Introduction
Diabetes mellitus is a worldwide health problem,
with global incidence estimated at >380 million cases and rising
(1). The International Diabetes
Federation estimates that by 2035, 592 million people will suffer
from diabetes (1). Among diabetic
patients, ~10% suffer from type 1 diabetes mellitus (T1DM). T1DM,
which primarily affects younger people, results in lifelong
dependency on exogenous insulin treatment for survival and has the
potential for serious complications, including cardiovascular
disease, chronic renal failure and eye damage (2). Although the etiology and pathogenesis
of T1DM remain to be fully elucidated, there is a consensus that
the autoimmune destruction of insulin-producing pancreatic islet
β-cells, as a result of environmental and genetic factors, is
critical for its development (3).
Vitamin D has been well-characterized as a regulator
of calcium-phosphorus metabolism and bone mineralization (4). While vitamin D exerts other
biological effects, its extra-skeletal activities have been
extensively investigated. Numerous epidemiological studies have
suggested that vitamin D may have a role in defense against
diabetes (5,6). Vitamin D deficiency is prevalent
among the diabetic population; early and long-term vitamin D
supplementation is associated with a decrease in the risk of
developing diabetes, and the incidence of T1DM is greater in areas
with fewer days of sunlight per year (7,8).
However, the underlying mechanisms of the involvement of vitamin D
in T1DM remain to be elucidated. The present study aimed to
investigate the role of vitamin D in the development of diabetes
and the underlying mechanisms.
Autophagy ('self-eating') is a catabolic process of
the lysosomal degradation pathway that enables metabolic turnover
and homeostasis (9). It is
characterized by the sequestration of cytoplasm and organelles to
form autophagosomes, which fuse with lysosomes to form
autophagolysosomes, resulting in the proteolysis of sequestered
material (10). It has been
reported that autophagy may influence diverse physiological
processes and affect the occurrence and outcome of numerous
diseases, including diabetes (11,12).
Previous studies have revealed that baseline autophagy is crucial
for the maintenance of the normal architecture of pancreatic islets
and intracellular insulin content (13,14).
In the present study, the role of vitamin D in the pathogenesis of
diabetes was investigated, in particular the direct effects of
vitamin D on pancreatic β-cells. The results revealed, for the
first time to the best of our knowledge, that vitamin D enhances
autophagy while inhibiting apoptosis of streptozotocin
(STZ)-treated β-cells, increases insulin secretion and increases
resistance of β-cells to cellular stress encountered during
diabetes.
Materials and methods
Animals and experimental protocol
A total of 40 C57BL/6J male mice (age, 10 weeks)
were provided by Shanghai Laboratory Animal Center, Chinese Academy
of Sciences (Shanghai, China). All mice were maintained at 23±2°C
in 50±5% relative humidity under a 12-h light/dark cycle, and had
free access to food and water. The T1DM mouse model was induced by
multiple intraperitoneal injections of low-dose STZ (Sigma-Aldrich,
St. Louis, MO, USA) as described previously (15). Briefly, the experimental mice
received intraperitoneal injections of freshly prepared 40 mg/kg
STZ [dissolved in 0.1 M citrate buffer (Sigma-Aldrich)] for 5
consecutive days. The first day of STZ administration was
designated as day 1 of the study. Body weight, food and water
intake, and plasma glucose were monitored weekly for 21 days. Blood
was collected from the tip of the murine tail vein, and non-fasting
blood glucose concentrations were measured by the glucose oxidase
method as described previously (16). Mice were considered diabetic when
their glucose levels were >16.7 mmol/l. To investigate the
effect of vitamin D on the course of the STZ-induced T1DM mouse
model, 1,25(OH)2D3 (Sigma-Aldrich), the
physiologically active metabolite of vitamin D, was administered
intraperitoneally 1 h prior to STZ injection at a dose of 5
µg/kg (dissolved in peanut oil), and every second day until
sacrifice. Mice in the control group were left untreated. Mice were
sacrificed by cervical dislocation on day 21; pancreases were
removed for histological examination and plasma separated from
blood by centrifugation at 1,500 × g, 4°C for 10 min was stored at
−80°C for the insulin assay. The protocol for the present study was
in accordance with the Principles of Laboratory Animal Care and
approved by the Administration Committee of Laboratory Animals of
Soochow University (Suzhou, China).
Histopathological analysis of pancreatic
islets
The pancreases were dissected out, fixed in 10%
formalin for 2 h and embedded in paraffin. Sections (5–10
µm) were stained with hematoxylin and eosin as previously
described (17). Histopathological
evaluation was performed in a blinded fashion. The severity of
insulitis was determined by the extent of cellular infiltration and
the presence of islet atrophy was analyzed under light microscopy
(magnification, ×400).
