Introduction
Peripheral artery disease (PAD) affects 3–20% of the
population >50–55 years old and is associated with a high
cardiovascular (CV) mortality (1).
PAD remains a major clinical problem as it is considered a marker
of the extent of atherosclerosis. Furthermore, a large number of
PAD patients suffer from multiple arterial co-morbidities leading
to high CV mortality or a poor prognosis within a short time frame
(2–4).
In the United States of America, Medicare spends
~3.9 billion dollars annually on treatment associated with PAD;
this is more than that spent on other CV diseases, including
myocardial infarction, angina, aortic aneurysm and carotid disease,
or on high blood pressure, cigarette smoking, diabetes mellitus and
hypercholesterolemia (3).
Different pathophysiological mechanisms underlie the development of
atherosclerotic plaque and the subsequent development of CV
disease, these mechanisms include inflammation, platelet
activation, endothelial damage, the proliferation/apoptosis balance
of smooth muscle cells, and oxidative stress (5). The association between oxidative
stress and CV disease has been demonstrated by numerous previous
studies (6,7). These studies have demonstrated that
oxidative stress may have an etiological role and be used as a
biomarker for atherosclerosis. When oxidative stress occurs, cells
trigger a series of biochemical cascades in an attempt to
counteract the altered redox balance and restore homeostasis
(8). Among these biochemical
responses, the heme oxygenase (HO) system has been suggested to be
key (7,9). HO-1 is an intracellular enzyme that
catalyzes the breakdown of heme to carbon monoxide, ferrous iron
and biliverdin (10). However,
numerous other effects not directly associated with erythrocyte
metabolism but of potential relevance to the CV system have been
reported, including protection from ischemia/reperfusion (10,11),
blood pressure regulation (12),
inflammation (13–15) and in angiogenesis (16,17).
In addition, the presence of HO-1 has been demonstrated in various
intracellular and extracellular compartments, suggesting that this
protein may have other properties independent of its known
enzymatic activity (18–20). This is important as HO-1 is an
intracellular enzyme and the role of its presence in the plasma is
unclear. One physiological role may be in degrading excess free
heme in association with hemopexin and transferrin that may result
from a hemolytic or other process. Alternatively, HO-1 may be
released into the plasma from smooth muscle cells, cardiomyocytes,
leukocytes, monocytes/macrophages and/or endothelial cells that are
damaged by the effect of hypertension, oxidative stress and/or
chronic inflammation (8).
The observations described above suggest that HO-1
has a role in CV disease pathogenesis. However, the role of HO-1 in
PAD remains to be elucidated.
Thus, the present study aimed to measure serum
levels of HO-1 and other surrogate markers of oxidative stress,
including reduced glutathione, lipid hydroperoxides and
isoprostanes, in patients with PAD at the time of their first
diagnosis.
Patients and methods
Patients (n=27; male; mean age, 66±8 years) were
enrolled consecutively and examined in the non-invasive vascular
laboratory of the Medical Angiology Unit (Garibaldi Hospital,
Catania, Italy). Patients were diagnosed with PAD based on their
medical history of intermittent claudication and/or vasodilatation
therapeutic agents, and an ankle/brachial index (ABI) ≤0.9. The ABI
was obtained by measuring the arterial pressure at the posterior or
anterior tibial of the lower limbs and was divided by the brachial
arterial pressure. A pocket-sized Doppler equipped with an 8 MHz
pencil style probe (Sonomed SRL, Rome, Italy) was used to record
the ABI. The lowest value observed in one of the two peripheral
arteries of the lower limbs was considered. All the enrolled
patients met the criteria of stage II according to the Fontaine
classification of PAD, in which pain due to walking is intermittent
claudication (21). The mean value
of the free walking distance in all PAD patients was 347±170 m. The
mean ABI value of the PAD patients was 0.83±0.08; 0.85±0.7 in less
severe patients (stage IIa) and 0.78±0.5 in the more severe
patients (stage IIb). None of the PAD patients exhibited a higher
ABI (>1.3) than the previously mentioned values. Healthy male
volunteers (n=27) served as age-matched controls.
