Introduction
Renal cell carcinoma (RCC) is a type of malignant
tumor, which originates from renal tubular epithelial cells. It
poses a serious threat to human health, as its morbidity rate is
high among tumors of the urinary system (1,2). In
recent years, the morbidity and mortality rates of patients with
RCC have increased in China (3).
Radical nephrectomy is currently the most effective treatment for
RCC. The 5-year survival rate of patients following surgery is
currently <10%, despite efforts to improve it (2). Therefore, the development of novel
effective treatment would be of great significance for patients
with RCC. Notably, multiple microRNAs (miRNAs) have been identified
to be closely associated with the genesis and development of RCC,
including effects on proliferation, apoptosis, invasion and
metastasis, which may provide an alternative strategy for the early
diagnosis and prognosis of RCC.
Endogenous non-coding miRNAs have highly conserved
sequences; therefore, they are able to inhibit the expression of a
target gene by binding to the 3′-untranslated region of the mRNA
and, subsequently, inhibiting its translation. Over 700 human
miRNAs have been identified to participate in ~1/3 of human gene
expression. Additionally, the abnormal expression of certain miRNAs
in RCC had been confirmed by previous studies (4–6).
Gottardo et al (4)
identified 10 miRNAs, including miR-223, miR-26b, miR-221,
miR-103-1, miR-185, miR-23b, miR-203, miR-17-5p, miR-23a and
miR-205, that were highly expressed in RCC when compared with
normal nephridial tissue (4). Chow
et al (5) also determined
that the expression levels of 21 miRNAs were upregulated, and 12
miRNAs were downregulated, in RCC (5). Similarly, the abnormal expression of
86 miRNAs in RCC was investigated by Yi et al (6) using gene chip technology, where 48
miRNAs were highly expressed and 38 exhibited low expression levels
(6). Therefore, specific
regulation of the target gene by diverse miRNAs may contribute to
the improvement of RCC treatment.
miRNA-205 (miR-205) sequence was first calculated
from homogenous sequences of mice and Takifugu rubripes
using miRNA software (7). In
humans, miR-205 is located on the second intron of the long arm of
chromosome 1 and is not located near to other miRNAs. miR-205 has
been determined to be abnormally expressed in various types of
tumor. miR-205 is capable of acting as an oncogene and tumor
suppressor. As a tumor suppressor, it may regulate the malignant
biological behaviors of various types of tumor, including prostatic
carcinoma, squamous cell carcinoma of the head and neck and breast
carcinoma, via targeting the expression of various genes (8,9).
Previous studies have reported low expression levels of miR-205 in
RCC (10,11), with miR-205 levels important for
the regulation of RCC (11).
However, the detailed molecular mechanisms involved remain to be
elucidated. Therefore, the present study aimed to validate the low
expression levels of miR-205 in the tissue and cells of RCC,
investigate the effect of miR-205 on the malignant biological
behaviors of RCC, including cell proliferation, invasion and
metastasis, and determine the underlying molecular mechanisms.
Materials and methods
Specimen collection
Ethical approval and written informed consent was
obtained from the Drug Clinical Trials Ethics Committee, First
Affliated Hospital of Xiamen University (Fujian, China), and 20 RCC
and 20 normal tissues adjacent to carcinoma were collected from
patients that underwent laparoscopic radical nephrectomy in the
Department of Urology, First Afflicted Hospital of Xiamen
University (Xiamen, China), between May 2014 and May 2015. Patients
did not receive chemotherapy or radiotherapy prior to the operation
and pathological diagnosis was performed.
Materials and cell culture
The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit was obtained
from Keygen Biotech Co., Ltd. (Nanjing, China). Bicinchoninic acid
assay (BCA) kit was from Beyotime Institute of Biotechnology
(Nanjing, China). Lipofectamine 2000, TRIzol reagent and one-step
reverse transcription-polymerase chain reaction (RT-PCR) kit were
purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Methyl thiazolyl tetrazolium (MTT) was purchased from
Gibco (Thermo Fisher Scientific, Inc.). Rabbit anti-E2F
transcription factor 1 (E2F1; cat. no. 8120-1), anti-B-cell
lymphoma-2 (Bcl-2; cat. no. 1017-1), anti-E-cadherin (cat. no.
5409-1), anti-vimentin (cat. no. 2707-1) and GAPDH (cat. no.
5632-1) monoclonal antibodies were obtained from Epitomics
(Burlingame, CA, USA). Rabbit anti-phosphatase and tensin homolog
(PTEN; cat. no. 9552), anti-AKT serine/threonine kinase 1 (AKT;
cat. no. 9272) and anti-phosphorylated AKT (p-AKT; cat. no. 4060)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Horseradish peroxidase-conjugated goat
anti-rabbit antibody (cat. no. A0208) was obtained from Beyotime
Institute of Biotechnology, Inc. Transwell inserts were obtained
from Corning Incorporated (Corning, NY, USA).
Human RCC cell lines, ACHN, 769-p, and Caki-1, were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China; cat. nos. TCHu199, TCHu215 and
TCHu135, respectively). Normal human renal tubular epithelial cell
line HK-2 was purchased from American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS; GE Healthcare
Life Sciences).
