Introduction
Gastric carcinoma (GC) is considered to be the
fourth most common type of malignancy worldwide and ranks as the
second leading cause of cancer-associated mortality, following lung
cancer (1,2). Despite advances made in the treatment
of GC, including surgical resection, chemotherapy and radiation
therapy, the results are often disappointing, with a high
recurrence rate of 70% following successful resection (3). Furthermore, the survival of patients
is poor with a 28% 5-year overall survival rate. Thus, a deeper
understanding of the molecular mechanisms involved in controlling
the initiation and progression of GC is imperative.
Previous studies have increasingly focused on the
cancer stem cell (CSC) theory and its crucial function in the
development and progression of cancer (4–6). A
growing body of evidence has confirmed that CSCs comprise a small
proportion of cancer cells with the ability to initiate tumor
development and are recognized as the 'heartbeat' of cancer. The
existence of CSCs has been identified in several types of cancer,
including breast cancer, stomach cancer and glioma (7–9).
Accumulating evidence has demonstrated that CSCs are involved in
tumor aggressiveness, chemoresistance and metastasis, which may
explain the high frequency of carcinoma relapse (4). However, a greater understanding of
the underlying mechanisms associated with CSCs may provide
potential novel therapeutic strategies for cancer.
MicroRNAs (miRNAs) are a family of endogenous small
non-coding RNA molecules 21–24 nucleotides in length, which can
interact with the 3′-untranslated region (3′-UTR) of target-mRNAs
to induce translational repression and gene silencing. The
dysregulation of miRNAs has been shown to interfere with various
biological functions, including cell proliferation, invasion,
metastasis and differentiation (10,11).
Emerging evidence has demonstrated the abnormal expression of
miRNAs in several types of cancer, and has indicated that miRNAs
can function as oncogenes or tumor suppressors to participate in
carcinogenesis and progression (12–14).
Previous studies reported that miRNAs can regulate the function of
CSCs (15,16). One such miRNA, miR-483-5p was
demonstrated to be dysregulated in several cancers and identified
as a potential carcinoma biomarker in certain types of cancer,
including hepatocellular carcinoma, multiple myeloma and
adrenocortical tumors (17,18).
In lung adenocarcinoma, miR-483-5p can promote
epithelial-mesenchymal transition and enhance invasive and
metastatic properties (19).
However, the function and the underlying molecular mechanisms of
miR-483-5p in the development of GC remains unclear.
The present study analyzed the expression of
miR-483-5p in gastric CSCs (GCSCs) derived from the MKN-45 human
gastric cancer cell line. The effects of miR-483-5p on cell growth,
invasion and self-renewal of GCSCs, and the underlying molecular
mechanisms were investigated.
Materials and methods
Reagents
Unless otherwise stated, all reagents were obtained
from Sigma-Aldrich (St. Louis, MO, USA). The primary rabbit
monoclonal antibodies against human β-catenin (cat. no. ab32572),
Bcl-2 (cat. no. ab32124) and cyclin D1 (cat. no. ab134175) were
purchased from Abcam (Cambridge, MA, USA). The mouse monoclonal
antibody against matrix metalloproteinase 2 (MMP-2; cat. no.
sc-13594) and the rabbit polyclonal antibody Ki67 (cat. no.
sc-15402) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Culture of parental and spheroid
body-forming cells
MKN-45 human gastric cancer cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). Cells
were maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS; Sigma-Aldrich) and 1% penicillin-streptomycin
sulfate (Sigma-Aldrich). All cells were cultured in a humidified
atmosphere at 37°C with 5% CO2. Following the attachment
of cells, they were subsequently passaged upon reaching 70–80%
confluence. To obtain the spheroid bodies, the parental cells (100
cells/well) were plated in a 96-well plate and incubated with
serum-free RPMI-1640 medium containing 100 ng/ml epidermal growth
factor (EGF; Amyjet Scientific, Wuhan, China), 20 ng/ml human
fibroblast growth factor-2 (FGF-2; Amyjet Scientific), 2% B-27
supplement (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 1% N-2 supplement (Gibco; Thermo Fisher Scientific, Inc.).
After ~2 weeks, the primary spheroid body formation was evaluated
and quantified using an inverted microscope (IX70; Olympus
Corporation, Tokyo, Japan). When reaching a size of 200–500 cells
per spheroid body, they were then collected and dissociated by
trypsinization for passaging, and further amplification was
performed as previously described (20).
Identification of parental-derived GCSCs
by flow cytometric analysis
For flow cytometry, 80% confluent cells were
detached from culture plates using 0.25% trypsin. Following
centrifugation at 800 × g for 5 min, cells were resuspended in
Hanks' balanced salt solution (HBSS) containing 1 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco; Thermo
Fisher Scientific, Inc.) and 2% FBS. Cells were then filtered using
a 40-µm mesh filter (BD Biosciences, San Jose, CA, USA). To
identify the induced GCSCs, cells were plated onto glass coverslips
and fixed with 4% paraformaldehyde (Sigma-Aldrich). After rinsing
with phosphate-buffered saline (PBS; Sigma-Aldrich), cells were
permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 10
min. Following incubation with 1% (w/v) solution of bovine serum
albumin (BSA; Sigma-Aldrich) in PBS for 30 min to block the
non-specific binding. Subsequently, a mouse anti-human
CD44-allophycocyanin monoclonal antibody (BD Biosciences; cat. no.
559942), a common specific marker for GCSCs, was added at a
dilution of 1:400 at 37°C with 5% CO2. All samples were
stained with 2 ng/ml of 4′,6-diamidino-2-phenylindole
(Sigma-Aldrich) to label DNA. After 30 mins, cells were rinsed with
HBSS, then the collected samples were analyzed by flow cytometry
using a FACSAria flow cytometer (BD Biosciences). The results were
analyzed using software FlowJo version 7.2.4 (Tree Star Inc.,
Ashland, OR).
