Introduction
Diabetic nephropathy (DN) is one of the most severe
microvascular complications of type I and II diabetes, and is a
major cause of end-stage renal disease (1). One major pathological feature of DN
is increased proliferation of renal mesangial cells (RMCs).
Although the precise mechanism underlying the onset and progression
of DN has not yet been elucidated, several in vivo studies
have demonstrated a significant association between RMC expansion
and early stages of DN. Specifically, these studies showed that
hypercellularity in the mesangial cell population precedes
expansion of the extracellular matrix (ECM) and glomerular
sclerosis (2,3). Proliferation of RMCs is also
correlated with the degree of glycemic control, indicating that
abnormally high blood glucose levels may be a crucial risk factor
triggering DN (4). Few studies
have explored effective strategies for pharmacological intervention
of DN, and there is a critical need to identify drugs with the
potential to inhibit or control excessive proliferation of RMCs and
thus serve to impede the progression of DN.
Shenkang injection (SKI) is a patented Chinese
medicine, which is an extract composed of Rheum officinale,
Salvia miltiorrhiza, Carthamus tinctorius and radix
Astragali, and is used to treat chronic renal failure
(5). Several clinical reports have
shown that SKI can inhibit the production of factors that either
promote the synthesis of ECM (transforming growth factor-β1 and
connective tissue growth factor) or antagonize pathways responsible
for the degradation of ECM (tissue inhibitor of metalloproteinase-1
and plasminogen activator inhibitor-1) in the kidney (6). A clinical study showed that SKI
treatment significantly improved the clearance rate of serum
creatinine (7), while another
study showed that in patients diagnosed with early DN, SKI
treatment significantly reduced the levels of the urine protein
β2-microglobulin (8).
In the combined herbal medicine SKI, rhubarb is the
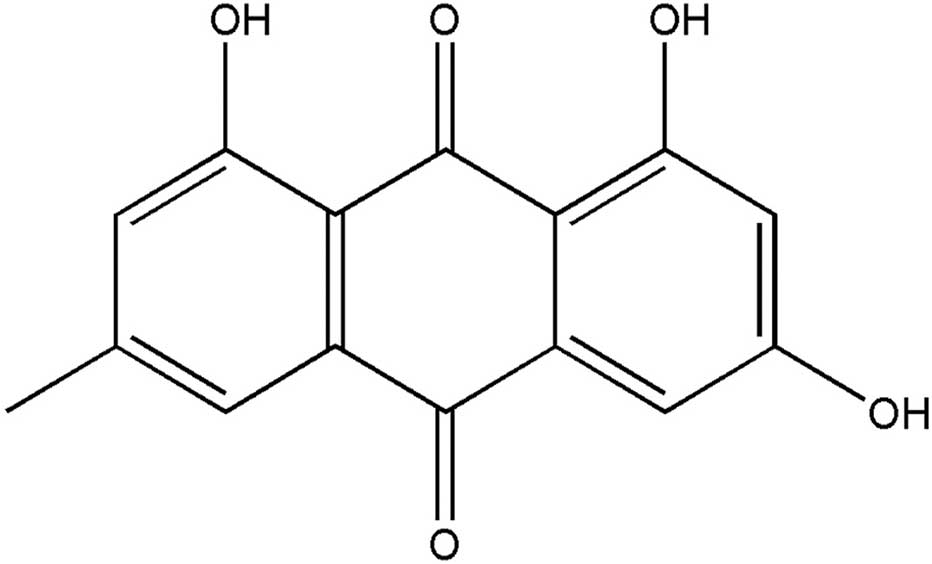
main ingredient, while emodin (EM; 3-methyl-1,6,8-trihydroxy
anthraquinone) (Fig. 1) is one of
the major active components. The present study aimed to assess
whether SKI or EM are suitable for the treatment of diabetic
nephropathy. In vivo studies showed that EM significantly
decreased the levels of blood glucose, triglycerides and total
serum cholesterol, while improving glucose tolerance and insulin
sensitivity (9–11). Furthermore, following
administration for eight weeks, renal lesions in rats were
significantly ameliorated and the levels of serum creatinine, urea
and 24-h urine protein were decreased (12). In vitro, EM markedly
suppressed high glucose-induced cell proliferation, reduced the
expression of fibronectin and collagen IV, decreased the
phosphorylation of p38 mitogen-activated protein kinase and
upregulated the expression of peroxisome proliferator-activated
receptor γ (13,14).
The present study assessed the effects of SKI and EM
on the pathology of DN and investigated the underlying mechanisms.
Specifically, the anti-proliferative and apoptotic effects of SKI
and its major component EM on high glucose-stimulated renal
mesangial cells (RMCs) were assessed.
Materials and methods
Cell culture and reagents
The well-characterized rat RMC line HBZY-1 was
obtained from The Chinese Center for Type Culture Collection
(Wuhan, China). Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; containing 5.6 mM or 25 mM glucose; Thermo Fisher
Scientific, Inc., Beijing, China) supplemented with 10% fetal calf
serum (FCS; Zhejiang Tianhang Biotechnology Co., Ltd., Zhejiang,
China), 10,000 U/ml penicillin and 10,000 µg/ml streptomycin
(Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2. RMCs between passages 3 and 10 were
used for all experiments. Following pre-incubation in DMEM
(containing 5.6 mM glucose) supplemented with 0.1% FCS for 24 h,
cells were divided into the following experimental groups: Normal
glucose (NG; 5.6 mM glucose); high glucose (HG; 25 mM glucose);
high glucose and different concentrations of SKI (HG + SKI-100
mg/l, 25 mM glucose + 100 mg/l SKI; HG + SKI-50 mg/l, 25 mM glucose
+ 50 mg/l SKI; HG + SKI-25 mg/l, 25 mM glucose + 25 mg/l SKI); high
glucose and different concentrations of EM (HG + EM-40 µM,
25 mM glucose + 40 µM EM; HG + EM-20 µM, 25 mM
glucose + 20 µM EM; HG + EM-10 µM, 25 mM glucose + 10
µM EM); mannitol (MN; 5.6 mM glucose and 19.4 mM mannitol).
