Introduction
Pigs are one of the most important large animal
models for xenotransplantation associated with various human
diseases, since pigs are physiologically similar to humans and are
widely available (1). Thus, pigs
serve as a biomedical animal model, and they have been genetically
modified (GM) and primarily produced using the technique of somatic
cell nuclear transfer (SCNT) (2).
Until recently, most genetically altered pigs were generated by
random insertions of expression cassettes into porcine fetal
fibroblasts (PFFs), or by editing the genome (3). Zinc finger nucleases (4–6) and
transcription activator-like effector nucleases, or ʻTALENsʼ
(7–9), have provided powerful tools to
generate specific gene modifications in pigs; however, the complex
design and generation greatly limit the application of these
systems. The so-called clustered regularly interspaced short
palindromic repeats (CRISPR) system, relying on CRISPR RNAs
(crRNAs) in combination with CRISPR-associated (Cas) proteins to
direct the degradation of complementary sequences present within
invading viral and plasmid DNA, has been demonstrated to be an
alternative strategy for precise gene editing (10,11).
Cas9 endonuclease, from the Streptococcus pyogenes type II
CRISPR/Cas system, functions as an RNA-guided endonuclease that
uses a dual-guide RNA, consisting of crRNA and trans-activating
crRNA (tracrRNA), for target recognition and cleavage by a
mechanism involving two nuclease active sites that together
generate double-stranded DNA (dsDNA) breaks, which requires the
recognition of a short trinucleotide proto-spacer adjacent motif
(PAM) sequence (-NGG) following the 20 bp crRNA target (12).
RNA-guided Cas9 nucleases have been successfully
used to generate GM pigs via the direct cytoplasmic injection of
Cas9 mRNA and single guide (sg)RNA into zygotes (13,14),
or through the modification of somatic cells followed by SCNT
(15). The CRISPR/Cas9 system has
been applied to genome-wide loss-of-function screening in human
cells (16,17) and mouse embryonic stem cells
(18), and it is superior as a
method to RNA interference (RNAi); however, the loss-of-function
genes have not been verified in vitro and the approach has
not been used in pig cells, particularly in primary cells.
Additionally, for certain genes that are lethal to embryos, it is
difficult to generate knockouts to study other functions.
In the present study, lentiviral cas9 was used to
infect pig primary fibroblasts in order to generate cell colonies
carrying the Cas9 protein. Subsequently, SCNT from pools of stably
nucleofected cell clones was used to generate inducible transgenic
expression cell lines for Cas9 protein. Thus, the Cas9 cell lines
are a convenient and useful tool for editing the pig genome and for
establishing pig models.
Materials and methods
Animal and recombinant DNA usage
The use of animals was approved by the Animal Care
and Use Committee of the South China Agricultural University,
Guangzhou, China. Recombinant DNA use was approved by the
Institutional Biosafety Committee. All surgical procedures were
performed under anesthesia, and all efforts were made to minimize
animal suffering.
Guide (g)RNA design and plasmid
construction
The lentiviral doxycycline-inducible FLAG-Cas9
(50661) and U6-sgRNA cloning vector (50662) plasmids were purchased
from Addgene, Inc. (Cambridge, MA, USA). Targeting sgRNA was
designed as previously described (16). An extra guanine was added to the 5′
end of the gRNA in which the first nucleotide was not guanine for
more efficient transcription by RNA polymerase III (19). These constructs were confirmed by
sequence analysis.
Virus production, transduction and
selection
Lentivirus of FLAG-Cas9 was produced by
co-transfection of the lentiviral transfer vector with the pMD2.G
and pPAX2 packaging plasmids (Addgene, Inc.) into 293FT cells using
Lipofectamine 3000 transfection reagent (Invitrogen Life
Technologies; Thermo Fisher Scientific, Waltham, MA, USA). The
virus-containing supernatant was collected 48 and 72 h following
transfection, cleared of cell debris by filtering through a 0.45
µm filter and concentrated by ultracentrifugation (Merck
Millipore, Darmstadt, Germany) at 4,000 g for 30 min at 4°C. PFFs
were cultured until they had reached 50–80% confluence in six-well
tissue culture plates, and were subsequently infected in media
containing 10 µg/ml polybrene (Sigma-Aldrich, St. Louis, MO,
USA) and concentrated lentivirus. At 12 h following infection, the
virus was removed and cells were selected with 2 µg/ml
puromycin (Sigma-Aldrich) for 5–7 days. Cell colonies were picked
and cultured in 48-well plates. After 2 days, the cell colonies
were subcultured and selected with 1 µg/ml puromycin for an
additional 10 days. Subsequently, a fraction of selected cells was
used for polymerase chain reaction (PCR) and reverse transcription
(RT)-PCR detection, and the other cells were frozen for future
use.