Oligonucleotide transfection
To specifically induce miR-483-5p expression in
GCSCs derived from MKN-45 cells, miR-483-5p mimics and scrambled
control oligonucleotides were synthesized and obtained from
Shanghai GenePharma Co., Ltd. (Shanghai, China). For transfection,
cells were seeded into 96-well plates and transfected with the
above oligonucleotides using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 48 h transfection, cells were
collected and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to evaluate the transfection
effectiveness.
Transfection of synthetic β-catenin small
interfering (si)RNA
Scrambled siRNA and siRNA targeting human β-catenin
fragments were purchased from Shanghai GenePharma Co., Ltd. For
siRNA transfection, cells with elevated miR-483-5p and control
cells were cultured in 24-well plates and then transfected with 2
µg/ml β-catenin siRNA mixed with 5 µl Lipofectamine
RNAi MAX (Invitrogen; Thermo Fisher Scientific, Inc.). Following
incubation at room temperature for 24 h, cells were collected and
the efficiency of the β-catenin siRNA transfection was confirmed by
western blot analysis.
RT-qPCR analysis
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then,
first-strand cDNA was obtained by RT using ImProm II Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) with oligo-dT
primers from 2 µg RNA. The obtained cDNA was subsequently
subjected to qPCR using an SYBR Premix Ex Taq II kit (Takara
Biotechnology Co., Ltd., Dalian, China) in a 20 µl reaction
mixture following the manufacturer's instructions. The
thermocycling conditions were as follows: Initiation at 95°C for 3
min; 40 cycles of 95°C for 15 sec and 60°C for 35 sec. The reaction
was conducted in an ABI 7900 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and the results were analyzed using SDS
2.3 software (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The specific primers for CD44, Oct4, Nanog, Sox2, CK14, CK18 and
miR-483-5p were used as previously described (20,21).
The quantity of PCR product was normalized with respect to U6 or
β-actin as the endogenous controls. The results were quantified
using the 2−ΔΔCq method (22).
Proliferation assays
Cell proliferation was measured using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays. Briefly, cells were plated in 96-well plates and treated as
described. Following washing with PBS, 20 µl of 5 mg/ml MTT
(Sigma-Aldrich) was added into each corresponding test well and
incubated for 5 h. Following removal of the supernatant, 200
µl dimethyl sulfoxide was introduced to dissolve the
formazan. The absorbance at 570 nm was detected to analyze cell
viability using a micro-ELISA reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Apoptosis analysis
The rate of apoptosis was assessed by Annexin
V-propidium iodide (PI) staining using the Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis Detection kit (Beyotime Institute
of Biotechnology, Haimen, China). Following preconditioning as
described, cells were lysed with lysis buffer (containing 10 mM
Tris, 10 mM EDTA and 0.5% Triton X-100, pH 7.5). Then, cells were
resuspended in binding buffer containing Annexin V-FITC and PI
according to the manufacturer's instructions. All specimens were
analyzed using a FACScan flow cytometer (BD Biosciences) and BD
FACSDiva 6.1.3 software (BD Biosciences).
Sphere formation assays
To determine the self-renewal ability of GCSCs,
sphere formation assays were performed. Briefly, cells were
cultured in ultra-low attachment 24-well plates (Corning Life
Sciences, Corning, NY, USA). Following transfection under the
indicated conditions, cells were incubated with serum-free RPMI
1640 medium (Biosera, Nuaille, France) containing 20 ng/ml EGF and
10 ng/ml bFGF. After 3 weeks, the number of spheroid colonies were
counted under a light microscope (CKX31; Olympus Corporation).
Cell invasion assays
Cell invasion assays were performed using 24-well
Transwell chambers (8-µm pore polycarbonate membrane; BD
Bioscience). Briefly, cells transfected with miR-483-5p mimics or
miR-con were cultured in serum-free RPMI 1640 medium. Subsequently,
the treated cells were seeded on the upper side of the membrane
pre-coated with diluted Matrigel (BD Biosciences) in PBS. Then,
medium containing 10% FBS was added into the lower chambers as a
chemoattractant. After 48 h incubation, the non-invading cells
inside the upper chamber were removed with a cotton swab. Cells
that had migrated through the membrane to the lower surface were
fixed with 4% paraformaldehyde, and then stained with 0.1% crystal
violet (Shanghai Shenggong Biology Engineering Technology Service,
Ltd., Shanghai, China). Quantification was performed by counting
the number of cells in six high-powered fields in the center of
each well under the CKX31 light microscope.
Western blotting
Cells were lysed with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology) and the protein
concentration was detected using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology). Following
electrophoresis using sodium dodecyl sulfate-polyacrylamide gel
electrophoresis with 12% polyacrylamide gel, the targeted proteins
were electroblotted onto a polyvinylidene difluoride membrane
(Merck Millipore, Darmstadt, Germany). Following incubation with 5%
non-fat milk to block the nonspecific binding, the membranes were
immunoblotted with primary antibodies against β-catenin (1:5,000),
cyclin D1 (1:10,000), Bcl-2 (1:1,000), Ki67 (1:1,000) and MMP-2
(1:1,000). After three washes with Tris-buffered saline with 0.05%
Tween 20 (Sigma-Aldrich) at room temperature, each blot was
incubated with goat anti-rabbit (1:1,000; cat. no. ab6789) and goat
anti-mouse (1:5,000; cat. no. ab97051) horseradish
peroxidase-conjugated secondary antibodies (Abcam) for 1 h. Signals
were detected with enhanced chemiluminescent detection reagent
(Beyotime Institute of Biotechnology). β-actin served as loading
control. The band intensity was scanned with the Gel Imaging System
(UVP Company, Upland, CA, USA) and quantified using ImageJ 1.32
software (National Institutes of Health, Bethesda, MD, USA). All
experiments were performed at least three times.