Cell culture and analysis of insulin
secretion
MIN6 mouse insulinoma β-cells (Shanghai Bioleaf
Biotech Co., Ltd., Shanghai, China) were maintained in Dulbecco's
modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with
15% fetal bovine serum (Sigma-Aldrich), 50 mg/l streptomycin and 75
mg/l penicillin at 37°C in an incubator containing 5%
CO2. Preliminary experiments revealed that treatment
with 5 mM STZ for 30 min resulted in significant destruction of
MIN6 cells. To evaluate the direct effect of vitamin D on
pancreatic β-cells, MIN6 cells were treated with 0.01 nM
1,25(OH)2D3 in DMEM for 24 h prior to STZ
administration. Control untreated MIN6 cells, MIN6 cells treated
with 5 mM STZ for 30 min, and MIN6 cells treated with
1,25(OH)2D3 prior to STZ treatment were
collected and incubated in low (2.8 mM) or high (20 mM) glucose
concentrations for 1 h. Insulin secretion was subsequently measured
using an enzyme-linked immunosorbent assay (ELISA) kit (BioExpress;
VWR International, Radnor, PA, USA) according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To evaluate the mRNA expression levels of the
autophagy markers, Beclin 1 and microtubule-associated
protein 1A/1B-light chain 3 (LC3) in MIN6 cells, RT-qPCR was
performed as previously described (18). Total RNA was extracted from MIN6
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and cDNA was synthesized with
the RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The mRNA expression levels of Beclin 1 and LC3 were
measured using the Platinum SYBR Green qPCR SuperMix-UDG 92
(Invitrogen; Thermo Fisher Scientific, Inc.) and an ABI7500
Real-Time system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). A total of 40 cycles were performed, under the following
conditions: denaturation at 95°C for 30 sec, annealing at 60°C for
34 sec and extension at 72°C for 30 sec. The primer sequences are
listed in Table I. Each reaction
was repeated three times and the mRNA expression levels of
Beclin 1 and LC3 were normalized to GADPH
quantification cycle (Cq) values using the comparative
quantification cycle 2−ΔΔCq method (19).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Sequence, 5′-3′
|
|---|
| Forward | Reverse |
|---|
| Beclin 1 |
TGCTGACGAATCTCAAGTGG |
GCTATACATGGCGTGCTGTG |
| LC3 |
CATGCCGTCCGAGAAGACCT |
TGAGCCGGACATCTTCCACT |
| GAPDH |
CCTTCATTGACCTCAACTACATG |
CTTCTCCATGGTGGTGAAGAC |
Western blotting
To determine the protein expression levels of Beclin
1 and B-cell lymphoma 2 (Bcl-2), markers of autophagy and apoptosis
regulation, respectively, cells were treated with lysis solution
(Promega Corporation, Madison, WI, USA), sonicated and centrifuged
at 8,000 × g, 4°C for 10 min. The supernatant was collected and
protein concentration determined using a bicinchoninic acid assay
(Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Proteins (45 µg) were loaded
onto 10% SDS-PAGE gels, subjected to electrophoresis (120 V for 65
min) and transferred onto polyvinylidene difluoride membranes.
Following blocking in 5% non-fat milk for 1 h at room temperature,
membranes were probed with mouse anti-β-actin antibody (1:5,000;
catalog no. AM1021B), rabbit anti-Bcl-2 antibody (1:600; catalog
no. AP1303A) or rabbit anti-Beclin 1 antibody (1:500; catalog no.
AP1818b) at 4°C overnight. Subsequently, the membrane was incubated
at room temperature for a further 2 h, washed in Tris-buffered
saline with Tween 20 three times and treated with horseradish
peroxidase-conjugated goat anti-mouse IgG (1:5,000; catalog no.
ASS1021) or goat anti-rabbit IgG (1:5,000; catalog no. ASR1038) at
room temperature for 90 min. All antibodies were obtained from
Abgent, Inc., San Diego, CA, USA. Protein bands were visualized
with the Enhanced Chemiluminescence Plus Western Blotting Detection
system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The
protein expression levels of Beclin 1 and Bcl-2 were normalized to
β-actin.
Apoptosis assay
Annexin V-Fluorescein Isothiocyanate Apoptosis
Detection Kit I (BD Biosciences, Franklin Lakes, NJ, USA) was used
for assessment of apoptosis. According to the manufacturer's
instructions, three groups of MIN6 cells were pelleted by
centrifugation at 600 × g for 5 min at room temperature, washed
once with ice-cold phosphate-buffered saline and resuspended in
binding buffer. Cell suspensions were then incubated with annexin V
and propidium iodide at 25°C for 5–15 min in the dark. Analysis was
performed using a flow cytometer (FC500; Beckman Coulter, Brea, CA,
USA) and Kaluza software version 1.2 (Beckman Coulter).
