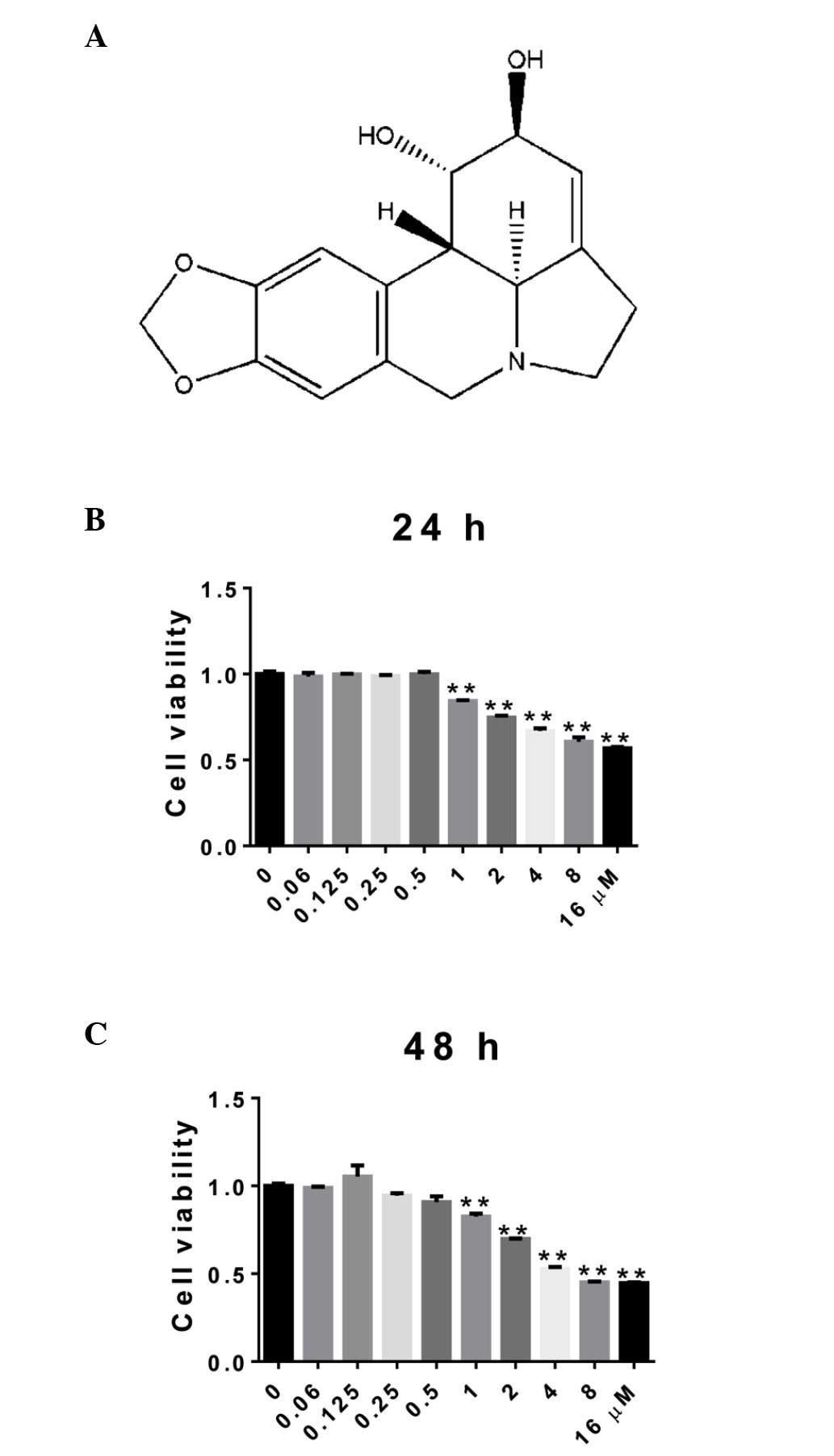
Introduction
Osteoarthritis (OA) is the most common type of joint
disease, and can cause chronic pain and disability, particularly in
the elderly population (1).
Progressive cartilage destruction is one of the most important
characteristics of OA (2).
Cartilage consists of chondrocytes and extracellular matrix (ECM).
Although the etiology of cartilage impairment remains to be fully
elucidated, imbalance in the anabolism and catabolism of cartilage
ECM is considered to be one of the predominant mechanisms of
cartilage degradation. The ECM of cartilage is predominantly
composed of type II collagen and aggrecan (3). Under normal conditions, the synthesis
and hydrolysis of these ECM proteins are in dynamic balance.
However, when OA begins to develop, the levels of inflammatory
cytokines, including interleukin-1β (IL-1β) and tumor necrosis
factor-α (TNF-α) increase, leading to the upregulation of matrix
degrading enzymes, including matrix metalloproteinases (MMPs) and a
disintergrin and metalloproteinases with thrombospondin motifs
(ADAMTSs) (4,5). This occurs through the nuclear factor
(NF)-κB and mitogen-activated protein kinase (MAPK) signaling
pathways (6). MMPs are
metal-dependent proteases, which are capable of degrading
components of the connective tissue ECM (7) and are major proteases in cartilage
degradation. MMP-3 and MMP-13 are among the most important
collagenases and aggrecanases during the progression of OA
(8,9), and increased expression levels of
these catabolic enzymes are considered the hallmark of cartilage
degeneration. Thus, the inhibition of MMPs may be a valuable
treatment strategy for OA.