Statistical analysis
All results are representative of at least three
experiments. Analysis was conducted using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). All data are presented as the
mean ± standard deviation. Significant differences between groups
was analyzed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of patient-derived
GCSCs
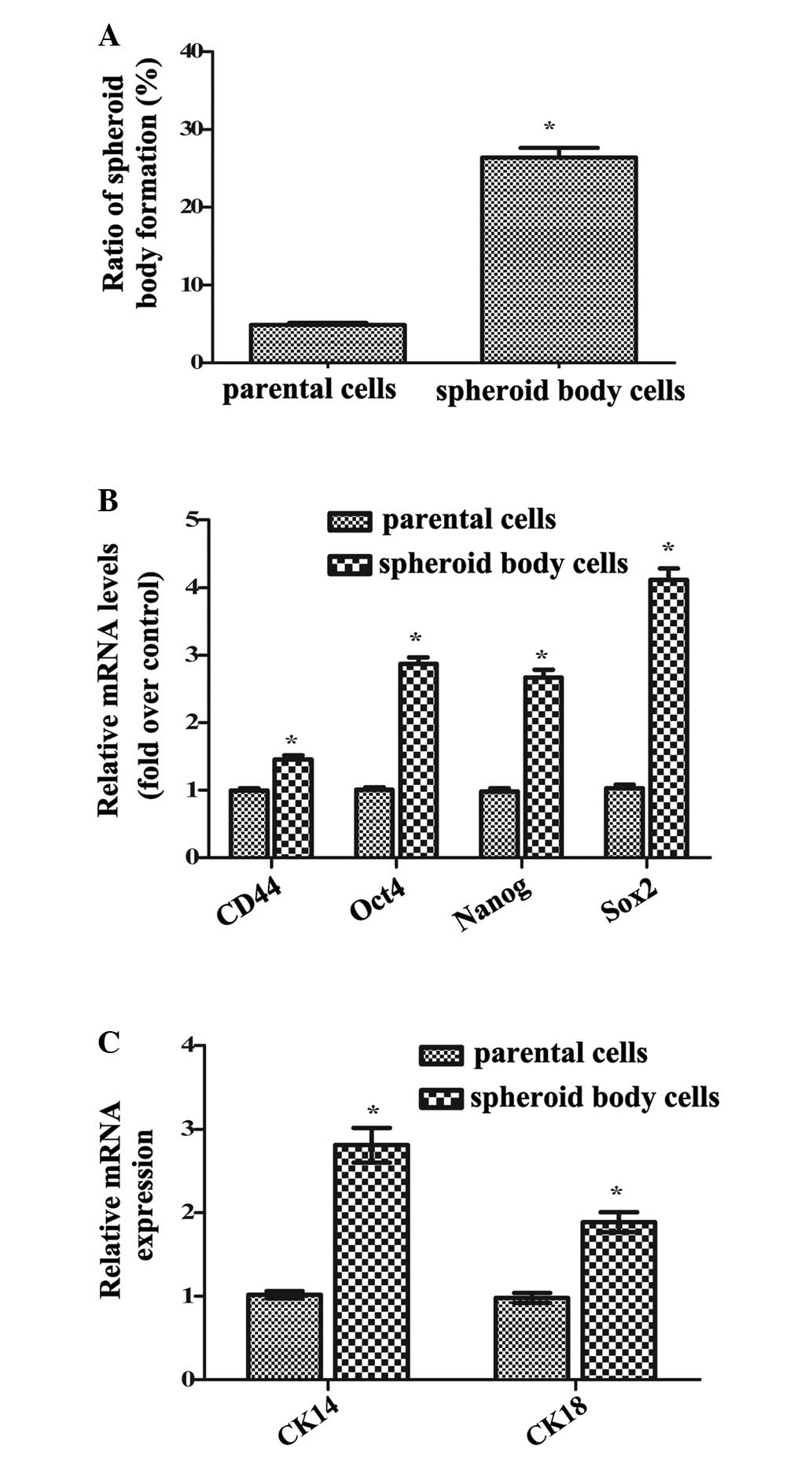
FACS-sorted CD44+ MKN-45 cells cultured
in serum-free medium formed non-adherent, three-dimensional
spheroid clusters, termed spheroid bodies. Notably, compared with
parental cells, the spheroid bodies exhibited significantly
increased self-renewal potential (P<0.05) detected by
tumorspheric generation (Fig. 1A).
Additionally, the mRNA levels of CD44, Oct4, Nanog and Sox2,
specific markers of GCSCs, were significantly increased in isolated
spheroid body cells compared with parental cells (P<0.05;
Fig. 1B). Furthermore, following
further attachment and incubation in serum-containing medium, the
spheroid body cells developed into elongated cells, and the mRNA
levels of cytokeratin 14 (CK14) and CK18, markers of cell
differentiation, were significantly upregulated compared with
parental cells (P<0.05; Fig.
1C). Thus, these results confirmed that isolated spheroid body
cells exhibited increased self-renewal and differentiation
potential, indicating the successful isolation of GCSCs from MKN-45
cells.
Level of miR-483-5p is elevated in
GCSCs
Numerous previous studies have suggested that
miR-483-5p expression is increased in several carcinomas and exerts
an important function in the development of cancer (19,23).
