Introduction
Chronic hepatitis B virus (HBV) infection is caused
by a complex interaction between a replicating noncytopathic virus
and aberrant host antiviral immunity (1,2).
Persistent infection causes chronic liver cell injury,
regeneration, inflammation, widespread DNA damage and insertional
dysregulation of cellular growth control genes. These factors
consequently result in the development of cirrhosis and
hepatocellular carcinoma (3,4).
Persistent HBV infection is characterized by a weak adaptive immune
response, which is presumed to be due to insufficient
CD4+ T-cell priming early in the infection, and the
subsequent development of an ineffective CD8+ T-cell
response (5,6).
Virus-specific CD8+ T cells are able to
efficiently control HBV replication predominantly by noncytolytic
mechanisms, and this effect is mediated by the secretion of
interferon (IFN)-γ and tumor necrosis factor (TNF)-α cytokines,
thus limiting viral spread to uninfected cells and reducing the
degree of immunopathology required to clear the infection (3,7).
Virus-infected cells, or cells that have undergone malignant
transformation, are eliminated by cytotoxic T lymphocytes (CTLs),
which recognize antigenic peptide epitopes in complex with major
histocompatibility complex class I (MHC I) molecules at the cell
surface (7). The majority of these
peptides are derived via proteasomal degradation in the cytosol,
and are subsequently translocated into the lumen of endoplasmic
reticulum (ER) in an energy-consuming manner by the transporter
associated with antigen processing (TAP), which delivers peptides
onto MHC I molecules as final acceptors. Previous studies have
suggested that the molecular chaperone tapasin, which mediates the
binding of TAP, stabilizes peptide-receptive MHC I conformation by
facilitating peptide exchange and allowing more peptides to be
translocated into the ER, thus enhancing specific MHC class
I-restricted CTL activity (8,9). The
loading of antigen-derived peptides onto MHC class I molecules for
presentation to CTLs is a key process in the adaptive immune
response, which has an important role in HBV clearance (9).
Our previous study demonstrated that the expressed
and purified fusion protein cytoplasmic transduction peptide
(CTP)-HBV core antigen (HBcAg)18–27-Tapasin could enter
the cytoplasm of bone marrow-derived dendritic cells (BMDCs),
promoting BMDC maturation and efficiently enhancing the T-cell
immune response in vitro (1). Furthermore, the
CTP-HBcAg18–27-Tapasin fusion protein could enhance the
percentage of CTLs, induce robust specific CTL activity, reduce
apoptosis of CD8+ T cells and inhibit HBV replication
in vivo (10,11). It has also been demonstrated that
chronic HBV (CHB) infection is associated with reduced
antigen-presenting capacity and insufficient CTL production
(12). The molecular chaperone
tapasin, which mediates binding of TAP, has an important role in
endogenous antigen processing and presentation, and is able to
induce specific CTL responses (13). The present study aimed to determine
whether tapasin is associated with CHB infection. The present study
investigated whether tapasin mRNA expression was correlated with
impaired CD8+ T-cell function in peripheral blood
mononuclear cells (PBMCs) from patients with CHB.
Materials and methods
Reagents and antibodies
The anti-CD8α (cat. nos. 11-0086 and 17-0086) and
IFN-γ (cat. no. 11-4724) fluorescent antibodies and corresponding
isotype controls (cat. nos. 17-4724, 12-4714 and 11-4724) were
obtained from eBioscience, Inc. (San Diego, CA, USA). The Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit was
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for IFN-γ,
TNF-α and interleukin (IL)-2 were obtained from R&D Systems,
Inc. (Minneapolis, MN, USA). Ionomycin, monensin and phorbol
12-myristate 13-acetate (PMA) were purchased from Sigma-Aldrich
(Merck Millipore, Darmstadt, Germany).
Patients
Peripheral heparinized blood samples were obtained
from patients with CHB infection (n=27) and acute HBV infection
(AHB; n=20). All patients were positive for HBV surface antigen
(HBsAg) and HBV envelope antigen. All patients were confirmed to be
negative for other viral infections, including hepatitis C,
hepatitis D and human immunodeficiency virus. The presence of other
liver diseases, including alcoholic, metabolic or autoimmune
hepatitis, was ruled out. Patients had not received any antiviral
treatment or immunotherapy for 6 months prior to blood collection.
Healthy controls (n=26) (age-, gender- and race-matched) had no
evidence of prior exposure to HBV (HBsAg-negative). All donors
provided written informed consent. The clinical characteristics of
the patients are listed in Table
I. Experiments and procedures were conducted in accordance with
the Helsinki Declaration of 1975, and were approved by the Human
Ethics Committee of Shanghai Jiaotong University, School of
Medicine (Shanghai, China).
 | Table IClinical characteristics of patients
with chronic HBV and acute HBV. |
Table I
Clinical characteristics of patients
with chronic HBV and acute HBV.
| Characteristic | Chronic HBV
patients | Acute HBV
patients | Healthy
controls |
|---|
| Number | 27 | 20 | 26 |
| Age (years)a | 35±9 | 36±15 | 29±5 |
| Gender (M/F) | 24/3 | 15/5 | 17/9 |
| ALT (IU/l)a | 164±101.8 | 2207±616.5 | 23±7.6 |
| HBV-DNA
(copies/ml) |
1.32×103–6.74×107 |
5.68×104–8.6×107 | Negative |
Cell isolation
PBMCs were obtained from fresh heparinized blood by
Ficolle Isopaque gradient centrifugation. Briefly, peripheral
venous blood (10 ml) samples were collected from patients in a
heparinized test tube. The entire blood sample was diluted to a
final volume of 20 ml in phosphate-buffered saline (PBS). The
suspension was then poured into 10 ml Ficoll-Biocoll separating
solution (Dakewe Biotech Co., Ltd., Beijing, China) in a conical
tube. Following density gradient centrifugation (1,007.1 × g, 20°C,
20 min), PBMCs were isolated. The isolated cells were carefully
collected and washed twice with sterile PBS (566.5 × g, 20°C, 10
min,) and re-suspended in RPMI-1640 (Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cell viability was determined
by trypan blue staining and cells were counted; cell viability
should always be >95%. The final cell density was adjusted to
5-6×106 cells/ml. PBMCs from healthy volunteers were
isolated in the same manner. Furthermore, serum samples were
obtained from 5 ml blood, which was centrifuged for 5 mins at
2,389.5 × g to obtain ~2 ml plasma. The samples were added to a
24-well culture plate and maintained at −20°C for subsequent
experiments.
