Introduction
Severe acute pancreatitis (SAP) is a common acute
abdominal disease, which can lead to early mortality of patients
due to associated systemic inflammatory response syndrome (SIRS)
and multiple organ dysfunction syndrome (MODS) (1). SAP-associated failure of the
gastrointestinal tract, which is characterized by endotoxin release
from the intestinal lumen, and translocation of enteric bacteria to
extraintestinal sites and systemic circulation, leads to increased
intestinal permeability and release of inflammatory mediators and
cytokines, which has a pivotal role during SAP. Bacteremia,
infected necrosis, organ failure and mortality are all associated
with intestinal barrier dysfunction in the early stages of SAP
(2).
The mechanisms underlying SAP-induced intestinal
mucosal injury have yet to be fully elucidated. Previous studies
have suggested that intestinal mucosal injury is a complex
pathophysiological process that involves several factors, including
inflammatory mediators, oxidative stress and intestinal
hypoperfusion (3,4). Therefore, it is clinically imperative
to study the association between injury of the intestinal mucosal
barrier and SAP. In addition, microcirculation disturbance,
ischemic reperfusion injury, excessive release of inflammatory
mediators and apoptosis may also have important roles in damaging
the intestinal mucosal barrier (5). Microcirculation disturbance results
in gut barrier dysfunction via the production of reactive oxygen
species (ROS) by xanthine oxidase (XO), and hypoxanthine
accumulation in intestinal tissue (6). It has previously been reported that
increased ROS generation and a dysregulated antioxidant defense
system in intestinal mucosal cells is involved in intestinal
mucosal dysfunction in rats with SAP (6).
Nicotinamide adenine dinucleotide phosphate (NADPH)
oxidase (NOX) is considered a potential source of oxidants in the
injured intestine in SAP. NOX produces a large amount of ROS
predominantly in professional phagocytes, such as neutrophils and
macrophages (7). NOX is a
multicomponent enzyme; the classical phagocytic NOX is comprised of
the membrane-bound subunits gp22phox and gp91phox (also termed
NOX2), as well as the cytosolic subunits, including gp67phox and
gp47phox (7). NOX has a crucial
role in various biological processes, including host defense,
signal transduction, mitogenic growth, hormone synthesis,
apoptosis, angiogenesis, and oxidative modification of the
extracellular matrix and extracellular proteins (8). In addition, NOX is involved in
various types of disease, including atherosclerosis, cancer,
diabetes, defective immune function and inflammatory conditions,
such as inflammatory bowel disease (8). Previous studies have demonstrated
that NOX is involved in mitogen-activated protein kinases (MAPK),
phospholipase A2 and nuclear factor (NF)-κB activation, and
cytokine expression, and therefore, pathogenesis of pancreatitis
(9,10). Experimental studies have suggested
that the source of ROS is NOX in stimulated neutrophils in cerulein
pancreatitis in vivo (11).
Therefore, treatments designed to modulate the production of ROS by
NOX enzymes may provide a novel therapeutic approach for the
treatment of some of these conditions.
Apocynin is a selective NOX inhibitor, which
exhibits low toxicity, and may therefore be considered a promising
potential therapy for asthma, arthritis, and neurological and
cardiovascular diseases via its antioxidant and anti-inflammatory
effects. In addition, apocynin has been used in several
experimental studies associated with ischemic reperfusion injury
(12,13).
At present, the protective effects of apocynin on
the intestinal mucosal barrier in rats with SAP have yet to be
investigated. The present study hypothesized that NOX is involved
in the pathogenesis of SAP-associated acute intestinal injury, and
aimed to evaluate the effects of the NOX inhibitor apocynin on
SAP-associated intestinal mucosal injury. The investigation of the
effects of apocynin on SAP-associated intestinal injury may provide
a novel basis for the treatment of SAP.
Materials and methods
Animals
A total of 60 male adult Sprague Dawley rats (age,
7–8 weeks; weight, 200–250 g) were obtained from Hubei Experimental
Animal Center (Wuhan, China). The rats were housed in a
climate-controlled room with an ambient temperature of 23°C and
were maintained under a 12:12 h light-dark cycle. The rats were fed
standard laboratory chow, given ad libitum access to water,
and were randomly assigned to four groups (n=15/group): Sham
operation group (SO), SAP group, apocynin treatment (APO) group and
drug control (APO-CON) group. All animal study procedures complied
with international guidelines for the care and use of laboratory
animals, and were approved by the Animal Ethics Committee of Wuhan
University (Wuhan, China).
SAP induction and sample collection
The rats were fasted 12 h prior to the experiment,
however drinking water remained available. The SAP model was
induced by a standardized pressure-controlled retrograde infusion
of 5% sodium taurocholate (1 ml/kg) into the biliopancreatic duct.
In the SO and APO-CON groups, an incision was made in the abdomen
of the rats under chloral hydrate (10%, 30 mg/kg; Aoxin Chemical
Factory, Yangzhou, China) anesthesia and was subsequently closed.
Following the operation, all rats received subcutaneous infusion of
sterile saline (2 ml/kg) to compensate for anticipated fluid loss.
In the APO group, 10% dimethyl sulfoxide (DMSO) containing apocynin
(50 mg/kg; Selleck Chemicals, Houston, TX, USA) was injected very
slowly through the femoral vein 30 min prior to SAP induction. In
the SO and SAP groups, 10% DMSO solution (2 ml/kg) was administered
via femoral vein 30 min prior to the operation. In the APO-CON
group, 10% DMSO containing apocynin (50 mg/kg) was injected very
slowly through the femoral vein 30 min prior to the sham
operation.