Controls were recruited from healthy volunteer blood
donors, regularly attending the transfusion center of the
University of Catania Hospital (Catania, Italy). Body mass index
(BMI) was calculated, for patients and controls, as
kg/m2. Obesity was diagnosed when the BMI was >30.
Controls were healthy individuals with no known risk factors for
PAD, including diabetes or dyslipidaemia.
The adopted procedures were in agreement with the
Helsinki Declaration of 1975, as revised in 1983 and were approved
by the Ethics Council and Institutional Review Boards of the
Garibaldi Hospital, University of Catania. All subjects provided
informed consent. Participants did not suffer from recent coronary
acute syndrome, heart failure, chronic or acute renal failure,
active cancer, chronic liver disease or immunologic diseases.
Blood sampling
Venepuncture was conducted using an antecubital vein
at the time of diagnosis and enrollment. The blood was collected in
vacutainers and distributed in 0.5 ml aliquots. The serum samples,
obtained by centrifugation at 3,000 × g for 15 min at 4°C, were
stored at ‒80°C until analysis (22).
Lipid hydroperoxide (LOOH)
determination
LOOH levels were evaluated following the oxidation
of Fe2+ to Fe3+ in the presence of xylenol
orange (23). The assay mixture
contained, in a total volume of 1 ml/100 ml of plasma sample, 100
mmol/l xylenol orange, 250 mmol/l ammonium ferrous sulfate, 90%
methanol, 4 mmol/l butylated hydroxytoluene and 25 mmol/l
H2SO4. Following incubation for 30 min at
room temperature, the absorbance was measured using a U2000 Hitachi
spectrophotometer (Hitachi, Ltd., Tokyo, Japan) at a wavelength of
560 nm. Calibration was obtained using hydrogen peroxide (0.2–20
mmol/l). The limit of detection for this assay is ~0.25 nmol/l.
Measurement of glutathione (GSH)
Plasma levels of GSH were measured using a
spectrophotometric assay based on the reaction of thiol groups with
2,2-dithio-bis-nitrobenzoic acid at a wavelength of 412 nm
(εM=13,600 M-1 cm-1, where εM is a wavelength-dependent molar
absorptivity coefficient) (23).
Measurements were performed in duplicate.
Measurement of HO-1, adiponectin and
isoprostanes
A commercially available HO-1 (human) ADI-EKS-800
ELISA kit (Stressgen Biotechnologies Corporation, Victoria, BC,
Canada) was used to measure HO-1 concentration. The assay was
performed according to the manufacturer's protocol as previously
described (24,25). Briefly, each plasma sample was
incubated with anti-HO-1, anti-rabbit immunoglobulin G and
horseradish peroxidase conjugates, in successive order. Absorbance
was measured at a wavelength of 450 nm, and HO-1 concentration was
calculated from a standard curve generated with purified proteins.
The limit of detection as specified by the manufacturer was
0.78±0.65 ng/ml. Each measurement was performed in triplicate, and
means were reported. Similarly, isoprostanes and adiponectin were
determined using the 8-Isoprostane ELISA kit and the adiponectin
(Human) EIA kit (Cayman Chemical Company, Ann Arbor, MI, USA)
according to the manufacturer's protocol.
Statistical analysis
Statistical analyses were performed using
Statview® 5.0 (SAS Institute Inc., Cary, NC, USA).
Univariate analyses were performed using Student's unpaired t-test
for numeric variables, whereas the differences in the prevalence
for nominal variables were analyzed using the χ2 test.
Correlation analyses were performed using the Pearson rank
correlation method.
