Introduction
Mesenchymal stem cells (MSCs), a heterogeneous cell
population, serve important roles in cancer. They migrate to the
tumor sites, differentiate into cancer-associated fibroblasts
(CAFs) and are involved in the formation of the tumor
microenvironment (1). These cells
exert promoting effects on tumor progression by secreting soluble
cytokines, immune regulation and remodeling the tumor extracellular
matrix (2,3).
Exosomes, nanoscale particles secreted by numerous
types of cells, deliver various signal molecules ranging from
proteins, mRNAs and non-coding RNAs [microRNA (miR) and lncRNA]
between cells (4,5). Exosomes have been reported to be
involved in reprogramming tumor behaviors, including growth, immune
escape, angiogenesis, metastasis and drug resistance (6–8).
Exosomes derived from breast cancer cells can transfer miR-105
targeting ZO-1 and destroy the tight junctions between endothelial
cells to promote breast cancer metastasis (9). Exosomes derived from tumor stromal
cells may carry mRNAs to activate the signal transducer and
activator of transcription 1/NOTCH3 signaling pathway though
retinoic acid-inducible gene 1, to induce the radiation resistance
in breast cancer cells (10).
Therefore, exosomes are major messengers in cellular communication.
However, the effects of exosomes derived from MSCs (MSC-ex) on
cancer cells remain to be elucidated.
Gastric cancer has high morbidity and mortality rate
worldwide (11). Revealing the
causes and potential mechanisms for the development of gastric
cancer is of great significance. Our previous study revealed that
MSC-ex facilitated gastric cancer growth in vivo (12) and exosomes derived from gastric
cancer cells stimulated CAF differentiation of MSCs (13). In the present study, the effects of
MSC-ex on the malignant properties of gastric cancer cells were
investigated. It was found that MSC-ex promoted the proliferative
and metastatic potential of gastric cancer cells ex vivo.
MSC-ex induced the epithelial-mesenchymal transition (EMT) and
cancer stemness in gastric cancer cells. It was further
demonstrated that MSC-ex activated the protein kinase B (Akt)
signaling pathway in gastric cancer cells. These findings provided
novel clues for understanding the role of MSC-ex in gastric
cancer.
Materials and methods
Cell culture
MSCs were isolated from human umbilical cords and
the experimental protocols were approved by the Ethics Committee of
Jiangsu University (Jiangsu, China). Fresh umbilical cords were
collected from consenting mothers and were rinsed twice in
phosphate-buffered saline, containing penicillin and streptomycin.
The washed cords were cut into sections of 1–3 mm2
following the removal of the cord vessels and were cultured in low
glucose Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.), 1% penicillin and
streptomycin at 37°C with 5% CO2. When fibroblast-like
cells reached 80% confluence, the cultures were trypsinized and
passaged into new flasks for further expansion. Human fetal lung
fibroblasts (HFL-1) and HGC-27 gastric cancer cells were purchased
from Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). The HFL-1 cells were cultured in
minimal essential medium-α, supplemented with 15% FBS. HGC-27 cells
were cultured in high glucose DMEM containing 10% FBS. The medium
was changed every 3 days.
Isolation and characteristics of
exosomes
MSCs and HFL-1 cells were cultured in serum-free
medium. After 48 h, the cell culture medium was collected and the
exosomes were isolated using density gradient centrifugation, as
previously described (14).
Exosomes were stored at −70°C until use. Exosomes derived from
HFL-1 cells were used as an exosomal control. Size distribution
within exosome reparations was analyzed by measuring the rate of
Brownian motion using a NanoSight LM10 system equipped with a fast
video capture and particle-tracking software (Nanoparticle Tracking
Analysis, version 2.3; NanoSight, Amesbury, UK).
Transwell migration and invasion
assays
Briefly, HGC-27 cells (5×104 cells/200
µl) suspended in serum-free medium were loaded into the
upper compartment of a Transwell chamber. The lower chamber was
filled with 500 µl 10% FBS-DMEM containing exosomes derived
HFL-1 cells (HFL-ex) or MSC-ex (80 µg/l) in the presence or
absence of LY294002 (50 µM/ml; Sigma-Aldrich, St. Louis, MO,
USA). Following culture at 37°C in a humidified atmosphere of 5%
CO2 for 8 h, the cells in the upper membrane were wiped
with a wet Q-tip. The cells that had migrated through the membrane
(8 µm pore size) were fixed with 4% paraformaldehyde and
stained with crystal violet. For the invasion assay, the upper
compartment of a Transwell chamber was covered with 40 µl
matrigel and the protocol was similar to the migration assay, with
the exception that the chamber was cultured at 37°C in a humidified
atmosphere of 5% CO2 for 24 h. The cells were observed
under a microscope and at least 6 fields of cells were assessed for
each group. Each assay was repeated three times.
Western blot analysis
The cells were collected and lysed in
radioimmunoprecipitation lysis buffer, supplemented with proteinase
inhibitors. Equal quantities of protein (200 µM) were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and were subsequently transferred onto a
polyvinylidene difluoride membrane. The membranes were blocked in
5% (w/v) non-fat milk at room temperature. Following blocking, the
membranes were incubated with the primary antibodies for 16 h at
4°C and secondary antibodies for 1 h at 37°C. The primary
antibodies were as follows: Anti-N-cadherin (1:1,000; cat. no.
ab98952; Abcam, Cambridge, MA, USA), anti-E-cadherin (1:500; cat.
no. sc-7870; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-Oct4 (1:800; cat. no. 21424), anti-Sox2 (1:500; cat. no.