Mannitol was purchased from Sigma-Aldrich (St. Louis, MO, USA). SKI
(0.3 g/ml) was supplied by Xi'an Shiji Shengkang Pharmaceutical
Industry Co., Ltd. (Xi'an, China) and the presence of EM was
confirmed using high-performance liquid chromatography. EM was
purchased from the National Food and Drug Testing Institute
(Beijing, China).
Cell proliferation assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used as a qualitative index of cell viability. All cells
were treated with 20 µl MTT (5 mg/ml; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) after 12, 24 or 48 h of
culture under the different experimental conditions stated above,
and further cultured for another 4 h. Next, they were lysed using
dimethylsulfoxide (0.15 ml/well; Sigma-Aldrich). Once the formazan
crystals dissolved completely, the optical density was measured at
490 nm using a Microplate Reader Model M680-UV Spectrophotometer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell cycle analysis
The cell cycle distribution of cells in the
different treatment groups was analyzed using flow cytometry. After
24 h of culture under the different experimental conditions,
(0.5–1)×106 cells were harvested by enzymatic digestion
with trypsin, washed twice with phosphate-buffered saline (PBS) and
fixed in 70% ethanol at 20°C. The fixed cells were re-suspended in
500 µl PBS and RNase A (100 µg/ml; Takara Bio, Inc.,
Otsu, Japan) and incubated at 37°C for 1 h. Next, the cells were
treated with propidium iodide (PI; 50 µg/ml; Takara Bio,
Inc.) for 30 min. The DNA content of 2×105 cells from
each experimental group was determined using a flow cytometer
(LSRFortessa; Becton Dickinson, San Jose, CA, USA), and the data
were analyzed using Mod Fit LT 2.0 software (Verity Software,
Topsham, ME, USA).
Apoptosis assay
Apoptotic cells were identified using an Annexin
V/PI apoptosis kit (Keygen Biotech, Nanjing, China) and flow
cytometry. After 24 h of culture under the different experimental
conditions, (0.5–1)×106 cells were harvested and
re-suspended in 500 µl binding buffer. Next, the cells were
incubated with 5 µl Annexin V-fluorescein isothiocyanate
(FITC) and 5 µl PI (50 mg/ml) for 15 min in the dark and
immediately analyzed by flow cytometry. Data from at least
2×105 cells of each sample were acquired and analyzed
using Cell Quest software, version 7.5.3 (Becton Dickinson). In the
PI vs. FITC scatter plot, the percentage of cells in the lower
right quadrant of (early apoptotic cells), upper right quadrant
(late apoptotic cells), upper left quadrant (necrotic cells) and
lower left quadrant (live cells) was calculated for comparison.
Transmission electron microscopy
(TEM)
To further assess the occurrence of apoptosis, the
morphology of the cells was observed by TEM. After 24 h of culture
under different experimental conditions, cells were collected by
centrifugation (1,080 × g, 3 min), washed twice with PBS and fixed
in freshly made 1% paraformaldehyde with 2% glutaraldehyde (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) for 24 h. Next,
the samples were treated with 1% osmium tetroxide (Beijing CoWin
Biotech Co., Ltd., Beijing, China) for 2 h, dehydrated using a
graded ethanol series and embedded in araldite. Ultra-thin sections
were prepared, stained with uranyl acetate (Shanghai Jianglai
Biotechnology Co., Ltd., Shanghai, China) and lead citrate and
observed by TEM (JEM-101; Jeol Electron Inc, Tokyo, Japan).
Western blot analysis
Cells cultured under the different experimental
conditions for 24 h were harvested and washed with ice-cold PBS.
Whole-cell protein extracts were obtained by lysing the cells with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentrations were
determined using the bicinchonic acid method (Beyotime Institute of
Biotechnology). Total proteins (50 µg/lane) were then
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, transferred onto nitrocellulose membranes (0.45
µm; Bio-Rad Laboratories, Inc.) and blocked with 5% skimmed
milk in Tris-buffered saline (pH 7.6; TBS) at room temperature. The
membranes were then incubated overnight at 4°C with primary
antibodies against B-cell lymphoma 2 (bcl-2)-associated X protein
(bax; rabbit monoclonal; 1:2,000 dilution; cat. no. ab32503; Abcam,
Cambridge, MA, USA), bcl-2 (rabbit monoclonal; 1:1,000 dilution;
cat. no. ab32124; Abcam), caspase-8 (rabbit polyclonal; 1:2,000
dilution; cat. no. ab25901; Abcam), caspase-6 (rabbit monoclonal;
1:1,000 dilution; cat. no. P55212; Epitomics; Abcam), caspase-3
(rabbit monoclonal; 1:1,000; cat. no P42574; Epitomics; Abcam),
cleaved caspase-3 (rabbit monoclonal; 1:500 dilution; cat. no.
AC033; Beyotime Institute of Biotechnology) and β-actin (rabbit
polyclonal; 1:1,000; cat. no. AP0060; Bioworld Technology, Inc.,
St. Louis Park, MN, USA). Subsequent to washing with TBS three
times, the membranes were incubated with the anti-rabbit or
anti-mouse IgG antibodies conjugated with horseradish peroxidase
(1:2,000 dilution; Beyotime Institute of Biotechnology) for 1.5 h
at room temperature. Subsequent to washing the membranes three
times, the resulting immune complexes were detected using enhanced
chemiluminescence kits (Thermo Fisher Scientific, Inc.).
Immunolabeled bands were further quantified using the Gel Doc™ XR
and Lab image 4.0.1 software (Bio-Rad Laboratories, Inc.). All
values were normalized to the absorbance of the internal control
(β-actin).