Multivariate analysis (by multiple regression model)
was performed in order to determine the effect of clinical and
laboratory parameters on the presence or severity of PAD. Two
different models of multivariate analysis for the presence and for
the severity of PAD were built, aiming to test whether HO-1 was an
independent predictor of the presence or the severity of PAD,
respectively, with the following dependent variables: HO-1,
hypertension, diabetes, ABI, smoking, age, dyslipidemia, obesity
and free walking distance. All data are expressed as mean and
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
The major clinical characteristics of the study
subjects are summarized in Table
I; the co-administration of other therapeutic agents for CV
prevention was considerable, and included anti-hypertensive and
lipid-lowering agents, in addition to aspirin. This was a potential
limitation of the present study. As presented in Table I, no significant differences in
LOOH, GSH and adiponectin levels were observed between the PAD
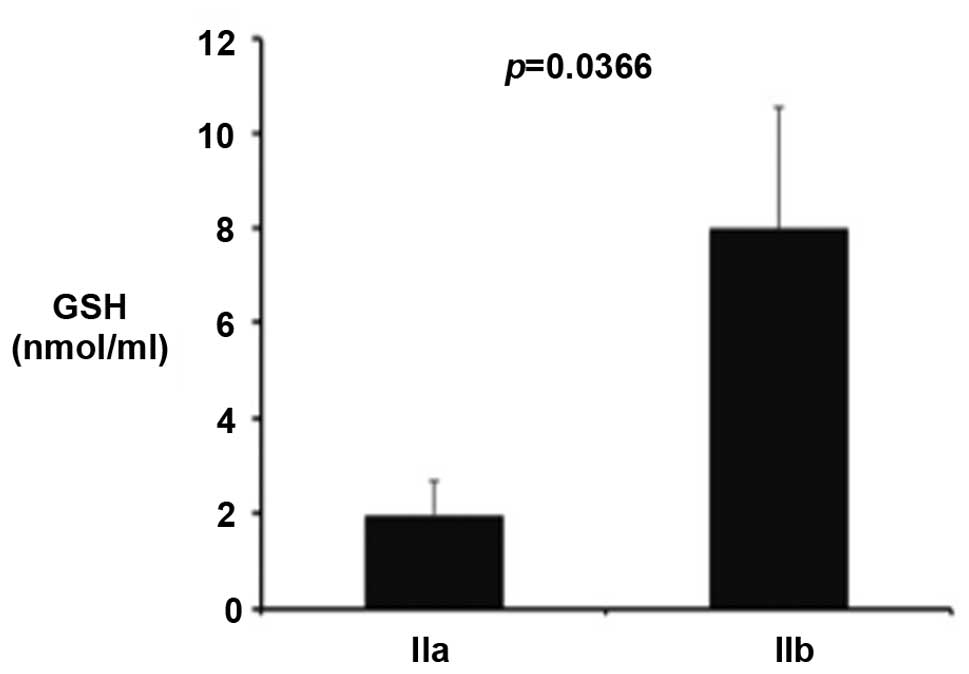
patients and controls. However, a significant increase in GSH level
was observed in stage IIb patients when compared with stage IIa
patients (Fig. 1; P<0.036).
Furthermore, a significant decrease in plasma protein levels of
HO-1 was observed in PAD patients when compared with the controls.
Notably, this reduction was not dependent on the stage of the
disease or levels of oxidative stress biomarkers. A multivariate
analysis (multiple regression) was also performed in order to test
whether HO-1 was an independent predictor of the presence or the
severity of PAD. It was observed that HO-1, hypertension, smoking
and dyslipidemia were independent predictors of the presence of PAD
(P<0.0001, P<0.0001, P=0.0082, and P=0.0013 respectively). In
addition, ABI was the only independent predictor of PAD severity
(P<0.0001).
 | Table IClinical and laboratory data in PAD
patients and controls. Data are expressed as the mean ± standard
deviation or as percentages. |
Table I
Clinical and laboratory data in PAD
patients and controls. Data are expressed as the mean ± standard
deviation or as percentages.