27015), anti-Lin28B (cat. no. 21626), anti-phosphorylated (p-)Akt
(1:500; cat. no. 11501), anti-t-Akt (1:500; cat. no. 21501) (all
from Signalway Antibody LLD, College Park, MD, USA), anti-Vimentin
(1:1,000; cat. no. BS1491), anti-Twist (1:500; cat. no. BS60412),
anti-CD63 (1:500; cat. no. BS3474) (all from Bioworld Technology,
Louis Park, MN, USA) and anti-glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; 1:2,000; cat. no. CW0100A; Cwbio, Beijing,
China). The blots were then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse (1:5,000; cat. no. CW102, 1:5,000)
and goat anti-rabbit (1:5,000; cat. no. CW103) secondary antibodies
(Cwbio). The signal was visualized using HRP substrate (EMD
Millipore, Billerica, MA, USA) and analyzed using the LAS 4000 mini
fluorescence/chemiluminescence imaging analysis system (GE
Healthcare Life Sciences, Chalfont, UK).
Cell colony-forming assay
HGC-27 cells pre-treated with HFL-ex or MSC-ex (80
µg/l) in the presence or absence of LY294002 (50
µM/ml) for 7 days were collected and seeded into ultra low
adhesion 6-well plates (1×103 cells/well). The cells
were incubated at 37°C in a humidified atmosphere with 5%
CO2 in serum-free medium containing growth factors,
HFL-ex, MSC-ex and LY294002. The medium was changed every 3 days.
The results are the mean values of three independent experiments
with three replicate plates in each.
Soft agar assay
A 2 ml agar mixture (L-DMEM + 0.6% agar) was coated
onto a 6-well plate as the bottom layer. A 2 ml agar medium mixture
(L-DMEM with 10% FBS + 0.3% agar) containing 1×103
HGC-27 cells pre-treated with HFL-ex or MSC-ex (80 µg/l) in
the presence or absence of LY294002 (50 µM/ml) was added for
7 days as the top layer. After the base layer had solidified, the
top layer was added. The plate was incubated for 14 days at 37°C in
humidified atmosphere with 5% CO2. Colony cultures were
imaged and quantified.
Statistical analysis
All data are expressed as mean ± standard deviation.
SPSS software (SPSS, Inc., Chicago, IL, USA). The means of
different treatment groups were compared by Student's t-test or
two-way analysis of variance followed by least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of exosomes
Exosomes derived from human MSCs and HFL-1 cells
(MSC-ex and HFL-ex, respectively) exhibited a round or oval
morphology, with a diameter of 79±30 nm. as determined by using
NanoSight visual nanoparticle tracking analysis. Western blot
analyses revealed the positive expression of the exosomal marker
CD63 in MSC-ex and HFL-ex (Fig.
1).
MSC-ex induce the EMT in gastric cancer
cells
Our previous study demonstrated that tumor cells
treated with hMSC supernatant underwent EMT (15). Whether MSC-ex had similar effects
on gastric cancer cells was investigated in the present study. EMT
phenotypes are associated with an enhanced migration and invasion
ability. Cell migration and invasion was first examined using a
Transwell system. Compared with the control and HFL-ex-treated
group, the MSC-ex-treatment markedly promoted the migration and
invasion of HGC-27 cells (Fig. 2A and
B). The number of migrating HGC-27 cells was revealed to
increase by 2–3-fold (Fig. 2A) and
the number of invading cells increased by 8-fold in MSC-ex
treatment group (Fig. 2B). Western
blot analysis revealed that the expression of N-cadherin, Vimentin
and Twist was increased in a time-dependent manner in HGC-27 cells
pre-treated with MSC-ex. By contrast, the expression of E-cadherin
was notably downregulated by MSC-ex (Fig. 2C). The protein expression levels of
E-cadherin, N-cadherin, Vimentin and Twist were not significantly
altered in HGC-27 cells pre-treated with HFL-ex.