Cytokine level measurement
The serum concentrations of IFN-γ, IL-2 and TNF-α
were detected by Quantikine ELISA kits (DTA00C, DIF50 and D2050;
R&D Systems) according to the manufacturer's protocols. The
cytokine concentrations in the samples were determined from
standard curves. Data are expressed as pg/ml.
Lymphocyte proliferation activity
assay
The PBMCs (1×106 cells/ml) were cultured
in 96-well culture plates at a final volume of 100 µl in the
presence of 5 µg/ml phytohemagglutinin (PHA) solution at
37°C, in an atmosphere containing 5% CO2, for 48 h.
Subsequently, 10 µl Cell Counting kit-8 solution (Beyotime
Institute of Biotechnology, Haimen, China) was added to the plates
at 37°C in an atmosphere containing 5% CO2 for 4 h
(14). Absorbance was finally
measured at a wavelength of 450 nm.
Assessment of apoptosis ex vivo
As aforementioned, PBMCs (1×106 cells/ml)
were cultured in 6-well plates at 37°C. After washing with PBS,
cells were stained with saturating concentrations of
allophycocyanin-conjugated anti-CD8α McAb (cat. no. 17-0086) and
isotype control (cat. no. 17-4724) for 15 min. Annexin V-FITC and
propidium iodide staining (Invitrogen; Thermo Fisher Scientific,
Inc.) was then conducted according to the manufacturer's protocol,
except that all staining was performed on ice. The percentage of
cells highly stained with Annexin V among the antigen-specific
CD8+ T cells was determined by flow cytometry.
Fluorescence analyses were performed on a COULTER EPICS XL Flow
Cytometer (Beckman Coulter, Inc., Brea, CA, USA) using Expo32-ADC
software.
Intracellular staining for IFN-γ
To evaluate the percentage of IFN-γ-secreting cells
in the CD8+ T-cell population from PBMC samples, single
cell (1×106 cells/ml) suspensions were analyzed by flow
cytometry. PBMCs were stimulated in the presence of 10 µg/ml
HBcAg for 6 h. After incubation for 3 h, 1 µg/ml ionomycin,
25 µg/ml PMA and 1.7 µg/ml monensin (Sigma-Aldrich;
Merck Millipore) were added and the cells were incubated for a
further 3 h (15). After washing
with PBS, cells were stained with phycoerythrin-labeled anti-CD8α
McAb (cat. no. 11-0086) and isotype control (cat. no. 11-4724) for
15 min. The cells were then fixed with Fix and Perm reagent A and B
(BD Biosciences) and were incubated for 30 min with saturating
concentrations of FITC-conjugated anti-IFN-γ McAb (cat. no.
12-7319; eBioscience, Inc.). Subsequently, the cells were washed
twice with PBS and were analyzed by flow cytometry. Fluorescence
analyses were performed on a COULTER EPICS XL Flow Cytometer using
Expo32-ADC software 1.0 (Beckman Coulter, Brea, CA, USA).
RNA and cDNA preparation from PBMCs
Peripheral blood was collected in sodium
citrate-containing cell preparation tubes. PBMCs were separated by
density gradient centrifugation. Subsequently, total RNA was
extracted from the PBMCs (5×105) using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc,) and
was treated with RNase-free DNase (Qiagen GmbH, Hilden, Germany) to
remove genomic DNA contamination. RNA (1 µg) was reverse
transcribed to cDNA using a reverse transcription (RT) system kit