An increasing number of natural compounds have been
shown to protect articular cartilage (10–12).
Lycorine (LY) is a natural alkaloid, which is extracted from
flowers and bulbs of Amaryllidaceae species (13). In previous studies, LY was found to
exhibit potential antitumor effects against ovarian cancer, myeloid
leukemia and melanoma with low toxicity (14–16).
In addition, LY has been shown to suppress
lipopolysaccharide-induced inflammation via inhibition of the MAPK
signaling pathway in a macrophage cell line (17). As MMPs are induced by
proinflammatory cytokines through the NF-κB and MAPK signaling
pathways, LY may be involved in reducing the expression of MMP and
preventing cartilage degeneration. The present study was designed
to examine the protective role of LY and its underlying mechanism
of action in cultured rat articular chondrocytes and in a mouse
anterior cruciate ligament transection (ACLT) model. An
understanding of the effects of lycorine on cartilage and its
associated mechanism may develop a novel, suitable therapy to treat
osteoarthritis and other cartilage-associated diseases.
Materials and methods
Ethical approval
All experiments performed in the present study were
approved by the Ethics Committee of Sir Run Run Shaw Hospital
(Hangzhou, China).
Primary rat articular chondrocyte
culture
The animal experiments in the present study were
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (18). The present study was approved by
the ethics committee of Sir Run Run Shaw Hospital, (Hangzhou,
China). Sprague-Dawley rats (male; weight, ~200 g; age, 2 months)
were supplied by the laboratory animal center of Zhejiang
University and were housed at ~24°C, with free access to food and
water were used in the current study. The bilateral knee joints of
rats (n=3/experiment) were separated and finely diced into small
pieces measuring no more than 1 mm3. The tissues were
treated with 0.2% collagenase (Sigma-Aldrich, St. Louis, MO, USA)
for 4 h at 37°C. Following centrifugation at 800 × g for 5 min at
37°C, the supernatant was placed in Dulbecco's modified Eagle's
medium (Haining Jinuo Biomedical Technology Co., Ltd., Hangzhou,
China) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
atmosphere containing 5% CO2 at 37°C. To sacrifice the
rats at 4 weeks following ACLT surgery, they were administered 5 ml
4% chloral hydrate by intraperitoneal injection.
Cell viability assay
The cytotoxic effects of LY in the rat chondrocytes
were determined using a Cell Counting Kit-8 (CCK-8) assay (Dojindo,
Molecular Technologies, Inc. Kumamoto, Japan). The chondrocytes
were plated in 96-well plates at a density of 1×104
cells/well in triplicate. The cells were then treated with
different concentrations of LY (0, 0.06, 0.125, 0.25, 0.5, 1, 2, 4,
8 or 16 Mm; Sigma-Aldrich) for 24 or 48 h at 37°C. Subsequently, 10
µl of CCK-8 buffer was added to each well, and the plates
were incubated for an additional 2 h at 37°C. The absorbance was
measured at a wavelength of 450 nm (650 nm reference) using an
ELX800 absorbance microplate reader (Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The primary rat chondrocytes were seeded into 6-well
plates at a density of 1×104 cells/well and treated with
10 ng/ml IL-1β (Sigma-Aldrich) and 0, 0.05, 0.1, 0.2 or 0.4
µM LY for 24 h. Total RNA was extracted using an RNeasy Mini
kit (Qiagen, Valencia, CA, USA). Complementary DNA (cDNA) was
synthesized using 0.5 µg of RNA from each sample, 2
µl of 5X PrimeScript RT Master mix (Takara Bio, Inc., Otsu,
Japan), and 4 µl RNase-free dH2O in a total
volume of 10 µl. RT-qPCR was performed on an ABI Prism 7500
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using SsoFast
EvaGreen supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The total volume (20 µl) of each qPCR reaction contained 10
µl SsoFast EvaGreen supermix, 7 µl ddH2O,
2 µl cDNA and 10 µM of each of the forward and
reverse primers (Sangon Biotech Co., Ltd., Shanghai, China). The
RT-qPCR reaction settings were as follows: 95°C for 10 min
(activation), 40 cycles of 95°C for 10 sec, 60°C for 20 sec 72°C
for 20 sec (amplification) and 72°C for 1 min (final extension), as
previously described (19). The
quantity of each target was normalized to β-actin and calculated
using the 2−ΔΔCq method (20). The mouse primer sequences were as
follows:
β-actin, forward 5′-CCTCTATGCCAACACAGT-3′ and
reverse 5′-AGCCACCAATCCACACAG-3′; MMP-3, forward
5′-TTGTCCTTCGATGCAGTCAG-3′ and reverse 5′-AGACGGCCAAAATGAAGAGA-3′;
MMP-13, forward 5′-AGGCCTTCAGAAAAGCCTTC-3′ and reverse
5′-GAGCTGCTTGTCCAGGTTTC-3′
Western blotting
The primary rat chondrocytes were seeded into 6-well
plates at a density of 10×104 cells/well. To investigate
the effects of IL-1β and LY on the expression of MMPs, the cells
were treated with IL-1β (10 ng/ml) with or without LY (0, 0.05 or
0.4 µM) for 48 h at 37°C. To investigate the effects of
IL-1β and LY on the activation of the signaling pathways, the cells
were pre-treated with or without 0.4 µM LY for 2 h, and then
stimulated with IL-1β (10 ng/ml) for 0, 5, 10, 20, 30 or 60 min at
37°C. Total protein was extracted from the cultured cells using
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich). The
lysates were centrifuged at 12,000 × g for 15 min at 4°C, and the
supernatants were collected. Proteins were quantified using a BCA
kit (Bio-Rad Laboratories, Inc.). The mean concentration of total
protein was ~1 mg/ml in cell lysate and 20 µl of cell lysate
was loaded on the SDS-PAGE gels. The proteins were resolved by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes by
electroblotting (Bio-Rad Laboratories, Inc.). The membranes were
blocked in 5% nonfat dry milk in Tris-buffered saline with Tween
20, containing 50 mM Tris (pH 7.6), 150 mM NaCl and 0.1% Tween 20,
at room temperature for 1 h. The membranes were then incubated with
antibodies purchased from Santa Cruz Biotechnology, Inc., (Dallas,
TX, USA) including, MMP-3 (cat no. sc-30070), MMP-13 (cat no.