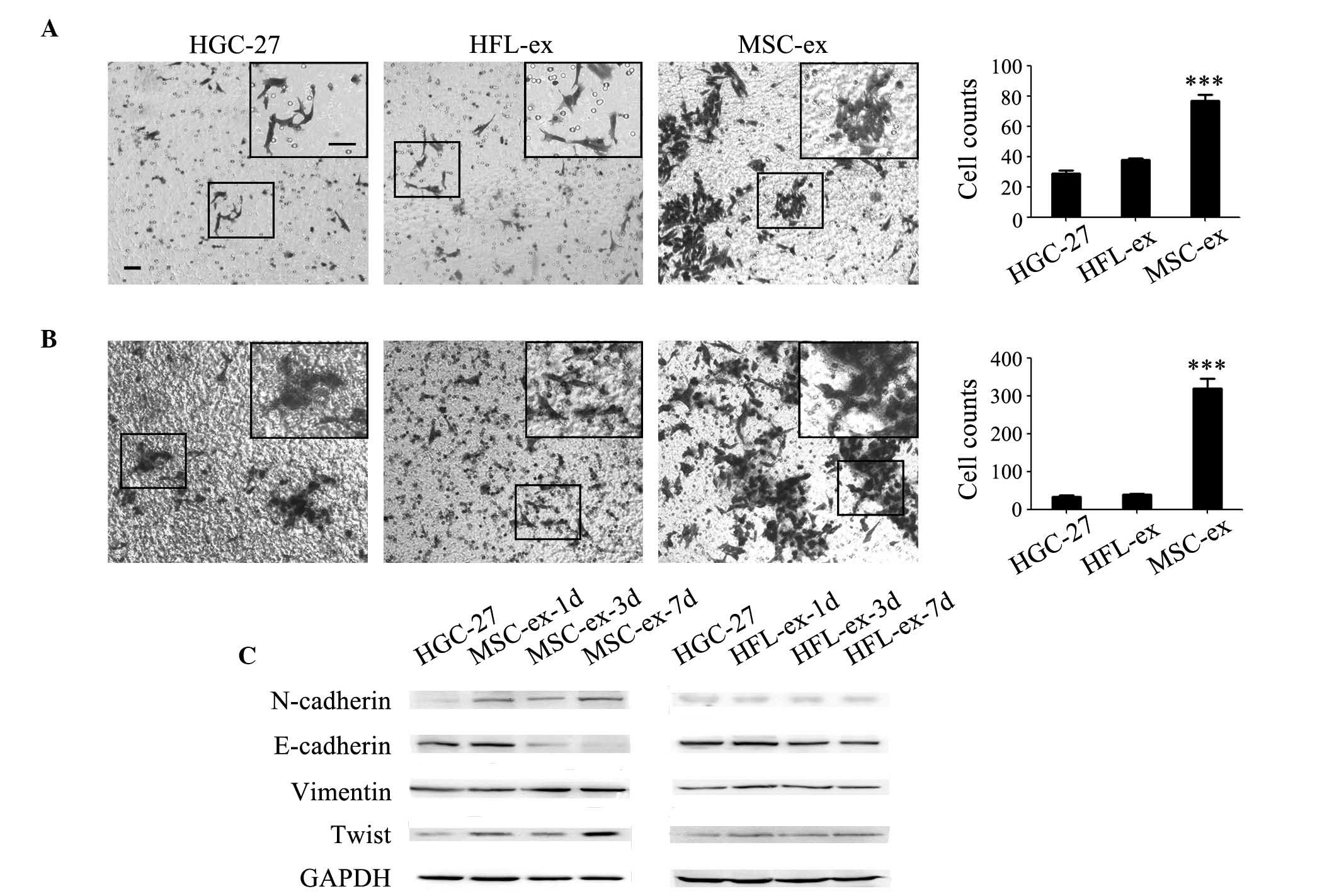 | Figure 2MSC-ex induce the EMT in gastric
cancer cells. MSC-ex promoted the (A) migration and (B) invasion of
HGC-27 cells. Representative images showing the migration of
control untreated HGC-27 cells, HFL-ex-treated cells and
MSC-ex-treated cells [magnification, ×100 (large panel);
magnification, ×200 (small panel); scale bar, 50 µm]. The
number of migrated cells was quantified. MSC-ex more effectively
enhanced HGC-27 cells penetration through the membranes. The data
are expressed as the mean ± standard deviation (n=3;
***P<0.001). (C) Western blot analysis of
EMT-associated proteins, including E-cadherin, N-cadherin, Vimentin
and Twist, were determined in HGC-27 cells pre-treated with MSC-ex
for 1, 3 and 7 d and HFL-ex for 1, 3 and 7 d. MSC-ex, mesenchymal
stem cell-exosomes; HFL-ex, human fetal lung fibroblast-exosomes;
EMT, epithelial-mesenchymal transition; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; d, days. |
MSC-ex confer stemness in gastric cancer
cells
The present results showed that MSC-ex can induce
the EMT in gastric cancer cells. Previous studies have reported
that the EMT serves a key role in generating cancer stem cells. To
explore whether MSC-ex can confer stem cell properties in gastric
cancer cells, the self-renewal ability of gastric cancer cells was
assessed in serum-free medium (Fig.
3A) and in semi-solid medium (Fig.
3B). HGC-27 cells in the MSC-ex treatment group formed tumor
spheres with a diameter of 20 µm in serum-free medium at the
first day and the sphere counts reached ~300 on the fifth day
(Fig. 3A). The tumor spheres with
a diameter of 50 µm were quantified on the fifth day, and
the sphere counts in MSC-ex treatment group was ~6-fold more
compared with that in the control group, and 2-fold in the HFL-ex
treatment group (Fig. 3A). The
results of soft agar assay revealed that MSC-ex promoted the
formation of HGC-27 cell spheres in semi-solid medium. HGC-27 cells
in the MSC-ex treatment group formed more and bigger colonies
compared with that in control and HFL-ex treatment groups (Fig. 3B). The spheres with a diameter of
20 µm in MSC-ex treatment group were ~2–3-fold more compared
with that in the other groups. The spheres with a diameter of 50
µm in the MSC-ex treatment group were at least 10-fold more
compared with that in the control group and ~5-fold more compared
with that in the HFL-ex treatment group (Fig. 3B). The expression levels of
stemness-associated proteins, including Oct4, Sox2 and Lin28B,
which are known to be sufficient to reprogram somatic cells to
pluripotent stem cells, were found to be notably increased in
MSC-ex pre-treated HGC-27 cells compared with the HFL-ex
pre-treated HGC-27 cells, as determined by western blotting
(Fig. 3C). The expression of these
proteins in HGC-27 cells was upregulated by MSC-ex in a
time-dependent manner.