(Promega Corporation, Madison, WI, USA), according to the
manufacturer's instructions.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using SYBR® Premix
Ex Taq reagents (Takara Bio Inc., Otsu, Japan) on a LightCycler
(Roche Diagnostics, Basel, Switzerland). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as an internal reference. Primers
were designed by Primer Premier 5.0 (Premier Biosoft, Palo Alto,
CA, USA) according to the mRNA sequences of tapasin retrieved from
GenBank (http://www.ncbi.nlm.nih.gov/genbank/), and were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The PCR
primer sequences were as follows: Tapasin, forward
5′-GAGTGTTGGTTCGTGGAGGAT-3′, reverse, 5′-TGGCTGTGGTCGCAAGAGG-3′;
and GAPDH, forward 5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse
5′-GGGGTCATTGATGGCAACAATA-3′. The PCR cycling conditions were as
follows: 30 sec at 95°C followed by 40 cycles of 95°C for 5 sec and
60°C for 30 sec, and a final step of 95°C for 15 sec, 60°C for 1
min and 95°C for 15 sec. The expression levels of the target gene
were calculated using the 2−ΔΔCq method (16). Three parallel reactions of each
sample and internal controls were run.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and were analyzed by SPSS 16.0 software (SPSS Inc.,
Chicago, IL, USA). One-way analysis of variance and post-hoc least
significant difference test were used to determine the statistical
significance in comparison to the control. The linear regression
and bivariate correlation tests were used to analyze correlations
among the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
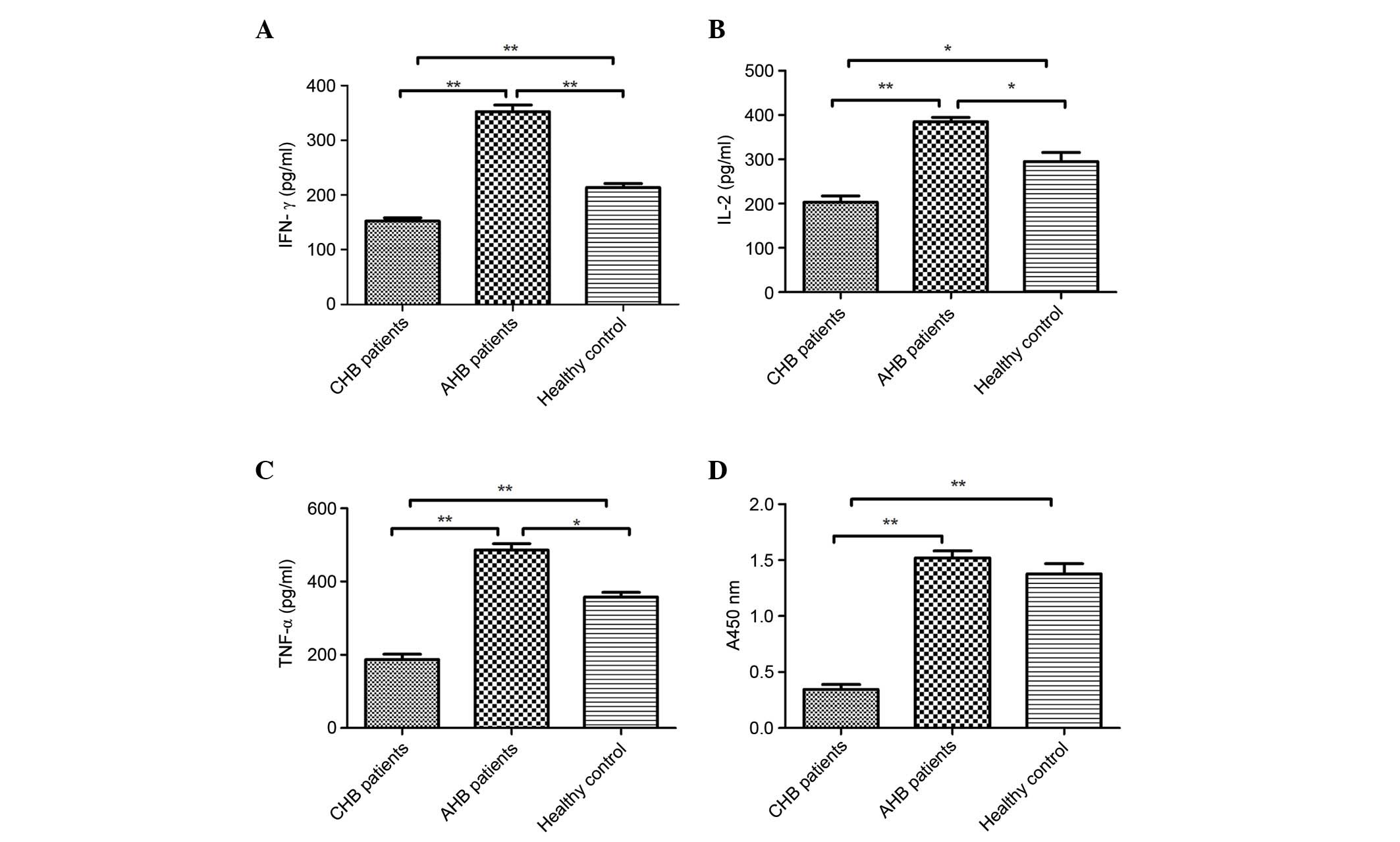
Cytokine serum levels (IFN-γ, IL-2 and
TNF-α) and lymphocyte proliferative activity
The inability of CD8+ T cells to produce
three cytokines is a hallmark of functional exhaustion (17,18).
Therefore, the present study analyzed the levels of the following
cytokines: IFN-γ, IL-2 and TNF-α in the serum of all groups. As
shown in Fig. 1A–C, IFN-γ
(152.63±5.43 pg/ml), TNF-α (187.12±14.68 pg/ml) and IL-2
(202.84±14.41 pg/ml) levels were significantly lower in the serum
of patients with CHB compared with in those with AHB (IFN-γ,
352.64±12.03 pg/ml; TNF-α, 485.91±17.45 pg/ml; and IL-2,
384.73±10.17 pg/ml) and the normal control group (IFN-γ,
213.52±7.25 pg/ml; TNF-α, 358.21±12.71 pg/ml; and IL-2,
295.23±20.35 pg/ml). These findings suggest that cytokine levels
(IFN-γ, IL-2 and TNF-α) in the serum of patients with CHB are
generally low, and dysregulation of cellular immunity exists in
these patients.
The present study used PHA-induced lymphocyte
proliferation to assess the proliferative activity of lymphocytes
in the various groups. As shown in Fig. 1D, the proliferative activity of
lymphocytes in patients with CHB (0.34±0.05%) was lower than in the
normal control group (1.38±0.09%) and AHB group (1.52±0.06%)
(P<0.01), thus suggesting that lymphocytes in patients with CHB
have functional defects. Furthermore, there were no significant
differences in the proliferative activity of lymphocytes between
the AHB and healthy control groups (P>0.05).