Statistical analysis
Data are expressed as the mean ± standard deviation
from at least three experiments. Statistical analyses were
performed in SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
Comparisons between groups were conducted using one-way analyses of
variance and chi-square tests, followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
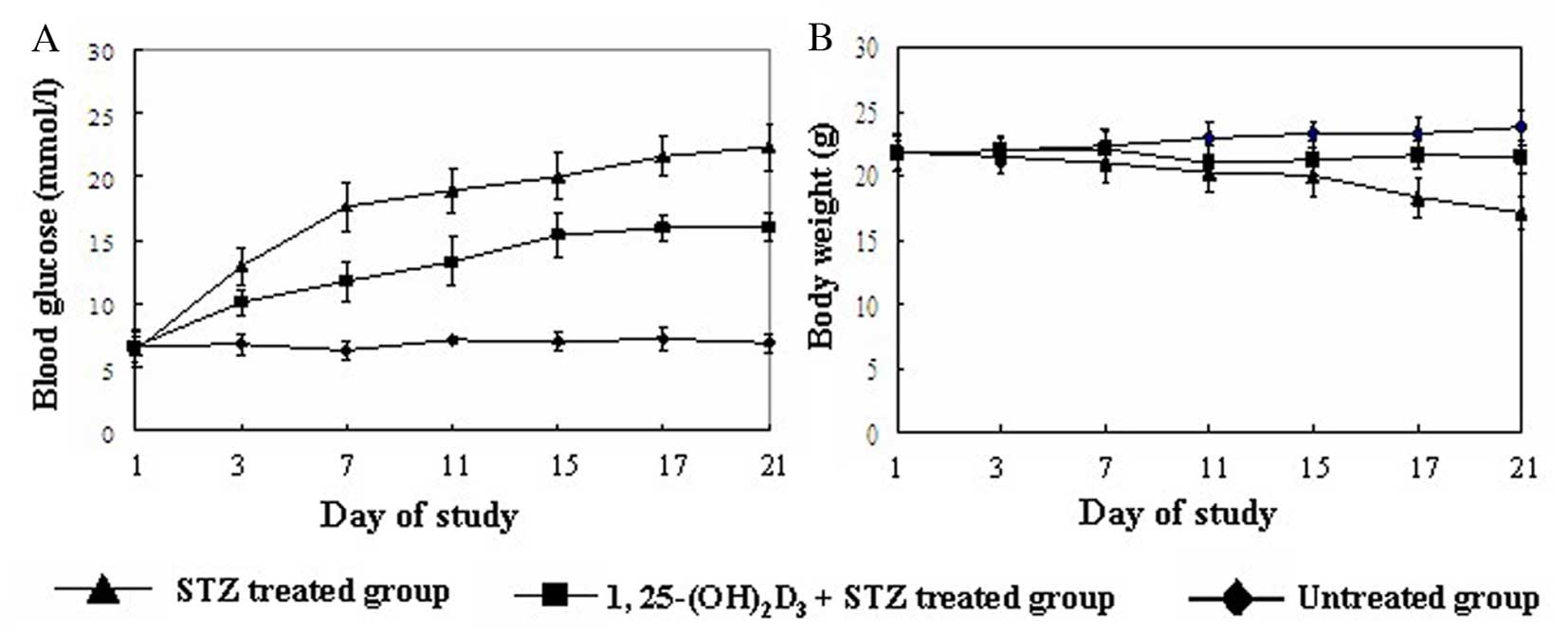
Vitamin D reduces the incidence of T1DM
and increases insulin secretion in the mouse model
To investigate the role of vitamin D in diabetes,
its effect on the incidence of diabetes and mouse phenotypes was
investigated. Treatment with 1,25(OH)2D3
markedly improved diabetes in mice, suggested by gradually
decreased blood glucose and increased body weight (Fig. 1). Mice presented with progressive
hyperglycemia following STZ treatment, and by 1 week following the
initial injection of STZ their plasma glucose levels were all
>16.7 mmol/l and diabetes was therefore defined. Administration
of 1,25(OH)2D3 significantly reduced the
incidence of diabetes (Table II).
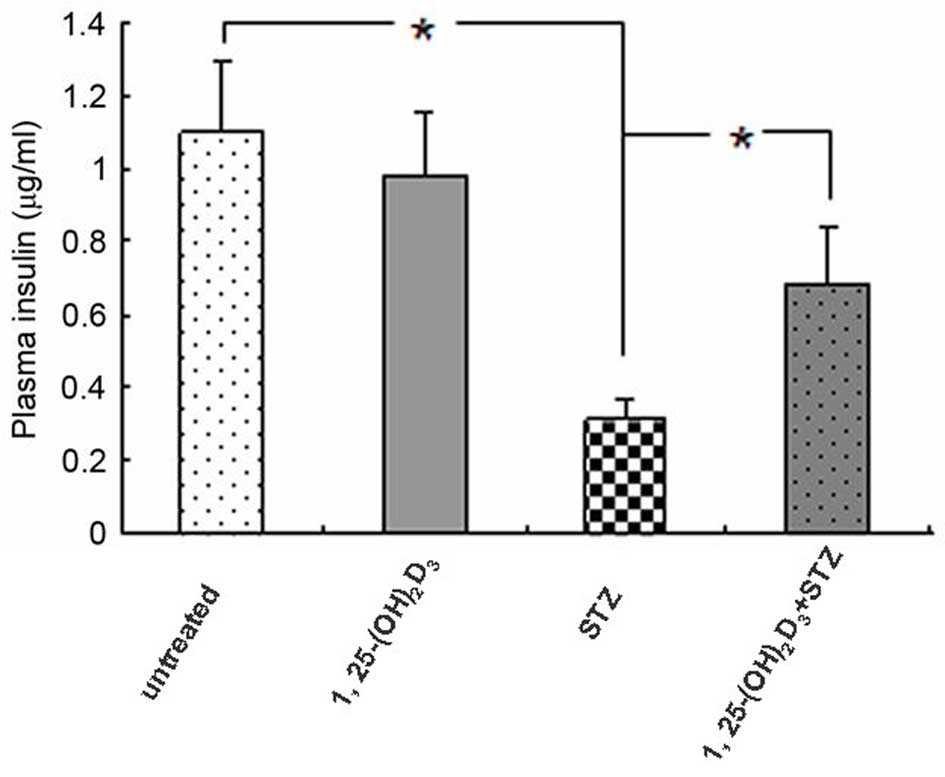
To evaluate pancreatic β-cell function with regard to insulin
secretion, mouse plasma insulin levels were measured on day 21. As
presented in Fig. 2, plasma
insulin levels were significantly decreased in STZ-treated mice,
and 1,25(OH)2D3 inhibited the STZ-induced
insulin reduction (P=0.033), suggesting a possible protective
effect against β-cell damage. Administration of
1,25(OH)2D3 alone had no effect on insulin
secretion compared with the untreated control group.