A total of 12 h after SAP induction, rats from each
group were anesthetized with 10% chloral hydrate (30 mg/kg), the
abdomen was opened, and pancreatic and ileum tissues adjacent to
the cecum were rapidly collected. All rats were sacrificed by the
collection of blood samples (5 ml) were from the left ventricle of
the heart. The samples were centrifuged at 300 × g for 15 min at
4°C, and the serum was stored at −20°C until further analysis. Half
of each pancreatic and ileal sample was fixed with 40 g/l
formaldehyde, embedded in paraffin and cut into 5 mm sections. The
other half was stored at −80°C for further use.
Biochemical assays
The serum levels of amylase (AMY) and lipase (LIP)
were measured according to the manufacturer's instructions, using
an automatic biochemistry analyzer (model AU600; Olympus
Corporation, Tokyo, Japan). Serum tumor necrosis factor (TNF)-α
(cat. no. BP-E40017), interleukin (IL)-6 (cat. no. BP-E30419) and
IL-1β (cat. no. BP-E30419) levels were determined using
enzyme-linked immunosorbent assay (ELISA) kits (Biomart, Co.,
Toronto, Canada) according to the manufacturer's protocols. For
diamine oxidase (DAO) detection, a rat DAO ELISA kit (cat. no.
RA20156; Shanghai DaWeiKe Biotechnology, Shanghai, China) was used
according to the manufacturer's protocol. Tissue malondialdehyde
(MDA), a marker of lipid peroxidation, and superoxide dismutase
(SOD) levels were measured using commercial MDA (cat. no. A003-1)
and SOD (cat. no. A001-1) assay kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions.
Histopathological evaluation
Paraffin-embedded samples from the pancreas and
ileum were sectioned (5 µm), stained with hematoxylin and
eosin for 10 min at room temperature, and examined by an
experienced pathologist who was blinded to the sample identity.
Histopathological alterations to the pancreas were evaluated
according to the criteria proposed by Schmidt et al
(14). The histological grade of
intestinal mucosal damage was scored according to the standard
scale described by Chiu et al (15). Briefly, mucosal damage was graded
from 0 to 5 according to the following criteria: Grade 0, normal
mucosal villi; grade 1, development of subepithelial Gruenhagen's
space at the apex of the villus, often with capillary congestion;
grade 2, extension of the subepithelial space with moderate lifting
of the epithelial layer from the lamina propria; grade 3, massive
epithelial lifting down the sides of villi, possibly with a few
denuded tips; grade 4, denuded villi with the lamina propria and
dilated capillaries exposed, possibly with increased cellularity of
the lamina propria; and grade 5, digestion and disintegration of
the lamina propria, hemorrhage and ulceration.
Immunohistochemistry
NOX2, p38 MAPK and NF-κB protein expression was
detected in the intestinal mucosa by immunohistochemistry. The
paraffin-embedded ileal tissue sections were dewaxed according to
standard procedures (16).
Subsequently, the sections were dipped in a solution containing
0.01-mol/l citric acid for 1 min at 95°C, blocked with 5% bovine
serum (Jingmei BioTech Co., Ltd., Yencheng, China) for 30 min, and
were then incubated with rabbit anti-p38 (1:50; cat. no. sc-535),
anti-phosphorylated (p)-P38 (1:50; cat. no. sc-101759), anti-NF-κB
(1:50; cat. no. sc-8008) and anti-NOX2 (1:100; cat. no. sc-6706)
polyclonal antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) overnight at 4°C. Sections were then incubated with
biotin-labeled goat anti-rabbit immunoglobulin G (1:200; cat. no.
sc-2012; Santa Cruz Biotechnology, Inc.) for 15 min at 37°C, and
horseradish peroxidase-labeled streptavidin (Wuhan Feiyi Technology
Co., Ltd., Wuhan, China) for 30 min at 37°C. Finally, the specimens
were stained with diaminobenzidine, nuclear counter-stained with
hematoxylin, and images were captured under a Leica DM 2500
microscope (Leica Microsystems GmbH, Wetzlar, Germany). Brown
staining area represents cells that contain p38, p-p38, NF-κB and
NOX2. The positively stained cells were observed under a light
microscope (magnification, 400x) and were evaluated by two
pathologists in a blind manner.
Immunohistochemical staining was analyzed using
Image Pro-Plus (version 6.0; Media Cybernetics, Inc., Rockville,
MD, USA). Briefly, the positive staining area was selected as the
area of interest (AOI), and the area sum and integrated optical
density (IOD) of the AOI were selected as measurement parameters.
The target protein expression index equaled the quotient between
the IOD and the total AOI. Finally, statistical analysis of the
mean expression index for each duplicate was performed.
Transmission electron microscopy
(TEM)
Fresh ileal tissue samples were fixed in a mixture
of 2% formaldehyde and 2% glutaraldehyde in 0.1 mol/l cacodylate
buffer (pH 7.4) overnight. The samples were postfixed in 1%
OsO4 in the same buffer, en bloc-stained with 2%
aqueous uranyl acetate, dehydrated in ethanol, and embedded in
Poly/Bed 812 (Polysciences, Inc., Warrington, PA, USA). Ultrathin
sections (50 nm) were cut on a Leica EMUC7 ultramicrotome (Leica
Microsystems GmbH), stained with lead citrate, and alterations to
the intestinal epithelial cells and microvilli were examined under
a JEM-2010F electron microscope.