SCNT
Porcine SCNT was performed as described previously
(15,20). Briefly, cumulus-oocyte complexes
were aspirated from the ovaries and matured in maturation medium
for 42–44 h at 39°C, as described previously (21). Cumulus cells were removed by
repeated pipetting in 0.1% hyaluronidase (Sigma-Aldrich). Matured
oocytes with a first polar body were enucleated manually in the
presence of 7.5 mg/ml cytochalasin B (Sigma-Aldrich). A single
fibroblast cell that was identified for gene integration by
genotyping was microinjected into the perivitelline space of the
oocytes. The oocyte-donor cell complexes were cultured in PZM3
medium (21) at 39°C for 1.5 h,
and subsequently fusion and activation were performed with two
successive direct current pulses at 1.2 kV/cm for 30 µs
using an electrofusion instrument (model, CF-150/B; BLS Ltd.,
Budapest, Hungary). The reconstructed embryos were cultured in PZM3
medium at 39°C for 20 h and surgically transferred to the oviducts
of the embryo recipients that were estrous-synchronized. The
pregnancy status of the recipient sows was monitored by
ultrasonography at ~25 days following the embryo transfer.
Cas9-PFF generation and culture
PFFs were isolated from 30-day-old fetuses of cloned
pigs that were integrated with the Cas9 gene by tissue explant
culture, as described previously (22). First, the fetuses were washed in
phosphate buffered saline (PBS) containing 100 U/ml penicillin and
100 mg/ml streptomycin (Sigma-Aldrich China, Inc., Shanghai,
China), and subsequently the heads, tails, limbs and viscera were
removed under sterile conditions. Secondly, the residual tissues
were washed twice in PBS and cut into small pieces (≤1
mm3), pasted on to 100-mm cell culture dishes, and 5 ml
fetal bovine serum (FBS) was subsequently added. The culture dishes
were incubated in humidified 95% air with 5% CO2 at 39°C
for 24 h. Typically, the first outgrowing cells from the fetal
explants became visible within 24 h of incubation, at which point
the medium was replaced with Dulbecco modified Eagle's medium
(DMEM) supplemented with 2 mM glutamine, 100 units/ml penicillin
and 100 mg/ml streptomycin containing 10% FBS (Gibco™; Thermo
Fisher Scientific) for 2–3 days. The cultured cells were expanded
and subsequently cryopreserved for future use.
PCR and RT-PCR detection
Genomic DNA was isolated from selected cell colonies
or Cas9-PFFs and wild-type cells using a TIANamp Genomic DNA kit
[Tiangen Biotech (Beijing) Co., Ltd, Beijing, China]. The total RNA
of the cell colonies or Cas9-PFFs induced with 1 µg/ml
doxycycline for 48 h was extracted using an E.Z.N.A.®
Total RNA kit (Omega Bio-Tek, Inc., Norcross, GA, USA), following
the manufacturer's protocol and including DNase I (Takara
Biotechnology Co., Ltd., Dalian, China) treatment to remove the
genomic DNA. Complementary DNA (cDNA) was synthesized at 42°C for
60 min using a PrimeScript™ RT-PCR kit (Takara Biotechnology Co.,
Ltd.) with oligo (dT) primers. PCR was performed for genomic lever
and RT-PCR for gene transcripts of Cas9 in a programmed thermal
cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
following PCR and RT-PCR conditions were used: Initial denaturation
at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C
for 30 sec; annealing at 60°C for 30 sec and extension at 72°C for
1 min; and 10 min final extension at 72°C.
Indirect immunofluorescent assay
(IFA)
Treated and untreated control cells cultured on
cover slips were fixed with 4% paraformaldehyde for 10 min,
permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) for 15 min,
and blocked with 1% bovine serum albumin for 30 min. After rinsing
twice with PBS, a 1:200 dilution of the anti-FLAG monoclonal
antibody (cat. no. AE005; ABclonal Biotechnology Co., Ltd.,
Cambridge, MA, USA) was added to the cells, incubated overnight at
4°C, and subsequently washed thrice in the washing buffer. The
cells were then sequentially incubated with fluorescein
isothiocyanate-conjugated goat anti-mouse secondary antibody (cat.
no. 7076; Cell Signaling Technology, Inc., Danvers, MA, USA) at a
dilution of 1:50 for 1 h at 37°C. Following washing, the cells were
stained for DNA with 10 µg/ml Hoechst 33342 (Molecular
Probes) and viewed under a fluorescent microscope (IX71; Olympus
Corporation, Tokyo, Japan).
Western blotting
Protein extracts were obtained from Cas9 PFFs
induced with 1 µg/ml doxycycline for 72 h and wild-type
PFFs. The protein concentrations were determined using the Bradford
method (23). Primary anti-FLAG
monoclonal antibody (1:2,000 dilution; ABclonal Biotechnology) and
anti-mouse immunoglobulin G horseradish peroxidase (1:3,000
dilution; Cell Signaling Technology, Danvers, MA, USA) were used
for the western blot analysis. Western blotting was performed as
previously reported (24).
Inverse PCR and integration analysis
To analyze FLAG-Cas9 insertion sites, 2 µg
transgenic pig genomic DNA was digested with XhoI, purified
on a DNA purification column (Omega Bio-Tek, Inc.), and eluted with
20 µl doubly distilled (dd)H2O. Eluted DNA was
self-ligated in a 400 µl reaction system, including 400
units T4 ligase at 16°C overnight, prior to being purified on a DNA
purification column and eluted with 20 µl ddH2O.