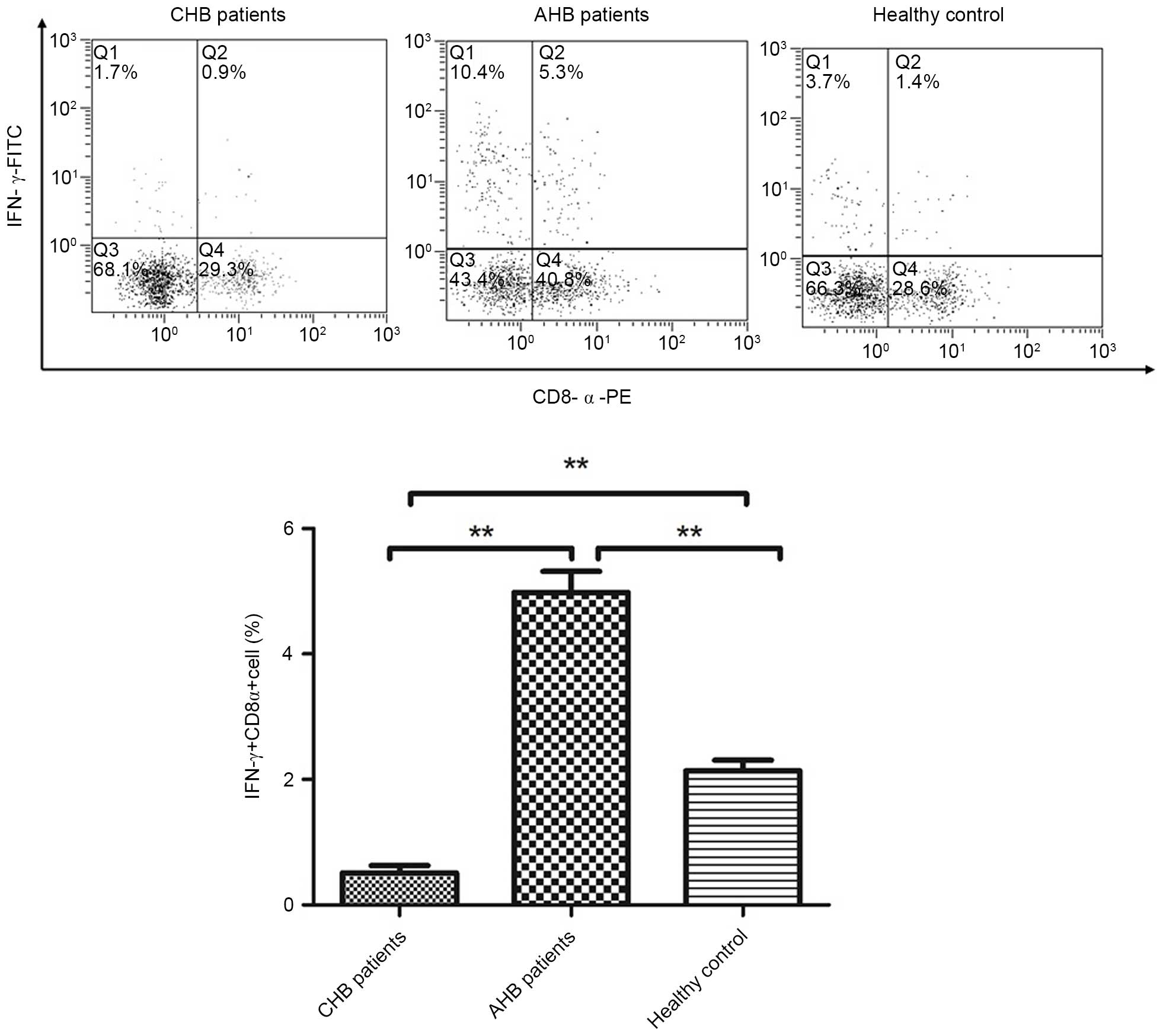
Percentage of IFN-γ-secreting cells in
the CD8+ T-cell population
The intracellular expression of IFN-γ in the
CD8+ T-cell population was detected in patients and
healthy controls using flow cytometry (Fig. 2). Secretion of IFN-γ in
CD8+ T cells was lower in patients with CHB (0.51±0.11%)
compared with in the normal control group (2.14±0.17%) and AHB
group (4.98±0.33%) (P<0.01), thus suggesting that
CD8+ T-cell function is defective in patients with
CHB.
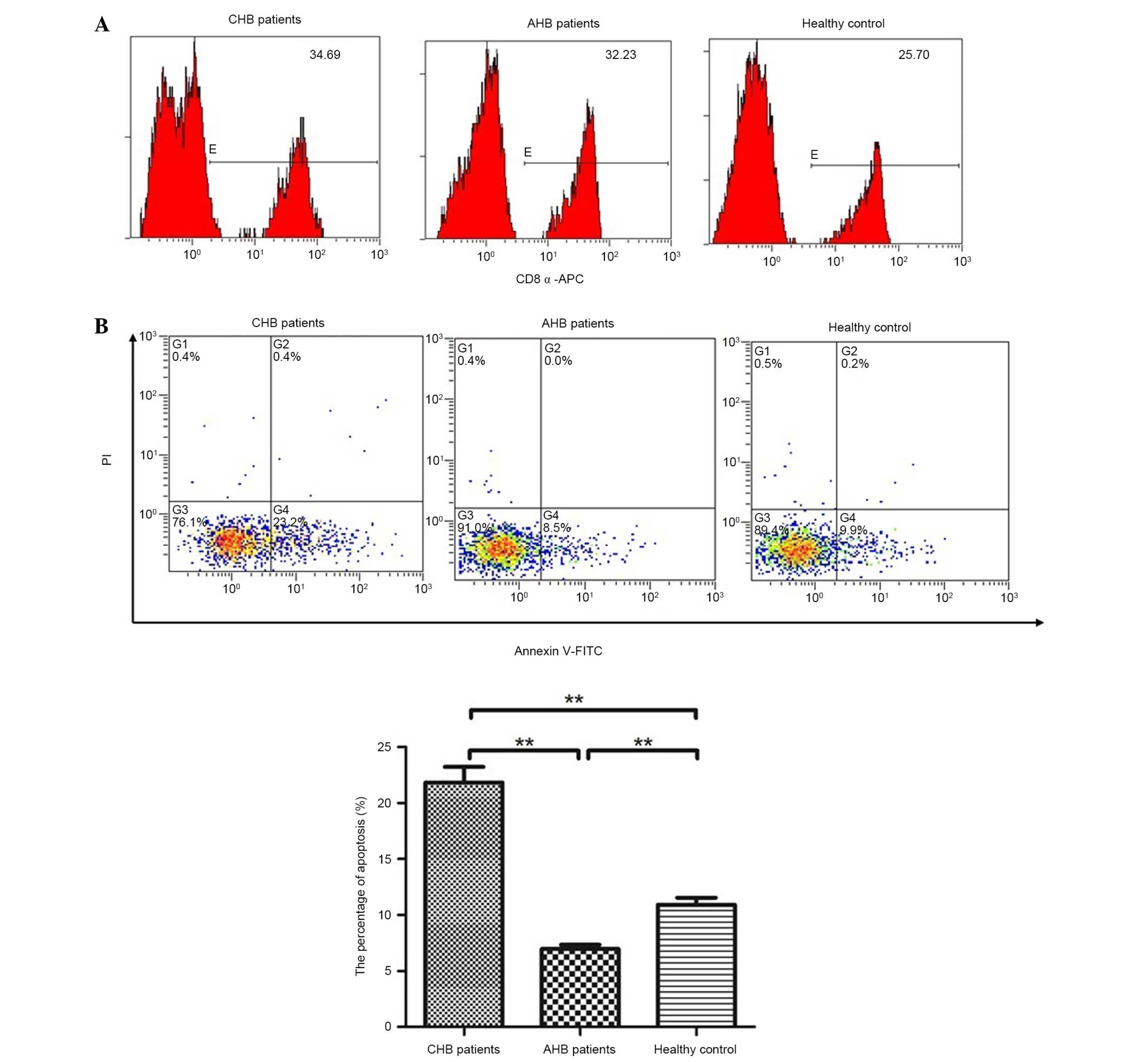
Apoptotic ratio of CD8+ T
cells
The apoptotic ratio of CD8+ T cells was
analyzed in patients and healthy controls by flow cytometry. The
number of cells stained with CD8-APC, Annexin V-FITC and PI was
counted by flow cytometry. As shown in Fig. 3, apoptosis of CD8+ T
cells was significantly reduced in patients with AHB (6.96±0.39%).
