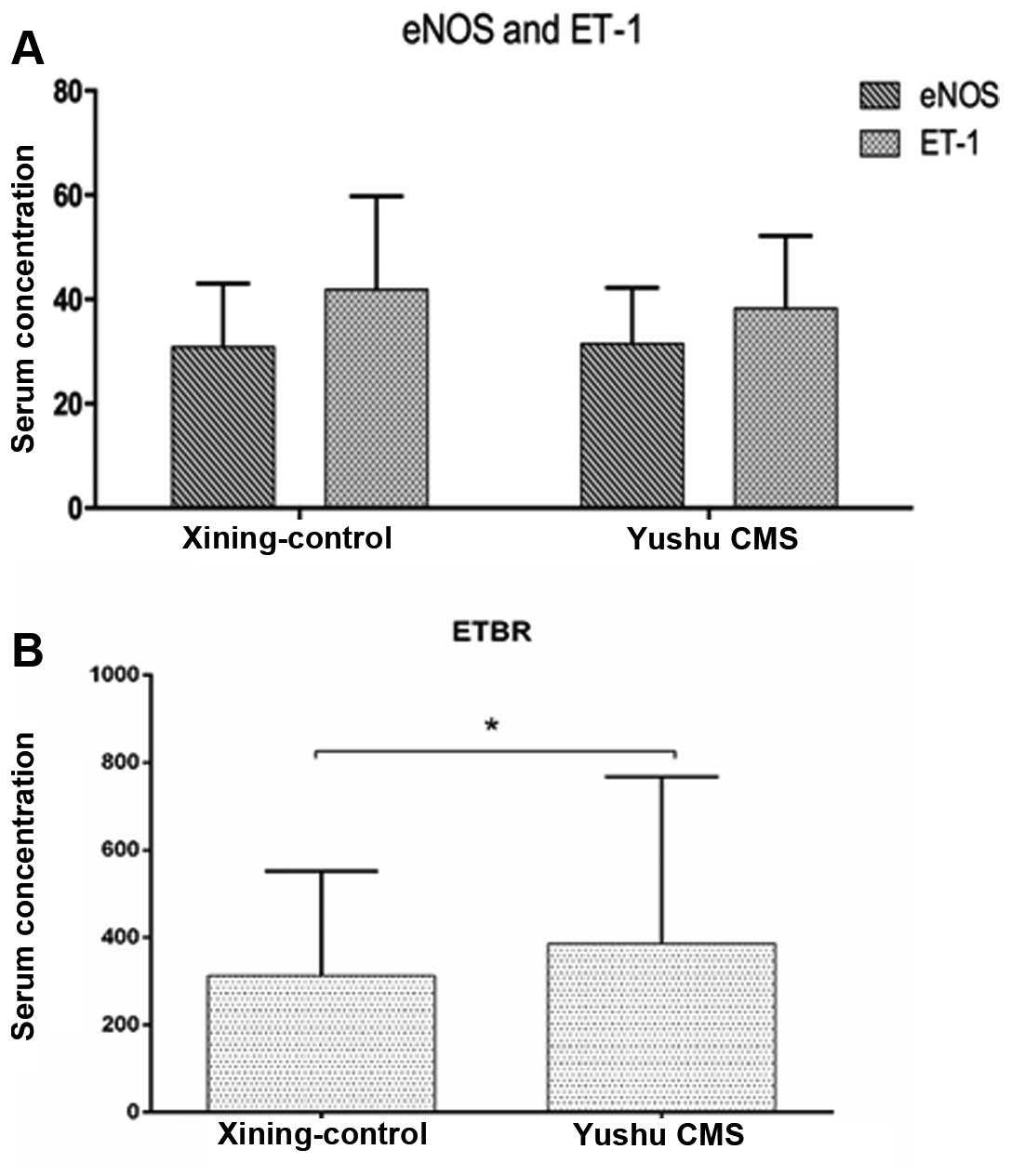
significantly lower compared to the healthy controls (Fig. 2). The serum levels of eNOS and ET-1
in the CMS group increased slightly, albeit there was no
statistical difference compared to the healthy control group
(P>0.05) (Fig. 3A). The
quantity of ETBR expression in the CMS group was significantly
higher than the control group. The difference was statistically
significant (P<0.05) (Fig. 3B
and Table II).