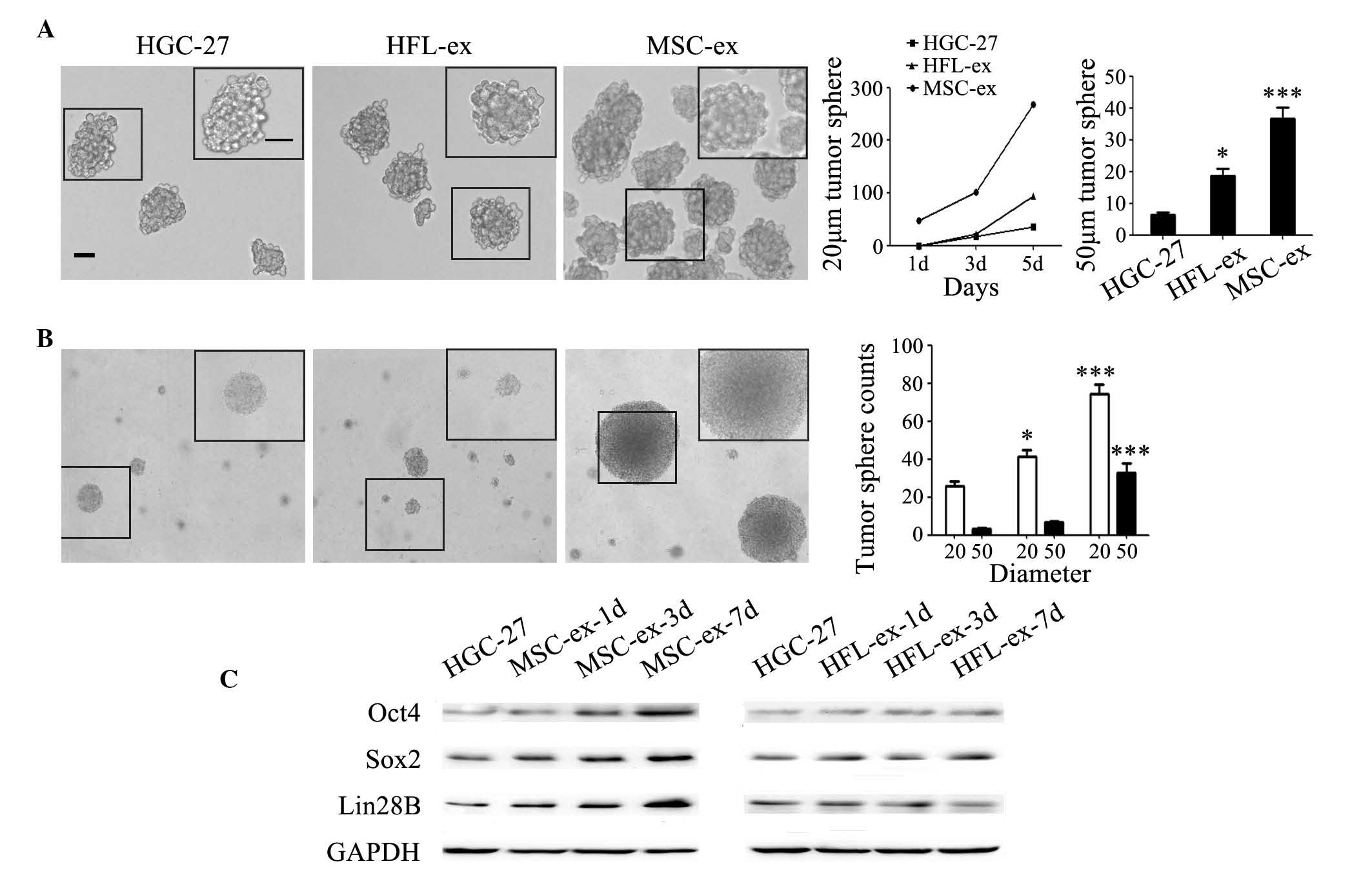 | Figure 3MSC-ex confer stemness in gastric
cancer cells. (A) MSC-ex enhanced the colony formation of HGC-27
cells in serum-free medium. Representative images of cells
manifesting the colonies of control untreated HGC-27 cells and
cells pre-treated with either HFL-ex and MSC-ex [magnification,
×100 (large panel); magnification, ×200 (small panel); scale bar,
50 µm]. The formation rate of tumor spheres with a diameter
of 20 µm or 50 µm was quantified at the fifth day.