Statistical analysis
Statistical analyses were carried out using SPSS
statistical software (PASW Statistics for Windows, version 18.0;
SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ±
standard deviation. One-way analysis of variance and
least-significant difference test was used to investigate
differences among the experimental groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of NOX inhibition on pancreatic
injury and intestinal mucosal dysfunction in SAP
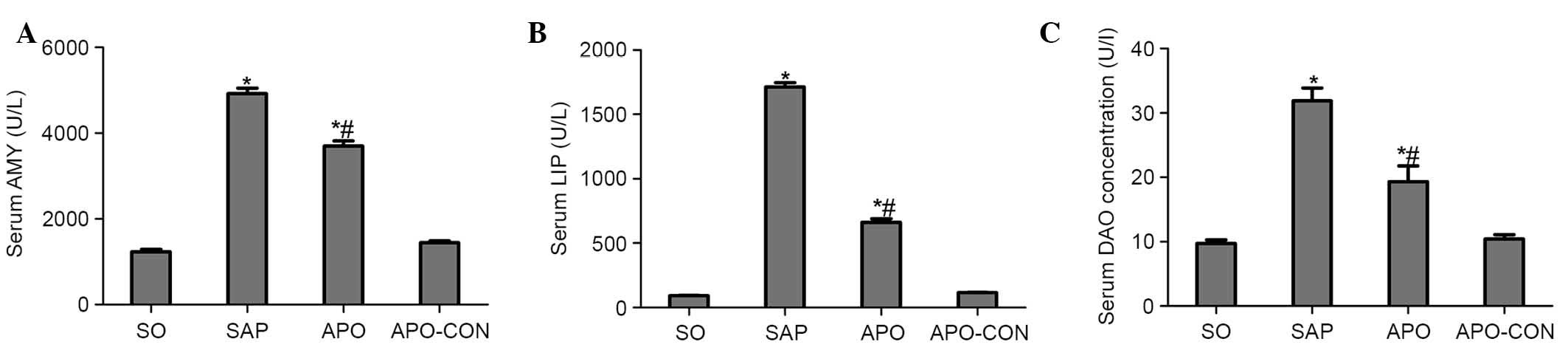
As shown in Fig. 1,
significant hyperamylasemia and hyperlipasemia developed following
induction of SAP, thus suggesting that the SAP model was
successfully induced. Treatment with apocynin reduced AMY and LIP
levels in the APO group compared with the SAP group (P<0.05;
Fig. 1A and B). In addition, rats
subjected to SAP exhibited a significant increase in DAO levels
(P<0.05), indicating that the rats experienced aggravated
intestinal dysfunction. Apocynin treatment resulted in a
significant decrease in the serum levels of DAO 12 h after SAP