However, apoptosis was increased in the patients with CHB
(21.85±1.39%), which is consistent with the aforementioned results.
Therefore, apoptosis of CD8+ T lymphocytes in patients
with CHB may lead to functional defects.
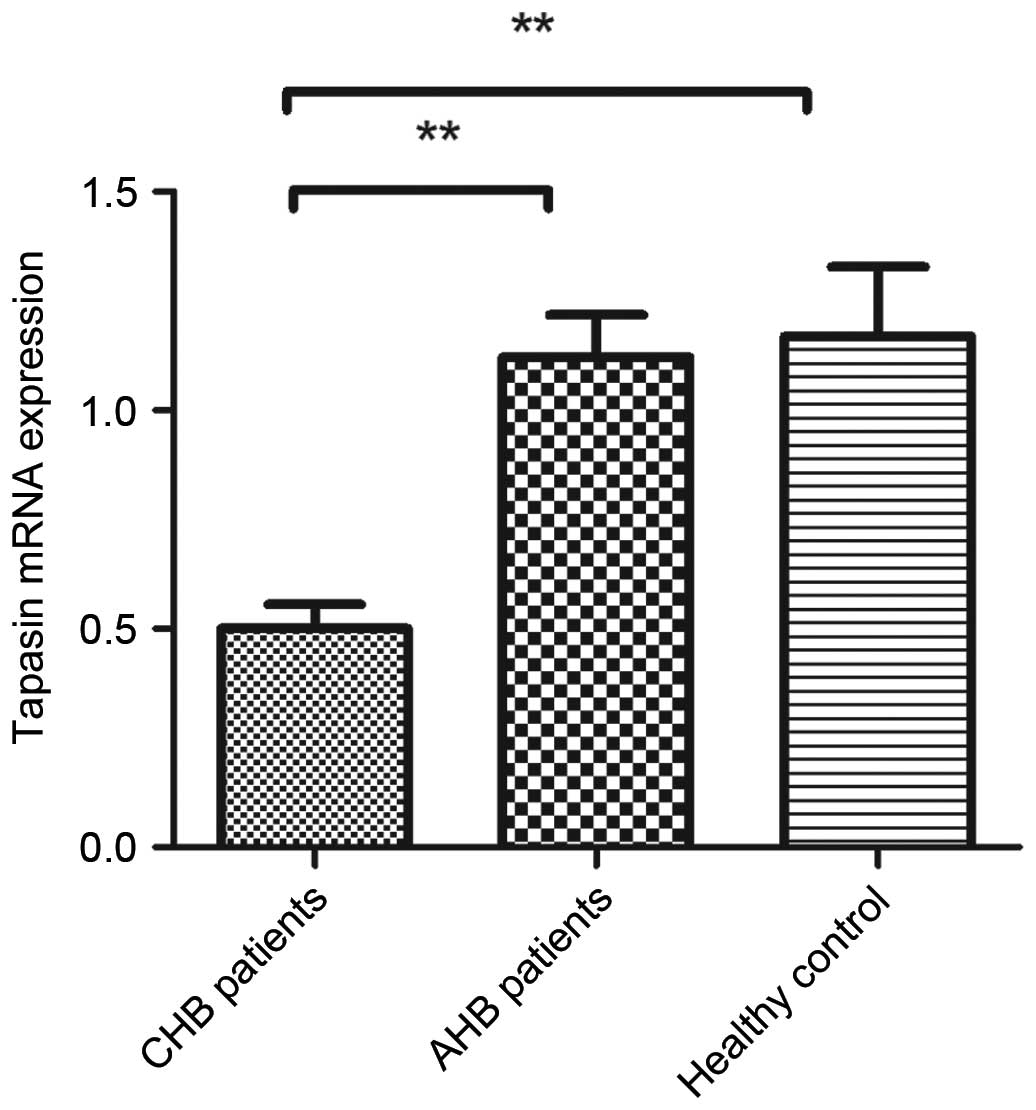
Quantification of tapasin mRNA expression
in PBMCs from patients and healthy controls by qPCR
To explore underlying mechanisms, combined with our
previous studies, the mRNA expression levels of tapasin were
detected in PBMCs from patients and healthy controls using RT-qPCR.
The expression levels of tapasin were significantly downregulated
in patients with CHB (Fig. 4);
however, tapasin expression was significantly unregulated in
patients with AHB. Furthermore, there were no significant
differences in the mRNA expression levels of tapasin between the
AHB and healthy control groups (P>0.05).