The resulting circular DNA underwent nested PCR, and was amplified
using the specific primers, LTR-1+P1 and LTR-2+P2 (for primer
sequences, see Table I). The PCR
products were cloned into pMD-18T, a simple cloning vector (Takara
Biotechnology Co., Ltd.) and sequenced. Sequences were aligned to
the sequence of the donor vector, pCW-Cas9, and the Sus
scrofa (wild pig) genomic DNA sequence database (Build
Sscrofa10.2) using the National Center for Biotechnological
Information's Basic Local Alignment Search Tool (ʻBLASTʼ).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name | Direction | Description | Sequences
(5′–3′) |
|---|
| Cas9-1f | Forward | Cas9 gene PCR |
ACAAGCTGATCCGGGAAGTG |
| Cas9-1r | Reverse | and RT-PCR
primers |
CTGTCTGCACCTCGGTCTTT |
| β-actin-f | Forward | Pig actin
detection |
GTGCGGGACATCAAGGAGAA |
| β-actin-r | Reverse | RT-PCR primers |
GTCACCTTCACCGTTCCAGT |
| LTR1 | Forward | First IPCR |
GAGGGATCTCTAGTTACCAGAGTCACA |
| P1 | Reverse | Primers |
GAAGAATCGCAAAACCAGCAAGAAAAG |
| LTR2 | Forward | Secondary IPCR |
AGCCAGAGAGCTCCCAGGCTCAGATC |
| P2 | Reverse | Primers |
CATAATGATAGTAGGAGGCTTGGTAGG |
Results
Assessment of doxycycline-inducible
FLAG-Cas9 in porcine primary cells
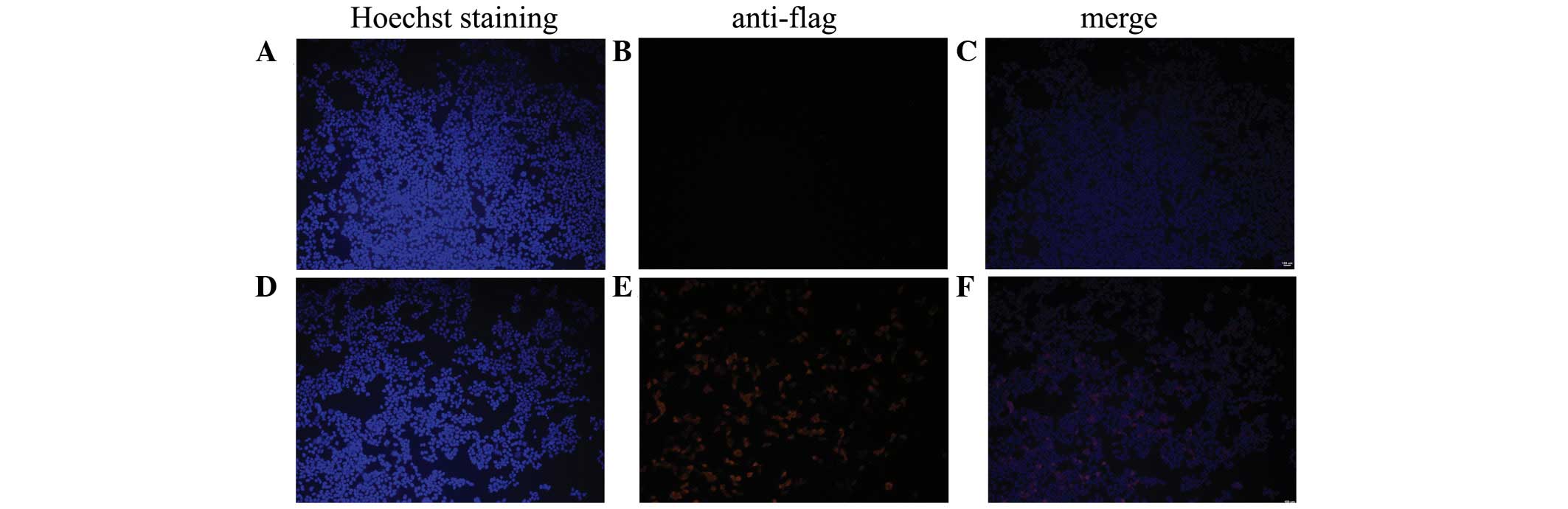
To determine doxycycline-inducible FLAG-Cas9 gene
expression in porcine primary cells, the plasmid-expressed Cas9 was
transfected into porcine fibroblasts and 293T cells as a control.
Cas9 expression was confirmed at 48 h using an IFA. The results
demonstrated that the tet-on system, which expresses FLAG-Cas9, is
able to function properly in porcine fibroblasts (Fig. 1), although it was weaker compared
with 293T cells (Fig. 2).