 | Table IIEffect of vitamin D on the incidence
of STZ-induced diabetes in mice on specific days following the
initiation of STZ treatment. |
Table II
Effect of vitamin D on the incidence
of STZ-induced diabetes in mice on specific days following the
initiation of STZ treatment.
| Group | Incidence of
diabetes, %
|
|---|
| Day 7 | Day 14 | Day 21 |
|---|
| STZ-treated | 100 | 100 | 100 |
| 1,25-(OH)2D3-
and | 0 | 20 | 40 |
| STZ-treated
-treatment | | | |
| P-value | 0.007 | 0.039 | 0.045 |
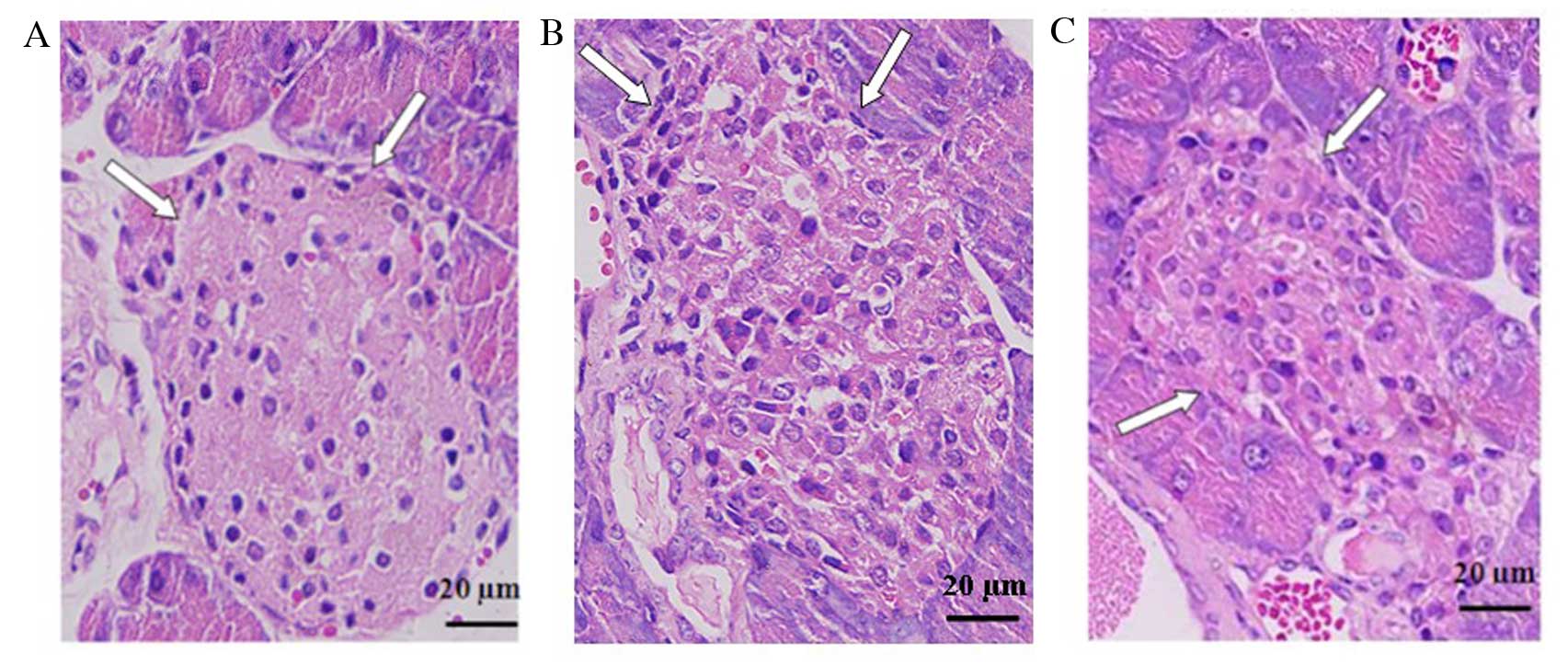
Vitamin D relieves insulitis of diabetic
mice
Histological analysis of the pancreas of STZ-induced
diabetic mice revealed cellular infiltration in and around islets,
distorted islets and β-cell degeneration. In accordance with the
declined incidence of diabetes, the administration of
1,25(OH)2D3 prior to STZ treatment markedly
reduced the infiltration of inflammatory cells and other
histopathological changes in pancreatic islets (Fig. 3). These results indicate that
vitamin D ameliorated islet lesions and β-cell destruction.