Tapasin expression is positively
correlated with IFN-γ production in CD8+ T cells
It is well known that IFN-γ-secreting
CD8+ T cells are essential for protective immunity
against viral infections. Therefore, the correlation between
tapasin expression and CD8+ T-cell activity was
investigated. As shown in Fig. 2,
compared with the normal control and AHB groups, the
CD8+ T-cell frequency in patients with CHB was
significantly decreased, thus indicating a compromised antiviral
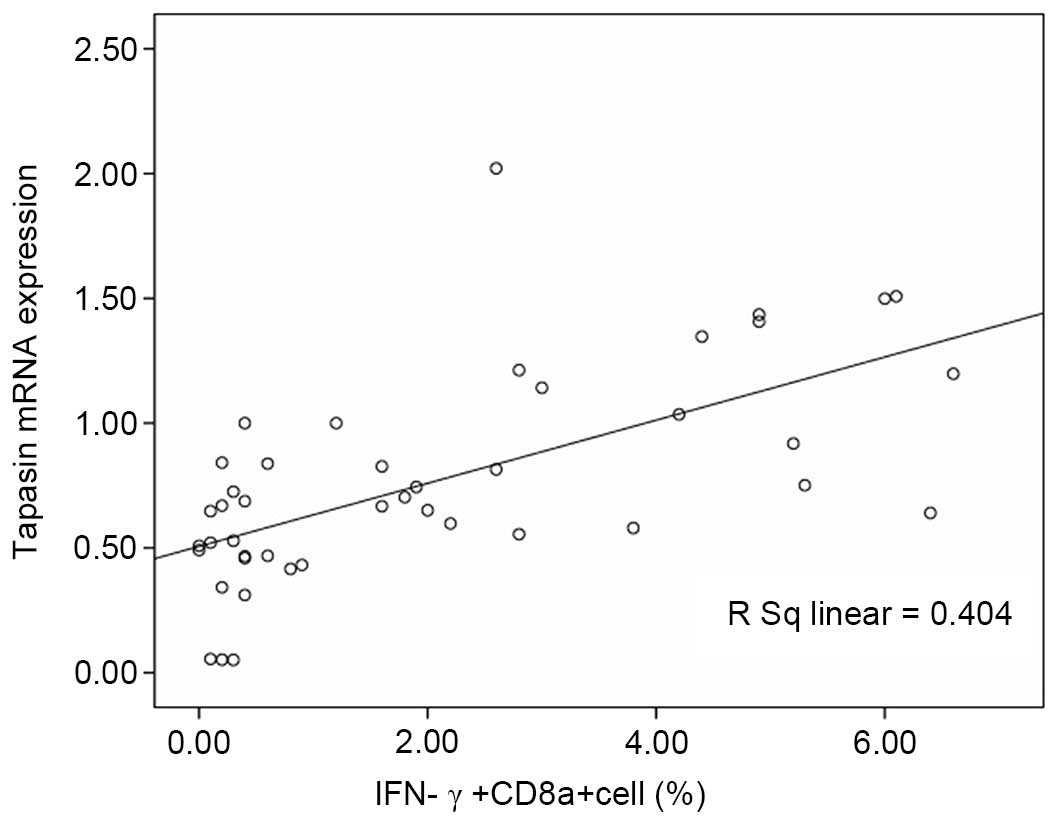
immune response. Furthermore, a positive correlation was detected
between the proportion of IFN-γ+CD8+ T cells
and tapasin mRNA expression (Fig.
5; F=28.513, P=0.001; r=0.636, P=0.001).
Tapasin mRNA expression is inversely
correlated with the apoptotic ratio of CD8+ T cells
During CHB, there is a continuum of T-cell
proliferation and apoptosis, and the balance between these cellular
processes controls the abundance of virus-specific CD8+
T cells. As shown in Fig. 3,
compared with the normal control and AHB groups, the percentage of
apoptotic CD8+ T cells was significantly increased in
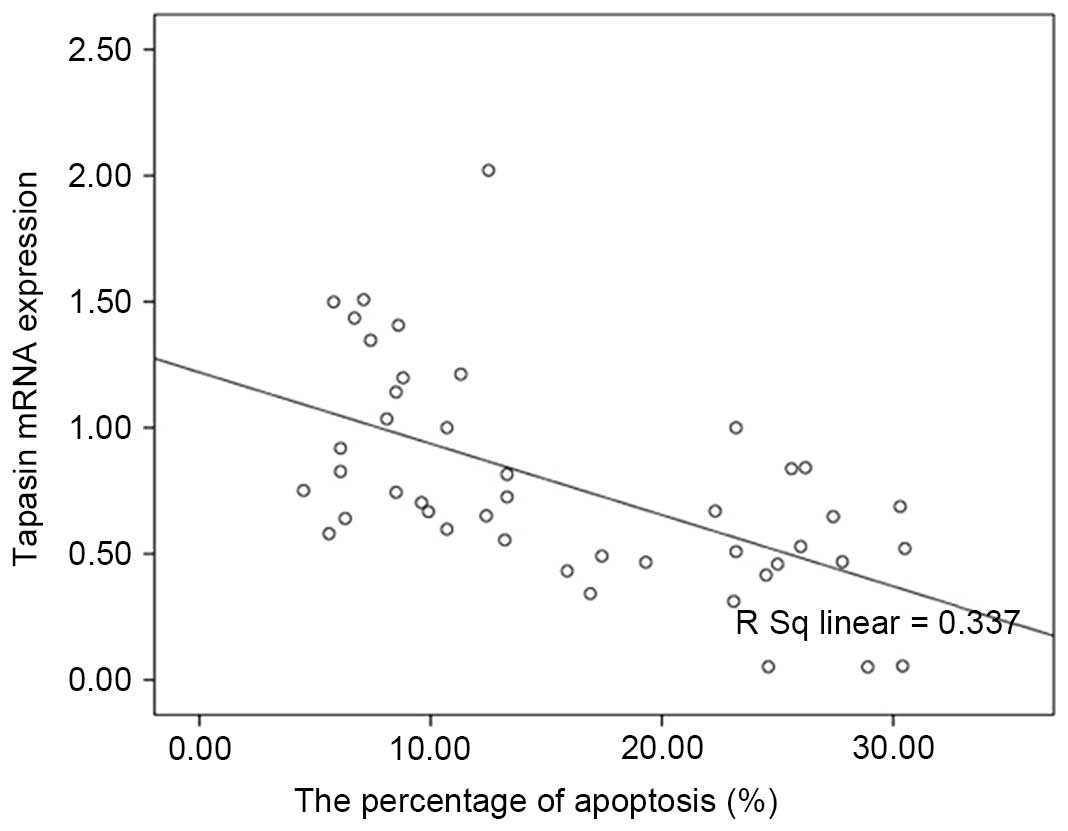
patients with CHB. In addition, an inverse correlation was detected
between CD8+ T-cell apoptosis and tapasin mRNA
expression (Fig. 6; F=21.391,
P=0.001; r=−0.581, P=0.001).