Statistical analysis
Differences between experimental groups were tested
for statistical significance using one-way analysis of variance
followed by Tukey's test. GraphPad Prism, version 6.0 (GraphPad
Software, Inc., La Jolla, CA, USA) was used for the analysis. All
values are expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
SKI and EM inhibit RMC proliferation
induced by HG
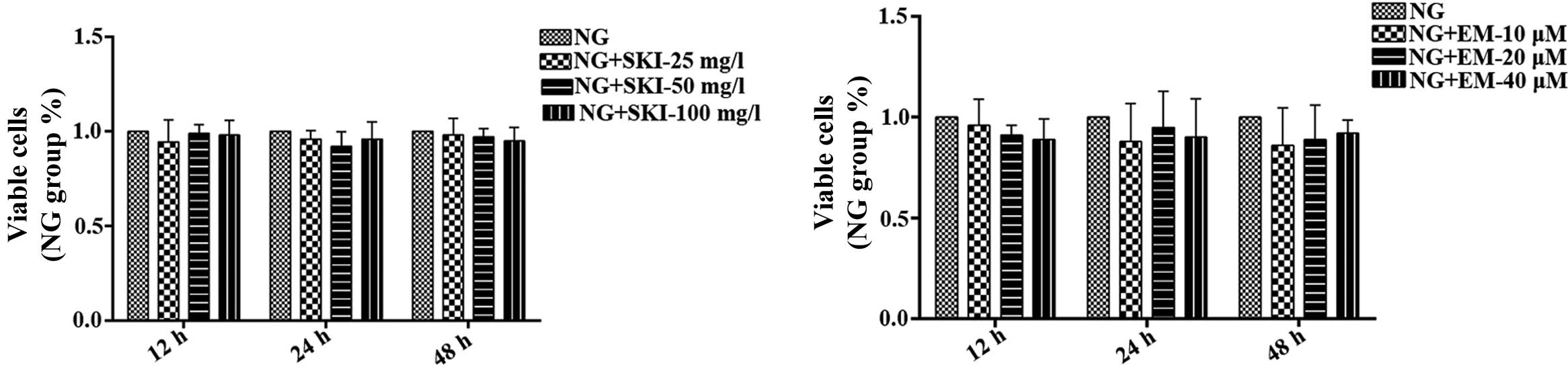
To determine the effects of SKI and EM on the
proliferation of RMCs under normal glucose conditions, an MTT assay
was performed, revealing that the drugs did not affect RMCs
(Fig. 2). Next, the effects of SKI
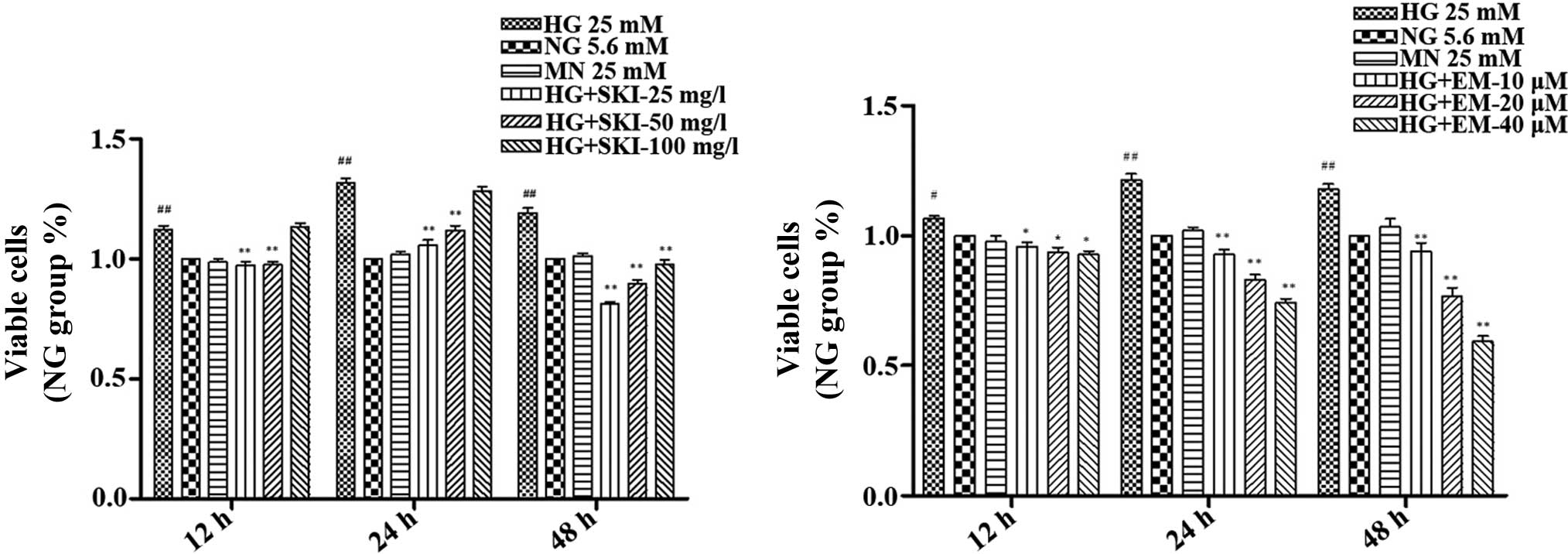
and EM on RMCs cultured under HG conditions were assessed (Fig. 3). The results showed that in
comparison to the NG group, 25 mM glucose (HG) increased the
proliferation of RMCs after 12, 24 and 48 h of culture, which was
significantly inhibited by treatment with 25 and 50 mg/l SKI.
However, a concentration of 100 mg/l SKI only significantly
inhibited the HG-induced increase in cell division at 48 h. In
addition, EM dose- and time-dependently inhibited HG-induced RMC
proliferation at concentrations of 10, 20 and 40 µM.
Finally, exposure to 25 mM mannitol, an osmotic control, did not
alter the growth rate of the RMCs. This suggested that HG-induced
RMC proliferation was not a consequence of high osmolarity
(Fig. 3).