 | Table IIComparison of levels of CVR, CVRI,
eNOS, ET-1 and ETBR values in CMS and healthy controls. |
Table II
Comparison of levels of CVR, CVRI,
eNOS, ET-1 and ETBR values in CMS and healthy controls.
| Group | No. of cases | CVR | CVRI | eNOS
(μg/μl) | ET
(μmol/l) | ET-RA/R (pg/ml) |
|---|
| CMS | 26 | 3.8±3.0 | 3.5±2.9 | 39.5±10.8 | 42.0±20.3 | 386.1±181.6 |
| Control | 23 | 6.4±6.9 | 7.1±7.5 | 38.3±13.9 | 41.9±17.9 | 312.3±138.1 |
| t-value | | 2.336a | 3.084a | 0.474 | 0.039 | 2.317a |
| P-value | | 0.011 | 0.001 | 0.325 | 0.484 | 0.011 |
Correlation analysis results
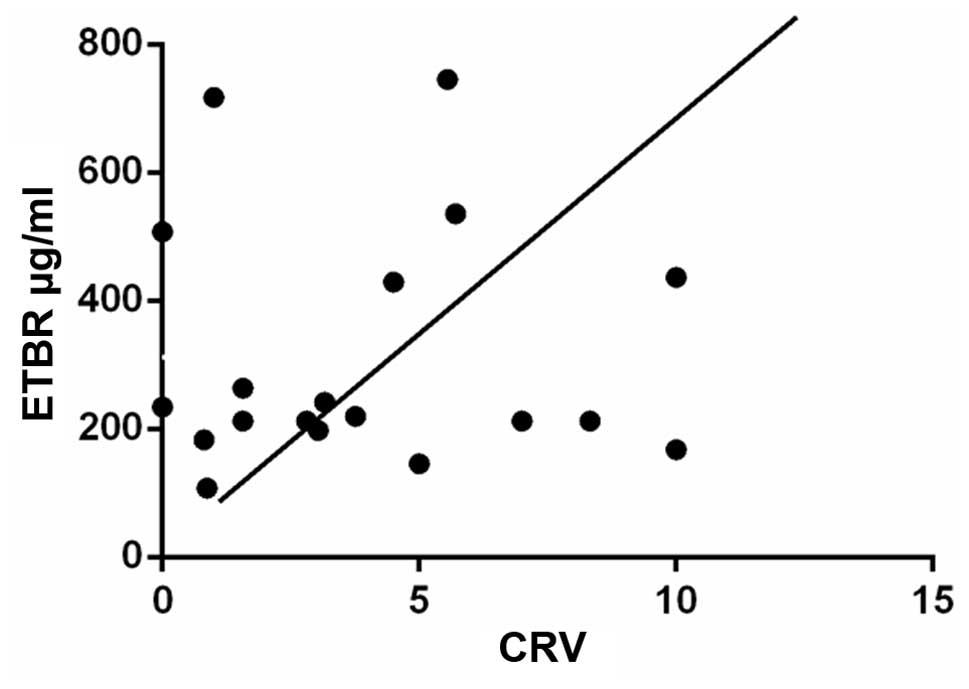
No correlation of the content of serum levels of
ET-1 and ETBR and CVR (P>0.05) were observed in the healthy
control group. No correlation was observed between the content of
ET-1 and CVR (P>0.05), while an obvious correlation was evident
between the ETBR and CVR content. The r-value was 0.378 for the CMS
group (Fig. 4).
Discussion
The molecular mechanisms of chronic plateau sickness
are not clear at present. Previous findings have shown that in a
high-altitude environment, the cold, low pressure, and low oxygen
under intense stimulation experienced can cause pituitary-adrenal
medulla hyperfunction and increases of aldosterone and ADH, which
can cause increasing peripheral resistance, sodium and water
retention, and at the same time cause polycythemia (16). Excessive RBC can cause
significantly increased hematocrit, blood viscosity and blood flow
resistance, slowing the blood flow and sedimentation, and tissue
and blood are discharged (17).
