Introduction
Lung cancer is a common and highly frequent cause of
cancer-associated mortality worldwide (1,2). In
addition, the occurrence of lung cancer between mice and humans is
similar, particularly adenocarcinoma (3). The incidence and outcome of lung
injury and inflammation are associated with the nature of the
precipitating disease, and individual susceptibility (4). Genetic susceptibility has been
reported to have an important role in the induction and development
of lung injury in humans (5) and
animals (6), and various mouse
strains display a variation in susceptibility to lung tumor
induction. In current lung cancer research, a urethane model of
lung cancer has been widely used, particularly in BALB/c mice
(7). Previous studies have
demonstrated that C57BL/6J mice are not very sensitive to
urethane-induced lung cancer (8,9).
However, with the extensive application of C57BL/6J mice in lipid
metabolism-related research, it appears important to establish a
lung cancer model based on C57BL/6J mice.
It has previously been reported that chronic
inflammation is associated with an increased risk of several types
of human cancer (10). The lung is
vulnerable to various chemical and biological insults, and
persistent exposure to these factors may induce the release of
several inflammatory cytokines from inflammatory cells,
consequently leading to chronic inflammation and an increased risk
of lung cancer (11,12). Inflammatory cells in the tumor
microenvironment may release cytokines to directly stimulate
oncogenic signaling in cancer cells, including nuclear factor
(NF)-κB, thus promoting cancer survival and proliferation (12). The present study designed an
experimental model, in which C57BL/6J mice received numerous
injections of urethane. The present study aimed to determine
whether inflammation has a role in this lung cancer model. The
results demonstrated that accumulation of lung inflammation was
associated with the occurrence of lung cancer in C57BL/6J mice.
Materials and methods
Animals and experimental design
A total of 40 male C57BL/6J mice (age, 6–8 weeks,
weight, 12.40–14.79 g; 20 per group) were obtained from the animal
facility of Chongqing Medical University (Chongqing, China) and
maintained in a controlled environment (12-h light/dark cycle,
ad libitum access to food and water) at the laboratory
animal facility of Chongqing Medical University (Chongqing, China).
All methods used in the present study were approved by the Animal
Care and Use Committee at Chongqing Medical University. After 3
weeks of adaptive feeding, the mice were randomly divided into two
groups: The urethane group, which received intraperitoneal (i.p)
injections of urethane (1 mg/g body weight; U2500; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) freshly dissolved in 0.9%
saline; or the control group, which received an equal volume of
saline. The mice were treated with urethane or saline once a week
for 10 consecutive weeks. At various time points, mice were
sacrificed using pentobarbital sodium (0.2 mg/g), blood was
collected by cardiac puncture. Simultaneously, bronchoalveolar
lavage fluid (BALF) and lung tissues were carefully collected.
Tumorigenesis assessments were conducted after a 15-week latency
period, as described previously (13). Mice were sacrificed at various time
periods: Experimental week 13 (following continuous i.p injections
of urethane or saline for 10 weeks) or experimental week 28 (final
point) for further studies (Fig.
1A).
Histology and immunohistochemistry
Excised mouse lungs were inflation-fixed in 4%
neutral buffered paraformaldehyde overnight at 4°C prior to
paraffin embedding. Tissues were sectioned (5 µm thick) and
mounted on slides. For histological analysis, sections were
counterstained with hematoxylin and eosin. For immunohistochemistry
(IHC), sections were incubated with rabbit polyclonal anti-cluster
of differentiation (CD)68 (cat. no. BA0461; 1:200; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) and rat anti-mouse
lymphocyte antigen 6G (cat. no. 551459; 1:200; BD Biosciences,
Franklin Lakes, NJ, United States) overnight at 4°C. The following
day, sections were washed three times in phosphate-buffered saline
(PBS) and were incubated with biotinylated anti-rabbit or anti-rat
immunoglobulin G secondary antibodies using streptavidin/peroxidase
link detection kit (cat. no. SP-9001; OriGene Technologies, Inc.,
Beijing, China) and Polink-1 HRP detection system for rat primary
antibody (cat. no. PV-6004; OriGene Technologies, Inc.); at 37°C
for 30 min. Tissues were then washed three times in PBS and were
incubated with horseradish peroxidase (HRP)-conjugated streptavidin
for 15 min. The tissues were washed a further three times in PBS
and were developed in 3,3′-diaminobenzidine (OriGene Technologies,
Inc., Beijing, China) for 3–5 min until a brown color appeared.