Generation of transgenic PFF
colonies
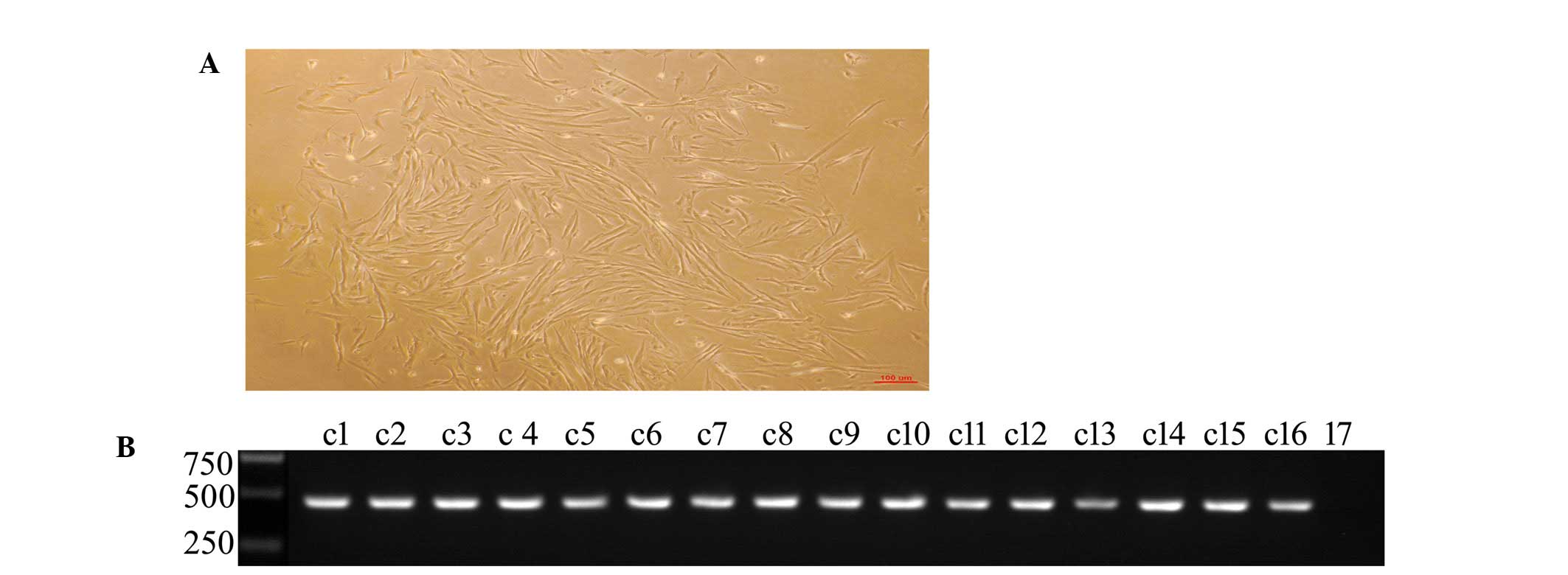
Following selection and culture for 20 days, 16 PFF
colonies were obtained (Fig. 3A),
and whether or not the target gene had been integrated into the
porcine genome was subsequently determined. The results of the
genomic DNA PCR analysis revealed that all colonies had integrated
the Cas9 gene (Fig. 3B).
Generation of FLAG-Cas9 PFFs via
SCNT
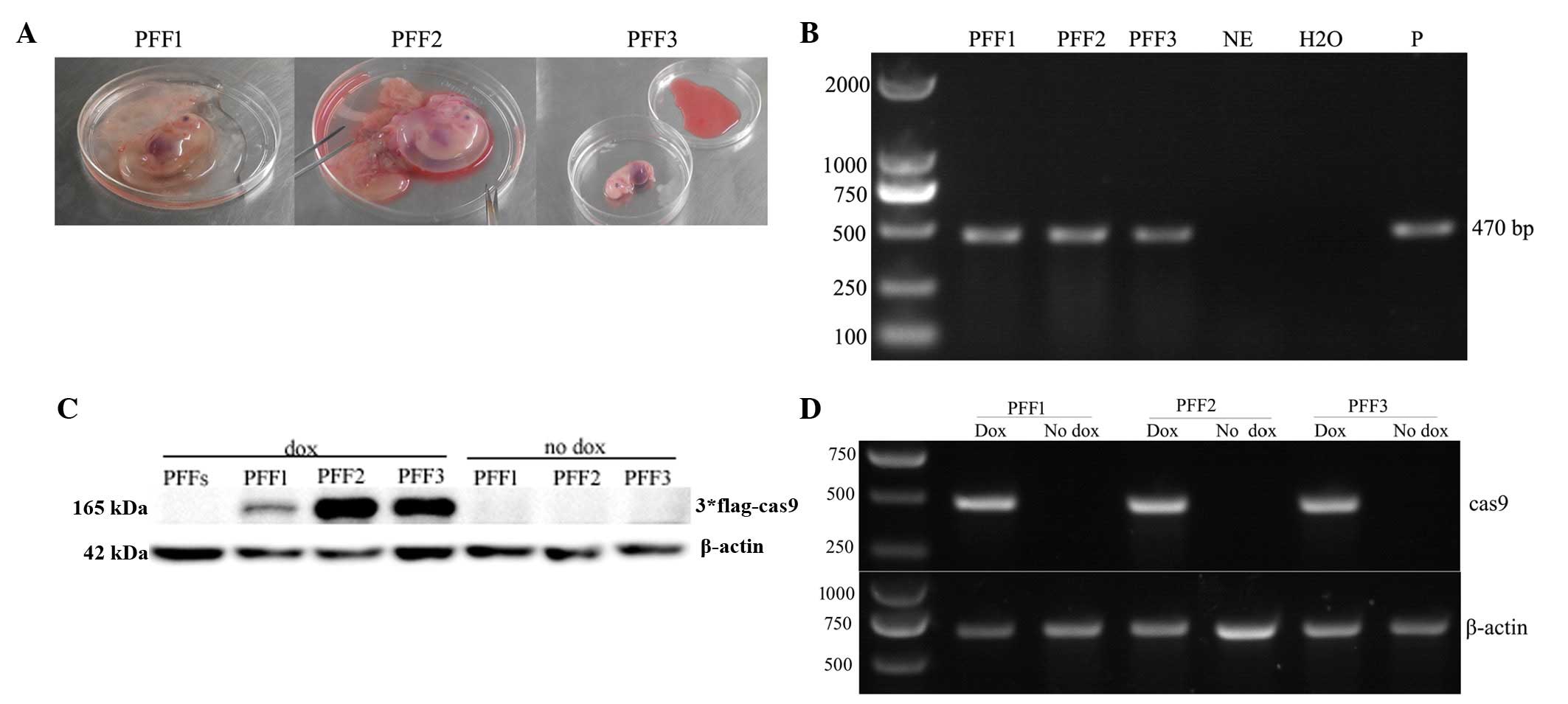
Eleven transgenic PFF colonies containing the Cas9
gene were selected as donors for SCNT. A total of 835 reconstructed
embryos were introduced into three surrogate mothers. Among the
surrogate mothers, one was revealed to be pregnant by
ultrasonography 25 days following embryo transfer, and the other
two were not apparently pregnant (Table II). Therefore, the pregnant
surrogate mother was sacrificed at 30 days, and three normal
fetuses were obtained (Fig. 4A).
The genomic DNA PCR analysis demonstrated three Cas9-positive PFFs
(Fig. 4B). RT-PCR and western blot
analyses indicated that three Cas9-positive PFFs successfully
exhibited mRNA and protein expression of the Cas9 gene (Fig. 4C and D). However, the FLAG-Cas9
gene was expressed at a lower level in PFF1 compared with PFF2 and
PFF3 (Fig. 4C).
 | Table IISomatic cell nuclear transfer results
for the generation of FLAG-Cas9 PFFS. |
Table II
Somatic cell nuclear transfer results
for the generation of FLAG-Cas9 PFFS.
| Target gene | Cell pool | Transferred
embryos | No. recipients | No. (%)
pregnancies | No. 30 day
fetuses | No. (%) transgenic
PFFs |
|---|
| Cas9 | Clo1-4 | 280 | 1 | 0 | 0 | 0 |
| Clo5-7 | 270 | 1 | 0 | 0 | 0 |