Preparation of miRNA
miR-205 mimics and the negative control were
synthesized by GenePharma Co., Ltd., (Shanghai, China). The
sequences were as follows: miR-205 mimics, forward
5′-UCCUUCAUUCCACCGGAGUCUG-3′, reverse 5′-GACUCCGGUGGAAUGAAGGAUU-3′;
and negative control, forward 5′-UUCUCCGAACGUGUCACGUTT-3′, reverse
5′-ACGUGACACGUUCGGAGAATT-3′. The mix ratio of the miRNA and
Lipofectamine 2000 reagent for gene transfection was defined
according to the manufacturer's protocol.
miR-205 expression level in RCC tissues
and cells
Total RNA of RCC tissues and cells was extracted
using TRIzol reagent in an RNase-free environment following to the
manufacturer's protocol. The reverse transcription from RNA to cDNA
and PCR amplification were performed using the one-step RT-PCR kit.
The primers used are presented in Table I and were added to 25 µl PCR
reaction system. The thermocycling parameters were defined as
follows: Denaturation for 45 sec at 94°C, annealing for 45 sec at
59°C, elongation for 60 sec at 72°C for 35 cycles. The
amplification products (5 µl) were loaded on a 2% (w/v)
agarose gel for separation by gel electrophoresis. A ChemiDoc XRS
gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using Goldview dye for visualization and analysis of
electrophoresis strips. Image Pro Plus version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used for
quantification.
 | Table IPrimers for reverse
transcription-polymerase chain reaction. |
Table I
Primers for reverse
transcription-polymerase chain reaction.
| Gene | Primer (5′-3′) | bp |
|---|
| miR-205 | F
ACACTCCAGCTGGGTCCTT | 72 |
| R
CTCAACTGGTGTCGTGGA |
| GADPH | F
AGCCACATCGCTCAGACA | 314 |
| R
TGGACTCCACGACGTACT |
Cell viability
ACHN cells were seeded at a density of
2.0×104 cells/well in 96-well plates. When cell
confluence reached 50%, miR-205 mimics and the negative control
were transfected into cells using Lipofectamine 2000. After 48 h,
20 µl MTT (5 mg/ml) was added into each well and cells were
cultured for a further 4 h. Culture media was discarded and 150
µl dimethyl sulfoxide was added into each well. When the
crystal was fully dissolved, absorbance was determined at 560 and
630 nm (reference wavelengths) using a microplate reader (Infinite
F200/M200; Tecan, Männedorf, Switzerland). The relative cell
viability was calculated. Cells without any treatment served as
control.
Cell apoptosis
ACHN cells were seeded at a density of
1.0×104 cells/well in 6-well plates. When cell
confluence reached 50%, miR-205 mimics and the negative control
were transfected into cells using Lipofectamine 2000. The Annexin
V-FITC/PI apoptosis detection kit was used to determine the cell
apoptosis according to the manufacturer's protocol. Briefly,
following 24 h of incubation, the cells were digested with 0.25%
(w/v) trypsin, washed with phosphate-buffered saline and collected
with a centrifugation at 800 × g for 5 min. Cells were then
resuspended in 500 µl binding buffer. Cell suspension was
mixed with 5 µl Annexin V-FITC and subsequently with 5
µl PI. After 15 min incubation at room temperature in the
dark, cell apoptosis was determined using a flow cytometer and
CellQuest Pro version 6.0 software (FACScan; BD Biosciences,
Franklin Lakes, NJ, USA).
Cell migration
ACHN cells were seeded at a density of
1.0×104 cells/well in 6-well plates. When cell
confluence reached 50%, miR-205 mimics and the negative control
were transfected into cells with Lipofectamine 2000. Next, cells
were digested and transferred into the upper chamber of Transwell
insert without Matrigel. DMEM supplemented with 5% (v/v) FBS was
added into the lower chamber of the Transwell insert. Cells were
incubated for 24 h. The Transwell insert was removed, washed, fixed
in paraformaldehyde, and stained with crystal violet. The number of
cells in the lower chamber of 5 visual fields was counted with a
light microscope (Eclipse TS100; Nikon Corporation, Tokyo, Japan).
The average number of cells in every visual field represented the
migration capacity of cells.
Cell invasion
ACHN cells (1.0×104 cells/well) were
transfected with miR-205 mimics and the negative control. Matrigel
was uniformly distributed onto the membrane of the Transwell insert
(5 µm) prior to addition of the cells. Then Transwell assay
was performed in a similar manner to the aforementioned cell
migration assay. The average number of cells in every visual field
in the lower chamber represented the invasion capacity of
cells.
Western blotting
ACHN cells (1.0×104 cells/well) were
transfected with miR-205 mimics and the negative control. After 48
h the cells were collected and lysed with radioimmunoprecipitation
assay lysis buffer. A 30 sec agitation using a vortex was performed
every 10 min. After 40 min, the mixture was centrifuged for 10 min
at 7,000 × g and 4°C. The supernatant was carefully collected to
obtain the total protein. BCA kit was used to determine the protein
content. Protein (30 ng) was loaded onto a sodium dodecyl
sulphate-polyacrylamide gel in order to perform electrophoresis.