The data are expressed as the mean ± standard deviation
(*P<0.05, ***P<0.001). (B) MSC-ex
promoted the formation of HGC-27 cells spheres in semi-solid
medium. Representative images of cells manifesting the colonies of
control untreated HGC-27 cells and cells pre-treated with HFL-ex
and MSC-ex in soft agar. Tumor spheres with a diameter of 20
µm and 50 µm were quantified after 2 weeks. The data
are expressed as the mean ± standard deviation
(*P<0.05, ***P<0.001). (C) Western blot
analysis of stemness-associated proteins, including Oct4, Sox2 and
Lin28B, were determined 1, 3 and 7 d following pre-treatment with
either MSC-ex or HFL-ex. MSC-ex, mesenchymal stem cell-exosomes;
HFL-ex, human fetal lung fibroblast-exosomes; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; d, days; Oct, octamer-binding
transcription factor 4; Sox, sex determining region Y-box 2. |
MSC-ex activate the Akt signaling pathway
to promote the progression of gastric cancer cells
To determine the mechanisms by which MSC-ex promote
the progression of gastric cancer cells, the present study examined
the activation of Akt in MSC-ex pre-treated HGC-27 cells. As shown
in Fig. 4A, the expression of
p-Akt gradually increased in a time-dependent manner (Fig. 4A). Akt is one of the key downstream
signaling proteins of the PI3K pathway. A specific inhibitor of
PI3K, LY294002, was used to inhibit the activation of PI3K. It was
found that the increased Akt phosphorylation following treatment
with MSC-ex was abrogated by LY294002 (Fig. 4A). To further confirm that Akt
activation is responsible for the progression of gastric cancer
cells by MSC-ex treatment, HGC-27 cells were treated with MSC-ex in
the presence or absence of LY294002. The increased expression of
N-cadherin, Vimentin, Twist and the decreased expression of
E-cadherin were reversed by LY294002 (Fig. 4B). The enhanced migration (Fig. 4C) and invasion (Fig. 4D) of MSC-ex-treated HGC-27 cells
were also inhibited by LY294002. In addition, the increased sphere
counts and the sphere size caused by MSC-ex were inhibited by
LY294002 both in serum-free medium (Fig. 5A) and in semi-solid medium
(Fig. 5B). The formation rate of
tumor spheres with a diameter of 20 µm in the LY294002 group
exhibited no difference with that in the control group (Fig. 5A). The number of tumor spheres with
a diameter of 50 µm in the LY294002 group was even lower
compared with that in the control group at the fifth day after
treatment (Fig. 5A). The number of
formed HGC-27 cell colonies in soft agar decreased to the normal
level (Fig. 5B). The induced
expression levels of Oct4, Sox2 and Lin28B proteins in HGC-27 cells
were also significantly decreased by LY294002 (Fig. 5C).
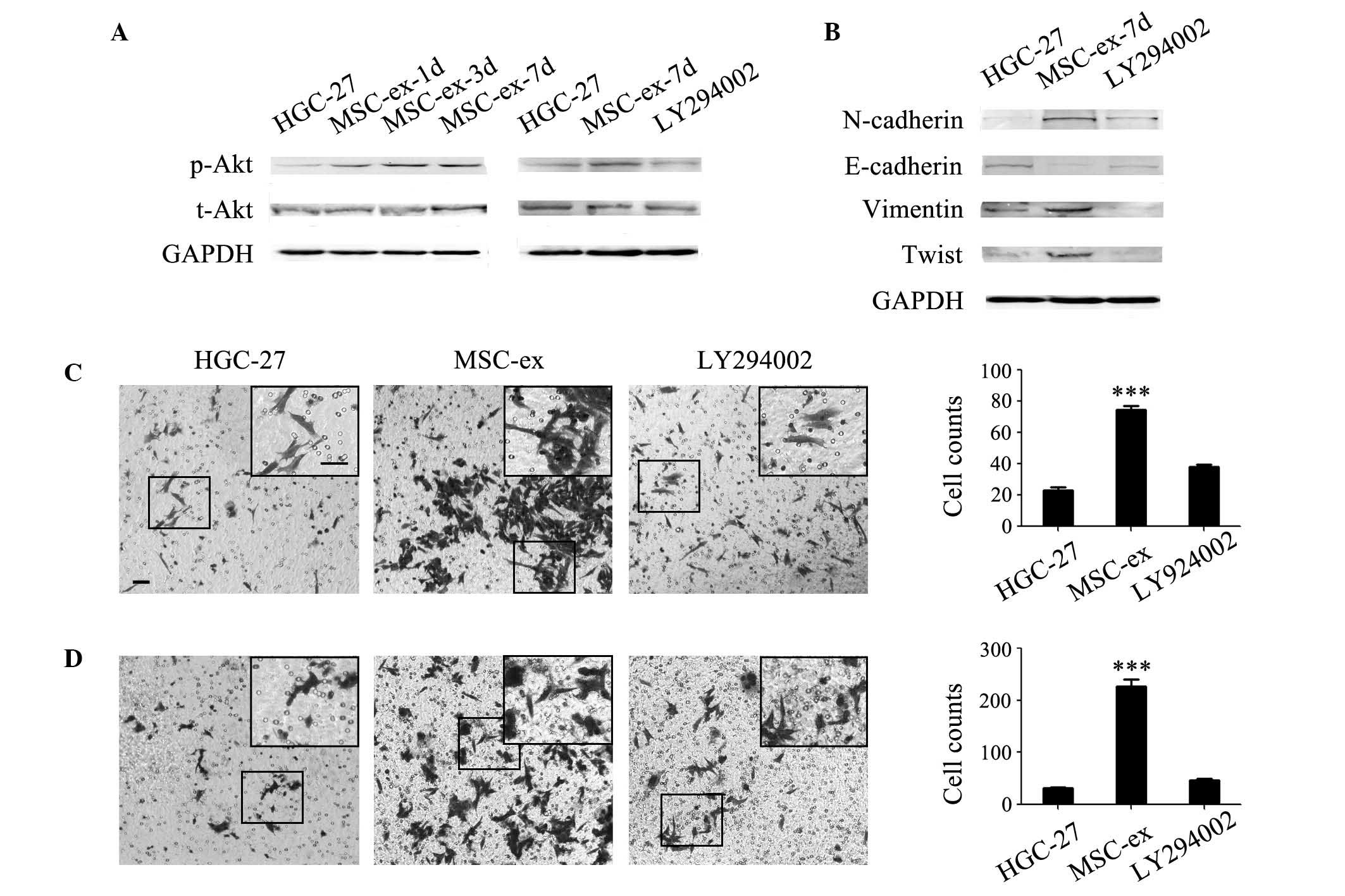 | Figure 4MSC-ex induce the EMT in gastric
cancer cells via the Akt signaling pathway. (A) The expression of
p-Akt gradually increased in a time-dependent manner in HGC-27
cells pre-treated with MSC-ex for 1, 3 and 7 d, and the activation
was inhibited by LY294002 as determined by western blotting. (B)
LY294002 inhibited the increased expression levels of N-cadherin,