induction (P<0.05; Fig.
1C).
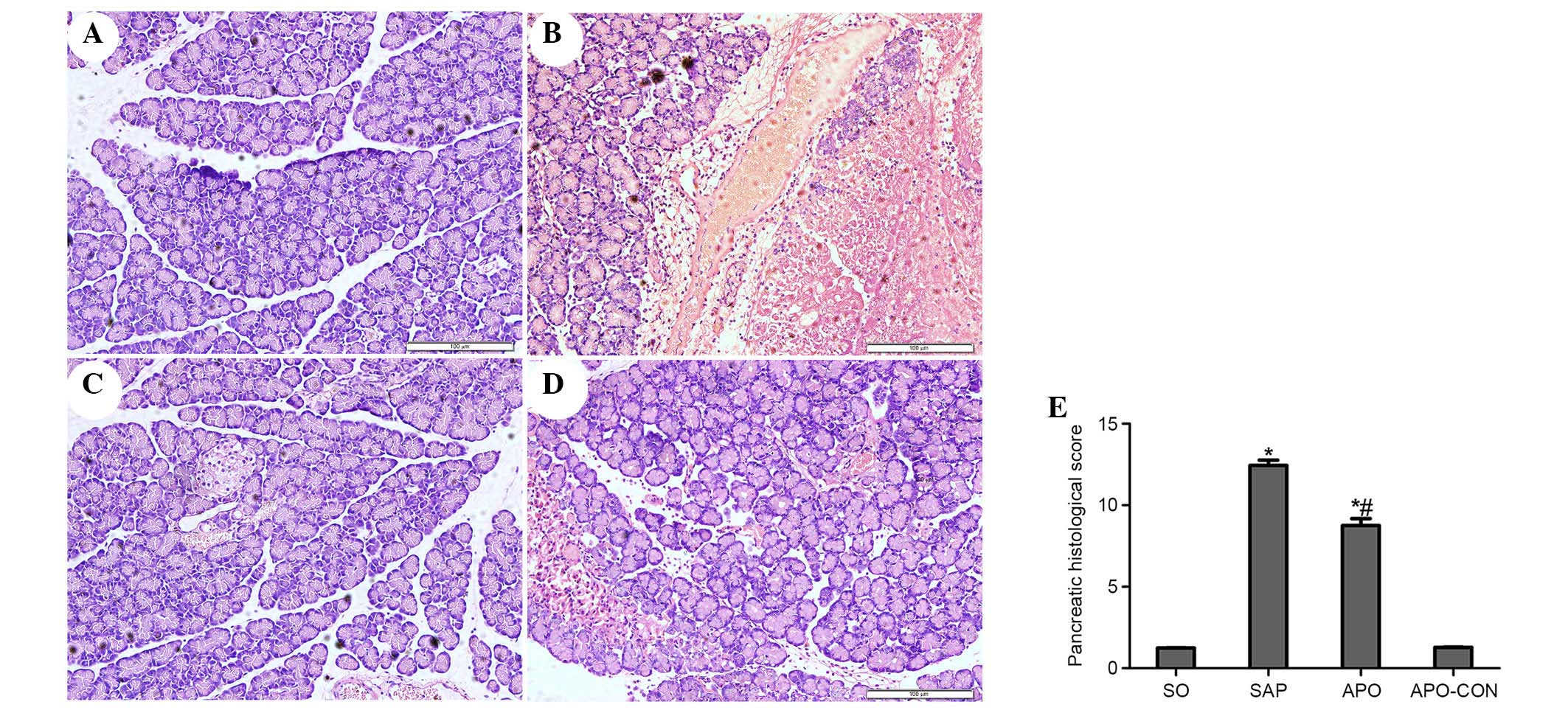
Inhibition of NOX attenuates pancreatic
and intestinal histopathology
Representative histological sections are presented
in Figs. 2 (pancreas) and 3
(intestinal tissue). No morphological alterations were observed in
the SO and APO-CON groups. Interstitial edema, acinar cell necrosis
and infiltration of inflammatory cells were observed in the SAP
group (Fig. 2). Treatment with APO
markedly decreased pancreatic histological injuries (P<0.05;
Fig. 2). The SO and APO-CON groups
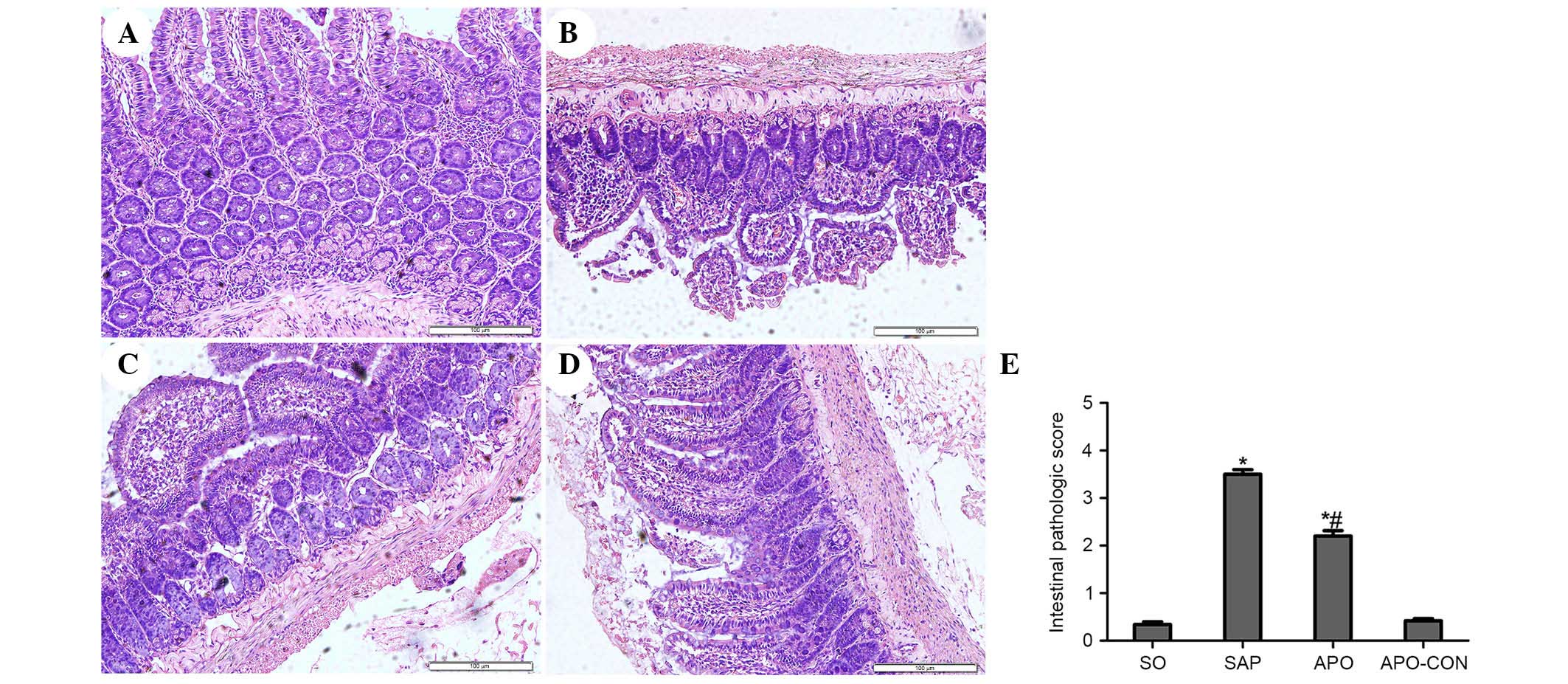
exhibited normal histological structure of intestinal mucosal
tissue (Fig. 3). Conversely,
characteristic signs of intestinal injuries, including edema,
villose exfoliation, degeneration of mucosal cells, mucosal cell
necrosis, bleeding, and leukocytic infiltration were observed in
the SAP group (Fig. 3). Intestinal
issues obtained from the rats treated with apocynin were shown to
have milder histological features and lower pathological scores
compared with the SAP group (P<0.05) (Fig. 3).