Proteins were transferred to a polyvinylidene difluoride membrane
using the wet transfer method. The membrane was blocked with 5%
(w/v) non-fat dry milk at room temperature for 1 h. The membrane
was immersed in the primary antibodies (dilution, 1:100) buffer
overnight at 4°C. Next, it was rinsed and immersed in a secondary
antibody buffer (dilution, 1:200) for 2 h at room temperature. The
membrane was rinsed again and enhanced chemiluminescent solution
(Beyotime Institute of Biotechnology, Inc.) was dropped onto the
membrane followed by exposure on a gel imaging system (ChemiDoc
XRS; Bio-Rad Laboratories, Inc.). QuantityOne version 4.6.2
software (Bio-Rad Laboratories, Inc.) was used to determine the
gray values of proteins. GAPDH was used as a reference gene.
Western blotting analysis was repeated three times.
Statistics analysis
Differences were analyzed using Student's t-test.
All experiments were repeated at least three times and data were
expressed as mean ± standard deviation. Statistical analysis was
performed by t-test using SPSS 17.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
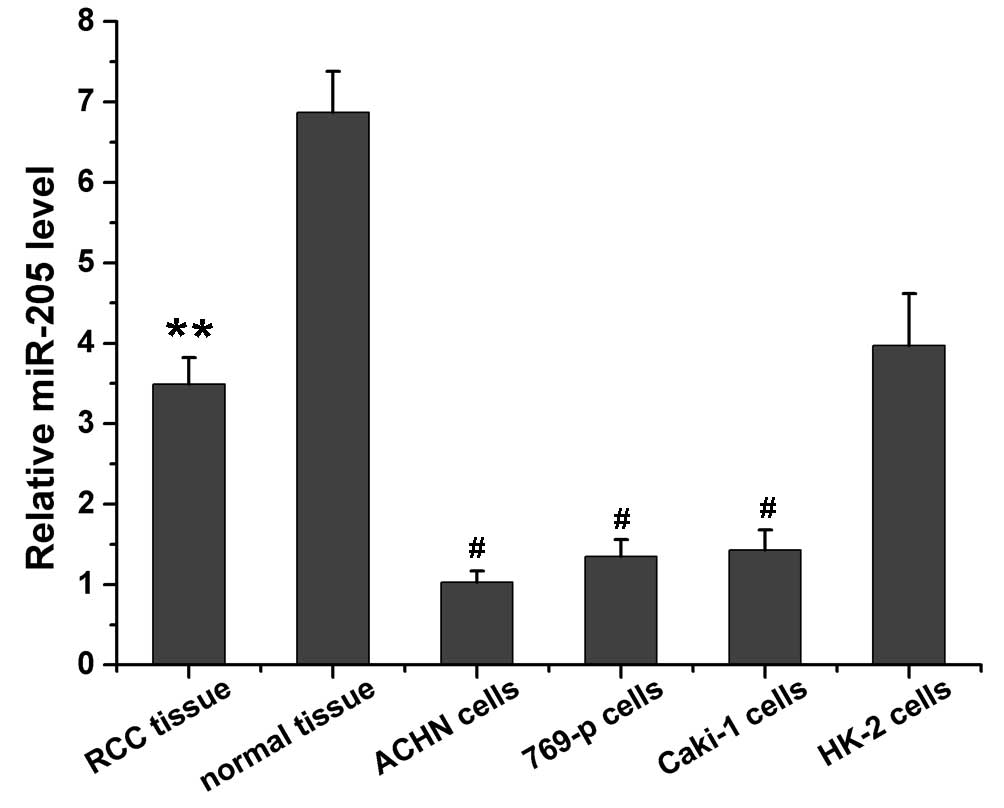
miR-205 level in RCC tissues and
cells
miR-205 expression levels in RCC tissue, normal
tissue adjacent to carcinoma, RCC cells, and HK-2 normal renal
cells were evaluated using RT-PCR and results are presented in
Fig. 1. It was determined that the
miR-205 expression level in normal tissue adjacent to carcinoma was
~2-fold higher than that in RCC tissue (P=0.0036). The miR-205
expression level in HK-2 normal renal cells was significantly
higher compared with RCC cells, including ACHN (P=0.01), 769-p
(P=0.011) and Caki-1 (P=0.012). The ACHN cells expressed the lowest
levels of miR-205 among the cell lines.