sc-30073), extracellular signal-regulated kinase (ERK; cat no.
sc-154), c-Jun N-terminal kinase (JNK; cat no. sc-571), P38 (cat
no. sc-535), inhibitor of NF-κB (IκB)-α (cat no. sc-847),
phosphorylated (p)-ERK (cat no. sc-7383) (Thr202/Tyr204), p-JNK
(cat no. sc-6254) (Thr183/Tyr185) and p-p38 (cat no. sc-7973)
(Thr180/Tyr182) overnight at 4°C. All antibodies used were
polyclonal, raised in rabbit and rat was the target-species. The
primary antibodies were used at a dilution of 1:1,000 and the
secondary antibody were diluted 1:2,000 and membranes were incubate
with the respective secondary antibody for 2 h at room temperature.
Protein bands were developed using a horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody
(Abcam, Cambridge, MA, USA) and were detected using electrochemical
luminescence reagent (EMD Millipore, Billerica, MA, USA). The
protein bands were visualized using the LAS-4000 Science Imaging
System (Fujifilm, Tokyo, Japan).
Mouse ACLT model
A total of 18 8-week-old C57BL/6 mice were
randomized into three groups (n=6 per group) as follows: Sham
group, mice subjected to sham surgery+phosphate-buffered saline
(PBS); Vehicle group, mice subjected to ACLT and PBS, and LY group,
mice subjected to ACLT and 2.5 mg/kg LY. The ACLT and sham surgical
procedures were performed on the right knees of the mice. Animals
were anesthetized with 4% chloral hydrate with the dose of 10
ml/Kg. The operation were performed on the right knees. An incision
was formed in the skin laterally to the knee in the ACLT and sham
groups. ACL was transected using a surgical scalpel after the joint
capsules were opened in ACLT group. In the LY group, 2.5 mg/kg LY
dissolved in 50 µl PBS was injected intraperitoneally every
other day for 4 weeks. In the Sham and Vehicle groups, the same
volume of PBS was injected. All animals were sacrificed 4 weeks
following surgery. The right knee joints were fixed in 4%
paraformaldehyde (Sigma-Aldrich) for histological analysis.
Histological analysis
The fixed mouse joints were decalcified in 10% EDTA
for 3 weeks and then embedded in paraffin. In each specimen, three
serial sections (4-µm thick) embedded in paraffin were cut
using a Leica RM2235 microtome (Leica Microsystems GmbH, Wetzlar,
Germany). To observe cell density and morphology, matrix
degeneration and proteoglycan content, the sections were stained
with hematoxylin and eosin (H&E; Sigma-Aldrich) and safranin O
(SO)-fast green (Sigma-Aldrich). The specimens were examined and
images were captured using a high-quality Nikon AZ100,
multi-purpose zoom microscope.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. The results were analyzed using SPSS software for
Windows, version 16.0 (SPSS, Inc., Chicago, IL, USA). Student's
t-test was used to compare between two groups. P<0.05 was
considered to indicate a statistically significant difference. Grey
level analysis was performed using Image J software (version 1.48;
National Institutes of Health, Bethesda, MD, USA)
Results
Effect of LY on cell viability in rat
articular chondrocytes
The cell viability of the chondrocytes treated with
LY was assessed to determine which concentrations were not
cytotoxic using a CCK-8 assay. The cells were treated with LY at
concentrations ranging between 0 and 16 µM for 24 and 48 h.
As shown in Fig. 1, cell viability
was unaffected by LY at concentrations <0.5 µM, which
indicated that these doses did not have a cytotoxic effect on the
rat articular chondrocytes.