| Parameter | PAD (n=27) | P= | Controls (n=27) |
|---|
| Age, years | 66±8 | ns | 65±9 |
| BMI
(kg/m2) | 29±1 | ns | 29±2 |
| Obesity (%) | 9 (33) | ns | 9 (23) |
| Diabetes, n (%) | 10 (37) | – | / |
| Smoking, n (%) | 22 (81) | ns | 20 (74) |
| Dyslipidemia, n
(%) | 16 (59) | – | / |
| Hypertension, n
(%) | 19 (70) | – | / |
| Use of statins, n
(%) | 15 (56) | – | / |
| Use of aspirin, n
(%) | 24 (89) | – | / |
| PAD stage IIa, n
(%) | 13 (48) | – | / |
| Pain-free walking
distance (meters) | 347±170 | – | / |
| Ankle-brachial
index | 0.83±0.08 | – | / |
| Lipid
hydroperoxides (nmol/ml) | 6.8±14.2 | ns | 8.3±14.9 |
| Glutathione
(nmol/ml) | 5.1±7.6 | ns | 6.9±9.1 |
| Heme oxygenase-1
(ng/ml) | 0.8±0.7 | <0.0001 | 3.4±1.3 |
| Lactic acids
(mM) | 0.11±0.01 | 0.0123 | 0.10±0.01 |
| Isoprostanes
(pg/ml) | 3.8±4.8 | <0.0001 | 120±91 |
| Adiponectin
(µg/ml) | 1.4±0.2 | ns | 1.5±0.5 |
Discussion
Oxidative stress is the result of an imbalance
between the generation of reactive oxygen species and the
antioxidant defense system. Although appropriate epidemiological
markers to measure oxidative stress are lacking, certain markers
have been examined specifically with respect to PAD. In the present
study, various biomarkers were evaluated to detect the level of
oxidative stress.
The results of the present study indicated there was
no significant difference in GSH content in PAD patients when
compared with healthy controls. These data may appear inconsistent
with previous data that demonstrated a marked increase in oxidative
stress markers in CV disease (26). However, it should be considered
that oxidative stress triggers a series of compensatory biochemical
mechanisms in order to maintain the redox balance. Thus, it may be
that patients increase GSH synthesis via the upregulation of its
enzymatic synthesis, such as GSH reductase and gamma glutamyl
cysteine synthase. The findings of the present study are consistent
with this hypothesis, they indicate that GSH is increased in the
group of patients with more severe disease (stage IIb) compared
with the milder form. Similarly, the level of isoprostanes in stage
IIb patients are not significantly different when compared with
stage IIa patients, suggesting that the more severe patients
exhibit a balanced maintenance of the systemic redox balance
accompanied by a concomitant upregulation of GSH production.
Regarding lipid peroxidation, LOOH may notably be transformed into
more oxidized products, including malondialdehyde, which is a
downstream product of lipid peroxidation and is not measured by the
spectrophotometric assay used in the current study. Isoprostanes,
the other lipid peroxidation biomarker used in the present study,
may appear inconsistent with data presented in previous studies
(27,28). Since their identification, levels
of F2-isoprostanes have been recommended as a reliable
biomarker for measuring in vivo lipid oxidation and
oxidative stress (27). Previous
studies demonstrated higher levels of urinary 8-iso-prostaglanding
F2α in patients with chronic lower limb ischemia
compared with healthy controls (28,29).
One previous study performed multivariate adjustment. Following
adjustment for age, gender, diabetes, hypertension, BMI,
creatinine, low-density lipoprotein, triglyceride, high-sensitivity
C-reactive protein and homocysteine, an increment of every 10 pg/ml
in plasma 8-iso-PGF2α was associated with an increased
risk of 11% for lower limb ischemia (28). Furthermore, the present study also
evaluated the levels of adiponectin in PAD patients and healthy
controls. Results from the present study indicated there was no
significant difference in adiponectin levels between the two
groups, however, a previous study (30) demonstrated that low adiponectin
levels were associated with an increase in PAD incidence.
The current study indicated there was a significant
reduction in HO-1 levels in PAD patients when compared with healthy
controls. The protective properties of HO-1 are multifactorial and
likely due to degradation of pro-oxidant heme, the generation of
antioxidant biliverdin and bilirubin, and the production of carbon
monoxide, which is a potent vasodilator with anti-inflammatory
effects (7,9). Furthermore, previous studies have
also observed reduced serum HO-1 in lung disease (31). The presence of HO-1 has recently
been demonstrated in various intracellular and extracellular
compartments, suggesting that this protein may exhibit other
functions independently of its known enzymatic activity (18,20,32).