Vimentin and Twist, and decreased the expression of E-cadherin in
HGC-27 cells pre-treated with MSC-exosomes for 7 d, as detemrined
by western blotting. (C) LY294002 reversed the migration of HGC-27
cells enhanced by treatment with MSC-ex. Representative images
demonstrated the migration of control untreated HGC-27 cells and
cells pre-treated with MSC-ex in the presence or absence of
LY294002 [magnification, ×100 (large panel); magnification, ×200
(small panel); scale bar, 50 µm]. The number of migrated
cells was quantified and the data are expressed as the mean ±
standard deviation (***P<0.001). (D) LY294002
inhibited the invasion of HGC-27 cells, which was enhanced by
treatment with MSC-ex. Representative images manifesting the
invasion of control untreated HGC-27 cells and MSC-ex-treated
HGC-27 cells in the presence or absence of LY294002 [magnification,
×100 (large panel); magnification, ×200 (small panel); scale bar,
50 µm]. The number of migrated cells was quantified and the
data are expressed as the mean ± standard deviation
(***P<0.001). MSC-ex, mesenchymal stem cell-exosomes;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; p-,
phosphorylated; t-, total; Akt, protein kinase B; EMT,
epithelial-mesenchymal transition; d, days. |
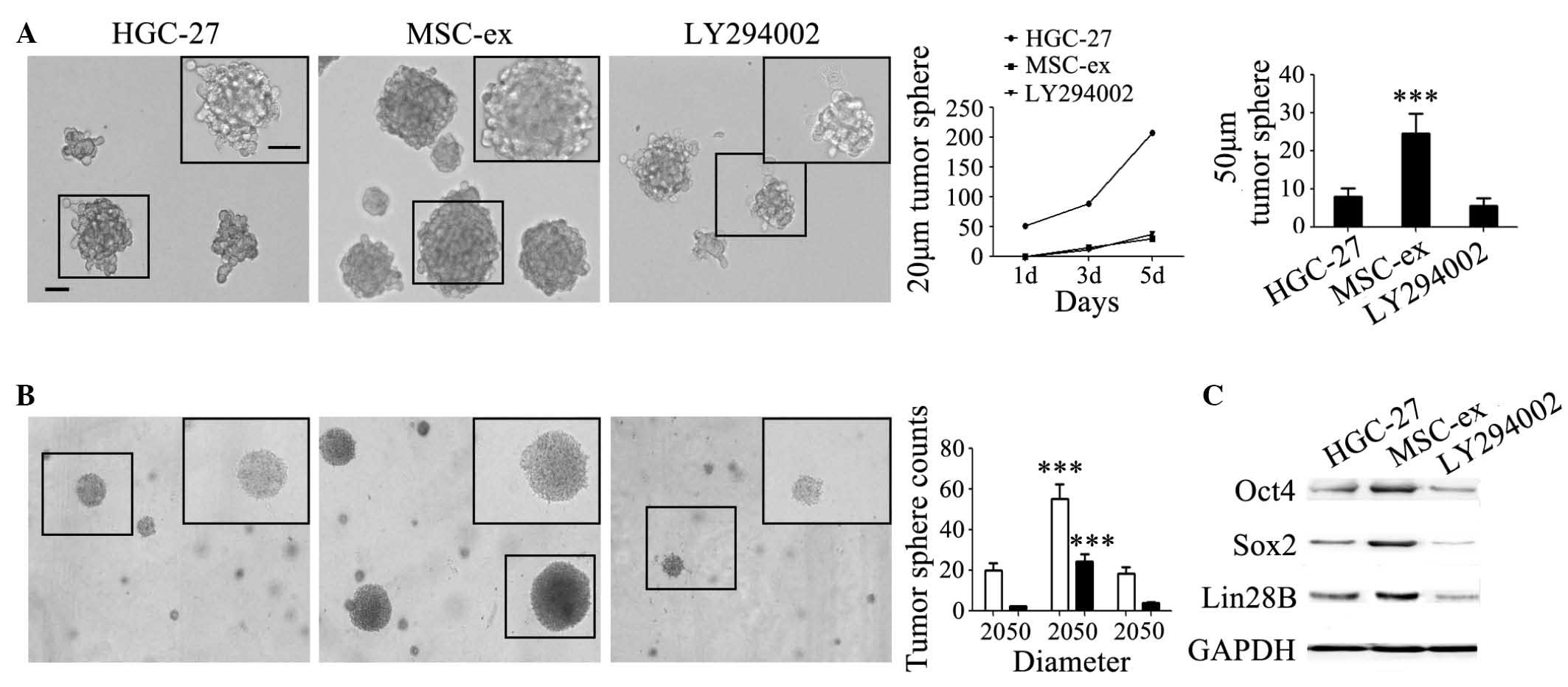 | Figure 5MSC-ex confer stemness in gastric
cancer cells via the Akt signaling pathway. (A) The colony
formation of HGC-27 cells in serum-free medium enhanced by MSC-ex
was abrogated by LY294002. Representative images manifesting the
colonies of control untreated HGC-27 cells MSC-ex-pre-treated cells
for 7 d in the presence or absence of LY294002 [magnification, ×100
(large panel); magnification, ×200 (small panel); scale bar, 50
µm]. The formation rate of tumor spheres with a diameter of
20 µm and 50 µm ere quantified at the fifth day. The
data are expressed as the mean ± standard deviation
(***P<0.001). (B) The colony formation of HGC-27
cells in semi-solid medium promoted by MSC-exosomes was abrogated
by LY294002. Representative images manifesting the colonies of
control untreated HGC-27 cells and MSC-ex-pre-treated cells for 7 d
in the presence or absence of LY294002 in soft agar. The tumor
spheres with a diameter of 20 µm and 50 µm were
quantified after 2 weeks. The data are expressed as the mean ±
standard deviation (***P<0.001). (C) LY294002
inhibited the increased expression of stemness-associated proteins,
Oct4, Sox2 and Lin28B in HGC-27 cells pre-treated with MSC-ex for 7
d, as determined by western blotting. MSC-ex, mesenchymal stem
cell-exosomes; HFL-ex, human fetal lung fibroblast-exosomes; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; d, days; Oct,
octamer-binding transcription factor 4; Sox, sex determining region
Y-box 2; Akt, protein kinase B. |
Discussion
Numerous previous studies have shown that MSCs can
promote the growth of different tumor types via secreting a variety
of factors involved in the formation of tumor blood vessels,
inducing the infiltration of inflammatory cells, or involved in the
tumor microenvironment by trans-differentiation to CAF-like cells.