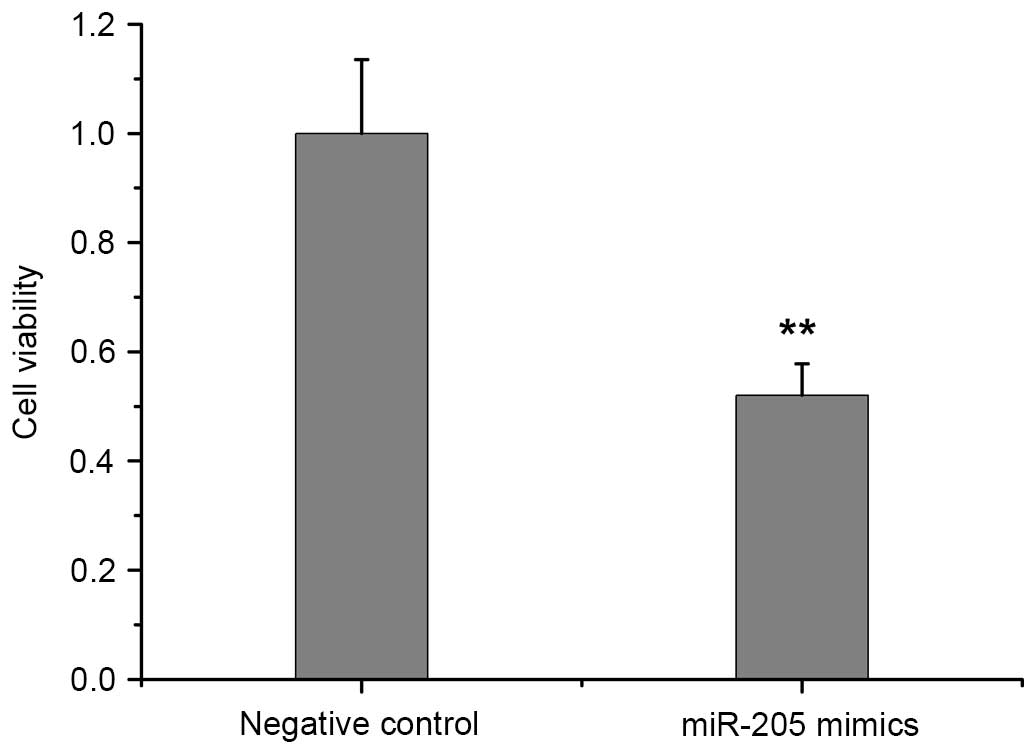
miR-205 reduces RCC cell viability
Fig. 2 shows the
viability of ACHN cells transfected with miR-205 mimics and the
negative control, which was determined by MTT assay. When compared
with the negative control, the elevation of intracellular miR-205
expression induced by the miR-205 mimics significantly reduced the
proliferation of ACHN cells (P=0.0059; Fig. 2).
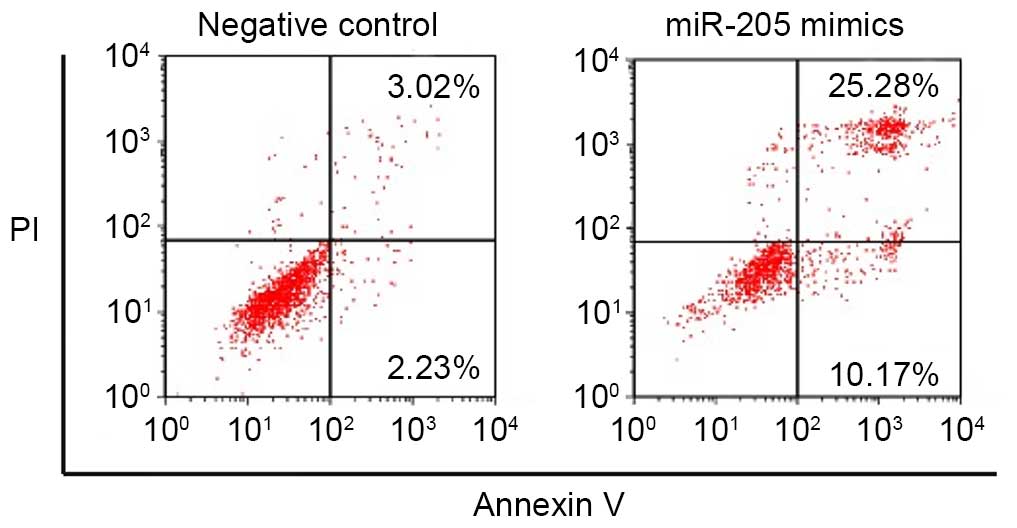
miR-205 increases the apoptotic rate of
RCC cells
Annexin V-FITC/PI staining was used to assess the
apoptotic rate of negative control RCC cells and those transfected
with miR-205 mimics. Fig. 3
presents the apoptosis of ACHN cells transfected with miR-205
mimics and the negative control. Following the transfection of
miR-205 mimics, the number of apoptotic ACHN cells was increased
compared with the negative control group, suggesting that the
upregulation of miR-205 expression induced the apoptosis of RCC
cells.