| Clo8-11 | 285 | 1 | 1 | 3 | 3 |
Identification of FLAG-Cas9 integration
sites in the transgenic PFFs genome
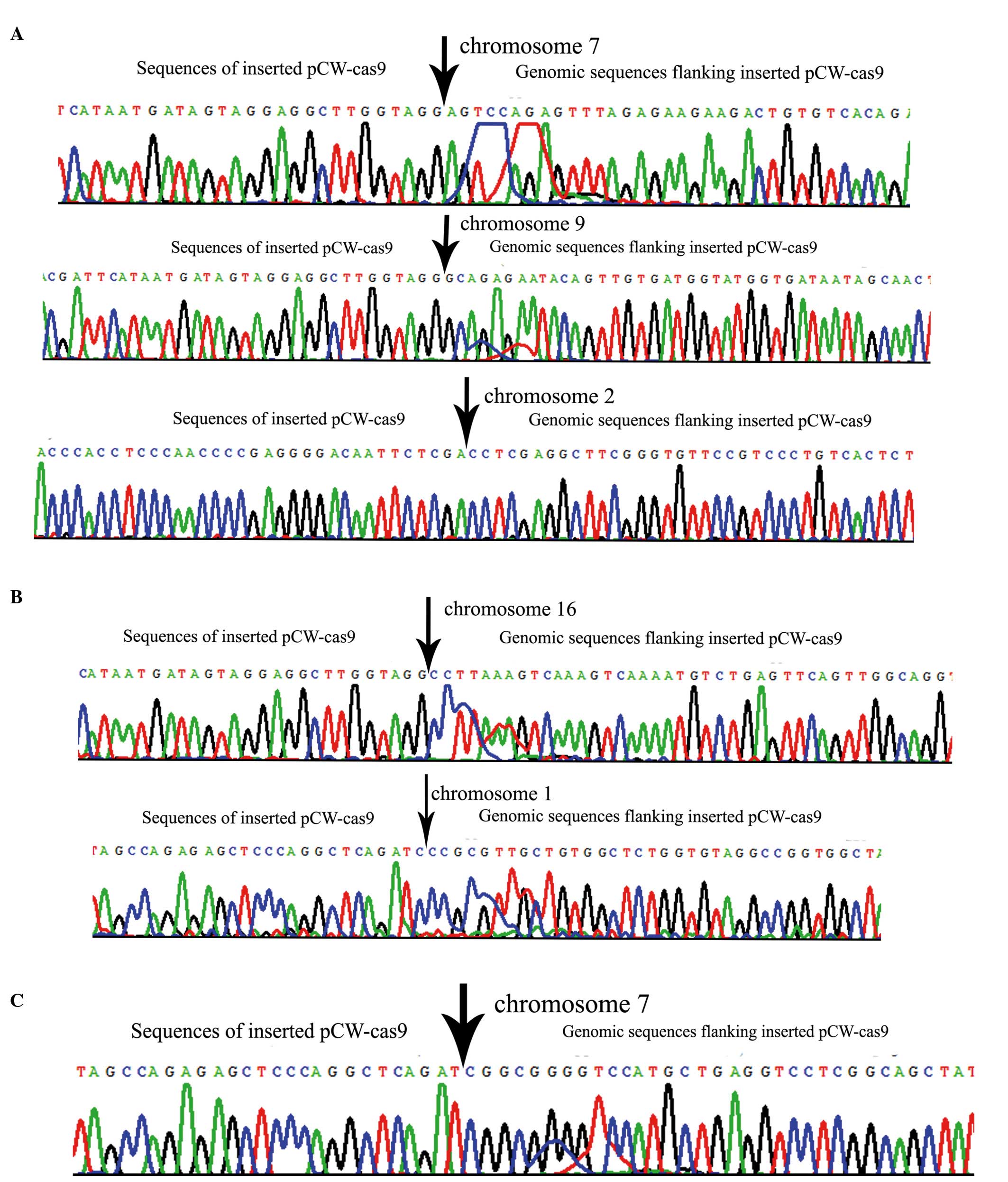
To analyze FLAG-Cas9 integration, inverse PCR was
used to identify the lentiviral vector insertion sites in the
genome of transgenic PFFs expressing Cas9. Three integration sites
were identified in the transgenic PFF1 genome, integrated on
chromosomes 2, 7 and 9 (Fig. 5A),
one of which was located near to known functional genes. Two
integration sites (on chromosomes 1 and 16) were identified in the
transgenic PFF2 (Fig. 5B), and one
integration site was identified in PFF3 (Fig. 5C), none of which were located near
to known functional genes.
Discussion
Cas9 is a dsDNA endonuclease that uses a crRNA guide
to specify the site of cleavage, and is an essential component of
the CRISPR/Cas9 system (11,25).
The cell lines expressing Cas9 have provided a convenient system
with which to generate targeted mutations of any gene by simple
transfection with sgRNA (26). In
the present study, three doxycycline-inducible flag-cas9 PFFs were
successfully generated through SCNT, which conditionally expressed
Cas9 endonuclease. Consistently with previous reports (27,28),
the transfection efficiency of the doxycycline-inducible FLAG-Cas9
vector was lower in porcine fibroblasts; however, transgenic PFF1
expressed less Cas9 protein compared with the other transgenic
PFFs, which may be associated with integration sites on the PFF
genome.
The CRISPR/Cas9 system was initially used to edit
the genomes of prokaryotic and eukaryotic organisms, including
deletion and insertion by DNA double-stranded break (DSB) repair