However, the importance of miR-483-5p in GC remains unclear. The
present study explored the expression of miR-483-5p in GCSCs. As
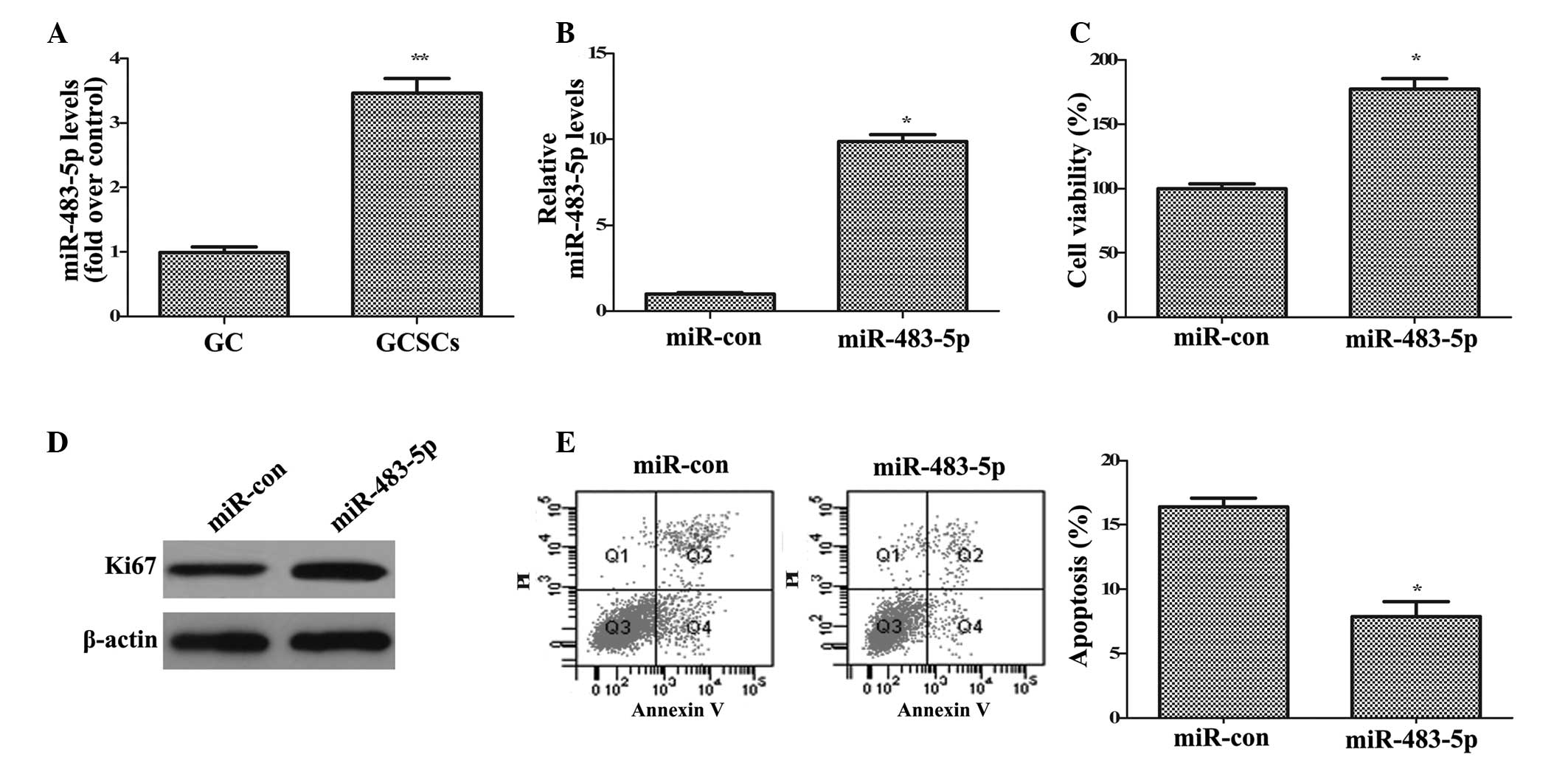
demonstrated in Fig. 2A, the
expression levels of miR-483-5p were significantly upregulated in
GCSCs compared with GC cells (P<0.01), indicating that
miR-483-5p is important in the development of GC.
miR-483-5p overexpression promotes GCSC
growth
To investigate the function of miR-483-5p in the
tumorigenesis of GC, the effect of miR-483-5p on GCSC growth was
analyzed. The current study overexpressed miR-483-5p in GCSCs by
transfection with miR-483-5p mimics and measured the level with
RT-qPCR (Fig. 2B). Functional
analysis indicated that compared with control miR, miR-483-5p
upregulation induced a 1.77-fold increase in cell viability
(P<0.05; Fig. 2C) and increased
the expression of Ki67 (Fig. 2D),
a common marker of cell proliferation. Apoptosis analysis
demonstrated that the apoptotic rate was significantly reduced from
16.4 to 7.86% in control miR and miR-483-5p transfected cells,
respectively (P<0.05; Fig. 2E).
Together, these data indicated that miR-483-5p upregulation
enhances GCSC growth by promoting cell proliferation and
attenuating cell apoptosis.