Exosomes are important message transmitters between cells in the
tumor microenvironment. They contain specific information materials
from the source cells, transport proteins and nucleic acids to
receptor cells, trigger downstream signaling events or change
biological characteristics of the recipient cells. Our previous
study demonstrated that MSC-ex accelerated the expression of
α-smooth muscle actin, vascular endothelial growth factor (VEGF),
C-X-C chemokine receptor type 4, proliferating cell nuclear antigen
and increased vascular density in gastric tissues (12,15).
However, the effects of MSC-ex on the other aspects of gastric
cancer remain unclear. Therefore, the present study investigated
the effects of MSC-ex on the growth and metastatic potential of
HGC-27 cells ex vivo and assessed the underlying molecular
mechanism.
The present results revealed that MSC-ex enhanced
the migration and invasion of HGC-27 cells ex vivo,
increased the expression of mesenchymal indicators and decreased
the expression of epithelial indicator in HGC-27 cells. MSC-ex
induced the EMT in HGC-27 cells. The EMT serves an important role
in tumor invasion and metastasis. Cells experienceing the EMT are
more likely to metastasize. During the EMT, tumor cells lose their
epithelial cell polarity, gaining mesenchymal cell-like athleticism
and can infiltrate into adjacent tissues, and this is hypothesied
to be the initial stage of tumor metastasis (16). Therefore, MSC-ex promoted the
occurrence of the EMT in HGC-27 cells, which may increase the
metastatic capacity of gastric cancer cells.
During the procession of obtaining the EMT, the
self-renewal capacity of tumor cells can also be enhanced (17). MSC-ex enhanced the tumorigenicity
of HGC-27 cells in liquid and semi-solid matrix ex vivo. The
sphere formation rate was higher, tumor sphere counts were
increased and the tumor sphere size was bigger in MSC-ex
pre-treated HGC-27 cells. Western blot analysis revealed that the
expression of stemness-relevant indicators, including Oct4, Sox2
and Lin28B, increased in HGC-27 cells pre-treated with MSC-ex
compared with the control group. Therefore, MSC-ex may confer
stemness in HGC-27 cells.
Akt activation is present in numerous tumor tissues,
including pancreatic and breast cancer (18,19).
Akt is one of major downstream effectors of PI3K and activates a
plurality of signal phosphorylated substrate, exerting a notable
influence on cell growth and cell cycle progression. The aberrant
activation of the Akt signaling pathway in malignancies can
stimulate the proliferation and angiogenesis of tumor cells and is
an important target for cancer therapeutics (20). In the present study, MSC-ex induced
the phosphorylation of Akt, which was suppressed by using a
specific inhibitor of PI3K. The inhibitor LY294002 thereby
inhibited the EMT and self-renewal capacity of gastric cancer
cells. Therefore, MSC-ex promoted the development of gastric cancer
by activating Akt signaling pathway.
Yang et al (21) analyzed the cellular interaction
between MSCs and different cancer cells by direct co-culture and
found that they exchanged membrane proteins and altered
functionality during bidirectional interaction. Previous studies
have suggested various mechanisms elucidating the role of MSCs in
the tumor microenvironment. Lee et al (22) demonstrated that MSC-ex
downregulated the expression of VEGF and suppressed angiogenesis in
breast cancer cells by transferring antiangiogenic molecules,
including miR-16. Lin et al (23) found that MSC-ex promoted migration
via the Wnt signaling pathway in breast cancer cells. MSC-ex may
serve as a significant mediator of cell-to-cell communication in
the tumor microenvironment. It was demonstrated that MSCs promoted
migration, invasion and tumorigenicity in gastric cancer cells by
exosomes instead of the physical presence of MSCs. The results of
the present study provided novel evidence for the hypothesis that
MSCs may function remotely through exosomes rather than in close
proximity to cancer cells. The interaction between MSCs and gastric
cancer cells by exosomes also imply an exosome-based therapeutic
strategy. Interventions against the formation or release of
exosomes, the internalization of exosomes by target cells, or the
signaling cascades triggered by exosomes may improve the efficacy
of gastric cancer therapy.