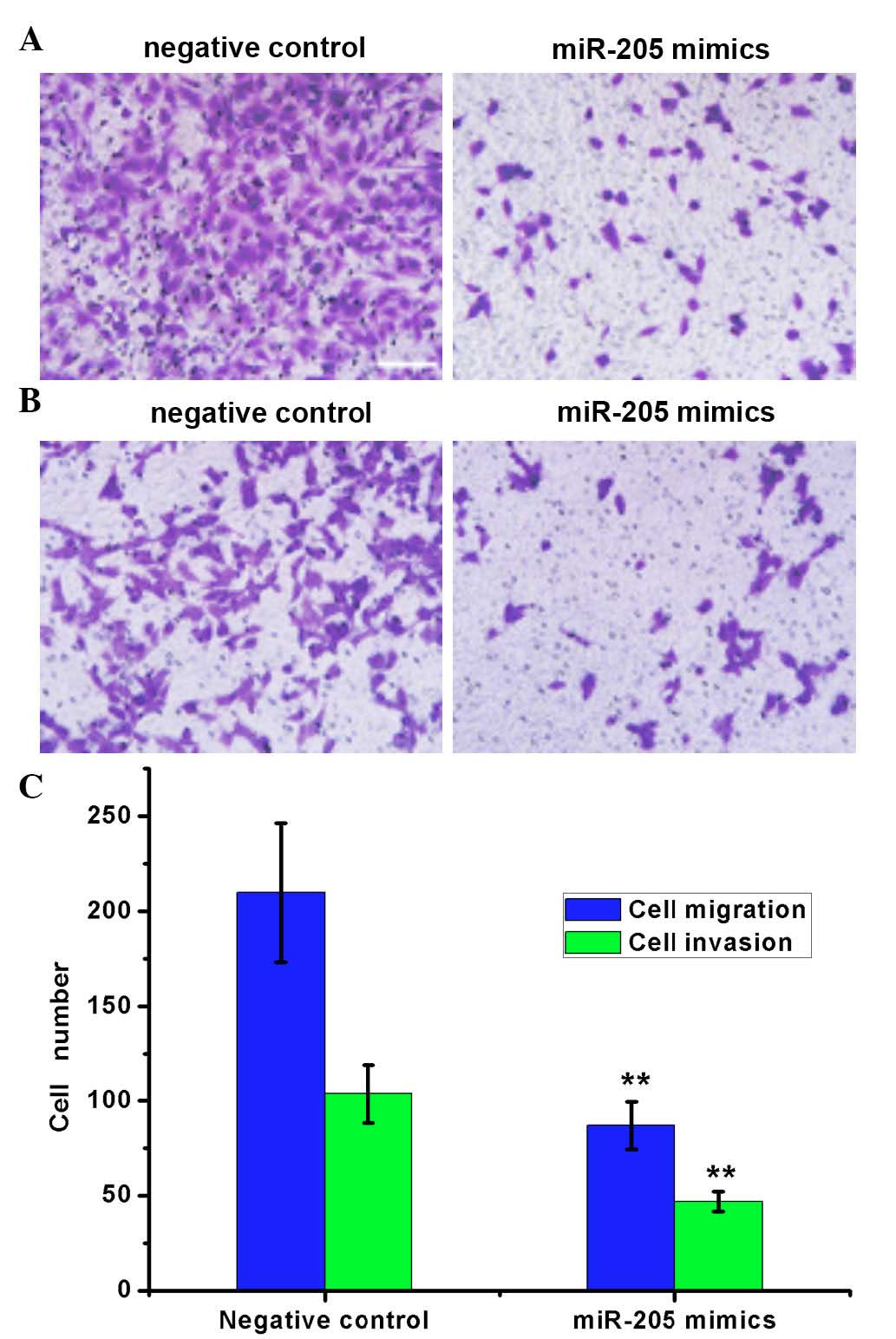
miR-205 reduces the migration and
invasion ability of RCC cells
The migration and invasion ability of ACHN cells
transfected with miR-205 mimics and the negative control were
evaluated by Transwell assay (Fig.
4). For cell migration, the number of cells migrating to the
lower chamber was significantly reduced in cells transfected with
miR-205 mimics (86.83±12.39) compared with the negative control
(209.75±36.48; P=0.0032). In the cell invasion assay, the number of
invading cells was significantly reduced in cells transfected with
miR-205 mimics (46.79±5.28) compared with the negative control
cells (103.56±15.43; P=0.0029). These results indicated that the
increased level of intracellular miR-205 may inhibit the migration
and invasion ability of RCC cells.
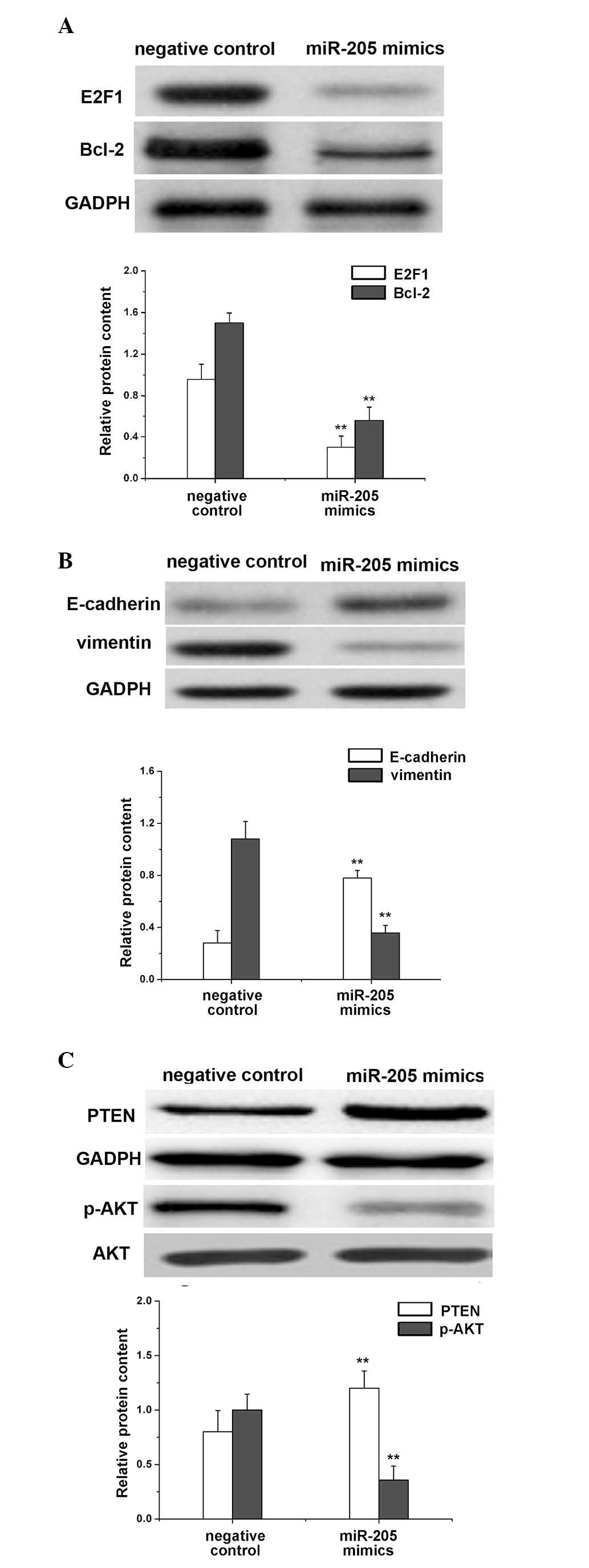
Effect of miR-205 on the expression
levels of E2F1, Bcl-2, E-cadherin, vimentin and PTEN/AKT in RCC
cells
The expression of these signaling molecules in ACHN
cells was affected by the transfection of miR-205 mimics and was
assessed by western blot analysis (Fig. 5). GADPH was used as an internal
control and similar blots of GADPH were observed in all groups,
validating the reliable determination protein expression levels.
Notably, fainter blots of E2F1 and Bcl-2 were observed in ACHN
cells transfected with miR-205 mimics comparing to their darker
blots in cells transfected with the negative control (Fig. 5A). This qualitative result was
verified by the quantification of the protein levels, indicating
that transfection with miR-205 mimics may significantly reduce the
expression levels of E2F1 (P= 0.0042) and Bcl-2 (P=0.0047) in RCC
cells compared with the negative control (Fig. 5A).
ACHN cells transfected with miR-205 mimics exhibited
higher expression levels of E-cadherin (P=0.0019) and reduced
expression levels of vimentin (P=0.0022) compared with the negative
control (Fig. 5B). Therefore,
miR-205 upregulated the expression of E-cadherin and downregulated
the expression of vimentin in RCC cells.
No significant difference between the AKT expression
level in ACHN cells transfected with miR-205 mimics and the
negative control was identified (data not shown). However, the
expression level of PTEN was significantly greater (P=0.0085;
Fig. 5C), and that of p-AKT was
significantly reduced (P=0.001; Fig.
5C), in cells transfected with miR-205 mimics compared with the
negative control. Therefore, the transfection of miR-205 mimics
into RCC cells increased the protein expression of PTEN and reduced
the phosphorylation of AKT.
Discussion
The abnormal expression of miR-205, which may be
associated with various types of tumor, is involved in
tumorigenesis and tumor progression. The present study demonstrated
that the expression of miR-205 in RCC tissue was reduced compared
with normal tissue adjacent. Previous studies have revealed that