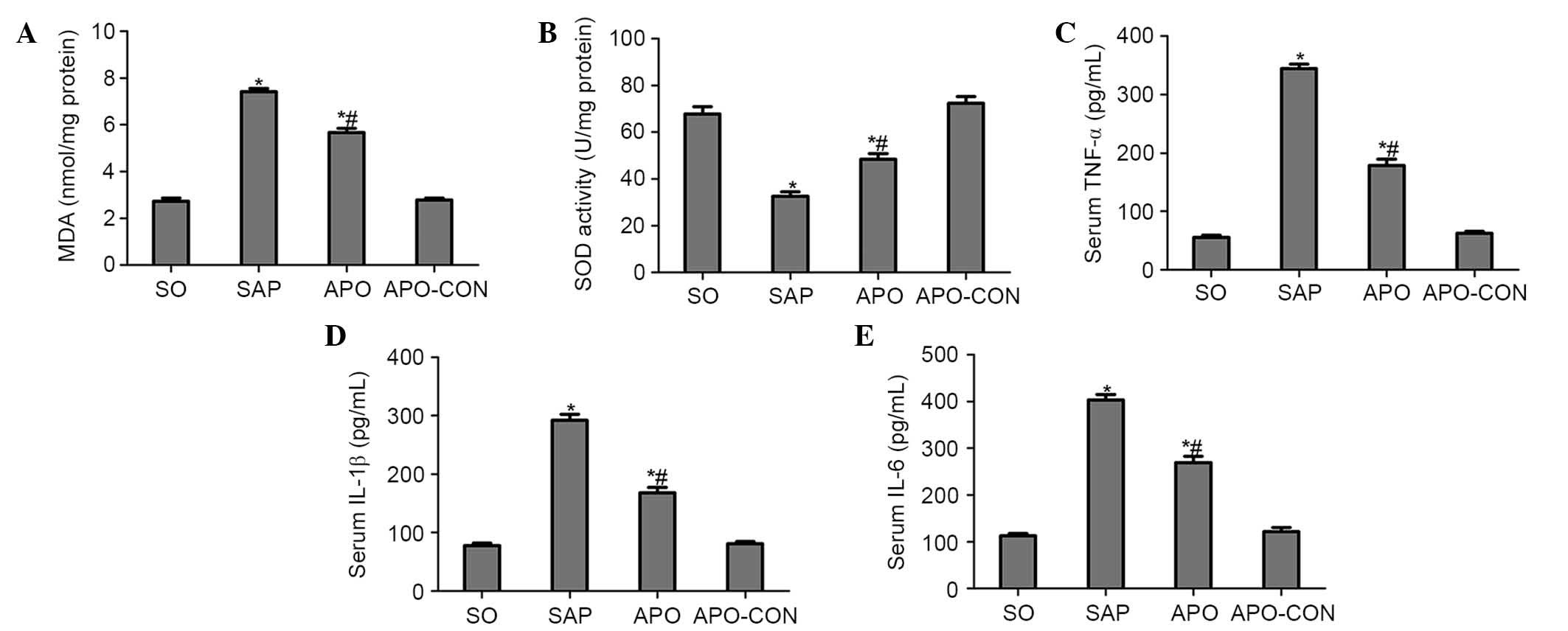
Apocynin treatment reduces oxidative
stress and production of proinflammatory cytokines
Intestinal tissue MDA levels were significantly
elevated in the SAP group compared with in the SO group (P<0.05;
Fig. 4A). Conversely, the
elevation was significantly inhibited by apocynin treatment
(P<0.05; Fig. 4A). SOD activity
was significantly depleted in the SAP group, probably as a result
of oxidative stress. Conversely, treatment with apocynin
effectively improved SOD activity in intestinal tissues (P<0.05;
Fig. 4B).
Serum concentrations of proinflammatory cytokines
were analyzed in order to evaluate the effects of NOX inhibition on
inflammatory process following SAP. As presented in Fig. 4, concomitant with the pathological
damage incited by SAP, serum levels of TNF-α, IL-1β and IL-6 were