In conclusion, the present findings suggested that
MSC-ex induce the EMT and confer stemness in gastric cancer cells
by activating the Akt signaling pathway. Exosomes may serve as a
novel mediators of the promoting role of MSCs in gastric cancer and
MSC-ex may represent a novel therapeutic target in gastric cancer
treatment.
Acknowledgments
The present study was supported by the Major
Research Plan of the National Natural Science Foundation of China
(no. 91129718), the Jiangsu Province's Project of Scientific and
Technological Innovation and Achievements Transformation (no.
BL2012055), the Jiangsu Province for Outstanding Sci-tech
Innovation Team in Colleges and Universities (no. SJK2013-10), the
Jiangsu Province's Outstanding Medical Academic Leader and Sci-tech
Innovation Team Program (no. LJ201117) and the Project Funded by
the Priority Academic Program Development of Jiangsu Higher
Education Institutions, Jiangsu Zhenjiang Science and Technology
Program (no. SH2012043).
References
|
1
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cukierman E and Bassi DE: The mesenchymal
tumor microenvironment: A drug-resistant niche. Cell Adh Migr.
6:285–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barcellos-de-Souza P, Gori V, Bambi F and
Chiarugi P: Tumor microenvironment: Bone marrow-mesenchymal stem
cells as key players. Biochim Biophys Acta. 1836:321–335.
2013.PubMed/NCBI
|
|
4
|
Simons M and Raposo G: Exosome-vesicular
carriers for intercellular communication. Curr Opin Cell Biol.
21:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pap E, Pállinger E, Pásztói M and Falus A:
Highlights of a new type of intercellular communication:
Microvesicle-based information transfer. Inflamm Res. 58:1–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taverna S, Flugy A, Saieva L, Kohn EC,
Santoro A, Meraviglia S, De Leo G and Alessandro R: Role of exosome
released by chronic myelogenous leukemia cells in angiogenesis. Int
J Cancer. 130:2033–2043. 2012. View Article : Google Scholar
|
|
7
|
Hood JL, San RS and Wickline SA: Exosome
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan
Y, Wang M, Zhu W, Qian H and Xu W: Exosomes derived from human
mesenchymal stem cells confer drug resistance in gastric cancer.
Cell Cycle. 14:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou W, Fong MY, Min Y, Somlo G, Liu L,
Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al:
Cancer-secreted miR-105 destroys vascular endothelial barriers to
promote metastasis. Cancer cell. 25:501–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang
L, Zhao C, Tao Y and Xu W: Circulating miR-17-5p and miR-20a:
Molecular markers for gastric cancer. Mol Med Rep. 5:1514–1520.
2012.PubMed/NCBI
|
|
12
|
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan
Y, Xu X, Wang M, Qian H and Xu W: Exosomes derived from human bone
marrow mesenchymal stem cells promote tumor growth in vivo. Cancer
Lett. 315:28–37. 2012. View Article : Google Scholar
|
|
13
|
Gu J, Qian H, Shen L, Zhang X, Zhu W,
Huang L, Yan Y, Mao F, Zhao C, Shi Y and Xu W: Gastric cancer
exosomes trigger differentiation of umbilical cord derived
mesenchymal stem cells to carcinoma-associated fibroblasts through
TGF-β/Smad pathway. PLoS One. 7:e524652012. View Article : Google Scholar
|
|
14
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W and Xu W: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate liver
fibrosis. Stem Cells Dev. 22:845–854. 2013. View Article : Google Scholar :
|
|
15
|
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan
Y, Mao F, Wu X and Xu WR: Mesenchymal stem cell-secreted soluble
signaling molecules potentiate tumor growth. Cell Cycle.
10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schlessinger J: Common and distinct
elements in cellular signaling via EGF and FGF receptors. Science.
306:1506–1507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zhang J, Xu K, Xiao Z, Sun J, Xu
J, Wang J and Tang Q: PTEN/PI3K/mTOR/B7-H1 signaling pathway
regulates cell progression and immuno-resistance in pancreatic
cancer. Hepatogastroenterology. 60:1766–1772. 2013.
|
|
19
|
Gargini R, Cerliani JP, Escoll M, Antón IM
and Wandosell F: Cancer stem cell-like phenotype and survival are
coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells.
33:646–660. 2015. View Article : Google Scholar
|
|
20
|
Davis WJ, Lehmann PZ and Li W: Nuclear
PI3K signaling in cell growth and tumorigenesis. Front Cell Dev
Biol. 3:242015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Otte A and Hass R: Human
mesenchymal stroma/stem cells exchange membrane proteins and alter
functionality during interaction with different tumor cell lines.
Stem Cells Dev. 24:1205–1222. 2015. View Article : Google Scholar :
|
|
22
|
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS,
Kim MK, Kim YG, Jang JY and Kim CW: Exosomes derived from
mesenchymal stem cells suppress angiogenesis by down-regulating
VEGF expression in breast cancer cells. PLoS One. 8:e842562013.
View Article : Google Scholar
|
|
23
|
Lin R, Wang S and Zhao RC: Exosomes from
human adipose-derived mesenchymal stem cells promote migration
through Wnt signaling pathway in a breast cancer cell model. Mol
Cell Biochem. 383:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|



