Vitamin D enhances insulin secretion of
STZ-treated MIN6 cells
To further investigate the in vivo data, the
effect of vitamin D on insulin secretion by MIN6 pancreatic β-cell
lines was assessed in vitro. Insulin secretion was
significantly decreased in STZ-treated MIN6 cells compared with the
untreated control group, and 1,25(OH)2D3
enhanced insulin secretion of STZ-treated cells in conditions of
low and high glucose concentrations (Table III).
 | Table IIIEffect of vitamin D on the insulin
secretion of STZ-treated MIN6 cells. |
Table III
Effect of vitamin D on the insulin
secretion of STZ-treated MIN6 cells.
| Group | Insulin secretion
(ng/ml)
|
|---|
| Low glucose | High glucose | High/low ratio |
|---|
| Untreated
control | 60.17±11.85 | 95.38±19.11 | 1.59±0.44 |
| STZ-treated | 39.95±8.13 | 42.66±9.05 | 1.07±0.19 |
| 1,25-(OH)2D3- and
STZ-treated | 49.82±5.99 | 60.37±13.32 | 1.21±0.21 |
| P-valuea | 0.044 | 0.033 | 0.041 |
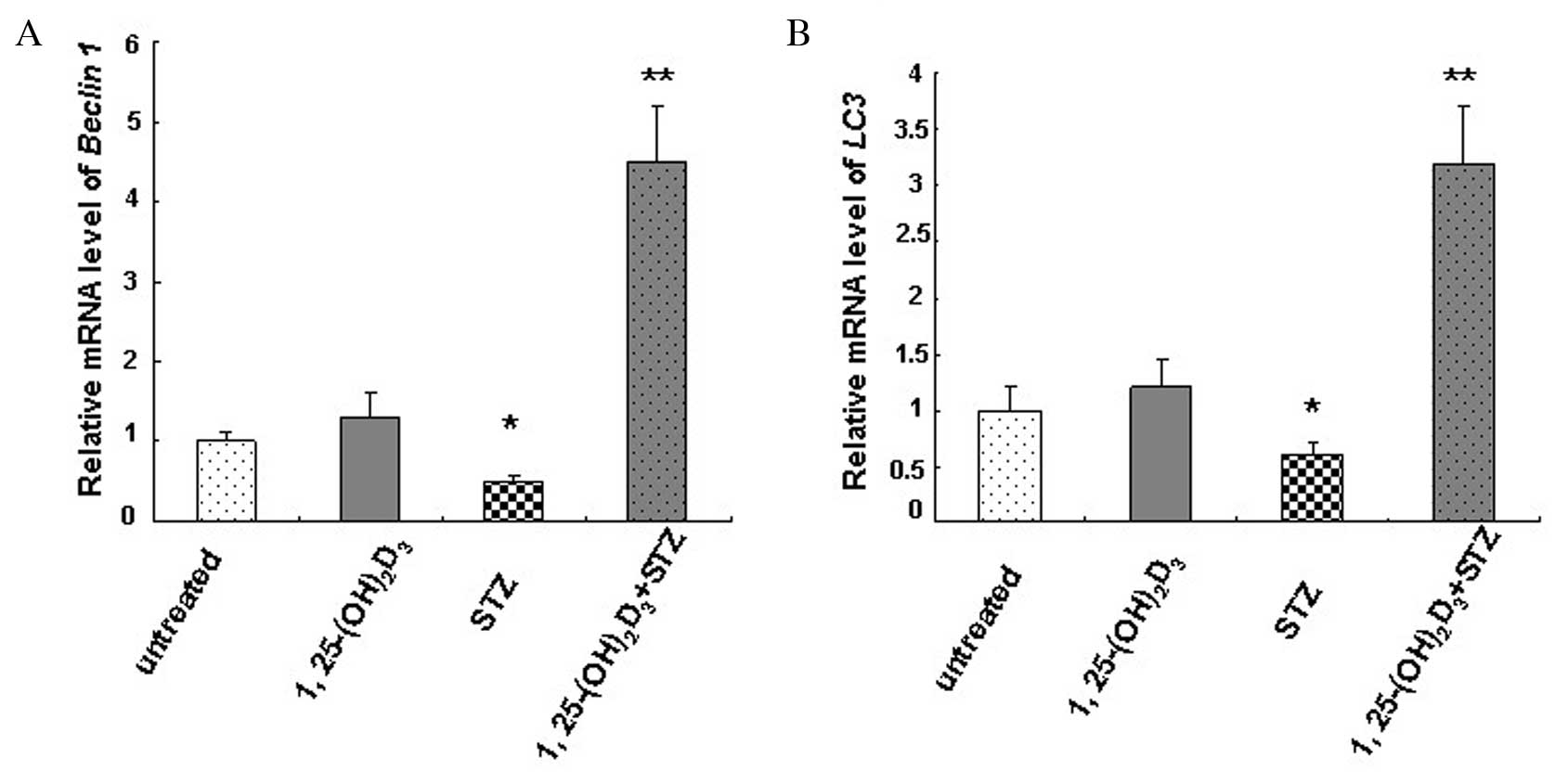
Vitamin D induces the expression of
Beclin 1, LC3 and Bcl-2
Relative mRNA expression levels of Beclin 1
(Fig. 4A) and LC3 (Fig. 4B) in MIN6 cells were determined by
RT-qPCR. The mRNA content of untreated MIN6 cells was designated as
1.0. The results revealed a significant decrease in Beclin 1
(0.5±0.08; P=0.032) and LC3 (0.6±0.1; P=0.047) mRNA
expression levels in the STZ-treated group compared with the
untreated control group, while 1,25(OH)2D3
significantly increased mRNA expression levels of Beclin 1
(4.5±0.7; P=0.009) and LC3 (3.2±0.5; P=0.007).