mechanisms. Cas9 endonuclease, with sgRNAs, was shown to be able to
modify single (29,30) or multiple (31) genes. Previously, the CRISPR/Cas9
system has been used to create pigs of one genetic strain or
multiple genetic modifications in a single pregnancy (32); however, the present study aimed to
directly generate modified organisms using Cas9 endonuclease and
targeted sgRNAs. In the present study, PFFs were generated
expressing stable tetracycline-regulated Cas9 endonuclease.
Subsequently, the genes of interest were specifically knocked out
using inducible Cas9, in association with targeting sgRNAs in pigs,
which is more convenient compared with the conventional CRISPR/Cas9
system. Premature microRNAs (miRNAs) exist as a classical stem-loop
structure. Therefore, DSBs could be generated using the CRISPR/Cas9
system. DSBs in the loop region may affect miRNA maturation during
processing by Drosha and Dicer, resulting in knockdown of the miRNA
in mammalian cells (33).
Therefore, doxycycline-inducible FLAG-Cas9 PFFs were able be used
for porcine genome editing and miRNA expression and regulation. The
CRISPR-associated protein Cas9 not only identifies genomic DNA and
generates sequence-specific dsDNA cleavage, but it also binds with
high affinity to single-stranded RNA (ssRNA) targets matching the
Cas9-associated guide RNA sequence when the PAM is presented in
trans as a separate DNA oligonucleotide, and stimulates
site-specific endonucleolytic cleavage of ssRNA targets, including
endogenous mRNA with PAM-presenting oligonucleotides (PAMmers)
(34). It is hypothesized that the
inducible Cas9 PFFs may be used to regulate porcine mRNA
expression.