Discussion
Persistent infection with HBV affects >360
million people worldwide and is a serious public health problem,
since HBV infection is a leading cause of cirrhosis and
hepatocellular carcinoma-associated mortality (19,20).
The T-cell response to HBV is vigorous, polyclonal and
multispecific in patients with AHB who successfully clear the
virus; however, the response is relatively weak and narrowly
focused in patients with CHB, particularly the HBV-specific
CD8+ T-cell response, which has an important role in the
process of HBV clearance (21). A
common factor underlying viral persistence in CHB infection is the
dysregulation of virus-specific T-cell responses (22). During CHB, there is a continuum of
T-cell proliferation and apoptosis; the balance between these
cellular processes is responsible for the abundance of
virus-specific CD8+ T cells (23,24).
The presentation of products of proteasomal
degradation on MHC I molecules at the cell surface is used to carry
information regarding the cellular proteome to CTLs and natural
killer cells, thus enabling them to monitor intracellular events,
and detect infection and tumorigenesis (25). Efficient antigen processing by MHC
I relies on the peptide-loading complex (PLC), of which
heterodimeric TAP is the center-piece. The PLC recruits the
peptide-receptive MHC I heavy-chain/β2-microglobulin
dimer via the adapter protein tapasin. Tapasin is a type I membrane
protein, which has critical roles in the expression and
presentation of antigens, and fulfills various functions within the
PLC. It has previously been indicated that the predominant function
of tapasin is the substantial enhancement of peptide loading. In
the absence of functional tapasin, the surface expression of mouse
H2 and human leukocyte antigen (HLA) class I molecules is usually
severely impaired. Therefore, these cells exhibit strongly reduced
levels of surface HLA class I molecules in the presentation of
antigens to the CD8+ T cells (9,26,27).
Tapasin acts as a peptide editor/facilitator, thus promoting
immunodominant peptide binding (28). Previous studies have reported that
peptide exchange is accelerated by tapasin and low-affinity
peptides dissociate faster (29,30).
For several human and mouse class I allotypes, tapasin increases
cell surface class I molecule expression (29). Therefore, tapasin may be considered
a key factor in overcoming immune suppression.
Our previous study demonstrated that tapasin
modification on the intracellular epitope HBcAg18–27 via
CTP could enter the cytoplasm of BMDCs, thus promoting BMDC
maturation, and efficiently enhancing the T-cell immune response
in vitro (1). Furthermore,
the CTP-HBcAg18–27-Tapasin fusion protein was able to
enhance the percentage of CTLs, induce robust specific CTL
activity, reduce apoptosis of CD8+ T cells and inhibit
HBV replication in vivo (10,11).
These findings suggested that tapasin may be a key factor in the
process of HBV clearance. Previous studies have reported that CHB
infection is associated with reduced antigen-presenting capacity
and insufficient production of CTLs. The molecular chaperone
tapasin, which mediates the binding of TAP, has an important role
in endogenous antigen processing and presentation, and the
induction of specific CTL responses (31,32).
Therefore, the present study aimed to determine whether tapasin is
associated with CHB infection. The present study demonstrated that
IFN-γ, TNF-α and IL-2 levels were significant reduced in the serum
of patients with CHB compared with in patients with AHB and normal
controls. In addition, the proliferative activity of lymphocytes
was lower in the CHB group compared with in the AHB and normal
control groups. The secretion of IFN-γ by CD8+ T cells
was also lower in patients with CHB compared with in the normal
control group and patients with AHB, and the percentage of
CD8+ T-cell apoptosis was significantly increased in
patients with CHB, as compared with in the other two groups. These
results suggested that the function of CD8+ T cells may
be defective in patients with CHB. To explore the underlying
mechanisms, the mRNA expression levels of tapasin were detected in
PBMCs from patients and healthy controls, and differences were
determined. The results revealed that tapasin expression was
significantly down-regulated in patients with CHB. In addition,
tapasin mRNA expression was positively correlated with IFN-γ
production by CD8+ T cells and was inversely correlated
with the apoptotic ratio of CD8+ T cells. These results
indicated a defective function of CD8+ T lymphocytes in
patients with CHB, which may be associated with tapasin expression.
These results may present the underlying mechanism of peripheral
lymphocyte tolerance in patients with CHB.
In conclusion, increased apoptosis and decreased
IFN-γ secretion by CD8+ T cells in patients with CHB may
reduce cellular immune function, and these effects may be
associated with tapasin expression. The results indicated that
decreased tapasin gene expression is closely associated with CHB
and suggest an important role for tapasin in the pathogenesis of
CHB.
Acknowledgments
The present study was supported by grants from the
Education Committee of Scientific Research Innovation Foundation of
Shanghai (grant no. 1522013).
References
|
1
|
Chen X, Liu H, Tang Z, Yu Y and Zang G:
The modification of Tapasin enhances cytotoxic T lymphocyte
activity of intracellularly delivered CTL epitopes via cytoplasmic
transduction peptide. Acta Biochim Biophys Sin (Shanghai).
45:203–212. 2013. View Article : Google Scholar
|
|
2
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chisari FV, Isogawa M and Wieland SF:
Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris).
58:258–266. 2010. View Article : Google Scholar
|
|
4
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thimme R, Wieland S, Steiger C, Ghrayeb J,
Reimann KA, Purcell RH and Chisari FV: CD8(+) T cells mediate viral
clearance and disease pathogenesis during acute hepatitis B virus
infection. J Virol. 77:68–76. 2003. View Article : Google Scholar :
|
|
6
|
Asabe S, Chattopadhyay PK, Roederer M,
Engle RE, Purcell RH and Chisari FV: The size of the viral inoculum
contributes to the outcome of hepatitis B virus infection. J Virol.