Tissues were then washed five times in water, were counterstained
with hematoxylin for 15–30 sec, were washed a further five times in
water, and were finally washed with serial dilutions of ethanol and
xylene. Inverted microscope was used for all observations.
BALF collection and multiplex antibody
array
BALF samples were collected and were analyzed, as
described in a previous study (14). Briefly, BALF collection was
performed using three aliquots of 1 ml 0.9% saline. The BALF
fraction was then separated by centrifugation at 400 × g for 10 min
at 4°C. Bronchoalveolar lavage cell numbers were counted by three
people blind to the experimental condition. The cell suspension was
stained with Diff-Quik stain kit (Shanghai Yuanye Biotechnology
Co., Ltd., Shanghai, China) in order to assess BALF inflammatory
cell types in cell smears. Multiplex antibody arrays
(Quantibody® Mouse Inflammation Array 1; Raybiotech,
Inc., Norcross, GA, USA) were conducted on BALF supernatant fluid
according to the manufacturer's protocol, and the amount of
inflammatory cytokines and chemokines were determined based on a
logarithmic regression standard curve.
Western blot analysis
Proteins were extracted from lung tissue homogenates
using the KeyGEN Nuclear and Cytoplasmic Protein Extraction kit
(Nanjing Keygen Biotech Co., Ltd., Nanjing, China). Protein
concentration was quantified using a bicinchoninic acid assay kit
(Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) Equal
quantity (50 µg) of nuclear protein were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (12% resolving