SKI and EM inhibit cell cycle progression
of RMCs stimulated by HG
To further evaluate the mechanisms of the
anti-proliferative effects of SKI and EM, flow cytometric cell
cycle analysis of cells in the various treatment groups was
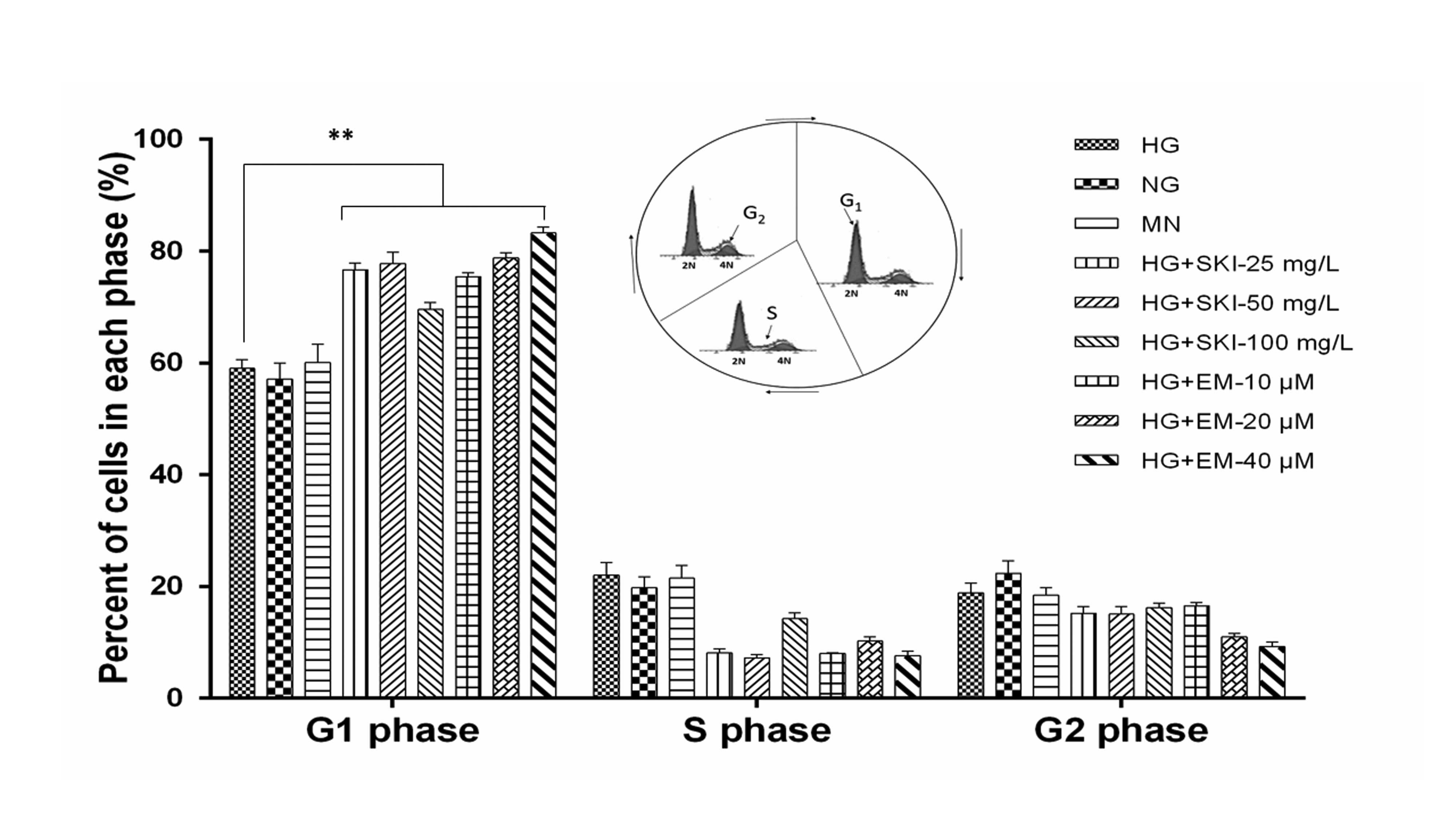
performed. As shown in Fig. 4, in
comparison to the HG group, treatment with 25, 50 or 100 mg/l SKI
increased the G1 phase population from 59.0±1.6 to 76.6±1.2,
77.7±2.1 and 69.5±1.3%, respectively. EM dose-dependently inhibited
cell cycle progression, as treatment with 25, 50 and 100 mg/l EM
led to an increase in the percentage of cells in G1 phase to
75.4±0.7%, 78.8±0.9% and 83.2±1.1%, respectively. In addition, it
was observed that in comparison with the MN and NG groups, HG
conditions promoted cell cycle progression (G1, 58.0±0.2%; S,
19.4±0.6%; G2, 22.6±0.4%). There was no notable difference between
the MN and NG groups, suggesting that changes in cell cycle
progression triggered by HG were not a result of the high
osmolarity in HG cultures. In conclusion, the results showed that
HG conditions promoted cell cycle progression and proliferation of
RMCs, which was significantly reversed by SKI and EM by causing
G1-phase arrest.
SKI and EM enhance the apoptotic rate of
RMCs exposed to HG
Next, the present study assessed whether SKI and EM
inhibited the increase in proliferation of RMCs under HG by
promoting apoptosis (programmed cell death). To address this
question, cellular morphology was first observed by TEM. As shown
in Fig. 5, cells in the MN and HG
groups did not display any changes in their typical morphology
compared to those in the NG group. However, following exposure to
different concentrations of SKI and EM for 24 h, obvious
morphological changes characteristic for apoptosis were observed in
these cells. These typically included chromatin condensation,
vacuolization in the mitochondria and degranulation in the
endoplasmic reticulum, as indicated in Fig. 5.