Loss-of-function screening is a powerful and
hypothesis-free approach to identify genes and pathways that
underlie biological processes. The CRISPR/Cas9 system was
considered to be an ideal tool for genetic screening, since RNAi
may only achieve a partial depletion of gene activity, and
knockout-based screens are difficult to manage in diploid mammalian
cells, for example, as has been applied to human and mouse cells
(17,18). PFFs are highly undifferentiated
cells compared with other cells retrieved from adult tissue, and
have been demonstrated to be the most effective donor cells for
SCNT (35,36). Therefore, the present study has
revealed that a loss-of-function pig model may be generated on the
basis of doxycycline-inducible flag-cas9 PFFs, and this function
may be verified in vitro.
In conclusion, three porcine fibroblast cell lines
that conditionally express Cas9 were established. These FLAG-Cas9
PFFs may be widely applicable in porcine genomic editing and the
regulation of gene expression. In our future work, these cells are
to be used with the intention of editing reproduction-associated
genes in pigs. The porcine fibroblast cell lines expressing Cas9
endonuclease may be productively used in SCNT procedures to
generate a porcine model of genomic modification, along with
targeting sgRNAs.
Acknowledgments
This study was supported by the National Basic
Research and Development Program of China (973 Program, nos.
2011CB944202 and 2010CB945001) and the National Natural Science
Foundation of China (no. 31402072).
References
|
1
|
Luo Y, Lin L, Bolund L, Jensen TG and
Sørensen CB: Genetically modified pigs for biomedical research. J
Inherit Metab Dis. 35:695–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tector AJ and Ford ML: Designing donors:
Nuclease-based genome editing in pigs. Am J Transplant. 15:32015.
View Article : Google Scholar
|
|
3
|
Tan W, Carlson DF, Lancto CA, Garbe JR,
Webster DA, Hackett PB and Fahrenkrug SC: Efficient nonmeiotic
allele introgression in livestock using custom endonucleases. Proc
Natl Acad Sci USA. 110:16526–16531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urnov FD, Rebar EJ, Holmes MC, Zhang HS
and Gregory PD: Genome editing with engineered zinc finger
nucleases. Nat Rev Genet. 11:636–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petersen B, Ahrens H, Herrmann D, Petkov
SG, Frenzel A, Hauschild J, Lucas-Hahn A, Hassel P, Ziegler M,
Baars W, et al: Generation of HHO-1/HA20/GGTA1-ko pigs by using
sleeping beauty transposon and zinc finger nucleases. Transgenic
Res. 23:8612014.
|
|
6
|
Tang L, Gonzalez R and Dobrinski I:
Germline modification of domestic animals. Anim Reprod. 12:93–104.
2015.PubMed/NCBI
|
|
7
|
Miller JC, Tan S, Qiao G, Barlow KA, Wang
J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al: A TALE
nuclease architecture for efficient genome editing. Nat Biotechnol.
29:143–148. 2011. View
Article : Google Scholar
|
|
8
|
Yao J, Huang J, Hai T, Wang X, Qin G,
Zhang H, Wu R, Cao C, Xi JJ, Yuan Z and Zhao J: Efficient
bi-allelic gene knockout and site-specific knock-in mediated by
TALENs in pigs. Sci Rep. 4:69262014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan D, Yuan J, Li X, Feng C, Long C, Cui
H, Wang F and Xu J: Efficient generation of GGTA1-null pigs via
TALENs. Transgenic Res. 22:2462013.
|
|
10
|
Wiedenheft B, Sternberg SH and Doudna JA:
RNA-guided genetic silencing systems in bacteria and archaea.
Nature. 482:331–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jinek M, Chylinski K, Fonfara I, Hauer M,
Doudna JA and Charpentier E: A programmable dual-RNA-guided DNA
endonuclease in adaptive bacterial immunity. Science. 337:816–821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sternberg SH, Redding S, Jinek M, Greene
EC and Doudna JA: DNA interrogation by the CRISPR RNA-guided
endonuclease Cas9. Nature. 507:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hai T, Teng F, Guo R, Li W and Zhou Q:
One-step generation of knockout pigs by zygote injection of
CRISPR/Cas system. Cell Res. 24:372–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whitworth KM, Lee K, Benne JA, Beaton BP,
Spate LD, Murphy SL, Samuel MS, Mao J, O'Gorman C, Walters EM, et
al: Use of the CRISPR/Cas9 system to produce genetically engineered
pigs from in vitro-derived oocytes and embryos. Biol Reprod.
91:782014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Xin J, Fan N, Zou Q, Huang J,
Ouyang Z, Zhao Y, Zhao B, Liu Z, Lai S, et al: Generation of
CRISPR/Cas9-mediated gene-targeted pigs via somatic cell nuclear
transfer. Cell Mol Life Sci. 72:1175–1184. 2015. View Article : Google Scholar
|
|
16
|
Wang T, Wei JJ, Sabatini DM and Lander ES:
Genetic screens in human cells using the CRISPR-Cas9 system.