gel and 5% stacking get) and were transferred to polyvinylidene
fluoride membranes (EMD Millipore, Bedford, MA, USA). The membranes
were then blocked for 1 h at 37°C in 5% non-fat milk, and were
incubated with phosphorylated (p)-NF-κB (cat. no. 3033S; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, United States)
primary antibodies overnight at 4°C. The primary antibody was
detected using a rabbit HRP-conjugated secondary antibody (cat. no.
BS13278; 1:10,000; Bioworld Technology, Inc., St. Louis Park, MN,
USA). The blots were visualized using a Chemiluminescent HRP
Substrate (EMD Millipore). Results were quantified using Image J
version 1.4 software (National Institutes of Health, Bethesda, MD,
USA) and are presented as optical density per square millimeter.
Lamin B-1 was used as control antibody (cat. no. BS3547; 1:1,000,
Bioword Technology, Inc.)
Statistical analysis
All experiments were conducted three times and are
reproducible. Data are presented as the mean ± standard error of
the mean. Statistical significance for each variable was estimated
using Student's t-test, or two-way analysis of variance followed by
least squares means multiple comparisons test using SAS version 9.2
(SAS Institute, Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of urethane on body weight and
lung tumor incidence in C57BL/6J mice
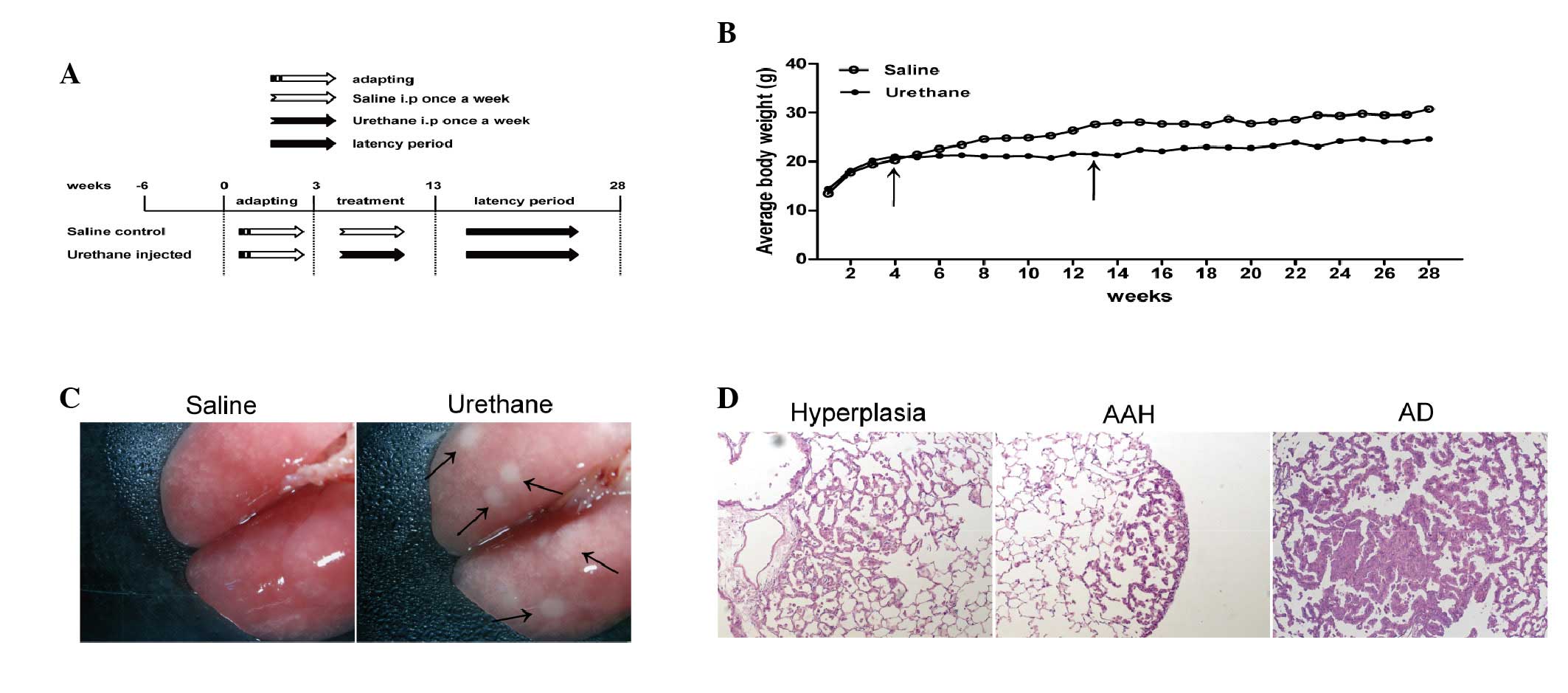
The body weights of the experimental mice were
detected once a week, up to week 28. Body weight was reduced in the
urethane-treated group compared with in the saline-treated control