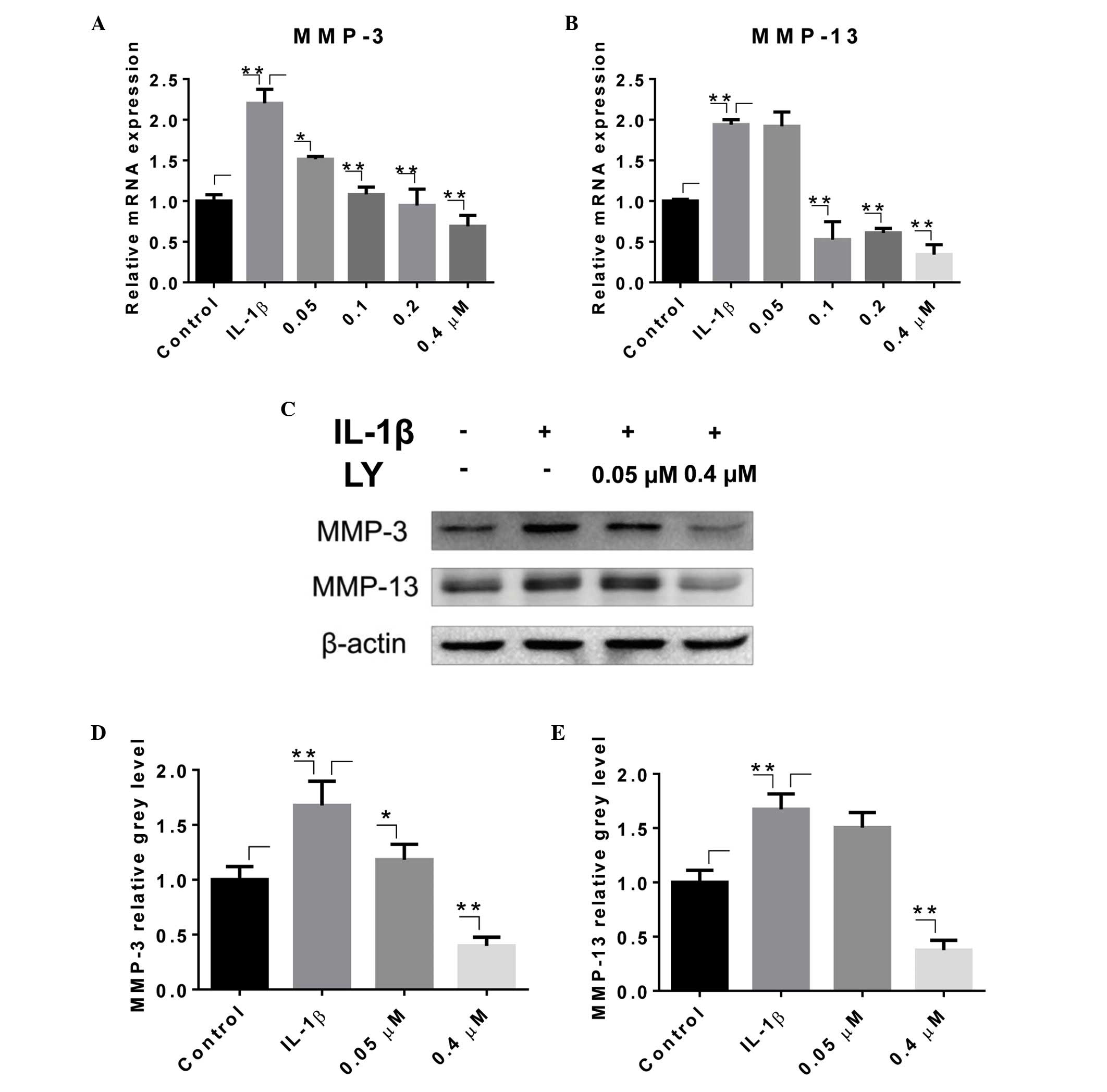
LY inhibits IL-1β-induced expression of
MMPs in rat articular chondrocytes
To determine the effect of LY on the catabolic
activities of chondrocytes, the rat articular chondrocytes were
treated with 0–0.4 µM LY and the inflammatory cytokine,
IL-1β, for 24 or 48 h. The expression levels of MMP-3 and MMP-13
were analyzed using RT-qPCR and Western blotting. Treatment with 10
ng/ml IL-1β resulted in upregulation of the mRNA levels of MMP-3
and MMP-13 (Fig. 2A and B).
However, the administration of LY led to the downregulation of the
expression levels of MMP-3 and MMP-13, which occurred in a
dose-dependent manner. The transcriptional levels of MMP-3 and
MMP-13 were significantly suppressed by 0.05 and 0.1 µM LY,
respectively. The levels of MMPs in the groups treated with higher
doses of LY (0.2 and 0.4 µM) were lower, compared with that
in the control group, which was not treated with IL-1β. The results
of the Western blotting were in accordance with those of the
RT-qPCR analysis (Fig. 2C–E). The
bands corresponding to the MMP proteins in the whole cell lysate
showed a significant upregulation of MMPs following treatment with
IL-1β. This effect was eradicated following treatment with 0.4
µM LY. Grey level analysis confirmed this observation.