miR-205 expression levels were reduced in ACHN cells compared with
HK-2 normal renal cells (10,11).
miR-205 has also been previously demonstrated to suppress the
expression of SRC proto-oncogene, LYN proto-oncogene and YES
proto-oncogene 1, which subsequently leads to the inhibition of
tumor development (11). The
current determined the expression levels of miR-205 using RT-PCR.
As ACHN cells exhibited the lowest miR-205 expression level they
were used as the model RCC cells in subsequent experiments.
The transfection of miR-205 mimics increased the
expression level of miR-205 in RCC cells. It was determined that
miR-205 inhibited the proliferation of RCC cells by inducing their
apoptosis, and reducing their migration and invasion ability. The
expression levels of certain signaling molecules that may be
involved in these processes were determined using western blot
analysis.
The anabolism of proteins in tumor cells is greater
than their catabolism, leading to continuous cell division in
tumors. The infinite proliferation of the tumor leads to gradual
disease progression. Therefore, reducing cell proliferation and
inducing apoptosis of tumor cells may be an effective way to
inhibit tumor development. As nuclear transcription factors, the
E2F protein family is important for the transcriptional control of
cell cycle. E2F1 facilitates the progression of cells from G1 to S
phase and, additionally, blocks cell apoptosis. A previous study
reported that the E2F1 protein expression level is high in
suprarenal epithelioma (12). Cell
apoptosis is also strictly regulated by various genes. The Bcl-2
family has been extensively investigated. Bcl-2 was initially
identified in patients with leucocythemia as a factor able to
promote the growth and proliferation of tumor cells. Additionally,
it has been established that it may be overexpressed in RCC tissue,
and may contribute to the genesis and development of RCC (13). Downregulation of the expression
levels of E2F1 and Bcl-2 by miR-205 may, therefore, facilitate the
apoptosis of RCC cells. A previous investigation using a luciferase
reporter gene in melanoma cells determined that E2F1 is a target
gene of miR-205 (14). An increase
in the miR-205 expression level in the previous study, directly
inhibited the expression of E2F1 and lead to reduced AKT
phosphorylation, a key factor in the phosphatidylinositol 3-kinase
(PI3K)/AKT signaling pathway (14). Therefore, the proliferation and
clone formation of melanoma cells was decreased and the apoptosis
of melanoma cells was increased by miR-205 (14). In addition, miR-205 was previously
demonstrated to inhibit the proliferation and cell cycle
progression of triple negative breast cancer (15). The present study confirmed that
elevation of intracellular miR-205 level inhibited the
proliferation of RCC cells and promoted their apoptosis,
potentially through downregulating the expression levels of E2F1
and Bcl-2.