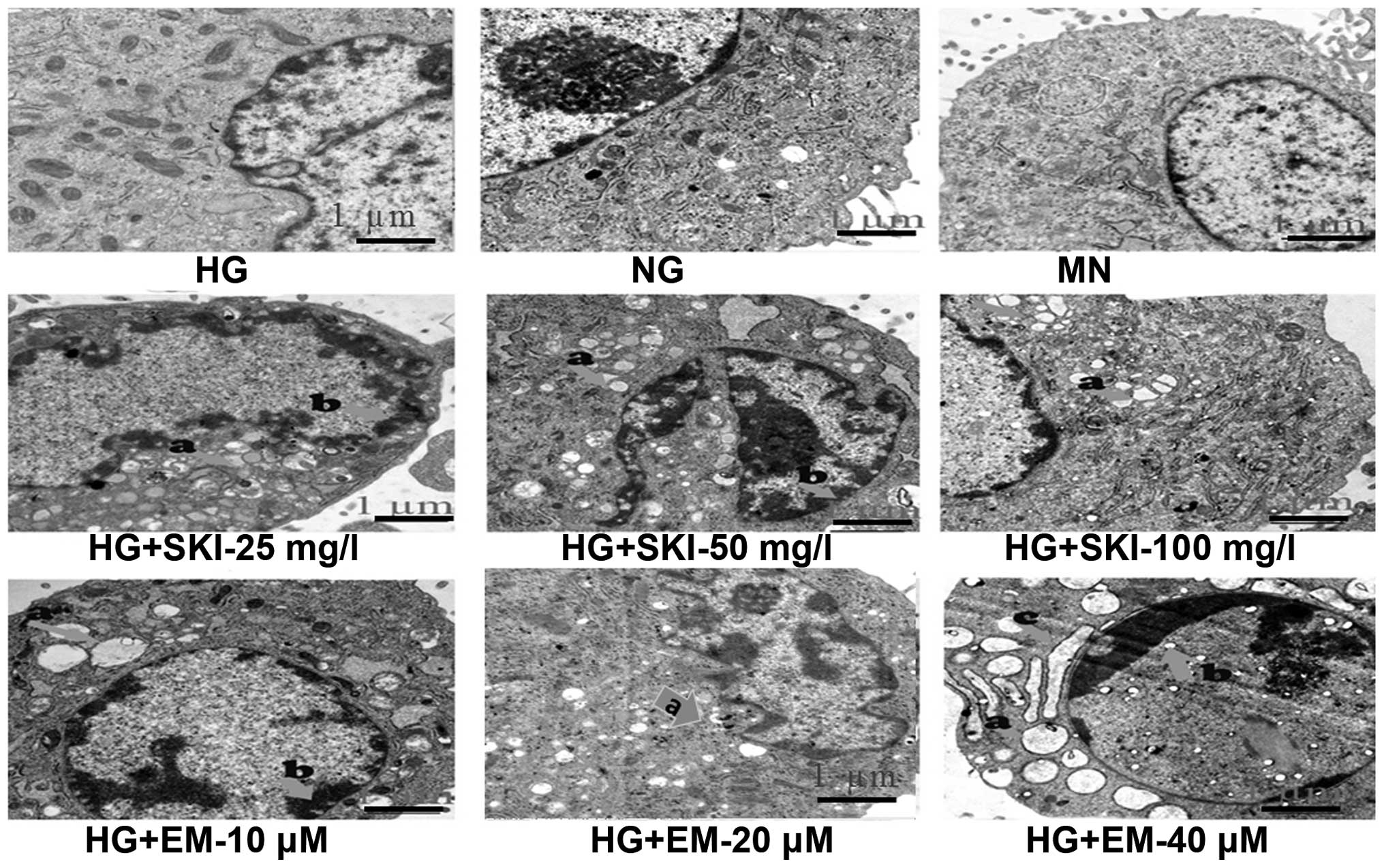 | Figure 5Effects of SKI and EM on morphological
characteristics of apoptosis (indicated by arrows; a, vacuolization
in the mitochondria; b, chromatin condensation; c, degranulation in
the endoplasmic reticulum) in renal mesangial cells cultured in
high glucose. Representative transmission electron microscopy
images (magnification, ×20,000) of cells exposed for 24 h are
shown. NG, normal glucose; HG, high glucose; SKI, Shenkang
injection; EM, emodin; MN, mannitol. |
To further confirm this observation, flow cytometric
analysis of RMCs in the various treatment groups was performed. As
shown in Fig. 6, SKI significantly
induced either late or early apoptosis in RMCs under HG;
furthermore, EM was found to dose-dependently induce apoptosis and
necrosis, while dose-dependently reducing the viability of the
cells. In addition, there was no obvious increase in cell death in
the MN, NG and HG groups.