group during and after urethane administration (Fig. 1B). The continuous i.p injection of
urethane over 10 weeks induced 100% lung tumor incidence at week 28
(no. of mice with tumors/no. of mice injected; data not shown), and
tumors were particularly evident (Fig.
1C). Histopathological analysis was conducted, and the lungs of
the urethane-treated group exhibited the following morphological
alterations: Epithelial hyperplasia, atypical adenomatous
hyperplasia (AAH) and adenoma (AD) (Fig. 1D); however, no adenocarcinomas were
observed at week 28.
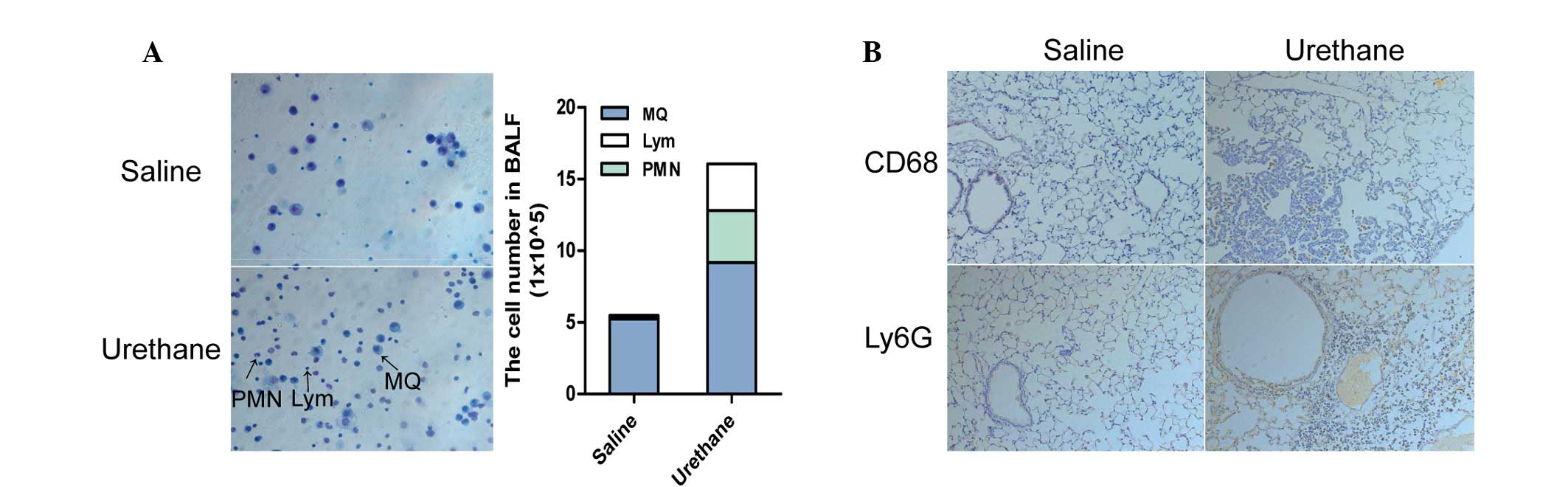
Effects of urethane on lung
inflammation
The number of immune cells in the BALF was
significantly different between the urethane-treated mice and
control mice at week 13. Urethane-treated mice exhibited an overall
increased number of cells in the BALF (data not shown), and the
number of macrophages, polymorphonuclear cells and lymphocytes were
increased to varying degrees, as determined by Diff-Quik staining
(Fig. 2A). Based on these results,
the present study aimed to determine whether lung tissues exhibited
the same changes. The results of IHC demonstrated that macrophages
and neutrophils were increased in the lung tissues of
urethane-treated mice compared with in saline-treated mice at week
28 (Fig. 2B).
To determine the circulating levels of cytokines and
chemokines in BALF at week 13, multiplex antibody arrays were
conducted. The expression levels of cytokines and chemokines in
BALF were significantly different between the two groups. Several