Thus, the patients with chronic plateau intracranial ischemia and
infarction identified were more than the healthy controls. Studies
on cerebrovascular hemodynamic for CMS patients are rare at present
(18). The aim of the present
study was to obtain cerebrovascular response data of CMS patients
to examine the effect of the plateau region on these patients
including stroke, and determine cardiovascular events that may be
useful in investigating this issue (19). In the present study, we found that
the number of RBCs, RBC deposits and hemoglobin levels were
significantly higher in CMS patients than the healthy controls. At
the same time, SaO2 was lower in CMS compared to the
healthy controls. There was obvious hypoxemia in CMS patients,
indicating excessive proliferation of RBCs and obvious hypoxemic
clinical manifestations in CMS patients (20). However, the mechanism of mutual
influence and RBCs of the CBF remains to be clarified, although the
existing literature offers the following as possible reasons: i)
The blood plasma viscosity altered the viscous friction force
causing an increase in cerebral vascular resistance; and ii)
arterial blood oxygen content was reduced in CMS patients, leading
to vascular relaxation response in order to maintain adequate
oxygen in the brain tissue (21).
Brain tissue is an important component of the
central nervous system and an organ relying primarily on oxygen to
function. (4) Additionally, it is
extremely sensitive to the change of blood oxygen partial pressure
(pO2). When the pO2 is decreased to 40–45
mmHg, it can cause cerebrovascular diastolic dysfunction (22). When the brain lacks oxygen, the
feedback brain artery expands to increase the brain blood supply,
but when cerebrovascular occurs at maximum expansion, its response
to CO2 stimulation is obviously decreased, CBF velocity
continues to increase and pO2 is reduced (22). Findings of previous studies have
confirmed that CBF under the condition of response to low oxygen is
a complicated pathophysiologic process that includes physical
mechanisms, involved in the regulation of metabolism at biochemical
and molecular levels (23). Thus,
adjustment of the CBF in the plateau low-oxygen environment is
complex, and cerebrovascular after a long period of low oxygen by
hypoxia under chronic injury condition can lead to cerebral
hemodynamic changes, thereby increasing the plateau cerebrovascular
disease (13). CVR was an
important indicator in the regulation of cerebrovascular diastole
and contraction, which can effectively response cerebrovascular
reserve force (4). The present
study employed TCD, and tested the reaction of CBF in CMS patients
and healthy controls. TCD is a non-invasive method that can
effectively detect intracranial arterial blood flow dynamic change
by detecting the arterial blood flow direction, blood flow
velocity, frequency spectrum form, while accurately reflecting
cerebral arterial stenosis, and cramps, such as ischemic
pathological state (24). The
present study found that CVR and CVRI were significantly reduced in
CMS patients compared to the healthy controls using TCD. The
results confirmed that the CBF reserve force was significantly
decreased in CMS patients compared to the healthy controls.
Cerebral hemodynamic change may occur due to
secretion vascular active factor adjustment of cerebrovascular
endothelial cells (25). Previous
findings suggest that, the change of CBF is controlled by vascular
smooth muscle tension, which is affected by nerve, body fluid,
metabolism, and various factors such as physical ones (26). Complex endothelial cells and the
nerve cell network are involved in vascular contraction and
relaxation factor release such as NO concentration, prostaglandins,
natriuretic peptide and its receptors and ET-1, and the
distribution of their receptors (27). These vessel endogenous substances
can be activated under different physiological stimulations, and by
changing the intracellular calcium ion concentration and regulating
potassium channels cause vascular smooth muscle contraction or
relaxation (28). eNOS catalytic
NO synthesis and vascular factors are released. Hypoxic conditions
in the HIF family induce the expression of NOS and increase
downstream genes, promoting the vascular endothelial cells to
produce NO. In addition, free diffusion to VSMCs stimulates the
synthesis of the cytoplasm soluble guanylate cyclase acid and cGMP,
resulting in vasodilatation (29).
The results of the present study have shown that there was no
significant difference between the concentration of ET-1 and eNOS
in the CMS patients and healthy controls, indicating that the
vascular regulating factor plays a role in long-term chronic
hypoxia in CMS patients.
ET is coupled to phospholipase C with GTP-binding
protein through the ETAR and ETBR receptors (23). ETAR can cause vasoconstriction
mainly through VSMCs, and activated ETAR1 can promote vascular
endothelial cells to release NO, which is involved in vascular
diastolic and expansion (30).