1,25(OH)2D3 treatment alone slightly
increased Beclin 1 and LC3 mRNA expression levels
compared with the untreated control group (Fig. 4). The results indicate an autophagy
deficiency in cells following STZ treatment and induction of
autophagy activity by vitamin D.
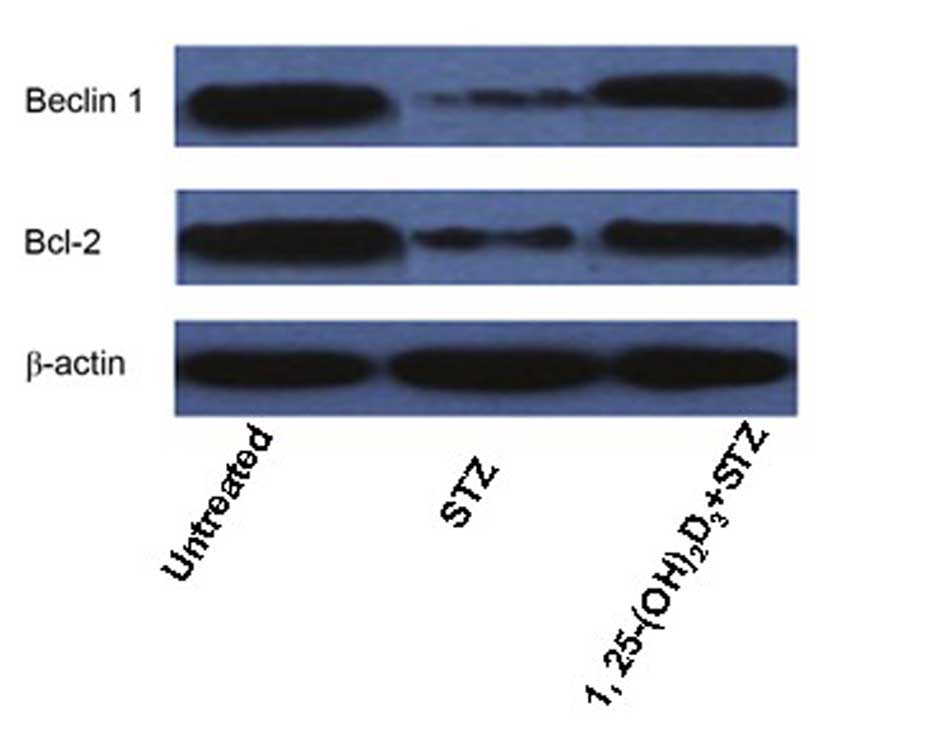
Western blotting revealed that the protein
expression levels of the autophagy and regulation of apoptosis
markers, Beclin 1 and Bcl-2, respectively, were markedly
downregulated in STZ-treated MIN6 cells compared with untreated
cells. This effect was attenuated by treatment with
1,25(OH)2D3 (Fig. 5).
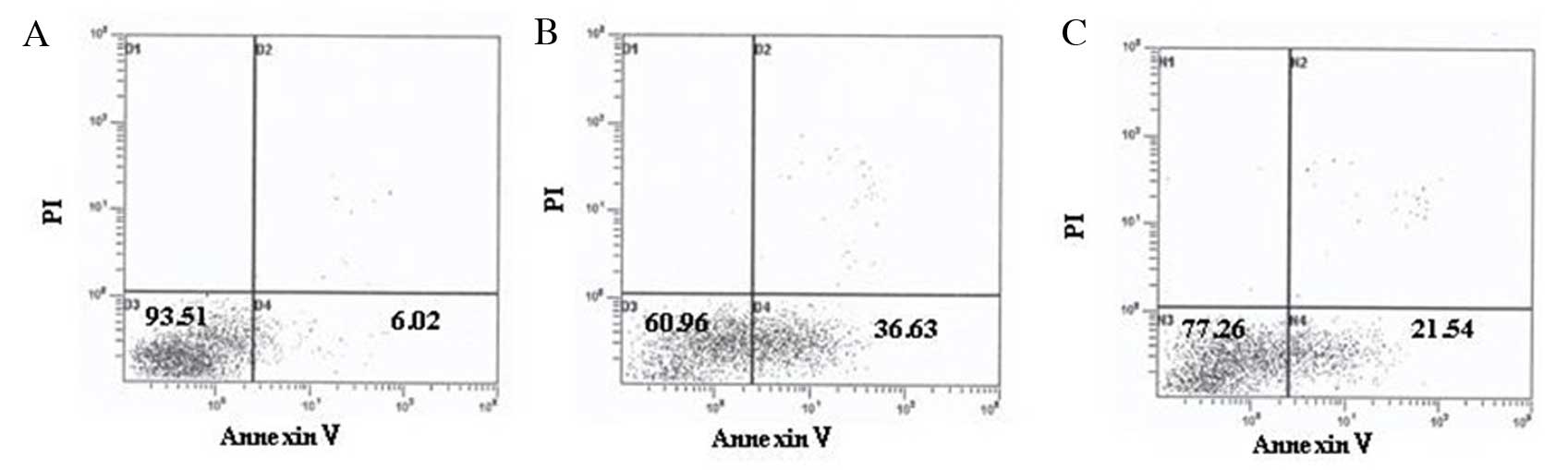
Vitamin D inhibits apoptosis of
STZ-treated MIN6 cells
As presented in Table
IV and Fig. 6, and consistent
with the western blotting results, apoptosis of STZ-treated MIN6
cells was markedly increased, and intervention with
1,25(OH)2D3 decreased the apoptosis rate
significantly.
 | Table IVEffect of vitamin D on the apoptosis
of STZ-treated MIN6 cells. |
Table IV
Effect of vitamin D on the apoptosis
of STZ-treated MIN6 cells.
| Group | Apoptosis rate
(%) | P-value |
|---|
| Untreated
control | 6.89±1.26 | 0.022a |
| STZ-treated | 37.96±7.51 | 0.026b |
| 1,25-(OH)2D3- and
STZ-treated | 20.77±3.18 | 0.033c |
Discussion
T1DM is a global disease of increasing incidence;
however, preventive measures and causal treatments remain lacking.