LY inhibits IL-1β-induced activation of
the NF-κB pathway and phosphorylation of JNK in rat articular
chondrocytes
To clarify the underlying mechanisms of the effect
of LY in the inhibition of cartilage catabolic activity, the
present study investigated key signaling pathways, including the
MAPK and NF-κB signaling pathways. The rat articular chondrocytes
were incubated with or without LY, and were then treated with IL-1β
for 0, 5, 10, 20, 30 and 60 min. As shown in Fig. 3A, the levels of p-JNK in the whole
cell lysate were significantly reduced in the groups pre-treated
with LY, indicating that LY significantly attenuated the
phosphorylation of JNK. The phosphorylation of ERK and P38 were not
significantly affected by LY, although delayed phosphorylation of
ERK was observed. The protein levels of IκB-α were reduced 20, 30
and 60 min following IL-1β treatment, which indicated the
degradation of IκB-α and activation of the NF-κB signaling pathway.
However, these changes were not observed in the groups pre-treated
with LY. The grey level quantitative analysis also confirmed that
LY effectively inhibited the IL-1β-induced phosphorylation of JNK
and activation of the NF-κB pathway (Fig. 3B–E).
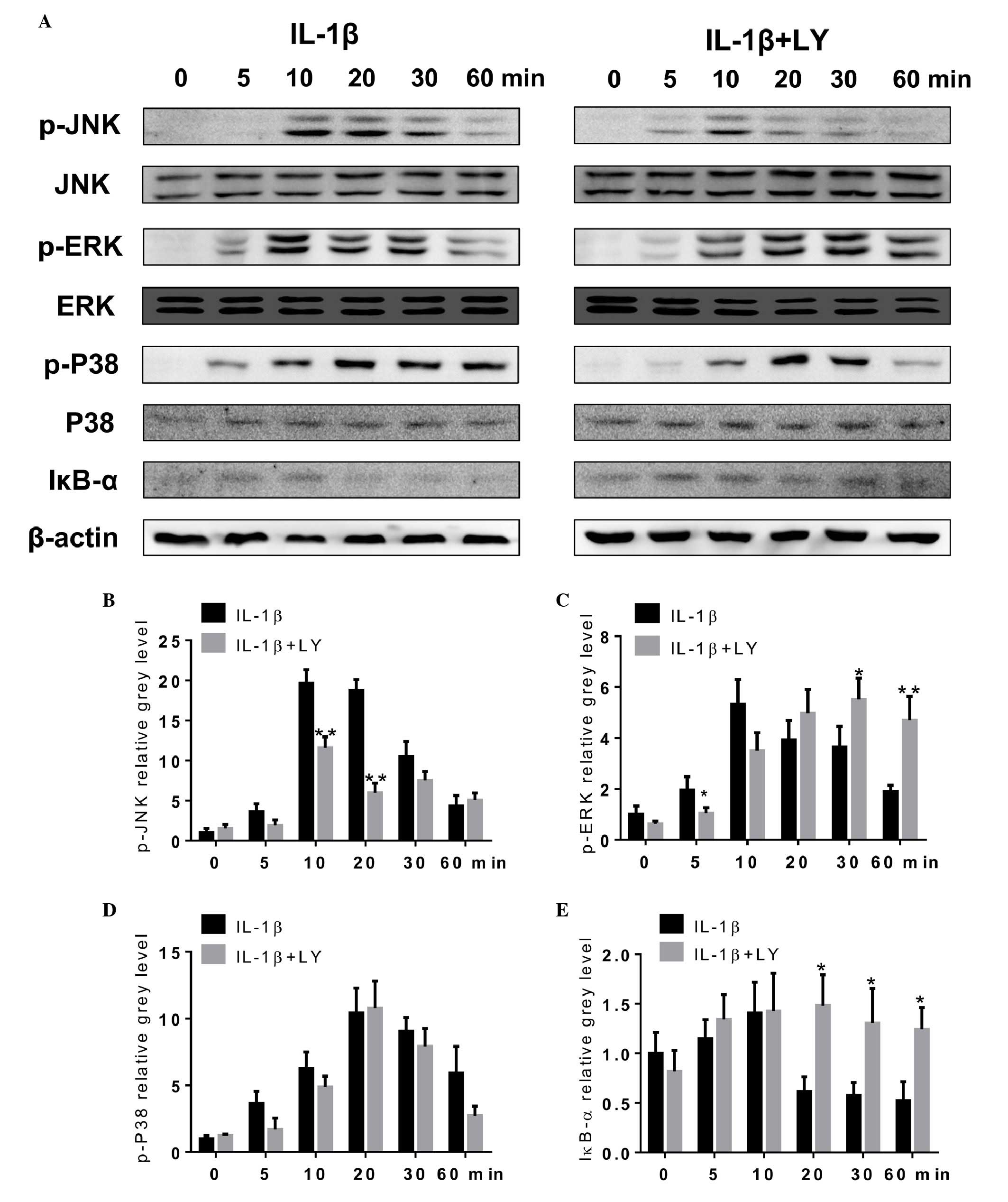 | Figure 3LY inhibits IL-1β-induced NF-κB
pathway activation and JNK phosphorylation in rat articular
chondrocytes. (A) Protein levels of p-JNK, JNK, p-P38, P38, p-ERK,
ERK, IκB-α and β-actin were determined in chondrocytes treated with