However, a higher concentration of ETR, affects the expression of
receptor under chronic low-oxygen stimulation, leading to a higher
expression (31). The correlation
analysis revealed there is no correlation between the content of
ET-1 and CVR, in the CMS patients or healthy controls, while there
is a significant correlation between the content of ETBR and CVR in
CMS patients. However, this result shows that ETBR expression
levels are associated with cerebral hemodynamics in CMS patients. A
cerebral hypoxia diastolic reaction is possible due to the high
expression of ETBR, rather than the result of the change of
ET-1.
In conclusion, cerebral circulation reserve capacity
was significantly lower in the CMS patients than the healthy
controls. The level of ET-1 and eNOS plasma was not significantly
different between the CMS patients and healthy controls. This
finding suggests the involvement of two vascular regulating factors
in patients with long-term chronic hypoxia. The high level of ETBR
expression observed in CMS patients compared to healthy controls
suggests that the cerebral hypoxia diastolic reaction is possible
due to ETBR, rather than ET-1 itself.
Acknowledgments
This study was supported in part by Qinghai Natural
Science Foundation of China (no. 2013-Z-921).
References
|
1
|
León-Velarde F, Maggiorini M, Reeves JT,
Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T,
Moore LG, et al: Consensus statement on chronic and subacute high
altitude diseases. High Alt Med Biol. 6:147–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Javaheri S: Hypoxemia lowers
cerebrovascular resistance without changing brain and blood [H+]. J
Appl Physiol (1985). 60:802–808. 1986.
|
|
3
|
Peterson EC, Wang Z and Britz G:
Regulation of cerebral blood flow. Int J Vasc Med.
2011:8235252011.PubMed/NCBI
|
|
4
|
Wolff CB: Cerebral blood flow and oxygen
delivery at high altitude. High Alt Med Biol. 1:33–38. 2000.
View Article : Google Scholar
|
|
5
|
Vicenzini E, Ricciardi MC, Altieri M,
Puccinelli F, Bonaffini N, Di Piero V and Lenzi GL: Cerebrovascular
reactivity in degenerative and vascular dementia: A transcranial
Doppler study. Eur Neurol. 58:84–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harer C and von Kummer R: Cerebrovascular
CO2 reactivity in migraine: Assessment by transcranial Doppler
ultrasound. J Neurol. 238:23–26. 1991. View Article : Google Scholar
|
|
7
|
Markus HS and Harrison MJ: Estimation of
cerebrovascular reactivity using transcranial Doppler, including
the use of breath-holding as the vasodilatory stimulus. Stroke.
23:668–673. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanagisawa M, Kurihara H, Kimura S, Tomobe
Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K and Masaki T: A novel
potent vasoconstrictor peptide produced by vascular endothelial
cells. Nature. 332:411–415. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simonson MS: Endothelins: Multifunctional
renal peptides. Physiol Rev. 73:375–411. 1993.
|
|
10
|
Liu Q and Huang Z: Endothelin and cerebral
vasospasm. Chongqing Med. 36:478–480. 2007.
|
|
11
|
Toda N and Ayajiki K: Phylogenesis of
constitutively formed nitric oxide in non-mammals. Rev Physiol
Biochem Pharmacol. 157:31–80. 2006.
|
|
12
|
Beall CM, Laskowski D and Erzurum SC:
Nitric oxide in adaptation to altitude. Free Radic Biol Med.