Overexpression of miR-483-5p induces GCSC
invasion and self-renewal
Based on the results of the current study, the
effects of miR-283-5p on GCSC invasion and self-renewal were
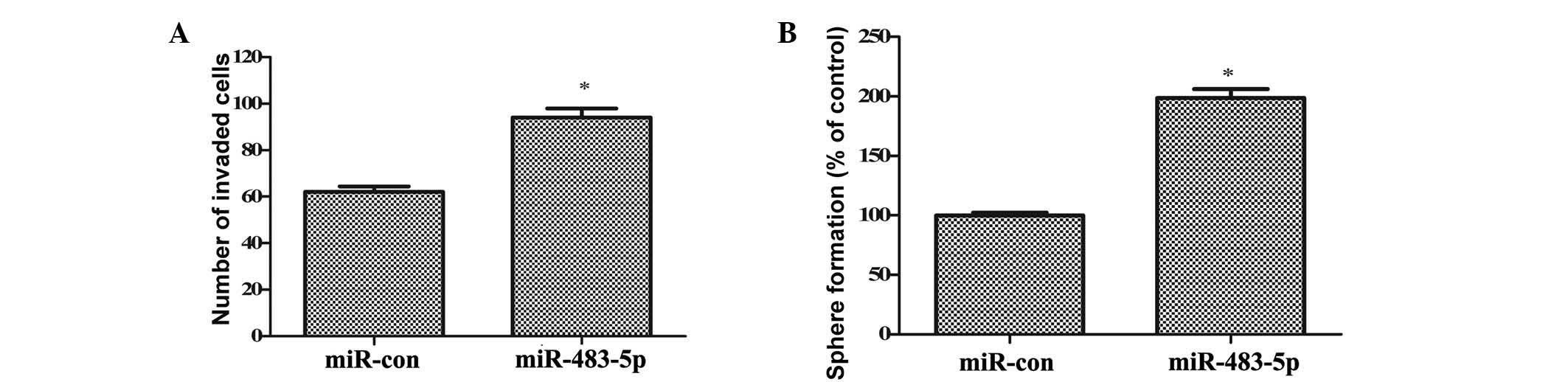
further investigated. As demonstrated in Fig. 3A, ectopic transfection of
miR-483-5p significantly increased the invasiveness of GCSCs
(P<0.05). The number of GCSCs that invaded the membrane was
increased from 62 to 94. The self-renewal ability of GCSCs, one of
the most important characteristics of CSCs, was analyzed by sphere
formation assays. Consistently, miR-483-5p overexpression induced a
1.98-fold increase in the number of spheres formed (P<0.05;
Fig. 3B). These results
corroborated that upregulation of miR-483-5p promotes the invasion
and self-renewal of GCSCs.
miR-483-5p induces the activation of
Wnt/β-catenin pathway
Numerous studies have demonstrated that the
abnormally high activation of the Wnt/β-catenin pathway is pivotal
for the initiation and development of various types of carcinoma
(24,25). To understand the underlying
mechanism involved in the effect of miR-483-5p on GCSC growth,
invasion and self-renewal, the Wnt/β-catenin pathway was
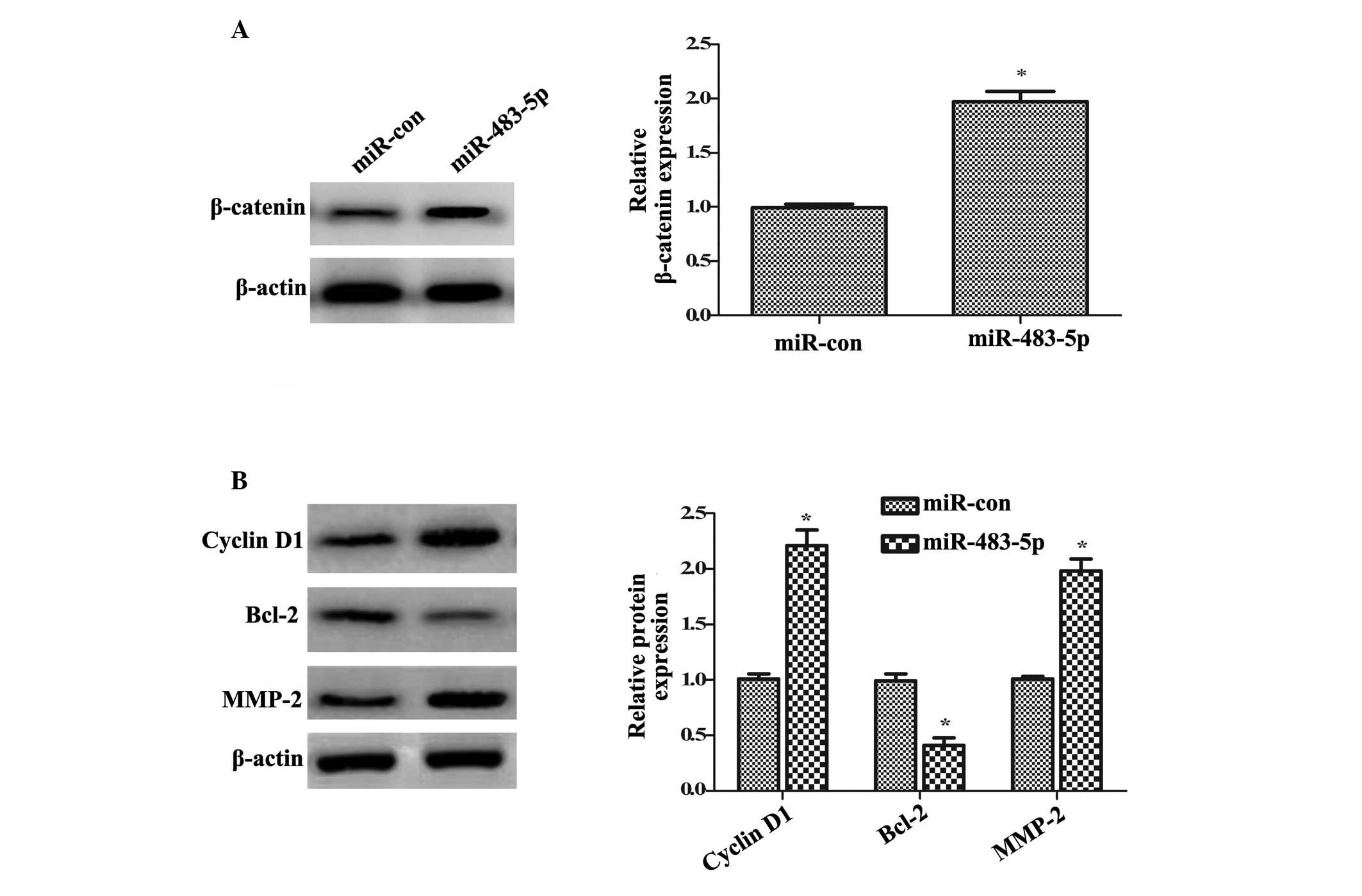
investigated. Western blotting demonstrated that transfection with
miR-483-5p mimics significantly upregulated the β-catenin protein
expression levels compared with control miR transfection
(~1.97-fold increase; P<0.05; Fig.