significantly increased compared with in the SO group (P<0.05;
Fig. 4C–E). However, the increased
levels of these cytokines following SAP induction were markedly
decreased by the pharmacological blockade of NOX, using the NOX
inhibitor apocynin (P<0.05; Fig.
4C–E).
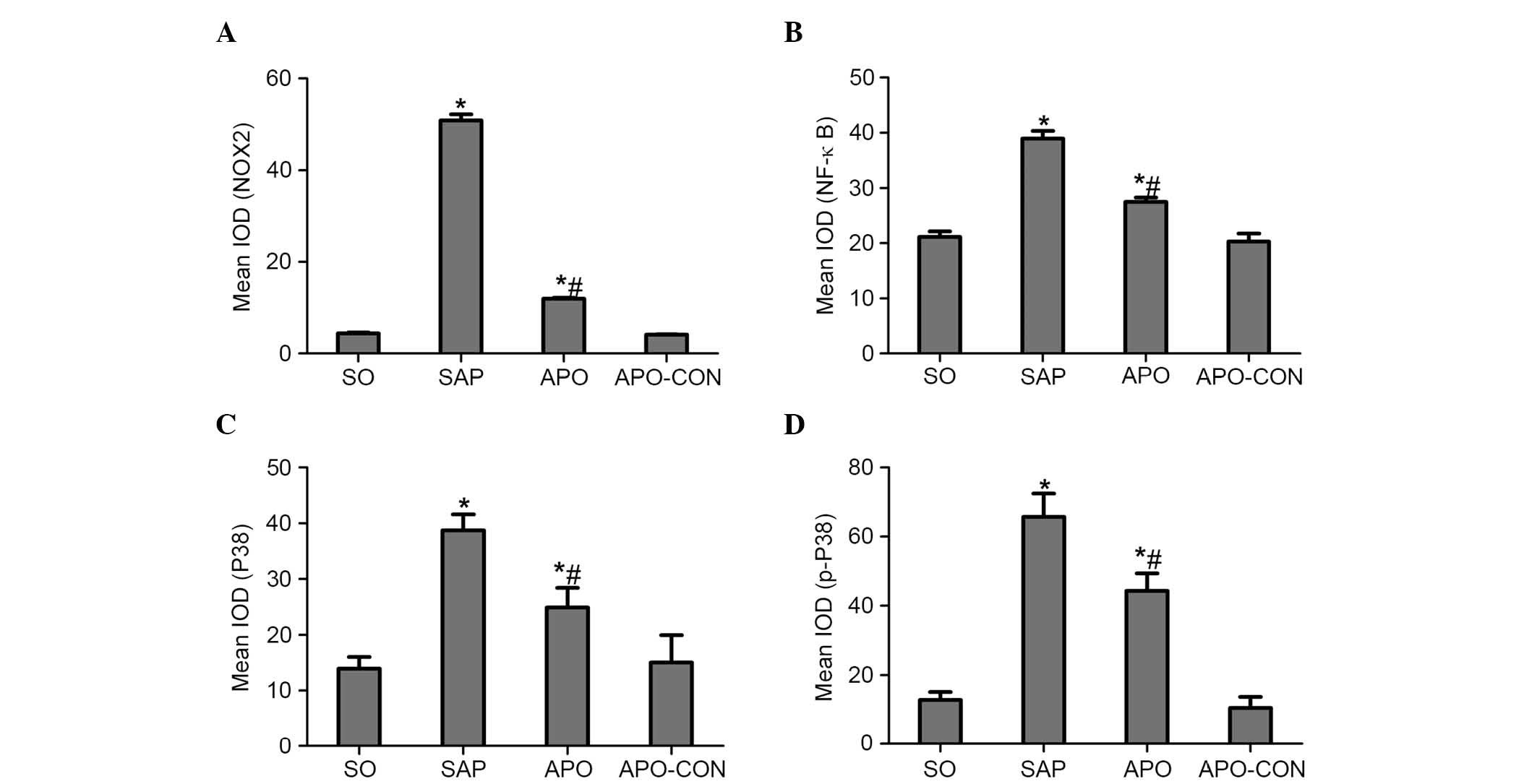
Inhibition of NOX reduces the expression
of p38 MAPK and NF-κB in intestinal mucosa after SAP
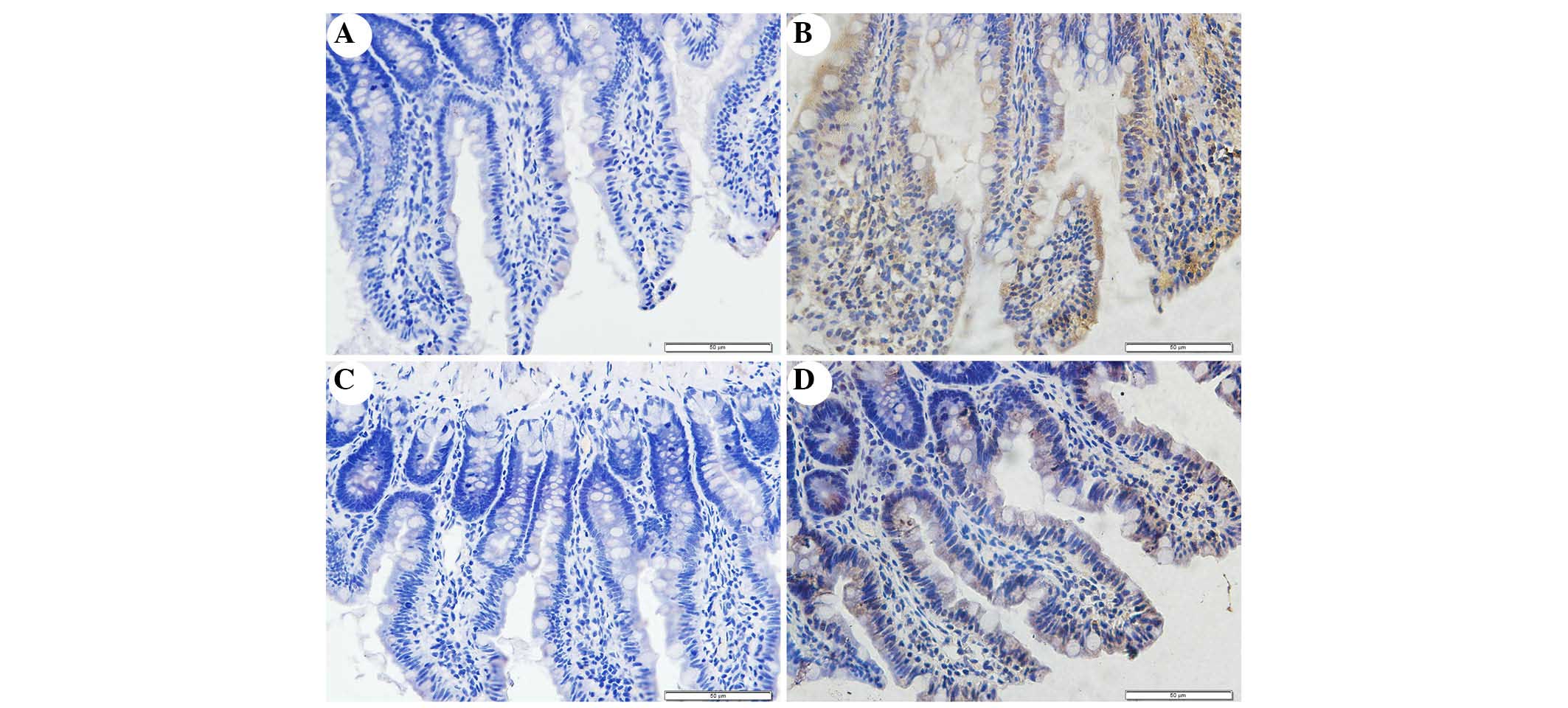
Immunohistochemical staining of NOX2 was used to
evaluate whether apocynin was able to successfully attenuate
SAP-induced NOX2 expression in intestinal tissue (Fig. 5). Intestinal tissue sections from
the SO and APO-CON rats exhibited little NOX2 expression (Fig. 5A and C). However, NOX2
immunoreactivity was enhanced in SAP rats (Fig. 5B) and was reduced in the intestinal
tissue of rats that had undergone apocynin pretreatment (Fig. 5D).