52:1123–1134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Z, Huang WD, Gao Q, Su ML, Yang YF,
Liu ZC and Zhu BH: Arnebin-1 promotes angiogenesis by inducing
eNOS, VEGF and HIF-1alpha expression through the PI3K-dependent
pathway. Int J Mol Med. 36:685–697. 2015.PubMed/NCBI
|
|
14
|
Kastrup A, Krüger G, Neumann-Haefelin T
and Moseley ME: Assessment of cerebrovascular reactivity with
functional magnetic resonance imaging: Comparison of CO(2) and
breath holding. Magn Reson Imaging. 19:13–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pfefferkorn T, von Stuckrad-Barre S,
Herzog J, Gasser T, Hamann GF and Dichgans M: Reduced
cerebrovascular CO(2) reactivity in CADASIL: A transcranial Doppler
sonography study. Stroke. 32:17–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hackett PH and Roach RC: High-altitude
illness. N Engl J Med. 345:107–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reinhart WH, Singh A and Straub PW: Red
blood cell aggregation and sedimentation: The role of the cell
shape. Br J Haematol. 73:551–556. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brugniaux JV, Hodges AN, Hanly PJ and
Poulin MJ: Cerebrovascular responses to altitude. Respir Physiol
Neurobiol. 158:212–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ainslie PN and Ogoh S: Regulation of
cerebral blood flow in mammals during chronic hypoxia: A matter of
balance. Exp Physiol. 95:251–262. 2010. View Article : Google Scholar
|
|
20
|
Qi Y, Cui S, Yang YZ, Ma Y, Li JZ and Ge
R: Detection of plasma VHL, HIF-1α, EPO levels and polymorphism
analysis of VHL 598C>T in CMS. Mod Prevent Med. 5:9442013.
|
|
21
|
Lucas SJ, Burgess KR, Thomas KN, Donnelly
J, Peebles KC, Lucas RA, Fan JL, Cotter JD, Basnyat R and Ainslie
PN: Alterations in cerebral blood flow and cerebrovascular
reactivity during 14 days at 5050 m. J Physiol. 589:741–753. 2011.
View Article : Google Scholar :
|
|
22
|
Duong TQ, Iadecola C and Kim SG: Effect of
hyperoxia, hypercapnia, and hypoxia on cerebral interstitial oxygen
tension and cerebral blood flow. Magn Reson Med. 45:61–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi S, Stocchetti N, Longhi L,
Balestreri M, Spagnoli D, Zanier ER and Bellinzona G: Brain oxygen
tension, oxygen supply, and oxygen consumption during arterial
hyperoxia in a model of progressive cerebral ischemia. J
Neurotrauma. 18:163–174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrante F, Fusco E, Calabresi P and
Cupini LM: Phyto-oestrogens in the prophylaxis of menstrual
migraine. Clin Neuropharmacol. 27:137–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Claydon VE, Norcliffe LJ, Moore JP, Rivera
M, Leon-Velarde F, Appenzeller O and Hainsworth R: Cardiovascular
responses to orthostatic stress in healthy altitude dwellers, and
altitude residents with chronic mountain sickness. Exp Physiol.
90:103–110. 2005. View Article : Google Scholar
|
|
26
|
Shmuel A, Yacoub E, Pfeuffer J, Van de
Moortele PF, Adriany G, Hu X and Ugurbil K: Sustained negative
BOLD, blood flow and oxygen consumption response and its coupling
to the positive response in the human brain. Neuron. 36:1195–1210.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robertson CS, Gopinath SP, Valadka AB, Van
M, Swank PR and Goodman JC: Variants of the endothelial nitric
oxide gene and cerebral blood flow after severe traumatic brain
injury. J Neurotrauma. 28:727–737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahnstedt H, Stenman E, Cao L, Henriksson M
and Edvinsson L: Cytokines and growth factors modify the
upregulation of contractile endothelin ET(A) and ET(B) receptors in
rat cerebral arteries after organ culture. Acta Physiol (Oxf).
205:266–278. 2012. View Article : Google Scholar
|
|
29
|
Xu X, Wang Z, Li Q, Xiao X, Lian Q, Xu W,
Sun X, Tao H and Li R: Endothelial nitric oxide synthase expression
is progressively increased in primary cerebral microvascular
endothelial cells during hyperbaric oxygen exposure. Oxid Med Cell
Longev. 2:7–13. 2009. View Article : Google Scholar :
|
|
30
|
Simão F, Pagnussat AS, Seo JH, Navaratna
D, Leung W, Lok J, Guo S, Waeber C, Salbego CG and Lo EH:
Pro-angiogenic effects of resveratrol in brain endothelial cells:
Nitric oxide-mediated regulation of vascular endothelial growth
factor and metalloproteinases. J Cereb Blood Flow Metab.
32:884–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Willie CK, Macleod DB, Shaw AD, Smith KJ,
Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, et al:
Regional brain blood flow in man during acute changes in arterial
blood gases. J Physiol. 590:3261–3275. 2012. View Article : Google Scholar : PubMed/NCBI
|