important cytokines and chemokines involved in lung inflammation
were detected (Fig. 3). BALF
levels of several important proinflammatory cytokines and
chemokines, including interleukin (IL)-6, regulated upon activation
normal T cell expressed and secreted (RANTES), thymus and
activation regulated chemokine (TARC) and TIMP metallopeptidase
inhibitor 1 (TIMP-1) were significantly increased in the
urethane-treated group. However, the levels of anti-inflammatory
cytokines and chemokines, such as IL-2, IL-4, IL-10 and IL-13 were
downregulated in BALF from the urethane-treated mice compared with
the controls. Furthermore, the levels of granulocyte-colony
stimulating factor (CSF), macrophage inflammatory protein (MIP)-1γ,
tumor necrosis factor receptor (TNFR)I and TNFRII were
significantly increased in urethane-treated mice, and the
expression levels of CD30 ligand, interferon γ, IL-1α, IL-1β, IL-7,
IL-15, IL-17, C-X-C motif chemokine 5, macrophage-CSF and MIP-1α
were significantly decreased compared with in the control
group.
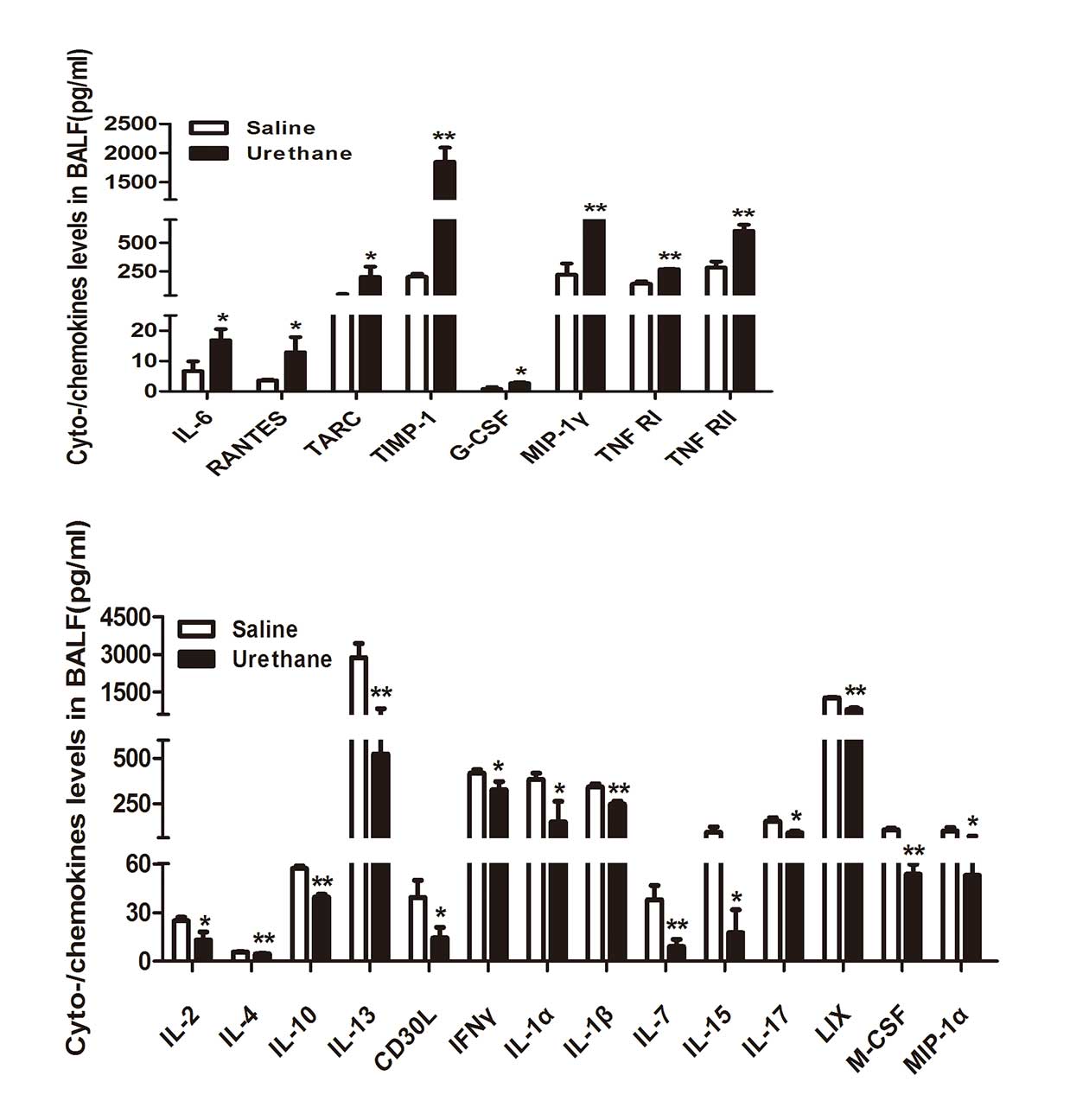 | Figure 3Multiplex antibody array
quantification of several important cytokines and chemokines in
bronchoalveolar lavage fluid (BALF) of the two groups (n=3/group).
Data are presented as the mean ± standard error of the mean.
Student's t test was used to determine significance.
*P<0.05, **P<0.01 vs. saline group. IL,
interleukin; RANTES, regulated upon activation normal T cell
expressed and secreted; TARC, thymus and activation regulated