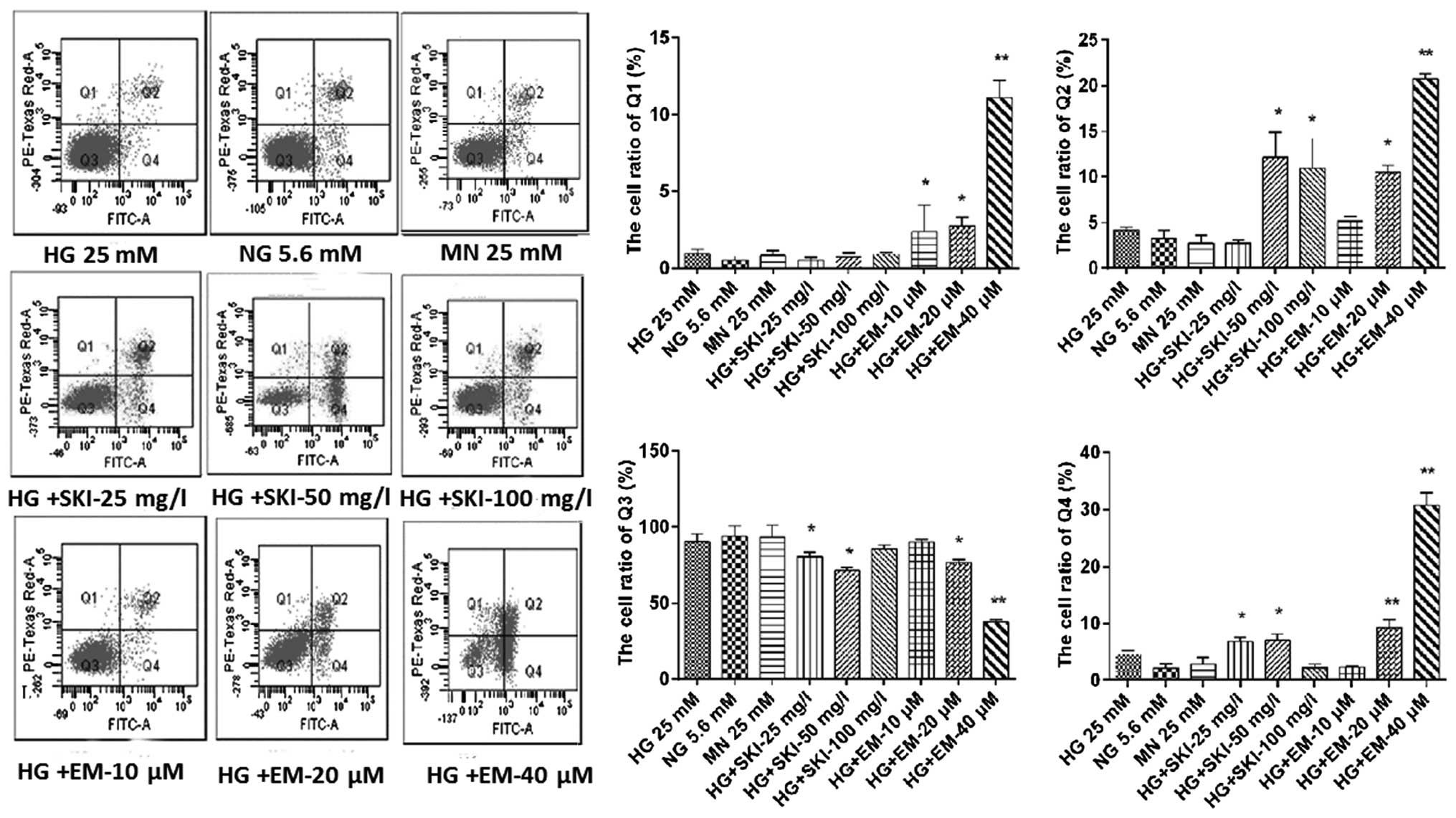 | Figure 6Effects of SKI and EM on renal
mesangial cell apoptosis under high-glucose conditions.
Representative flow cytometric dot plots of cells treated for 24 h
and stained with Annexin V/PI are shown. Cells in the quadrants
were quantified as follows: Q1, necrotic cells; Q2, late apoptotic
cells; Q3, normal cells; Q4, early apoptotic cells. Values from
five independent experiments are presented as the mean ± standard
deviation. *P<0.05 vs. HG group,
**P<0.01 vs. HG group. NG, normal glucose; HG, high
glucose; SKI, Shenkang injection; EM, emodin; MN, mannitol. |
Finally, western blot analysis was performed to
determine which components of the apoptotic pathway are impacted by
SKI and EM treatment. Incubation of RMCs with various
concentrations of SKI for 24 h under HG led to a significant
upregulation of bax, caspase-3, cleaved caspase-3, caspase-6 and
caspase-8 (Fig. 7A). Furthermore,
EM was found to dose-dependently increase the levels of all of
these proteins (Fig. 7B).
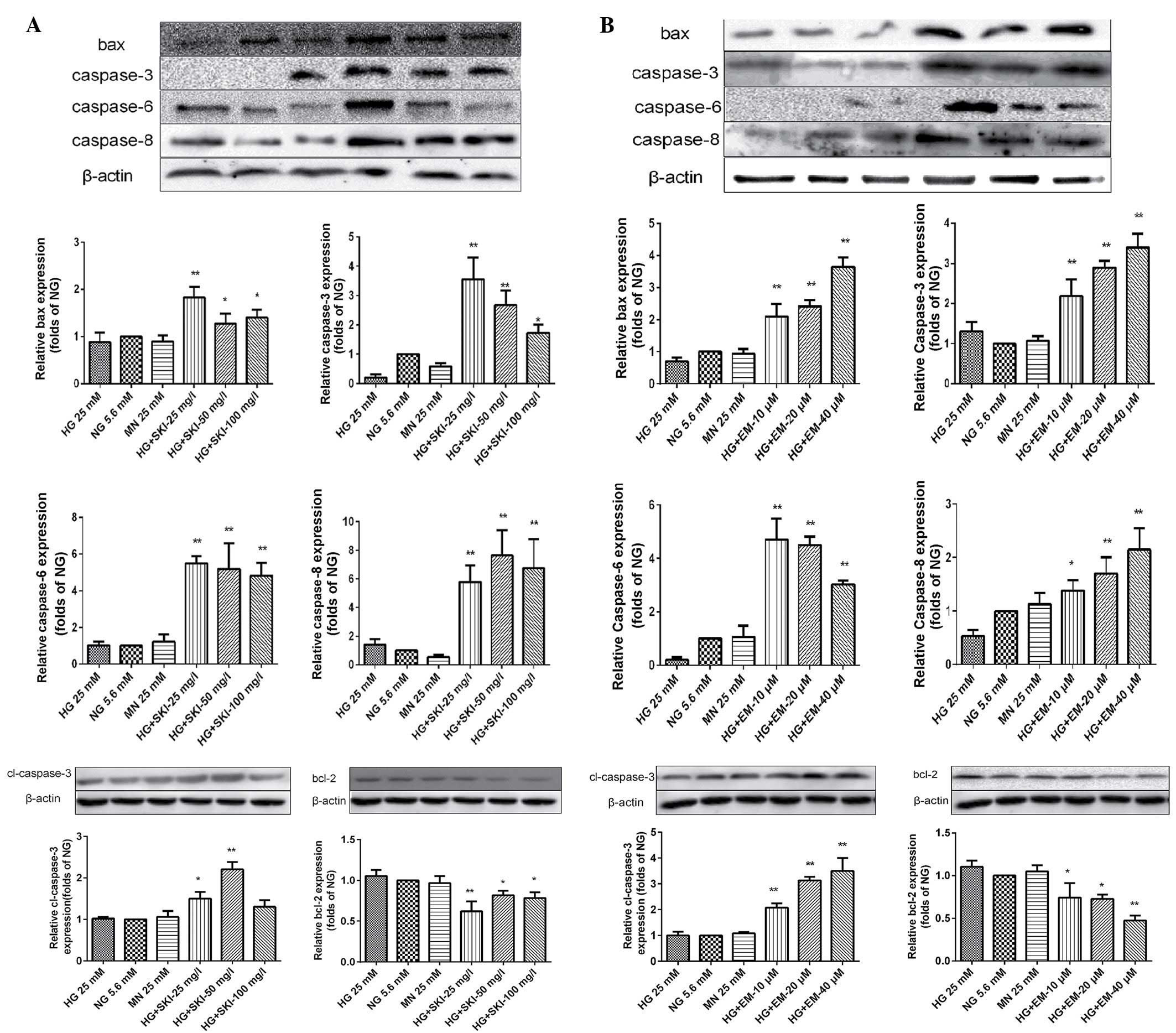 | Figure 7Effects of (A) SKI and (B) EM
treatment for 24 h on the protein expression of bax, caspase-3,
caspase-6, caspase-8, cleaved caspase-3 and bcl-2 under
high-glucose conditions as detected by western blot analysis.