83:9652–9662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Phillips S, Chokshi S, Riva A, Evans A,
Williams R and Naoumov NV: CD8(+) T cell control of hepatitis B
virus replication: Direct comparison between cytolytic and
noncytolytic functions. J Immunol. 184:287–295. 2010. View Article : Google Scholar
|
|
8
|
Leonhardt RM, Abrahimi P, Mitchell SM and
Cresswell P: Three tapasin docking sites in TAP cooperate to
facilitate transporter stabilization and heterodimerization. J
Immunol. 192:2480–2494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hulpke S, Baldauf C and Tampé R: Molecular
architecture of the MHC I peptide-loading complex: One tapasin
molecule is essential and sufficient for antigen processing. FASEB
J. 26:5071–5080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YY, Tang ZH, Zhang Y, Zhuo M, Zang
GQ, Chen XH and Yu YS: The fusion protein of CTP-HBcAg18-27-Tapasin
mediates the apoptosis of CD8(+)T cells and CD8(+) T cell response
in HLA-A2 transgenic mice. Hepat Mon. 14:e161612014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Tang Y, Zhang Y, Zhuo M, Tang Z,
Yu Y and Zang G: Tapasin modification on the intracellular epitope
HBcAg18-27 enhances HBV-specific CTL immune response and inhibits
hepatitis B virus replication in vivo. Lab Invest. 94:478–490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinke JW, Liu L, Turner RB, Braciale TJ
and Borish L: Immune surveillance by rhinovirus-specific
circulating CD4+ and CD8+ T lymphocytes. PLoS
One. 10:e01152712015. View Article : Google Scholar
|
|
13
|
Ashraf S, Nitschke K, Warshow UM, Brooks
CR, Kim AY, Lauer GM, Hydes TJ, Cramp ME, Alexander G, Little AM,
et al: Synergism of tapasin and human leukocyte antigens in
resolving hepatitis C virus infection. Hepatology. 58:881–889.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pei J, Tang Z, Zang G and Yu Y: Blockage
of Notch1 signaling modulates the T-helper (Th)1/Th2 cell balance
in chronic hepatitis B patients. Hepatol Res. 40:799–805. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crawford TQ, Ndhlovu LC, Tan A, Carvidi A,
Hecht FM, Sinclair E and Barbour JD: HIV-1 infection abrogates
CD8+ T cell mitogen-activated protein kinase signaling
responses. J Virol. 85:12343–12350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Rubinstein MP, Lind NA, Purton JF,
Filippou P, Best JA, McGhee PA, Surh CD and Goldrath AW: IL-7 and
IL-15 differentially regulate CD8+ T-cell subsets during
contraction of the immune response. Blood. 112:3704–3712. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Virgin HW, Wherry EJ and Ahmed R:
Redefining chronic viral infection. Cell. 138:30–50. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burns GS and Thompson AJ: Viral hepatitis
B: Clinical and epidemiological characteristics. Cold Spring Harb
Perspect Med. 4:a0249352014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie Q, Shen HC, Jia NN, Wang H, Lin LY, An
BY, Gui HL, Guo SM, Cai W, Yu H, et al: Patients with chronic
hepatitis B infection display deficiency of plasmacytoid dendritic
cells with reduced expression of TLR9. Microbes Infect. 11:515–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woltman AM, Op den Brouw ML, Biesta PJ,
Shi CC and Janssen HL: Hepatitis B virus lacks immune activating
capacity, but actively inhibits plasmacytoid dendritic cell
function. PLoS One. 6:e153242011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blackburn SD, Shin H, Haining WN, Zou T,
Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA and Wherry
EJ: Coregulation of CD8+ T cell exhaustion by multiple
inhibitory receptors during chronic viral infection. Nat Immunol.
10:29–37. 2009. View
Article : Google Scholar
|
|
23
|
Klenerman P and Hill A: T cells and viral
persistence: Lessons from diverse infections. Nat Immunol.
6:873–879. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ou R, Zhang M, Huang L and Moskophidis D:
Control of virus-specific CD8+ T-cell exhaustion and
immune-mediated pathology by E3 ubiquitin ligase Cbl-b during
chronic viral infection. J Virol. 82:3353–3368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neefjes J, Jongsma ML, Paul P and Bakke O:
Towards a systems understanding of MHC class I and MHC class II
antigen presentation. Nat Rev Immunol. 11:823–836. 2011.PubMed/NCBI
|
|
26
|
Papadopoulos M and Momburg F: Multiple
residues in the transmembrane helix and connecting peptide of mouse
tapasin stabilize the transporter associated with the
antigen-processing TAP2 subunit. J Biol Chem. 282:9401–9410. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cabrera CM: The double role of the
endoplasmic reticulum chaperone tapasin in peptide optimization of
HLA class I molecules. Scand J Immunol. 65:487–493. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tey SK and Khanna R: Host immune system
strikes back: Autophagy-mediated antigen presentation bypasses
viral blockade of the classic MHC class I processing pathway.
Autophagy. 8:1839–1841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen M and Bouvier M: Analysis of
interactions in a tapasin/class I complex provides a mechanism for
peptide selection. EMBO J. 26:1681–1690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wearsch PA and Cresswell P: Selective
loading of high-affinity peptides onto major histocompatibility
complex class I molecules by the tapasin-ERp57 heterodimer. Nat
Immunol. 8:873–881. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boulanger DS, Oliveira R, Ayers L, Prior
SH, James E, Williams AP and Elliott T: Absence of tapasin alters
immunodominance against a lymphocytic choriomeningitis virus
polytope. J Immunol. 184:73–83. 2010. View Article : Google Scholar
|
|
32
|
Sa Q, Woodward J and Suzuki Y: IL-2
produced by CD8+ immune T cells can augment their IFN-γ
production independently from their proliferation in the secondary
response to an intracellular pathogen. J Immunol. 190:2199–2207.
2013. View Article : Google Scholar : PubMed/NCBI
|