chemokine; TIMP-1, TIMP metallopeptidase inhibitor 1; M-/G-CSF,
macrophage-/granulocyte-colony stimulating factor; TNFRI/II, tumor
necrosis factor receptor I/II; CD30L, cluster of differentiation 30
ligand; IFNγ, interferon γ; LIX, C-X-C motif chemokine 5; MIP,
macrophage inflammatory protein. |
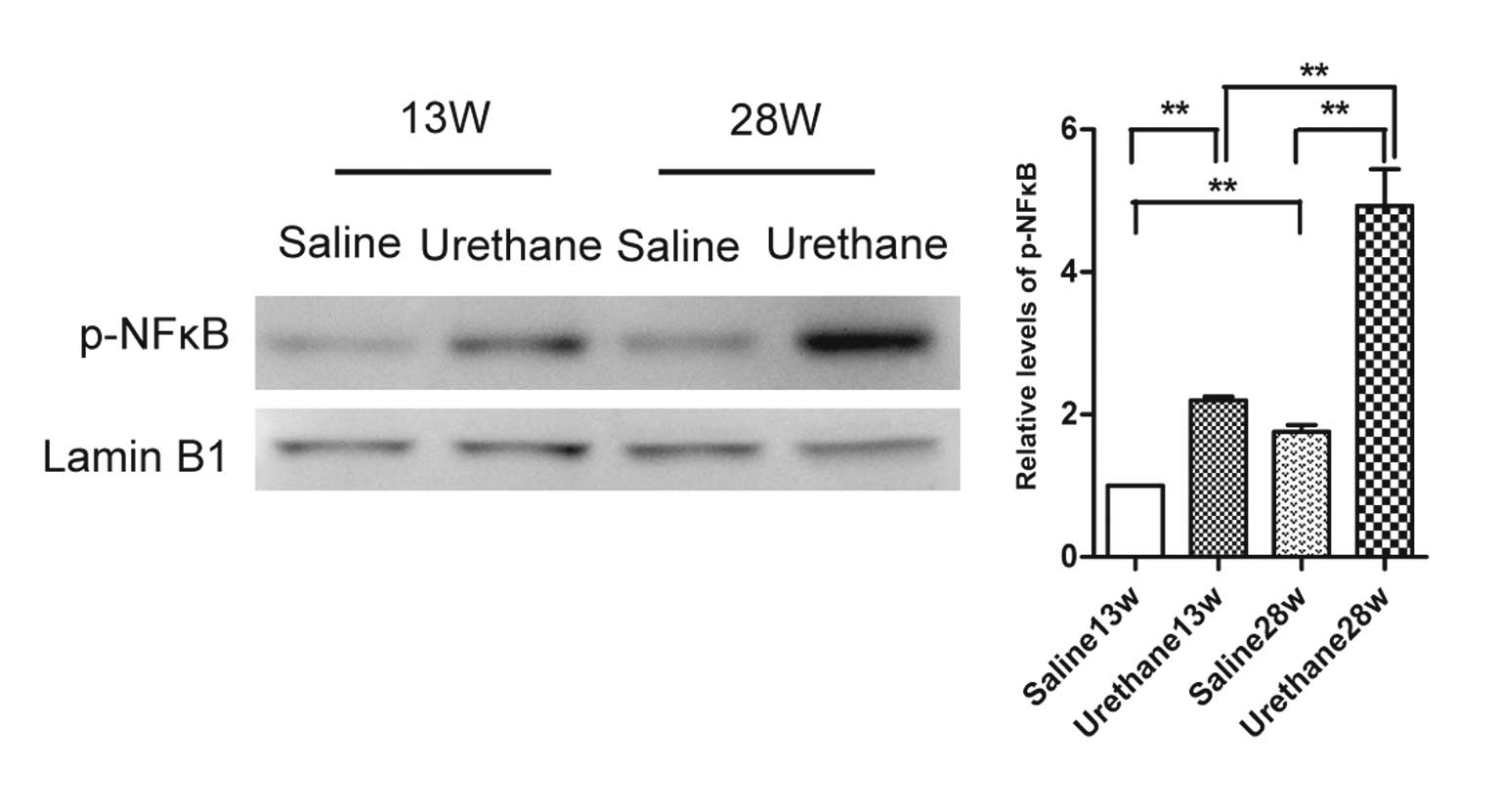
Expression of the transcription factor
NF-κB following urethane treatment
The expression levels of p-NF-κB were detected in
lung tissues from the urethane-treated and saline-treated mice by
western blotting at weeks 13 and 28 (Fig. 4). NF-κB activation was increased
following urethane treatment, and the expression levels increased
in a time-dependent manner.
Discussion
Genetic background has been reported to have an
important role in the induction and development of lung injury in
humans (5) and animals (6), and various species of mice exhibit
various levels of sensitivity to induced lung cancer. Miller et
al (13) reported that a
single dose of urethane does not induce a high incidence of AD in
B6 mice; however, 10 weekly urethane injections induced ~100% lung
tumor incidence. The present study was designed to explore whether
inflammation has a role in lung cancer induced by several
injections of urethane in C57BL/6J mice. The results demonstrated
that the continuous i.p administration of urethane over 10 weeks
induced 100% lung tumor incidence, which was consistent with a
previous study (13). AAH and AD
were the predominant morphological changes observed in the urethane
model; of which AD was shown to be similar to human lung
cancer.