Values from three independent experiments are presented as mean ±
standard deviation. *P<0.05 vs. HG group,
**P<0.01 vs. HG group. NG, normal glucose; HG, high
glucose; SKI, Shenkang injection; EM, emodin; MN, mannitol; bax,
bcl-2-associated X protein; bcl-2, B-cell lymphoma 2. |
All of these results indicated that SKI and EM
induced mitochondria-mediated apoptosis in RMCs under HG.
Discussion
SKI is a Traditional Chinese Medicine whose major
active component is EM. Although it is widely used to treat DN in
China, the precise molecular functions of its components and its
mechanism of action have remained to be elucidated. The present
study demonstrated the inhibitory effects of SKI and EM on
HG-induced proliferation of RMCs. Furthermore, the underlying
mechanisms were revealed to comprise inhibition of DNA synthesis
resulting from cell cycle arrest in G1 phase, as well as induction
of mitochondrial apoptosis in RMCs. Morphological changes in RMCs
were observed following exposure to different concentrations of SKI
and EM, including chromatin condensation, vacuolization in the
mitochondria and degranulation in the endoplasmic reticulum.
Furthermore, flow cytometric analysis revealed an increase in the
apoptotic rate of RMCs exposed to SKI and EM for 24 h under HG. The
underlying molecular mechanism was further elucidated using western
blot analysis, revealing an upregulation of the apoptotic proteins
bax, caspase-3, cleaved caspase-3, caspase-6 and caspase-8 in
addition to downregulation of bcl-2 in RMCs following incubation
with SKI and EM. These results revealed that SKI and EM can inhibit
HG-induced RMC proliferation by regulating cell cycle progression
and inducing apoptosis.
Studies have shown that a proliferative response of
RMCs subsequent to a variety of stimuli is associated with matrix
accumulation and the development of glomerulosclerosis, which
eventually leads to progressive renal disease. HG concentrations
were shown to contribute to uncontrolled proliferation of RMCs,
distal tubular epithelial cells and vascular smooth muscle cells
during diabetes (15–17). The present study used rat RMCs as
an in vitro model to study changes in cell proliferation
during the early stages of diabetic nephropathy. As clinical trials
have demonstrated that HG is the principal cause of renal damage in
type I and type II diabetes (18),
HG culture conditions were applied to stimulate RMC
proliferation.
During cell cycle progression, the transition from
G1 to S phase is essential for DNA synthesis, which is followed by
G2 phase and finally the M phase, in which mitotic cell division
takes place (19,20). In the present study, compared to
the NG group, HG conditions significantly promoted cell cycle
progression. However, treatment with different concentrations of
SKI and EM led to G1-phase arrest. Furthermore, in the MN group the
cell cycle distribution was not markedly affected, confirming that
HG-associated osmotic pressure was not involved in these effects.
These results suggested that HG induces RMC proliferation by
promoting cell cycle progression in RMCs, and that SKI and EM can
reverse this effect by arresting cells in G1 phase.
Apoptotic cell death is characterized by specific
biochemical and morphological changes, which can be identified
using several assays, including morphological analysis by
high-resolution microscopy, as well as flow cytometry and western
blot analysis. In the present study, TEM was used to examine
HG-induced RMCs treated with various concentrations of SKI and EM,
revealing the following morphological changes: Chromatin
condensation, vacuolization in the mitochondria and degranulation
in the endoplasmic reticulum. However, the MN, and HG groups did
not show any morphological abnormalities compared with the NG
group. Flow cytometry was further used to verify the increase in
the percentage of apoptotic cells in the SKI and EM groups. The
results clearly showed that SKI and EM induced apoptosis in RMCs
under HG conditions.
Bax is a pro-apoptotic protein that facilitates
apoptosis through an intrinsic, damage-induced pathway, and
amplifies apoptotic signaling upregulated via extrinsic,
receptor-mediated triggers (21).
The effect of bax mediated-apoptosis is largely dependent on the
concentration of bax and its inhibitor bcl-2. It is expressed in
viable cells and activated in response to pro-apoptotic stimuli.