LY (0 or 0.4 µM) and IL-1β (10 ng/ml) for 0, 5, 10, 20, 30
or 60 min. levels were analyzed using Western blotting. Grey levels
of (B) p-JNK, (C) p-ERK and (D) p-P38 were quantified and
normalized to total JNK, ERK and P38. The grey level of (E) IκB-α
was normalized to β-actin. Data are presented as the mean ±
standard error of the mean (*P<0.05 and
**P<0.01). LY, lycorine; IL-1β, interleukin-1β; JNK,
c-Jun N-terminal kinase; ERK, extracelluar signal-regulated kinase;
IκB-α, inhibitor of NF-κB; p-, phosphorylated. |
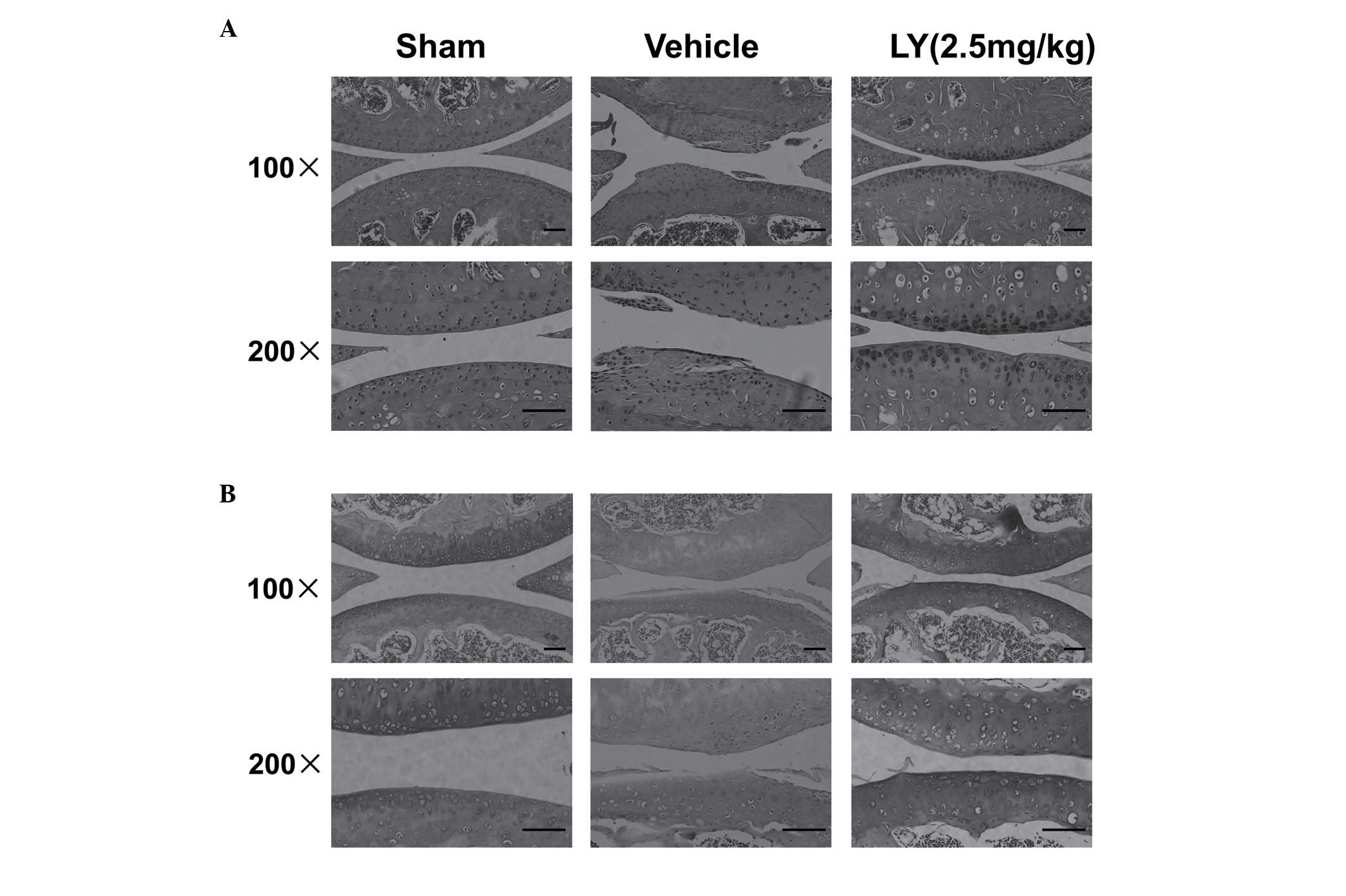
LY effectively protects cartilage in a
mouse ACLT model
To examine the protective role of LY in cartilage,
the present study further examined the effect of LY in a mouse ACLT
model. Following treatment with LY for 4 weeks, histological
analysis of the joint suggested that LY had a protective effect
against ACLT-induced cartilage destruction. H&E staining showed
severe cartilage destruction and fibrillation in the superficial
and mid layers of cartilage in the Vehicle group. Matrix
discontinuity was also observed in the Vehicle group (Fig. 4A). However, in the LY-treated
groups, cartilage destruction was less severe, compared with that
in the Vehicle group, although the cartilage surface remained
uneven. The SO staining showed that the cartilage matrix was
severely reduced in the superficial and deeper layers of cartilage
in the Vehicle group, and that the ECM was generally unaffected in
the LY-treated groups (Fig. 4B).