Inflammation has a key role in cancer. Raposo et
al (15) demonstrated that
inflammatory cells are able to produce reactive oxygen species and
reactive nitrogen intermediates, which increase the mutation rate
of cells, induce DNA damage and increase genomic instability; these
molecules also inactivate mismatch repair functions, thus
supporting tumor promotion. Due to the close association between
inflammation and cancer (16–19),
the present study aimed to evaluate whether inflammatory changes
appear prior to the appearance of hyperplasia or tumor. It was
determined that inflammatory changes occurred at week 13; however,
tumorigenesis was not observed until week 28. Redente et al
(20) reported that macrophages in
normal lungs were located in the alveolar interstitia and airways,
whereas macrophages adjacent to tumors and neutrophil accumulation
were observed in the lungs of urethane-treated A/J mice. In the
present study, compared with the saline control group,
urethane-treated mice exhibited increased numbers of macrophages
and neutrophils (Fig. 2) in BALF
and lung tissues. Among the inflammatory cell types in the lungs,
macrophages are the most abundant. Depending on microenvironmental
cues, these cells are able to stimulate inflammatory responses via
the secretion of proinflammatory cytokines, or suppress immune
responses by releasing high levels of anti-inflammatory cytokines.
IL-6 is a multifunctional cytokine that may regulate immune and
inflammatory responses, including control of the acute phase
response at the beginning of acute inflammation, regulation of
B-cell and T-cell differentiation and activation, and promotion of
cell growth and survival (21).
Other members of the IL family associated with lung cancer include
IL-2, IL-4 and IL-10 (22).
IL-2-activated NK cells are able to induce regression of
established lung tumors (22).
IL-4 is an anti-inflammatory cytokine, which reduces the production
of proinflammatory cytokines by monocytes and with direct
antiproliferative effects in some tumors (22). Additionally, it induces cathepsin
protease activity in tumor-associated macrophages to promote cancer
growth and invasion and stimulates 15-hydroxyprostaglandin
dehydrogenase activity mediated by janus kinase-signal transducer
and activator of transcription 6, mitogen-activated protein kinase,
phosphoinositide 3-kinase/Akt and protein kinase C signaling
pathways (22). IL-10 is an
immunosuppressive cytokine involved in carcinogenesis via immune
escape (22). RANTES is a C-C
chemokine that serves as a chemoattractant for various cells. Conti
and DiGioacchino (23) identified
RANTES as a mediator of inflammation. In the present study, the
expression levels of proinflammatory cytokines and chemokines,
including IL-6, RANTES, TARC and TIMP-1 were significantly
increased in BALF from urethane-treated mice, whereas the levels of
anti-inflammatory cytokines and chemokines, such as IL-2, IL-4,
IL-10 and IL-13 were down-regulated (Fig. 3). Chronic inflammation is a
characteristic phenotype of urethane-induced tumorigenesis. Narayan
and Kumar (24) reported that
urethane-induced lung tumorigenesis in BALB/c mice may be caused by
chronic lung inflammation.
The NF-κB transcription factor functions as a
crucial regulator of inflammation, the immune response, and cell
survival (25,26). In addition, NF-κB has been
implicated in cellular transformation and tumorigenesis, and has
been revealed to be activated at the early stages of carcinogenesis
(27,28). In mice treated with several
injections of urethane, NF-κB was activated in the lung tissues,
and its expression increased in a time-dependent manner (Fig. 4). These results were inconsistent
with those of Stathopoulos et al (28); however, C57BL/6 mice received
weekly i.p injections of urethane for only 4 weeks in this previous
study. The results of the present study suggested that the duration
of urethane injections may have an important role in the activation
of NF-κB.
In conclusion, the present study determined that the
accumulation of lung inflammation was associated with the
occurrence of lung cancer in C57BL/6J mice., Due to the extensive
application of C57BL/6J mice in lipid metabolism-associated lung
cancer research, multiple injections of urethane may be potential
experimental animal models.
Abbreviations:
|
BALF
|
bronchoalveolar lavage fluid
|
|
AAH
|
adenomatous hyperplasia
|
|
AD
|
adenoma
|
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of China (NSFC, grant no. 81071907), the
Natural Science Foundation of Chong Qing (CSTC, grant no.
2011BB5128) and the Program for New Century Excellent Talents in
University, China (grant no. NCET-10-0997).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malkinson AM: Molecular comparison of
human and mouse pulmonary adenocarcinomas. Exp Lung Res.
24:541–555. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nonas S, Miller I, Kawkitinarong K,
Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan
V, Garcia JG and Birukov KG: Oxidized phospholipids reduce vascular
leak and inflammation in rat model of acute lung injury. Am J
Respir Crit Care Med. 173:1130–1138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lagan AL, Melley DD, Evans TW and Quinlan
GJ: Pathogenesis of the systemic inflammatory syndrome and acute
lung injury: Role of iron mobilization and decompartmentalization.