This latter point is important as HO-1 is an intracellular enzyme,
thus, the underlying reason for its presence in the plasma is
unclear. Data from the present study demonstrates that HO-1
reduction is not dependent on other covariables and, in particular,
oxidative stress parameters (Table
II). In this context, it may be hypothesised that as our
previous data indicated that HO-1 protein expression is reduced in
experimental diabetes and as the majority of PAD patients in the
current study also had diabetes, reduced HO-1 levels in PAD may
reflect reduced intracellular HO-1 content in this group (33). In addition, previous studies have
also reported that HO-1 deletion in mice evokes resistance to
diet-induced insulin resistance and inflammation, markedly reducing
secondary diseases, including steatosis and liver toxicity
(34,35). Multivariate analyses were also
performed in order to test whether HO-1 was an independent
predictor of the presence or severity of PAD. It was observed that
HO-1 was independently associated with the presence of PAD.
Furthermore, hypertension, smoking and dyslipidemia were also
independent predictors of the presence of PAD, while ABI was the
only independent predictor of PAD severity. These findings suggest
that HO-1 may be useful in determining the presence of PAD.
 | Table IIPearson correlations in peripheral
artery disease patients. |
Table II
Pearson correlations in peripheral
artery disease patients.
| Parameter | LOOH | Glutathione | HO-1 | Lactic acids | Isoprostanes | Adiponectin |
|---|
| Age | −.074 | −.111 | −.125 | .026 | .112 |
−.457a |
| BMI | .123 | .220 | .151 | −.304 | −.227 | −.076 |
| PfWD | .084 | −.316 | .258 | −.100 | −.238 | −.178 |
| ABI | .238 | −.359 | .057 | .001 | .104 | −.095 |
| LOOH | – | −.194 | −.263 | .100 | .243 | −.227 |
| Glutathione | −.194 | – | −.253 |
−.525a | .045 | .016 |
| HO-1 | −.263 | −.253 | – | −.061 | .009 | .190 |
| Lactic acid | .100 |
−.525a | −.061 | – | .175 | −.242 |
| Isoprostanes | .243 | .045 | .009 | .175 | – | .008 |
| Adiponectin | −.227 | .016 | .190 | −.242 | .008 | – |
Therefore, it is possible to hypothesise that plasma
HO-1 reduction may also be part of the compensatory mechanisms to
maintain the cellular redox status. However, the sample size of the
cohort in the present study is small and this may represent a
potential limitation of the data, which is required to be confirmed
on a larger population.
In conclusion, the results of the present study
indicate that oxidative stress depends on the stage of PAD and that
the condition is followed by a concomitant antioxidant response,
which, however, is only partially sufficient to maintain the redox
balance. These findings suggest a biological basis for
antioxidants, such as polyphenols, to be tested as therapeutic
agents in appropriately designed prospective trials.
References
|
1
|
Abdulhannan P, Russell DA and
Homer-Vanniasinkam S: Peripheral arterial disease: A literature
review. Br Med Bull. 104:21–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch AT, Halverson SL, Treat-Jacobson D,
Hotvedt PS, Lunzer MM, Krook S, Rajala S and Hunninghake DB: The
minnesota regional peripheral arterial disease screening program:
Toward a definition of community standards of care. Vasc Med.
6:87–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramos R, Quesada M, Solanas P, Subirana I,
Sala J, Vila J, Masiá R, Cerezo C, Elosua R, Grau M, et al:
Prevalence of symptomatic and asymptomatic peripheral arterial
disease and the value of the ankle-brachial index to stratify
cardiovascular risk. Eur J Vasc Endovasc Surg. 38:305–311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q
and Hughes JP: Prevalence of peripheral arterial disease and risk
factors in persons aged 60 and older: Data from the National Health
and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc.
55:583–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishna SM, Moxon JV and Golledge J: A
review of the pathophysiology and potential biomarkers for
peripheral artery disease. Int J Mol Sci. 16:11294–11322. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siti HN, Kamisah Y and Kamsiah J: The role
of oxidative stress, antioxidants and vascular inflammation in
cardiovascular disease (a review). Vascul Pharmacol. 71:40–56.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barbagallo I, Galvano F, Frigiola A,
Cappello F, Riccioni G, Murabito P, D'Orazio N, Torella M, Gazzolo
D and Li Volti G: Potential therapeutic effects of natural heme
oxygenase-1 inducers in cardiovascular diseases. Antioxid Redox
Signal. 18:507–521. 2013. View Article : Google Scholar
|
|
8
|
Novo G, Cappello F, Rizzo M, Fazio G,
Zambuto S, Tortorici E, Marino Gammazza A, Corrao S, Zummo G, De
Macario EC, et al: Hsp60 and heme oxygenase-1 (Hsp32) in acute
myocardial infarction. Transl Res. 157:285–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbagallo I, Nicolosi A, Calabrese G,
David S, Cimino S, Madonia M and Cappello F: The role of the heme
oxygenase system in the metabolic syndrome. Curr Pharm Des.