It is widely accepted that genetic predisposition and environmental
factors contribute to the development of diabetes. Despite the
evidence that vitamin D deficiency is one of the environmental risk
factors linked to the development of T1DM (20–23),
the precise underlying mechanisms remain to be fully elucidated.
The present study used a well-established T1DM animal model of
STZ-induced diabetes and the insulinoma cell line, MIN6 to
investigate the potential involvement of vitamin D in the
pathogenesis of diabetes, with an emphasis on its direct effects on
pancreatic islet β-cells.
During T1DM development, β-cell injury is induced by
infiltrating immune cells, leading to a progressive impairment of
insulin production and ultimately resulting in apoptosis (24). In the present study, insulin
secretion by STZ-treated β-cells was decreased and pre-treatment
with 1,25(OH)2D3 promoted insulin secretion.
STZ-treated mice exhibited cellular infiltration in pancreatic
islets and β-cell apoptosis, while vitamin D significantly reversed
insulitis and protected β-cells against apoptosis. These results
demonstrated that vitamin D directly affects β-cells and increases
their resistance to cellular stress encountered during
diabetes.
Autophagy is an important mechanism underlying cell
stability and acts as a defense against cellular stress. The
degradation of unnecessary and impaired cellular components by
autophagy is essential for the maintenance of normal cellular
architecture (9,10). It has been verified that autophagy
is important for the survival and function of β-cells (12,14).
In the present study, the mRNA expression levels of LC3 and
Beclin 1 in STZ-treated MIN6 cells were reduced compared
with untreated cells, while 1,25(OH)2D3
enhanced expression levels. LC3 and Beclin 1 are essential proteins
involved in autophagy, and Beclin 1 is regarded as a marker of
autophagy initiation (25). The
results of the present study suggested an autophagy deficit of
β-cells in this diabetes model, and demonstrated that vitamin D may
induce autophagy and therefore accelerate the renewal of organelles
under hyper-glycemic conditions. Autophagy activation may serve as
a compensatory response in protection from apoptosis (26). MIN6 cell damage induced by STZ was
a dynamic process, and injured cells may release certain
pro-apoptotic factors that may be removed via vitamin D-induced
autophagy. In addition, expression levels of the anti-apoptotic
protein Bcl-2 in STZ-treated MIN6 cells was increased by vitamin D
treatment, suggesting the suppression of apoptosis by vitamin D. It
has been reported that Beclin 1, a critical mediator of autophagy,
may be regulated by Bcl-2. The formation of a Beclin 1/Bcl-2
complex affects autophagy and apoptosis (27,28).
Autophagy is a dynamic process; preliminary data from our
laboratory using the inhibitors of autophagy, 3-methyladenine or
bafilomycin A1 and the autophagy agonist, rapamycin
in vitro and in vivo, has revealed a complex
association between vitamin D-mediated autophagy and its protective
effects. This association is currently being further investigated
in our laboratory, and will be reported in a future
publication.
In conclusion, the results of the present study
demonstrate, for the first time to the best of our knowledge, that
vitamin D induces autophagy and suppresses apoptosis of pancreatic
β-cells, as well as preventing insulitis. These results suggest a
potential novel strategy for the treatment of diabetes via agents
enhancing autophagy in pancreatic β-cells. Future studies are
required to investigate the associations between autophagy,
apoptosis and inflammation, and provide novel insights into the
involvement of vitamin D in diabetes.
Acknowledgments
The authors would like to thank Professor Jian Tong
(Soochow University, Suzhou, China) for helpful discussions. The
present study was supported by the Natural Science Foundation of
Jiangsu Province (grant no. BK20151217).
References
|
1
|
Jeon JY, Ha KH and Kim DJ: New risk
factors for obesity and diabetes: Environmental chemicals. J
Diabetes Invest. 6:109–111. 2015. View Article : Google Scholar
|
|
2
|
Santos RX, Correia SC, Alves MG, Oliveira
PF, Cardoso S, Carvalho C, Duarte AI, Santos MS and Moreira PI:
Insulin therapy modulates mitochondrial dynamics and biogenesis,
autophagy and tau protein phosphorylation in the brain of type 1
diabetic rats. Biochim Biophys Acta. 1842:1154–1166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mauf S, Penna-Martinez M, Jentzsch T,
Ackermann H, Henrich D, Radeke HH, Brück P, Badenhoop K and
Ramos-Lopez E: Immunomodulatory effects of 25-hydroxyvitamin D3 on
monocytic cell differentiation and influence of vitamin D3
polymorphisms in type 1 diabetes. J Steroid Biochem Mol Biol.