4A). Furthermore, the significant upregulation of cyclin D1 and
Bcl-2 protein levels was demonstrated in miR-483-5p-overexpressing
cells compared with miR control cells (P<0.05; Fig. 4B), which are key targeted molecules
in the Wnt/β-catenin pathway and are involved in cell growth
(25). Additionally, compared with
miR control cells, the protein expression levels of MMP-2 were
significantly increased following miR-483-5p overexpression. Taken
together, the results of the present study suggested that
miR-483-5p induces activation of the Wnt/β-catenin pathway.
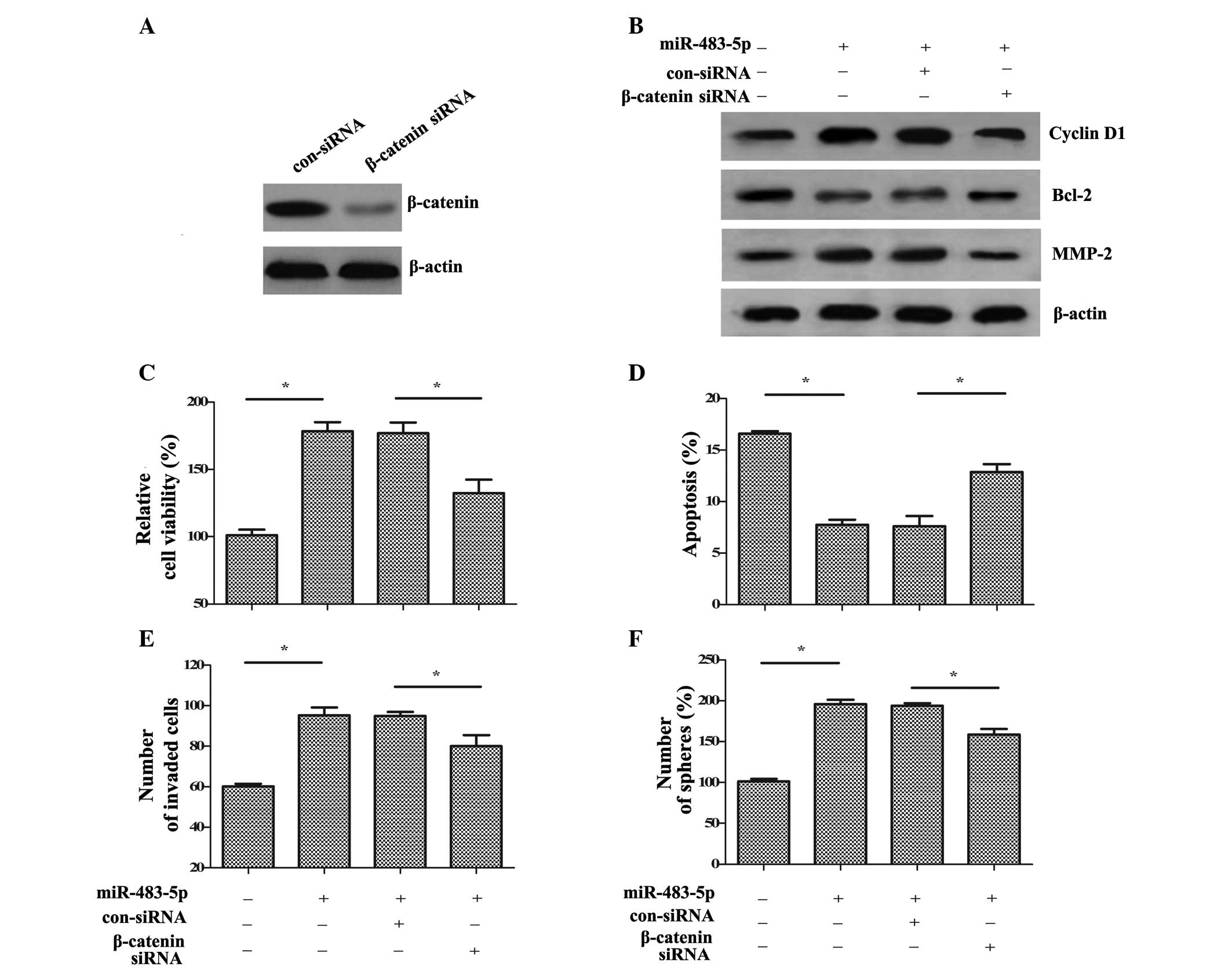
Wnt/β-catenin signaling is involved in
miR-483-5p-induced tumorigenesis
To elucidate the molecular mechanisms via which
miR-483-5p affects the biological function of GCSCs, a specific
β-catenin siRNA was synthesized. Following transfection with
β-catenin siRNA, the expression levels of β-catenin were markedly
reduced in GCSCs compared with control siRNA transfection (Fig. 5A). Additionally, the
miR-483-5p-induced increase in the downstream targets of cyclin D1,
Bcl-2 and MMP-2 were also markedly decreased compared with
miR-483-5p and control siRNA transfected cells (Fig. 5B). Functional assays demonstrated
that β-catenin silencing significantly reduced cell viability
compared with miR-483-5p transfection (P<0.05; Fig. 5C). Additionally, compared with
miR-483-5p and control siRNA transfected cells, the inhibitory
effect of miR-483-5p on cell apoptosis was ameliorated by β-catenin
siRNA (P<0.05; Fig. 5D).