20:4970–4974. 2014. View Article : Google Scholar
|
|
10
|
Li Volti G, Sacerdoti D, Di Giacomo C,
Barcellona ML, Scacco A, Murabito P, Biondi A, Basile F, Gazzolo D,
Abella R, et al: Natural heme oxygenase-1 inducers in hepatobiliary
function. World J Gastroenterol. 14:6122–6132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Volti G, Sorrenti V, Murabito P,
Galvano F, Veroux M, Gullo A, Acquaviva R, Stacchiotti A, Bonomini
F, Vanella L and Di Giacomo C: Pharmacological induction of heme
oxygenase-1 inhibits iNOS and oxidative stress in renal
ischemia-reperfusion injury. Transplant Proc. 39:2986–2991. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Volti G, Seta F, Schwartzman ML,
Nasjletti A and Abraham NG: Heme oxygenase attenuates angiotensin
II-mediated increase in cyclooxygenase-2 activity in human femoral
endothelial cells. Hypertension. 41:715–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kushida T, LiVolti G, Goodman AI and
Abraham NG: TNF-alpha-mediated cell death is attenuated by
retrovirus delivery of human heme oxygenase-1 gene into human
microvessel endothelial cells. Transplant Proc. 34:2973–2978. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sacerdoti D, Colombrita C, Ghattas MH,
Ismaeil EF, Scapagnini G, Bolognesi M, Li Volti G and Abraham NG:
Heme oxygenase-1 transduction in endothelial cells causes
downregulation of monocyte chemoattractant protein-1 and of genes
involved in inflammation and growth. Cell Mol Biol
(Noisy-le-grand). 51:363–370. 2005.
|
|
15
|
Marrazzo G, Bosco P, La Delia F,
Scapagnini G, Di Giacomo C, Malaguarnera M, Galvano F, Nicolosi A
and Li Volti G: Neuroprotective effect of silibinin in diabetic
mice. Neurosci Lett. 504:252–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Volti G, Sacerdoti D, Sangras B,
Vanella A, Mezentsev A, Scapagnini G, Falck JR and Abraham NG:
Carbon monoxide signaling in promoting angiogenesis in human
microvessel endothelial cells. Antioxid Redox Signal. 7:704–710.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Volti G, Wang J, Traganos F, Kappas A
and Abraham NG: Differential effect of heme oxygenase-1 in
endothelial and smooth muscle cell cycle progression. Biochem
Biophys Res Commun. 296:1077–1082. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tibullo D, Barbagallo I, Giallongo C, La
Cava P, Parrinello N, Vanella L, Stagno F, Palumbo GA, Li Volti G
and Di Raimondo F: Nuclear translocation of heme oxygenase-1
confers resistance to Imatinib in chronic myeloid leukemia cells.
Curr Pharm Des. 19:2765–2770. 2013. View Article : Google Scholar
|
|
19
|
Rizzo M, Abate N, Chandalia M, Rizvi AA,
Giglio RV, Nikolic D, Marino Gammazza A, Barbagallo I, Isenovic ER,
Banach M, et al: Liraglutide reduces oxidative stress and restores
heme oxygenase-1 and ghrelin levels in patients with type 2
diabetes: A prospective pilot study. J Clin Endocrinol Metab.
100:603–606. 2015. View Article : Google Scholar :
|
|
20
|
Li Volti G, Galvano F, Frigiola A,
Guccione S, Di Giacomo C, Forte S, Tringali G, Caruso M, Adekoya OA
and Gazzolo D: Potential immunoregulatory role of heme oxygenase-1
in human milk: A combined biochemical and molecular modeling
approach. J Nutr Biochem. 21:865–871. 2010. View Article : Google Scholar
|
|
21
|
Fontaine R, Kim M and Kieny R: Surgical
treatment of peripheral circulation disorders. Helv Chir Acta.