These histological results in the mouse ACLT model confirmed that
LY effectively prevented cartilage degradation.
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that IL-1β-induced upregulation of MMP-3
and MMP-13 was significantly suppressed by the administration of
the natural alkaloid, LY. In addition, the results showed that this
effect was mediated by inhibition of the JNK and NF-κB signaling
pathways. The protective effect of LY on articular cartilage was
also observed in the mouse ACLT model.
ECM provides tension and absorbs stress in normal
articular cartilage, and the dynamic balance between ECM synthesis
and degradation is maintained. Biomechanical signaling and
inflammatory signaling result in the secretion of IL-1β and TNF-α
in chondrocytes, and the recruitment of mononuclear inflammatory
cells (21,22). These inflammatory cytokines
stimulate the expression of proteases, including MMPs and ADAMTSs.
MMPs are the major mediators of cartilage turnover in OA, and are
able to degrade all components of the cartilage ECM (8,23).
Collagen turnover is almost exclusively mediated by MMPs with
collagenolytic ability, including MMP-1 and MMP-13 (8). In articular cartilage, collagenase
MMP-13 is the predominant rate-limiting factor in the degradation
of the most prevalent type of collagen, type II collagen (8). MMP-3 has 5–10 times more type II
collagen cleaving activity, compared with MMP-1. MMP-3 is another
important protease and is associated with the degradation of
aggrecan, which is the major type of proteoglycan (24). MMP-3 can also cleave other
non-collagen cartilage ECM components, including fibronectin,
elastin and laminin (8). In the
present study, the administration of LY significantly reduced the
IL-1β-induced expression of MMP-3 and MMP-13 at the mRNA and
protein levels. Considering the importance of MMP-3 and MMP-13 in
OA, it is reasonable to conclude that LY had a protective role in
the present in vivo study.
The synthesis of MMPs is predominantly regulated at
the transcriptional level by activator protein 1 (AP-1) and NF-κB
(25,26). AP-1 proteins are c-Fos/c-Jun
heterodimers or c-Jun/c-Jun homodimers. The MMP-1, MMP-3 and MMP-13
promoters contain a key binding site for AP-1 at −73 bp, which is
critical for the regulation of their transcriptional activity. The
binding activity of AP-1 is regulated by IL-1β and TNF-α through
signaling pathways, including the JNK, ERK and P38 pathways
(27). JNK can phosphorylate c-Jun
to activate the AP-1 DNA-binding complex and promote the expression
of MMPs (8). P38 can also activate
factors required for the synthesis of AP-1 (8). The stimulation of inflammatory
factors results in the phosphorylation and degradation of IκB,
which activates NF-κB and leads to its translocation into the
nucleus. Here, it directly binds to the MMP-1 and MMP-3 promoters,
and induces the expression of MMP1 and MMP-3 (26). NF-κB does not bind to the promoter
of MMP-13, however, NF-κB inhibition can lead to the downregulation
of MMP-13, which suggests that other mechanisms are involved in
regulating the expression of MMP-13 (8). The results of the present study
showed that activation of the JNK and NF-κB signaling pathways by
IL-1β was significantly inhibited by LY. Drugs, which are currently
used for the treatment of OA, including non-steroidal
anti-inflammatory drugs, are unable to stop the destruction of
connective tissues. Although substantial effort has been made over
several years to identify the inhibitors of MMPs, no effective
direct inhibitors have been found; therefore, targeting other
molecules in the MAPK or NF-κB pathways may offer a novel method
for the treatment for OA. LY is a promising novel drug, which can
effectively inhibit the synthesis of MMPs by targeting
inflammation-induced pathways.
In conclusion, the present study demonstrated that
LY inhibited the IL-1β-induced expression of MMP-3 and MMP-13,
which are pivotal in the progression of OA. The present study also
showed that this effect was mediated by inhibition of the JNK and
NF-κB pathways. The results obtained from the mouse ACLT model
showed that LY exerted a significant protective effect on
cartilage. Taken together, these findings suggested that LY merits
consideration as a therapeutic drug in the treatment of OA.
Acknowledgments
This study was supported by the Chinese National
Natural Science Foundation (grant nos. 81271971, 81301587 and
81472064), Natural Science Foundation of Zhejiang Province (grant
no. LZ15H060002) the Platform Major Project of Health and Family
Planning Commission of Zhejiang province (grant no. 2016145597 and
2015KYA133) and the Project of Education Department of Zhejiang
Province (grant no. Y201017108).