As important biological characteristics of tumor
cells, migration and invasion are primary causes of disease
recurrence following surgical intervention, chemotherapy and/or
radiotherapy. Epithelial-mesenchymal transition (EMT) is a crucial
process involved in migration and invasion. During EMT, tumor cells
lose their epithelial phenotype and acquire the phenotype of
mesenchymal cells, resulting in the loss of the polarity of
epithelial cells, reduced contact between surrounding and matrix
cells, decreased intercellular interaction and the increased cell
migration (16,17). Previous studies have determined
that the downregulated expression of E-cadherin, as a cell adhesion
molecule, and the upregulated expression of vimentin, a mesenchymal
marker, contribute to the molecular characteristics of EMT
(18,19). The decreased expression level of
E-cadherin and increased level of vimentin reduce intercellular
adhesion, and facilitate the invasion capacity of tumor cells from
the primary tumor site leading to their subsequent infiltration and
diffusion. Therefore, the migration and invasion of tumor cells may
be inhibited by reducing the expression levels of EMT-associated
proteins. In a previous study using 769-p RCC cells, vimentin was
overexpressed and the invasion ability of tumor cells was increased
(20). The elevated expression
level of miR-205 in melanoma cells and esophageal squamous cell
carcinoma cells may upregulate the expression of E-cadherin and
down-regulate the expression of vimentin, subsequently reducing the
migration and invasion of tumor cells (8,21).
Therefore, it was hypothesized that miR-205 may inhibit the
migration and invasion of RCC cells through a reduction in the
expression levels of E-cadherin and vimentin, which was verified in
the present study using a Transwell assay and western blotting
analysis following transfection of RCC cells with miR-205
mimics.
The PI3K/AKT signaling pathway is important for the
genesis and development of various human carcinomas by promoting
cell growth, apoptosis, cell cycle progression, angiogenesis, cell
migration and invasion. Consequently, the inhibition of the
PI3K/AKT signaling pathway may improve cancer therapy. PTEN
dephosphorylates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to produce
phosphatidylinositol (4,5)-bisphosphate (PIP2), thus, negatively
regulating the PI3K/AKT signaling pathway. In RCC, the inactivation
of PTEN has been observed to increase activation of the P3IK/AKT
signaling pathway (22). Zhang
et al (23) reported that
PTEN is a target gene of miR-205, and the transfection of miR-205
mimics into endometrial cancer cells enhanced the expression of
PTEN and phosphorylation of AKT, and inhibited the apoptosis of
tumor cells (23). However, in
non-small cell lung carcinoma and nasopharyngeal carcinoma cells,
the downregulated expression of miR-205 was identified to
significantly increase the expression level of PTEN and promote the
phosphorylation of AKT (24,25).
The effect of miR-205 on the expression of PTEN, phosphorylation of
AKT and the associated biological behavior is established, however,
the positive or negative regulation exerted by miR-205 on PTEN and
p-AKT is contradictory between distinct types of tumor cells. The
present study determined, through the use of a Transwell assay and
western blotting, that miR-205 promoted the apoptosis of RCC cells
and suppressed their proliferation, migration and invasion. miR-250
also increased the PTEN expression levels and inhibited the
phosphorylation of AKT.
In conclusion, miR-205 is important for the genesis
and development of RCC. The present study demonstrated that miR-205
was expressed in low levels in RCC tissues and cell lines; and this
may contribute to the aggressive biological behavior of RCC.
Following the transfection of miR-205 mimics into RCC cells,
miR-205 promoted the apoptosis of RCC cells and suppressed their
proliferation, migration and invasion, which may be mediated by
effects on the PTEN/AKT signaling pathway. E2F1, Bcl-2 and vimentin
were negatively regulated by miR-205 and E-cadherin was positively
regulated. The present study may provide future guidelines for the
development of miRNA-associated therapeutic agents for the
treatment of RCC.