Science. 343:80–84. 2014. View Article : Google Scholar
|
|
17
|
Shalem O, Sanjana NE, Hartenian E, Shi X,
Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG and
Zhang F: Genome-Scale CRISPR-Cas9 knockout screening in human
cells. Science. 343:84–87. 2014. View Article : Google Scholar :
|
|
18
|
Koike-Yusa H, Li Y, Tan EP,
Velasco-Herrera Mdel C and Yusa K: Genome-wide recessive genetic
screening in mammalian cells with a lentiviral CRISPR-guide RNA
library. Nat Biotechnol. 32:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doench JG, Hartenian E, Graham DB, Tothova
Z, Hegde M, Smith I, Sullender M, Ebert BL, Xavier RJ and Root DE:
Rational design of highly active sgRNAs for CRISPR-Cas9-mediated
gene inactivation. Nat Biotechnol. 32:1262–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Z, Xu Z, Zou X, Zeng F, Shi J, Liu D,
Urschitz J, Moisyadi S and Li Z: Pig transgenesis by piggyBac
transposition in combination with somatic cell nuclear transfer.
Transgenic Res. 22:1107–1118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng W, Yang D, Zhao B, Ouyang Z, Song J,
Fan N, Liu Z, Zhao Y, Wu Q, Nashun B, et al: Use of the 2A peptide
for generation of multi-transgenic pigs through a single round of
nuclear transfer. PLoS One. 6:e199862011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kues WA, Petersen B, Mysegades W, Carnwath
JW and Niemann H: Isolation of murine and porcine fetal stem cells
from somatic tissue. Biol Reprod. 72:1020–1028. 2005. View Article : Google Scholar
|
|
23
|
Zor T and Selinger Z: Linearization of the
Bradford protein assay increases its sensitivity: theoretical and
experimental studies. Anal Biochem. 236:302–308. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng T, Xue X and Fu J: Effect of OLIG1
on the development of oligodendrocytes and myelination in a
neonatal rat PVL model induced by hypoxia-ischemia. Mol Med Rep.
11:2379–2386. 2015.
|
|
25
|
Gasiunas G, Barrangou R, Horvath P and
Siksnys V: Cas9-crRNA ribonucleoprotein complex mediates specific
DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci
USA. 109:E2579–E2586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang M, Zhang L, Stevens J and Gibson G:
CRISPR/Cas9 mediated generation of stable chondrocyte cell lines
with targeted gene knockouts; Analysis of an aggrecan knockout cell
line. Bone. 69:118–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim B, Jin D, Kim H, Kim M and Park C:
Analysis of transgene intergration efficiency into porcine fetal
fibroblast using different transfection methods. Reprod Dev Biol.
33:113–117. 2009.
|
|
28
|
Blanton JR Jr, Bidwell CA, Sanders DA,
Sharkey CM, McFarland DC, Gerrard DE and Grant AL: Plasmid
transfection and retroviral transduction of porcine muscle cells
for cell-mediated gene transfer. J Anim Sci. 78:909–918. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang
L, Kang Y, Zhao X, Si W, Li W, et al: Generation of gene-modified
cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell
embryos. Cell. 156:836–843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z,
Zhang X, Zhang P and Huang X: Generation of gene-modified mice via
Cas9/RNA-mediated gene targeting. Cell Res. 23:720–723. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao Y, Guan Y, Wang L, Qiu Z, Liu M, Chen
Y, Wu L, Li Y, Ma X, Liu M and Li D: CRISPR/Cas-mediated genome
editing in the rat via direct injection of one-cell embryos. Nat
Protoc. 9:2493–2512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li P, Estrada JL, Burlak C, Montgomery J,
Butler JR, Santos RM, Wang ZY, Paris LL, Blankenship RL, Downey SM,
et al: Efficient generation of genetically distinct pigs in a
single pregnancy using multiplexed single-guide RNA and
carbohydrate selection. Xenotransplantation. 22:20–31. 2015.
View Article : Google Scholar
|
|
33
|
Zhao Y, Dai Z, Liang Y, Yin M, Ma K, He M,
Ouyang H and Teng CB: Sequence-specific inhibition of microRNA via
CRISPR/CRISPRi system. Sci Rep. 4:39432014.PubMed/NCBI
|
|
34
|
O'Connell MR, Oakes BL, Sternberg SH,
East-Seletsky A, Kaplan M and Doudna JA: Programmable RNA
recognition and cleavage by CRISPR/Cas9. Nature. 516:263–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee GS, Hyun SH, Kim HS, Kim DY, Lee SH,
Lim JM, Lee ES, Kang SK, Lee BC and Hwang WS: Improvement of a
porcine somatic cell nuclear transfer technique by optimizing donor
cell and recipient oocyte preparations. Theriogenology.
59:1949–1957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei H, Qing Y, Pan W, Zhao H, Li H, Cheng
W, Zhao L, Xu C, Li H, Li S, et al: Comparison of the efficiency of
Banna miniature inbred pig somatic cell nuclear transfer among
different donor cells. PLoS One. 8:e577282013. View Article : Google Scholar : PubMed/NCBI
|