Am J Physiol Lung Cell Mol Physiol. 294:L161–L174. 2008. View Article : Google Scholar
|
|
6
|
Moraes TJ, Zurawska JH and Downey GP:
Neutrophil granule contents in the pathogenesis of lung injury.
Curr Opin Hematol. 13:21–27. 2006. View Article : Google Scholar
|
|
7
|
Malkinson AM and Beer DS: Major effect on
susceptibility to urethan-induced pulmonary adenoma by a single
gene in BALB/cBy mice. J Natl Cancer Inst. 70:931–936.
1983.PubMed/NCBI
|
|
8
|
Bernert H, Sekikawa K, Radcliffe RA, Iraqi
F, You M and Malkinson AM: Tnfa and Il-10 deficiencies have
contrasting effects on lung tumor susceptibility: Gender-dependent
modulation of IL-10 haploinsufficiency. Mol Carcinog. 38:117–123.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doris K, Karabela SP, Kairi CA, Simoes DC,
Roussos C, Zakynthinos SG, Kalomenidis I, Blackwell TS and
Stathopoulos GT: Allergic inflammation does not impact
chemical-induced carcinogenesis in the lungs of mice. Respir Res.
11:1182010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartsch H and Nair J: Chronic inflammation
and oxidative stress in the genesis and perpetuation of cancer:
Role of lipid peroxidation, DNA damage, and repair. Langenbecks
Arch Surg. 391:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azad N, Rojanasakul Y and Vallyathan V:
Inflammation and lung cancer: Roles of reactive oxygen/nitrogen
species. J Toxicol Environ Health B Crit Rev. 11:1–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33(Suppl 1): S79–S84. 2013.
View Article : Google Scholar
|
|
13
|
Miller YE, Dwyer-Nield LD, Keith RL, Le M,
Franklin WA and Malkinson AM: Induction of a high incidence of lung
tumors in C57BL/6 mice with multiple ethyl carbamate injections.
Cancer Lett. 198:139–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lian X, Qin Y, Hossain SA, Yang L, White
A, Xu H, Shipley JM, Li T, Senior RM, Du H and Yan C:
Overexpression of Stat3C in pulmonary epithelium protects against
hyperoxic lung injury. J Immunol. 174:7250–7256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raposo TP, Beirão BC, Pang LY, Queiroga FL
and Argyle DJ: Inflammation and cancer: Till death tears them
apart. Vet J. 205:161–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanterman J, Sade-Feldman M and Baniyash
M: New insights into chronic inflammation-induced
immunosuppression. Semin Cancer Biol. 22:307–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonda TA, Tu S and Wang TC: Chronic
inflammation, the tumor microenvironment and carcinogenesis. Cell
Cycle. 8:2005–2013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albini A and Sporn MB: The tumour
microenvironment as a target for chemoprevention. Nat Rev Cancer.
7:139–147. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Porta C, Larghi P, Rimoldi M, Totaro MG,
Allavena P, Mantovani A and Sica A: Cellular and molecular pathways
linking inflammation and cancer. Immunobiology. 214:761–777. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Redente EF, Orlicky DJ, Bouchard RJ and
Malkinson AM: Tumor signaling to the bone marrow changes the
phenotype of monocytes and pulmonary macrophages during
urethane-induced primary lung tumorigenesis in A/J mice. Am J
Pathol. 170:693–708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014. View Article : Google Scholar
|
|
22
|
Cho WC, Kwan CK, Yau S, So PP, Poon PC and
Au JS: The role of inflammation in the pathogenesis of lung cancer.
Expert Opin Ther Targets. 15:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conti P and DiGioacchino M: MCP-1 and
RANTES are mediators of acute and chronic inflammation. Allergy
Asthma Proc. 22:133–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narayan C and Kumar A: Constitutive over
expression of IL-1β, IL-6, NF-κB, and Stat3 is a potential cause of
lung tumorgenesis in urethane (ethyl carbamate) induced Balb/c
mice. J Carcinog. 11:92012. View Article : Google Scholar
|
|
25
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stathopoulos GT, Sherrill TP, Cheng DS,
Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B
and Blackwell TS: Epithelial NF-kappaB activation promotes
urethane-induced lung carcinogenesis. Proc Natl Acad Sci USA.
104:18514–18519. 2007. View Article : Google Scholar : PubMed/NCBI
|