References
|
1
|
Abdellah A, Selma K, Elamin M, Asmae T,
Lamia R, Abderrahmane M, Sanaa el M, Hanan E, Tayeb K and
Noureddine B: Renal cell carcinoma in children: Case report and
literature review. Pan Afr Med J. 20:842015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kostrzewa M, Zyła M, Władziński J,
Stetkiewicz T, Stachowiak G and Wilczyński JR: Metastases of renal
clear cell carcinoma to ovary-case report and review of the
literature. Eur J Gynaecol Oncol. 36:219–222. 2015.
|
|
3
|
Ye DW and Zhang HL: Critical appraisal of
sorafenib in the treatment of Chinese patients with renal cell
carcinoma. Onco Targets Ther. 7:925–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow TF, Youssef YM, Lianidou E, Romaschin
AD, Honey RJ, Stewart R, Pace KT and Yousef GM: Differential
expression profiling of microRNAs and their potential involvement
in renal cell carcinoma pathogenesis. Clin Biochem. 43:150–158.
2010. View Article : Google Scholar
|
|
6
|
Yi Z, Fu Y, Zhao S, Zhang X and Ma C:
Differential expression of miRNA patterns in renal cell carcinoma
and nontumorous tissues. J Cancer Res Clin Oncol. 136:855–862.
2010. View Article : Google Scholar
|
|
7
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsushima K, Isomoto H, Yamaguchi N,
Inoue N, Machida H, Nakayama T, Hayashi T, Kunizaki M, Hidaka S,
Nagayasu T, et al: MiRNA-205 modulates cellular invasion and
migration via regulating zinc finger E-box binding homeobox 2
expression in esophageal squamous cell carcinoma cells. J Transl
Med. 9:302011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gandellini P, Folini M, Longoni N, Pennati
M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta
P, et al: miR-205 Exerts tumor-suppressive functions in human
prostate through down-regulation of protein kinase Cepsilon. Cancer
Res. 69:2287–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Z, Tang ZY, He Y, Liu LF, Li DJ and
Chen X: miRNA-205 is a candidate tumor suppressor that targets ZEB2
in renal cell carcinoma. Oncol Res Treat. 37:658–664. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majid S, Saini S, Dar AA, Hirata H,
Shahryari V, Tanaka Y, Yamamura S, Ueno K, Zaman MS, Singh K, et
al: MicroRNA-205 inhibits Src-mediated oncogenic pathways in renal
cancer. Cancer Res. 71:2611–2621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Fan Y, Chen W, Huang Q, Ai Q, Ni D,
Ma X and Zhang X: Effect of E2Fl knockdown on proliferation and
invasion of clear cell renal cell carcinoma cell line Caki-2. Zhong
Hua Shi Yan Wai Ke Za Zhi She. 333–335. 2013.In Chinese.
|
|
13
|
Li Y, Zhang J, Ge D, Yang Y and Yu J:
Expression and significance of E-cadherin and Bcl-2 in early renal
carcinoma and adjacent tissues of different levels of distance. J
Contemp Urol Reprod Onco. 108–111. 2011.In Chinese.
|
|
14
|
Dar AA, Majid S, de Semir D, Nosrati M,
Bezrookove V and Kashani-Sabet M: miRNA-205 suppresses melanoma
cell proliferation and induces senescence via regulation of E2F1
protein. J Biol Chem. 286:16606–16614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piovan C, Palmieri D, Di Leva G, Braccioli
L, Casalini P, Nuovo G, Tortoreto M, Sasso M, Plantamura I, Triulzi
T, et al: Oncosuppressive role of p53-induced miR-205 in triple
negative breast cancer. Mol Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar :
|
|
17
|
Ogunwobi OO and Liu C: Therapeutic and
prognostic importance of epithelial-mesenchymal transition in liver
cancers: Insights from experimental models. Crit Rev Oncol Hematol.
83:319–328. 2012. View Article : Google Scholar
|
|
18
|
Yamada S, Okumura N, Wei L, Fuchs BC,
Fujii T, Sugimoto H, Nomoto S, Takeda S, Tanabe KK and Kodera Y:
Epithelial to mesenchymal transition is associated with shorter
disease-free survival in hepatocellular carcinoma. Ann Surg Oncol.
21:3882–3890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou KZ, Fu ZQ and Gong H: Chemokine ligand
20 enhances progression of hepatocellular carcinoma via
epithelial-mesenchymal transition. World J Gastroenterol.
21:475–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang Y, Wei J, Cao J, Zhao H, Liao B, Qiu
S, Wang D, Luo J and Chen W: Protein expression of ZEB2 in renal
cell carcinoma and its prognostic significance in patient survival.
PLoS One. 8:e625582013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Tetzlaff MT, Liu A, Liegl-Atzwanger
B, Guo J and Xu X: Loss of microRNA-205 expression is associated
with melanoma progression. Lab Invest. 92:1084–1096. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hager M, Haufe H, Lusuardi L, Schmeller N
and Kolbitsch C: PTEN, pAKT, and pmTOR expression and subcellular
distribution in primary renal cell carcinomas and their metastases.
Cancer Invest. 29:427–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang G, Hou X, Li Y and Zhao M: MiR-205
inhibits cell apoptosis by targeting phosphatase and tensin homolog
deleted on chromosome ten in endometrial cancer Ishikawa cells. BMC
Cancer. 14:4402014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai J, Fang L, Huang Y, Li R, Yuan J, Yang
Y, Zhu X, Chen B, Wu J and Li M: miR-205 targets PTEN and PHLPP2 to
augment AKT signaling and drive malignant phenotypes in non-small
cell lung cancer. Cancer Res. 73:5402–5415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|