21:499–533. 1954.In German. PubMed/NCBI
|
|
22
|
Malaguarnera M, Vacante M, Giordano M,
Pennisi G, Bella R, Rampello L, Malaguarnera M, Li Volti G and
Galvano F: Oral acetyl-L-carnitine therapy reduces fatigue in overt
hepatic encephalopathy: A randomized, double-blind,
placebo-controlled study. Am J Clin Nutr. 93:799–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nibali L, Rizzo M, Li Volti G, D'Aiuto F,
Giglio RV, Barbagallo I, Pelekos G and Donos N: Lipid subclasses
profiles and oxidative stress in aggressive periodontitis before
and after treatment. J Periodontal Res. 50:890–896. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Volti G, Musumeci T, Pignatello R,
Murabito P, Barbagallo I, Carbone C, Gullo A and Puglisi G:
Antioxidant potential of different melatonin-loaded nanomedicines
in an experimental model of sepsis. Exp Biol Med (Maywood).
237:670–677. 2012. View Article : Google Scholar
|
|
25
|
Li Volti G, Salomone S, Sorrenti V,
Mangiameli A, Urso V, Siarkos I, Galvano F and Salamone F: Effect
of silibinin on endothelial dysfunction and ADMA levels in obese
diabetic mice. Cardiovasc Diabetol. 10:622011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rani V, Deep G, Singh RK and Palle K:
Pathogenesis and therapeutic strategies. Life Sci. 148:183–193.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niki E: Biomarkers of lipid peroxidation
in clinical material. Biochim Biophys Acta. 1840:809–817. 2014.
View Article : Google Scholar
|
|
28
|
Rossi P, Riutta A, Kuukasjärvi P, Vehmas
T, Mucha I and Salenius JP: Revascularization decreases
8-isoprostaglandin F2alpha excretion in chronic lower limb
ischemia. Prostaglandins Leukot Essent Fatty Acids. 71:97–101.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang Y, Wei P, Duke RW, Reaven PD, Harman
SM, Cutler RG and Heward CB: Quantification of
8-iso-prostaglandin-F(2alpha) and 2,3-dinor-8-iso-prostaglandin
F(2alpha) in human urine using liquid chromatography-tandem mass
spectrometry. Free Radic Biol Med. 34:409–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho DY, Cook NR, Britton KA, Kim E, Creager
MA, Ridker PM and Pradhan AD: High-molecular-weight and total
adiponectin levels and incident symptomatic peripheral artery
disease in women: A prospective investigation. Circulation.
124:2303–2311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato T, Saito Y, Inoue S, Shimosato T,
Takagi S, Kaneko T and Ishigatsubo Y: Serum heme oxygenase-1 as a
marker of lung function decline in patients with chronic silicosis.
J Occup Environ Med. 54:1461–1466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Q, Weis S, Yang G, Weng YH, Helston R,
Rish K, Smith A, Bordner J, Polte T, Gaunitz F and Dennery PA: Heme
oxygenase-1 protein localizes to the nucleus and activates
transcription factors important in oxidative stress. J Biol Chem.
282:20621–20633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abraham NG, Rezzani R, Rodella L, Kruger
A, Taller D, Li Volti G, Goodman AI and Kappas A: Overexpression of
human heme oxygenase-1 attenuates endothelial cell sloughing in
experimental diabetes. Am J Physiol Heart Circ Physiol.
287:H2468–H2477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jais A, Einwallner E, Sharif O, Gossens K,
Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard
K, et al: Heme oxygenase-1 drives metaflammation and insulin
resistance in mouse and man. Cell. 158:25–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang JY, Chiang MT, Yet SF and Chau LY:
Myeloid heme oxygenase-1 haploinsufficiency reduces high fat
diet-induced insulin resistance by affecting adipose macrophage
infiltration in mice. PLoS One. 7:e386262012. View Article : Google Scholar : PubMed/NCBI
|