References
|
1
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
2
|
Feldmann M: Pathogenesis of arthritis:
Recent research progress. Nat Immunol. 2:771–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: Cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Wang H, Yang H, Li J, Cai Q,
Shapiro IM and Risbud MV: Tumor necrosis factor-α- and
interleukin-1β-dependent matrix metalloproteinase-3 expression in
nucleus pulposus cells requires cooperative signaling via syndecan
4 and mitogen-activated protein kinase-NF-κB axis: Implications in
inflammatory disc disease. Am J Pathol. 184:2560–2572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy G, Hembry RM, Hughes CE, Fosang AJ
and Hardingham TE: Role and regulation of metalloproteinases in
connective tissue turnover. Biochem Soc Trans. 18:812–815. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar
|
|
9
|
Takaishi H, Kimura T, Dalal S, Okada Y and
D'Armiento J: Joint diseases and matrix metalloproteinases: A role
for MMP-13. Curr Pharm Biotechnol. 9:47–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng L, Wang W, Rong XF, Zhong Y, Jia P,
Zhou GQ and Li RH: Chondroprotective effects and multi-target
mechanisms of Icariin in IL-1 beta-induced human SW 1353
chondrosarcoma cells and a rat osteoarthritis model. Int
Immunopharmacol. 18:175–181. 2014. View Article : Google Scholar
|
|
11
|
Chen WP, Xiong Y, Shi YX, Hu PF, Bao JP
and Wu LD: Astaxanthin reduces matrix metalloproteinase expression
in human chondrocytes. Int Immunopharmacol. 19:174–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee WS, Lim JH, Sung MS, Lee EG, Oh YJ and
Yoo WH: Ethyl acetate fraction from Angelica sinensis inhibits
IL-1β-induced rheumatoid synovial fibroblast proliferation and
COX-2, PGE2 and MMPs production. Biol Res. 47:412014. View Article : Google Scholar
|
|
13
|
Zupkó I, Réthy B, Hohmann J, Molnár J,
Ocsovszki I and Falkay G: Antitumor activity of alkaloids derived
from Amaryllidaceae species. In Vivo. 23:41–48. 2009.PubMed/NCBI
|
|
14
|
Cao Z, Yu D, Fu S, Zhang G, Pan Y, Bao M,
Tu J, Shang B, Guo P, Yang P and Zhou Q: Lycorine hydrochloride
selectively inhibits human ovarian cancer cell proliferation and
tumor neovascularization with very low toxicity. Toxicol Lett.
218:174–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Dai HJ, Ye M, Wang SL, Xiao XJ,
Zheng J, Chen HY, Luo YH and Liu J: Lycorine induces cell-cycle
arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer
Cell Int. 12:492012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu R, Cao Z, Tu J, Pan Y, Shang B, Zhang
G, Bao M, Zhang S, Yang P and Zhou Q: Lycorine hydrochloride
inhibits metastatic melanoma cell-dominant vasculogenic mimicry.
Pigment Cell Melanoma Res. 25:630–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang J, Zhang Y, Cao X, Fan J, Li G, Wang
Q, Diao Y, Zhao Z, Luo L and Yin Z: Lycorine inhibits
lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7
cells through suppressing P38 and STATs activation and increases
the survival rate of mice after LPS challenge. Int Immunopharmacol.
12:249–256. 2012. View Article : Google Scholar
|
|
18
|
National Institutes of Health: Revised
guide for the care and use of laboratory animals. (NIH GUIDE 25).
P.T. 34. 1996
|
|
19
|
Chen S, Huang Y, Zhou ZJ, Hu ZJ, Wang JY,
Xu WB, Fang XQ and Fan SW: Upregulation of tumor necrosis factor α
and ADAMTS-5, but not ADAMTS-4, in human intervertebral cartilage
endplate with modic changes. Spine (Phila Pa 1976). 39:E817–E825.
2014. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Millward-Sadler SJ, Wright MO, Davies LW,
Nuki G and Salter DM: Mechanotransduction via integrins and
interleukin-4 results in altered aggrecan and matrix
metalloproteinase 3 gene expression in normal, but not
osteoarthritic, human articular chondrocytes. Arthritis Rheum.
43:2091–2099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caron JP, Fernandes JC, Martel-Pelletier
J, Tardif G, Mineau F, Geng C and Pelletier JP: Chondroprotective
effect of intraarticular injections of interleukin-1 receptor
antagonist in experimental osteoarthritis. Suppression of
collagenase-1 expression. Arthritis Rheum. 39:1535–1544. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonassar LJ, Frank EH, Murray JC, Paguio
CG, Moore VL, Lark MW, Sandy JD, Wu JJ, Eyre DR and Grodzinsky AJ:
Changes in cartilage composition and physical properties due to
stromelysin degradation. Arthritis Rheum. 38:173–183. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: A tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barchowsky A, Frleta D and Vincenti MP:
Integration of the NF-kappaB and mitogen-activated protein
kinase/AP-1 pathways at the collagenase-1 promoter: Divergence of
IL-1 and TNF-dependent signal transduction in rabbit primary
synovial fibroblasts. Cytokine. 12:1469–1479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mengshol JA, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases as therapeutic targets in arthritic
diseases: Bull's-eye or missing the mark? Arthritis Rheum.
46:13–20. 2002. View Article : Google Scholar : PubMed/NCBI
|