147:17–23. 2015. View Article : Google Scholar
|
|
4
|
Hoffmann MR, Senior PA and Mager DR:
Vitamin D supplementation and health-related quality of life: A
systematic review of the literature. J Acad Nutr Diet. 115:406–418.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sørensen IM, Joner G, Jenum PA, Eskild A,
Torjesen PA and Stene LC: Maternal serum levels of
25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes
in the offspring. Diabetes. 61:175–178. 2012. View Article : Google Scholar :
|
|
6
|
Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han
SF and Qin LQ: Vitamin D intake and risk of type 1 diabetes: A
meta-analysis of observational studies. Nutrients. 5:3551–3562.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Setty-Shah N, Maranda L and Nwosu BU:
Increased risk for vitamin d deficiency in obese children with both
celiac disease and type 1 diabetes. Gastroenterol Res Pract.
2014:5613512014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cadario F, Prodam F, Savastio S, Monzani
A, Balafrej A, Bellomo G and Bona G: Vitamin D status and type 1
diabetes in children: Evaluation according to latitude and skin
color. Minerva Pediatr. 67:263–267. 2015.PubMed/NCBI
|
|
9
|
Yoon SY and Kim DH: Alzheimer's disease
genes and autophagy. Brain Res pii. S0006–S8993. 2016.
|
|
10
|
Zhou Z, Wu S, Li X, Xue Z and Tong J:
Rapamycin induces autophagy and exacerbates metabolism associated
complications in a mouse model of type 1 diabetes. Indian J Exp
Biol. 48:31–38. 2010.PubMed/NCBI
|
|
11
|
Ding Y and Choi ME: Autophagy in diabetic
nephropathy. J Endocrinol. 224:R15–R30. 2015. View Article : Google Scholar
|
|
12
|
Lee MS: Role of islet β cell autophagy in
the pathogenesis of diabetes. Trends Endocrinol Metab. 25:620–627.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abe H, Uchida T, Hara A, Mizukami H,
Komiya K, Koike M, Shigihara N, Toyofuku Y, Ogihara T, Uchiyama Y,
et al: Exendin-4 improves β-cell function in autophagy-deficient
β-cells. Endocrinology. 154:4512–4524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watada H and Fujitani Y: Minireview:
Autophagy in pancreatic β-cells and its implication in diabetes.
Mol Endocrinol. 29:338–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amirshahrokhi K and Ghazi-Khansari M:
Thalidomide attenuates multiple low-dose streptozotocin-induced
diabetes in mice by inhibition of proinflammatory cytokines.
Cytokine. 60:522–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikegami M, Ikeda H, Ohashi T, Kai M, Osada
M, Kamei A and Kamei J: Olanzapine-induced hyperglycemia: Possible
involvement of histaminergic, dopaminergic and adrenergic functions
in the central nervous system. Neuroendocrinology. 98:224–232.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uemura M, Toda I, Kawashima W, Yoshimoto
G, Fang YR, Xu YJ, Liu Y, Zhang L and Takemura A: Morphological
study of the articular disc and capillary of the retrodiscal tissue
in a type 2 spontaneous diabetes mellitus rat model. Okajimas Folia
Anat Jpn. 92:53–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okada H, Senmaru T, Fukui M, Kondo Y,
Ishigami A, Maruyama N, Obayashi H, Yamazaki M, Nakamura N and
Hasegawa G: Senescence marker protein-30/gluconolactonase
deficiency exacerbates diabetic nephropathy through tubular injury
in a mouse model of type 1 diabetes. J Diabetes Investig. 6:35–43.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative CR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Wolden-Kirk H, Overbergh L, Christesen HT,
Brusgaard K and Mathieu C: Vitamin D and diabetes: Its importance
for beta cell and immune function. Mol Cell Endocrinol.
347:106–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wranicz J and Szostak-Węgierek D: Health
outcomes of vitamin D, Part II: Role in prevention of diseases.
Rocz Panstw Zakl Hig. 65:273–279. 2014.
|
|
22
|
Grant WB: Low vitamin D concentrations may
contribute to the increased risk of diabetes mellitus related to
shift work. Occup Environ Med. 72:1612015. View Article : Google Scholar
|
|
23
|
Gruber BM: The phenomenon of vitamin D.
Postepy Hig Med Dosw (Online). 69:127–139. 2015.
|
|
24
|
Liu L, Liu JL and Srikant CB: Reg2
protects mouse insulinoma cells from streptozotocin-induced
mitochondrial disruption and apoptosis. Growth Factors. 28:370–378.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wirawan E, Lippens S, Vanden Berghe T,
Romagnoli A, Fimia GM, Piacentini M and Vandenabeele P: Beclin1: A
role in membrane dynamics and beyond. Autophagy. 8:6–17. 2012.
View Article : Google Scholar
|
|
26
|
Zou MH and Xie Z: Regulation of interplay
between autophagy and apoptosis in the diabetic heart: New role of
AMPK. Autophagy. 9:624–625. 2013. View Article : Google Scholar :
|
|
27
|
Shi M, Cheng L, Zhang Z, Liu Z and Mao X:
Ferroferric oxide nanoparticles induce prosurvival autophagy in
human blood cells by modulating the Beclin 1/Bcl-2/VPS34 complex.
Int J Nanomedicine. 10:207–216. 2014.
|
|
28
|
Marquez RT and Xu L: Bcl-2:Beclin 1
complex: Multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|