Furthermore, the increased cell invasion (Fig. 5E) and self-renewal (Fig. 5F) induced by miR-483-5p
upregulation were significantly ablated following β-catenin siRNA
transfection compared with miR-483-5p and control siRNA transfected
cells (P<0.05). Therefore, the data of the current study
indicated that miR-483-5p may promote GCSC growth, invasion and
self-renewal via the Wnt/β-catenin pathway.
Discussion
GC ranks as the leading cause of tumor-related
mortality worldwide with a 5-year survival rate of only ~28%
(1). Despite the advances made in
modern medicine, the incidence of GC remains high and constitutes a
major health problem in developing countries with ~700,000
fatalities due to GC in 2012. CSCs have become an important field
in cancer research as their characteristic stemness properties may
provide novel strategies for the treatment of patients with cancer
(4,6). The current study demonstrates the
important finding that miR-483-5p levels were significantly
upregulated in GCSCs. Notably, increased miR-483-5p promoted GCSC
growth, invasion and self-renewal via the Wnt-β-catenin pathway.
Therefore, the present study may clarify the importance of
miR-483-5p in the development of GC.
miRNA dysregulation has previously been demonstrated
to be involved in the development and progression of practically
all types of cancer (12).
Emerging evidence has confirmed an association between miRNAs and
the pathological process of CSCs (13,26).
CSCs have been identified in various tumors, including GC, and
exhibit mesenchymal and progenitor cell properties, including
self-renewal and proliferative capacity (27,28).
Thus, CSCs may be associated with causing tumor initiation,
metastasis and recurrence, and may be a potential target for the
treatment of cancer. In the present study, the non-adherent
spheroid body-forming MKN-45 cells cultured in stem cell
conditioned medium exhibited increased self-renewal, levels of
GCSC-associated markers (CD44, Oct4, Nanog and Sox2) and cell
differentiation, indicating that the isolated cells possessed GCSC
properties. Further analysis demonstrated increased expression of
miR-483-5p in isolated GCSCs compared with GC cells, suggesting
that miR483-5p is important for the development of GCSCs.
miR-483-5p was previously demonstrated to be
overexpressed in several types of cancer, including multiple
myeloma, hepatocellular carcinoma and adrenocortical cancer
(17,19). Furthermore, circulating miR-483-5p
has previously been confirmed as a novel biomarker for the
diagnosis and survival prediction of patients with cancer,
indicating a pivotal function of miR-483-5p in the progression of
carcinoma (18,29). However, the underlying mechanism
remains undefined in GC. To investigate the function of miR-483-5p
in the development of GC, its effects on GCSC function were
analyzed. Upregulation of miR-483-5p expression in GCSCs resulted
in increased cell viability and reduced cell apoptosis, suggesting
that miR-483-5p promotes GCSC growth. Further analysis demonstrated
that miR-483-5p overexpression enhanced GCSC invasion. Notably,
miR-483-5p overexpression increased the number of spheroid bodies
formed, indicating that miR-483-5p is a positive regulator of GCSC
self-renewal ability, which is one of the most important
characteristics of CSCs. Thus, the data of the present study
suggest that miR-483-5p may act as an oncogene during GC
carcinogenesis by regulating GCSC function.
The canonical Wnt/β-catenin signaling pathway has
previously been demonstrated to be a major tumorigenesis pathway in
various types of carcinoma (23,30).
Convincing evidence indicates that Wnt/β-catenin signaling mediates
tumor initiation and progression by regulating cell proliferation,
invasion and metastasis. Emerging research has demonstrated the
importance of Wnt/β-catenin signaling in tumor stem and progenitor
cells (31). The abnormal
activation of the Wnt/β-catenin pathway has previously been
detected in CSCs from various types of carcinoma, including GC
(32,33). When Wnt/β-catenin signaling was
suppressed by chromobox 7, CSC proliferation, self-renewal and
tumor initiating ability was shown to be inhibited in breast cancer
(34). To further clarify the
underlying mechanism associated with the effects of miR-483-5p on
GCSC growth, invasion and self-renewal, the Wnt/β-catenin signaling
was investigated. In accordance with the hypothesis of the present
study, miR-483-5p upregulation increased the protein expression
levels of β-catenin, and upregulated cyclin D1, Bcl-2 and MMP-2
protein expression, which are common downstream molecules of
Wnt/β-catenin signaling involved in cell proliferation and
invasion. Further functional assays demonstrated that inhibiting
the pathway by β-catenin siRNA transfection attenuated the
increases in miR-483-5p-induced cell growth, invasion and
self-renewal ability. Thus, the data of the current study suggest
that miR-483-5p may enhance GCSC function via activation of the
Wnt/β-catenin pathway, inducing cell growth, invasion and
self-renewal.
In conclusion, the present study confirmed that
miR-483-5p is increased in GCSCs derived from MKN-45 cells.
Notably, miR-483-5p may be associated with GC maintenance by
regulating growth, invasion and self-renewal via Wnt/β-catenin
signaling. Accordingly, the results of the current study may
contribute to the understanding of how miR-483-5p regulates the
development and pathological progression of GC, and provide a
promising target for the treatment of GC.
References
|
1
|
Gomceli I, Demiriz B and Tez M: Gastric
carcinogenesis. World J Gastroenterol. 18:5164–5170.