Caspase-3, -6 and -8 are members of a family of cysteine proteases
originally discovered for their role in apoptosis. During this
process, caspases participate in signaling cascades where the
upstream initiator caspases activate the downstream executioner
caspases, which in turn cleave a specific subset of cellular
targets (22). To elucidate the
mechanism of action underlying EM and SKI-induced apoptosis in
RMCs, the expression levels of bax, bcl-2, caspase-3, cleaved
caspase-3, caspase-6, and caspase-8 were examined by western blot
analysis in the present study.
In conclusion, the present study demonstrated that
SKI and its major active component EM inhibited HG-stimulated
proliferation of RMCs by causing cell cycle arrest at G1
phase and inducing apoptosis. At the molecular level, the
underlying mechanism was shown to include upregulation of bax,
caspase-3, cleaved caspase-3, caspase-6 and caspase-8, and
downregulation of bcl-2. The present study supported the use of SKI
and EM for potential use as therapeutics for DN.
References
|
1
|
Giunti S, Barit D and Cooper ME: Diabetic
nephropathy: From mechanisms to rational therapies. Minerva Med.
97:241–262. 2006.PubMed/NCBI
|
|
2
|
Abboud HE: Mesangial cell biology. Exp
Cell Res. 318:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danesh FR, Sadeghi MM, Amro N, Philips C,
Zeng L, Lin S, Sahai A and Kanwar YS: 3-hydroxy-3-methylglutaryl
CoA reductase inhibitors prevent high glucose-induced proliferation
of mesangial cells via modulation of Rho GTPase/p21 signaling
pathway: Implications for diabetic nephropathy. Proc Natl Acad Sci
USA. 99:8301–8305. 2002. View Article : Google Scholar
|
|
4
|
Hodgkinson AD, Bartlett T, Oates PJ,
Millward BA and Demaine AG: The response of antioxidant genes to
hyperglycemia is abnormal in patients with type 1 diabetes and
diabetic nephropathy. Diabetes. 52:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye CH, Wu F, Liu TH and Li MQ: Compound
traditional chinese medicine preparation. Chinese Patent
CN103768525A. Filed January 17, 2014; issued May 7, 2014.
|
|
6
|
QY YC and Rui P: Antagonizing effects of
Shenkang Injection on renal interstitial fibrosis in model rat of
chronic aristolochic acid nephropathy. Chin Tradit Herb Drugs.
4:587–592. 2009.
|
|
7
|
Jiang ZW, Lv YY and Xia JL: The phase
clinical observation study of shengkang injection on chronic renal
failure. J China Med Univ. 40:941–945. 2011.
|
|
8
|
Du J, Chen H and Wang XB: Effect of
shenkang injection on hypertrophy and expressions of p21 and p27 in
glomerular mesangial cells of rats cultured in high glucose. Chin J
Integr Tradit West Med. 26(Suppl): 68–71. 2006.In Chinese.
|
|
9
|
Xue J, Ding W and Liu Y: Anti-diabetic
effects of emodin involved in the activation of PPARgamma on
high-fat diet-fed and low dose of streptozotocin-induced diabetic
mice. Fitoterapia. 81:173–177. 2010. View Article : Google Scholar
|
|
10
|
Yang LHLF: Effect of emodin and berberine
on gastrointestinal motility in type 2 diabetic rats. World Chin J
Digestol. 13:607–611. 2005.
|
|
11
|
Song B and Liu XZ: Emodin improves insulin
sensitivity in KKAy diabetic mice. Chinese PLA Postgrad Med.
32:1274–1276. 2011.
|
|
12
|
Wang J, Huang H, Liu P, Tang F, Qin J,
Huang W, Chen F, Guo F, Liu W and Yang B: Inhibition of
phosphorylation of p38 MAPK involved in the protection of
nephropathy by emodin in diabetic rats. Eur J Pharmacol.
553:297–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Jia L, Liu ZC, Zhang H, Zhang PJ,
Wan Q and Wang R: Emodin ameliorates high-glucose induced mesangial
p38 over-activation and hypocontractility via activation of
PPARgamma. Exp Mol Med. 41:648–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Liu W, Wang Q, Liu P, Deng Y, Lan T,
Zhang X, Qiu B, Ning H and Huang H: Emodin suppresses cell
proliferation and fibronectin expression via p38MAPK pathway in rat
mesangial cells cultured under high glucose. Mol Cell Endocrinol.
307:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arora MK and Singh UK: Molecular
mechanisms in the pathogenesis of diabetic nephropathy: An update.
Vascul Pharmacol. 58:259–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Pang S, Deng B, Qian L, Chen J,
Zou J, Zheng J, Yang L, Zhang C, Chen X, et al: High glucose
induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sodhi CP, Phadke SA, Batlle D and Sahai A:
Hypoxia and high glucose cause exaggerated mesangial cell growth
and collagen synthesis: Role of osteopontin. Am J Physiol Renal
Physiol. 280:F667–F674. 2001.PubMed/NCBI
|
|
18
|
Suzaki Y, Yoshizumi M, Kagami S, Nishiyama
A, Ozawa Y, Kyaw M, Izawa Y, Kanematsu Y, Tsuchiya K and Tamaki T:
BMK1 is activated in glomeruli of diabetic rats and in mesangial
cells by high glucose conditions. Kidney Int. 65:1749–1760. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye Y, Wang H, Chu JH, Chou GX, Chen SB, Mo
H, Fong WF and Yu ZL: Atractylenolide II induces G1 cell-cycle
arrest and apoptosis in B16 melanoma cells. J Ethnopharmacol.
136:279–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Wang P, Liu Q, Cheng X, Zhou Y and
Xiao Y: Cell cycle arrest and cell apoptosis induced by Equisetum
hyemale extract in murine leukemia L1210 cells. J Ethnopharmacol.
144:322–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghibelli L and Diederich M: Multistep and
multitask Bax activation. Mitochondrion. 10:604–613. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuentes-Prior P and Salvesen GS: The
protein structures that shape caspase activity, specificity,
activation and inhibition. Biochem J. 384:201–232. 2004. View Article : Google Scholar : PubMed/NCBI
|