2012.PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner B, Hoshen MB, Purim O, David MB,
Ashkenazi K, Marshak G, Kundel Y, Brenner R, Morgenstern S, Halpern
M, et al: MicroRNAs as a potential prognostic factor in gastric
cancer. World J Gastroenterol. 17:3976–3985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu G, Shen J, Ou Yang X, Sasahara M and Su
X: Cancer stem cells: The 'heartbeat' of gastric cancer. J
Gastroenterol. 48:781–797. 2013. View Article : Google Scholar
|
|
6
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conley SJ, Gheordunescu E, Kakarala P,
Newman B, Korkaya H, Heath AN, Clouthier SG and Wicha MS:
Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci USA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan K, Wu Q, Yan DH, Lee CH, Rahim N,
Tritschler I, DeVecchio J, Kalady MF, Hjelmeland AB and Rich JN:
Glioma cancer stem cells secrete Gremlin1 to promote their
maintenance within the tumor hierarchy. Genes Dev. 28:1085–1100.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Que T, Song Y, Liu Z, Zheng S, Long H, Li
Z, Liu Y, Wang G, Liu Y, Zhou J, et al: Decreased miRNA-637 is an
unfavorable prognosis marker and promotes glioma cell growth,
migration and invasion via direct targeting Akt1. Oncogene.
34:4952–4963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGirt LY, Adams CM, Baerenwald DA,
Zwerner JP, Zic JA and Eischen CM: miR-223 regulates cell growth
and targets proto-oncogenes in mycosis fungoides/cutaneous T-cell
lymphoma. J Invest Dermatol. 134:1101–1107. 2014. View Article : Google Scholar :
|
|
12
|
Chen PS, Su JL and Hung MC: Dysregulation
of microRNAs in cancer. J Biomed Sci. 19:902012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okada N, Lin CP, Ribeiro MC, Biton A, Lai
G, He X, Bu P, Vogel H, Jablons DM, Keller AC, et al: A positive
feedback between p53 and miR-34 miRNAs mediates tumor suppression.
Genes Dev. 28:438–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao B, Wang Z, Ali S, Ahmad A, Azmi AS,
Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S and Sarkar FH:
Metformin inhibits cell proliferation, migration and invasion by
attenuating CSC function mediated by deregulating miRNAs in
pancreatic cancer cells. Cancer Prev Res (Phila). 5:355–364. 2012.
View Article : Google Scholar
|
|
16
|
Liu C, Kelnar K, Vlassov AV, Brown D, Wang
J and Tang DG: Distinct microRNA expression profiles in prostate
cancer stem/progenitor cells and tumor-suppressive functions of
let-7. Cancer Res. 72:3393–3404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chabre O, Libé R, Assié G, Barreau O,
Bertherat J, Bertagna X, Feige JJ and Cherradi N: Serum miR-483-5p
and miR-195 are predictive of recurrence risk in adrenocortical
cancer patients. Endocr Relat Cancer. 20:579–594. 2013.PubMed/NCBI
|
|
18
|
Zhang Z, Ge S, Wang X, Yuan Q, Yan Q, Ye
H, Che Y, Lin Y, Zhang J and Liu P: Serum miR-483-5p as a potential
biomarker to detect hepatocellular carcinoma. Hepatol Int.
7:199–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen
J, Zhang Y, Lai P, Fan X, Zhou X, et al: miR-483-5p promotes
invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1
and ALCAM. Cancer Res. 74:3031–3042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J,
Chen R and Zhou Y: Spheroid body-forming cells in the human gastric
cancer cell line MKN-45 possess cancer stem cell properties. Int J
Oncol. 42:453–459. 2013.
|
|
21
|
Wang L, Shi M, Hou S, Ding B, Liu L, Ji X,
Zhang J and Deng Y: MiR-483-5p suppresses the proliferation of
glioma cells via directly targeting ERK1. FEBS Lett. 586:1312–1317.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-timequantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Wu H, Echt CS, Popp MP and Davis JM:
Molecular cloning, structure and expression of an
elicitor-inducible chitinase gene from pine trees. Plant Mol Biol.
33:979–987. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar
|
|
25
|
Yu T, Liu K, Wu Y, Fan J, Chen J, Li C,
Yang Q and Wang Z: MicroRNA-9 inhibits the proliferation of oral
squamous cell carcinoma cells by suppressing expression of CXCR4
via the Wnt/β-catenin signaling pathway. Oncogene. 33:5017–5027.
2014. View Article : Google Scholar
|
|
26
|
Brower JV, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Li C, Liang Y and Chen Z: Trastuzumab (herceptin) targets
gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar
|
|
28
|
Templeton AK, Miyamoto S, Babu A, Munshi A
and Ramesh R: Cancer stem cells: Progress and challenges in lung
cancer. Stem Cell Investigation. 1:92014.PubMed/NCBI
|
|
29
|
Qu X, Zhao M, Wu S, Yu W, Xu J, Xu J, Li J
and Chen L: Circulating microRNA 483-5p as a novel biomarker for
diagnosis survival prediction in multiple myeloma. Med Oncol.
31:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arensman M, Lay AR, Kulikauskas RM, Chien
AJ and Dawson DW: Wnt/β-catenin transcriptional activation promotes
tumorigenesis and predicts survival in pancreatic cancer. Cancer
Res. 73:40112013. View Article : Google Scholar
|
|
31
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai C and Zhu X: The Wnt/β-catenin pathway
regulates self-renewal of cancer stem-like cells in human gastric
cancer. Mol Med Rep. 5:1191–1196. 2012.PubMed/NCBI
|
|
33
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar
|
|
34
|
Kim HY, Park JH, Won HY, Lee JY and Kong
G: CBX7 inhibits breast tumorigenicity through DKK-1-mediated
suppression of the Wnt/β-catenin pathway. FASEB J. 29:300–313.
2015. View Article : Google Scholar
|