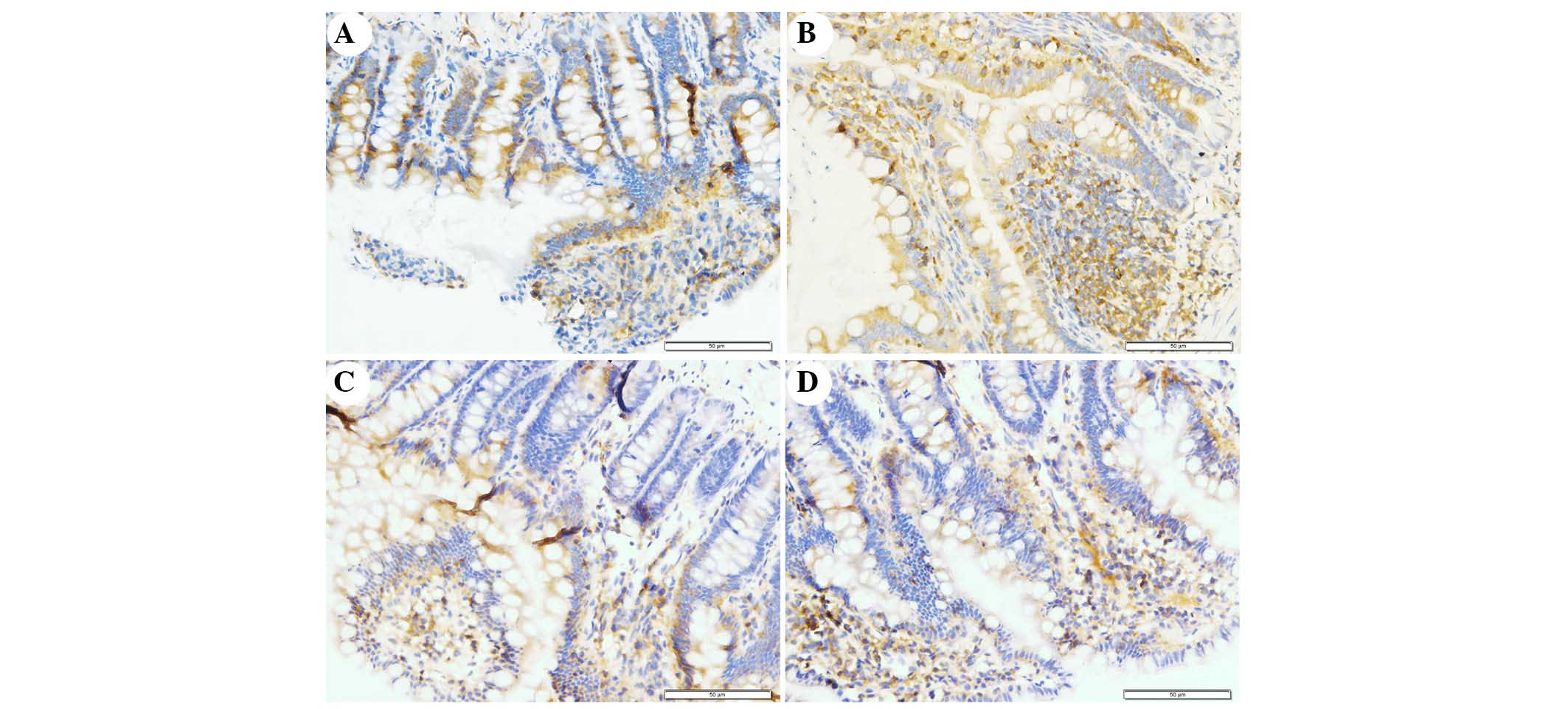
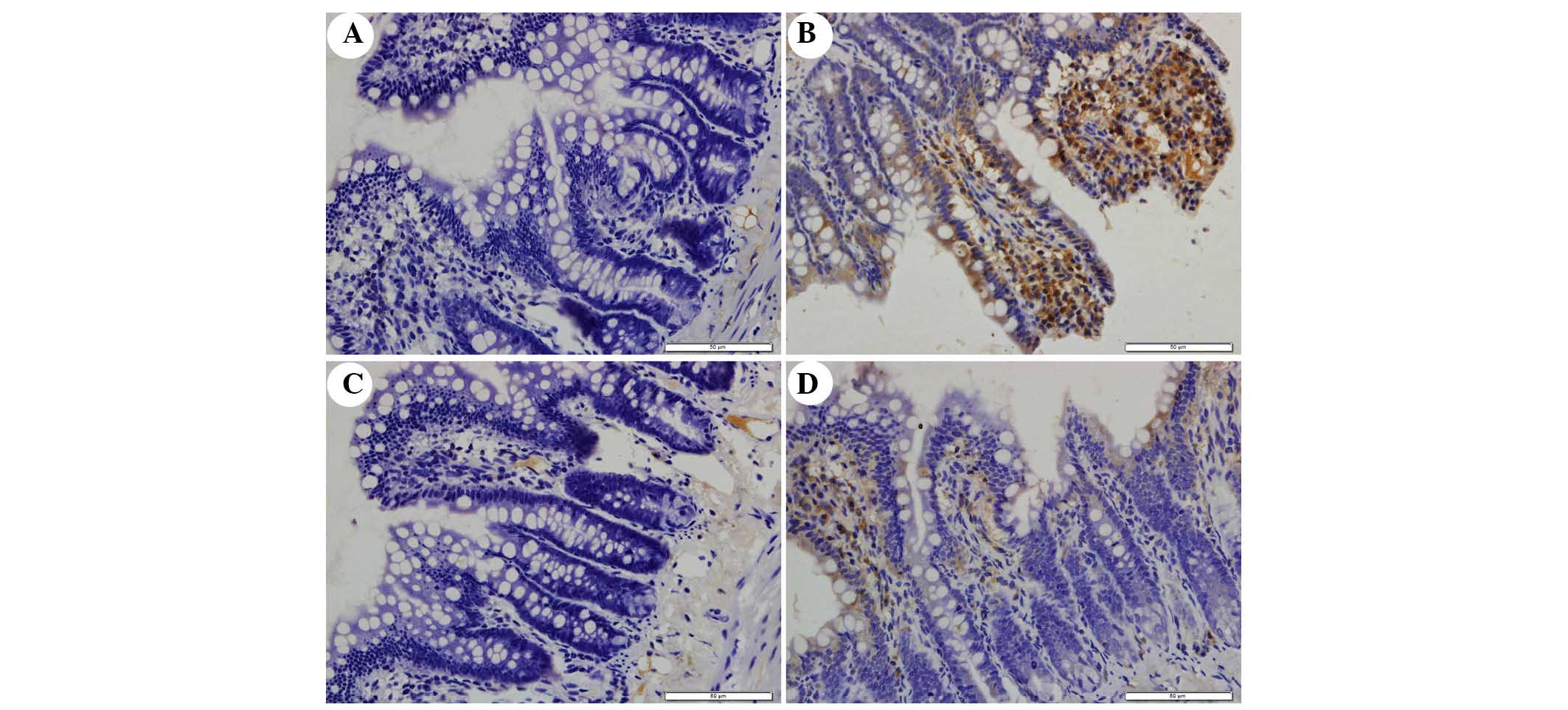
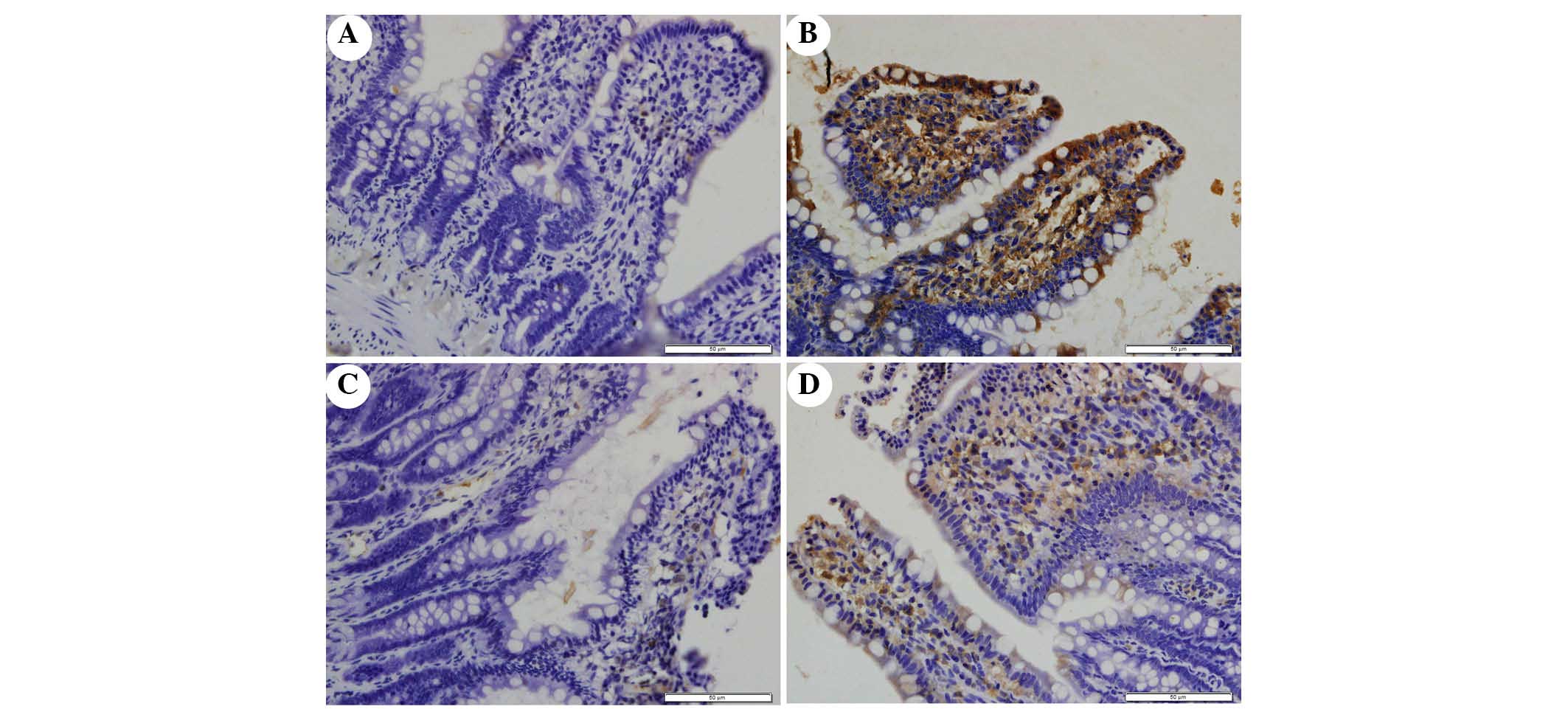
The present study determined the efficacy of
apocynin treatment on NF-κB, p38 MAPK and p-p38 MAPK expression in
intestinal mucosa using immunohistochemical assays (Figs. 6Figure 7–8). In the SO group, weak NF-κB expression
was detected in the cytoplasm (Fig.
6A), and the expression of p38 MAPK and p-p38 MAPK in the
cytoplasm was exceedingly weak (Figs.
7A and 8A). In the SAP group,
intense NF-κB p65 immunoreactivity was expressed in the nucleus
(Fig. 6B). Intestinal expression
of p38 MAPK and p-p38 MAPK was significantly increased in the
cytoplasm of the SAP group compared with in the SO group (Figs. 7B and 8B; P<0.05). In addition, inhibition of
NOX with apocynin significantly decreased the nuclear expression of
NF-κB and cytoplasmic expression of p38 MAPK in intestinal tissues
(Fig. 9; P<0.05).
Ultrastructural alterations to intestinal
mucosal cells, as detected under TEM
TEM analysis of intestinal mucosa demonstrated that
integrity of the intestinal villi and intestinal epithelial cells
(IECs) of the rats in the SO group was maintained throughout the
experiment. The mitochondria, endoplasmic reticulum, ribosomes and
other cellular organelles were normal following examination at a
magnification of ×20,000 (Fig. 10A
and B). The microvilli and IECs in the SAP group exhibited a
loss of integrity. Microvillus atrophy, enlarged cytoplasmic
vesicles, including endoplasmic reticulum and mitochondria, were
characteristics observed at a magnification of ×2,500 (Fig. 10C). The integrity of the
microvilli and IECs of rats in the APO group was significantly
improved (Fig. 10D). The
ultrastructural damage to mitochondria, endoplasmic reticulum,
ribosomes and other organelles was ameliorated by apocynin
treatment (Fig. 10D).
Ultrastructural changes in the APO-CON group were similar to the SO
group.
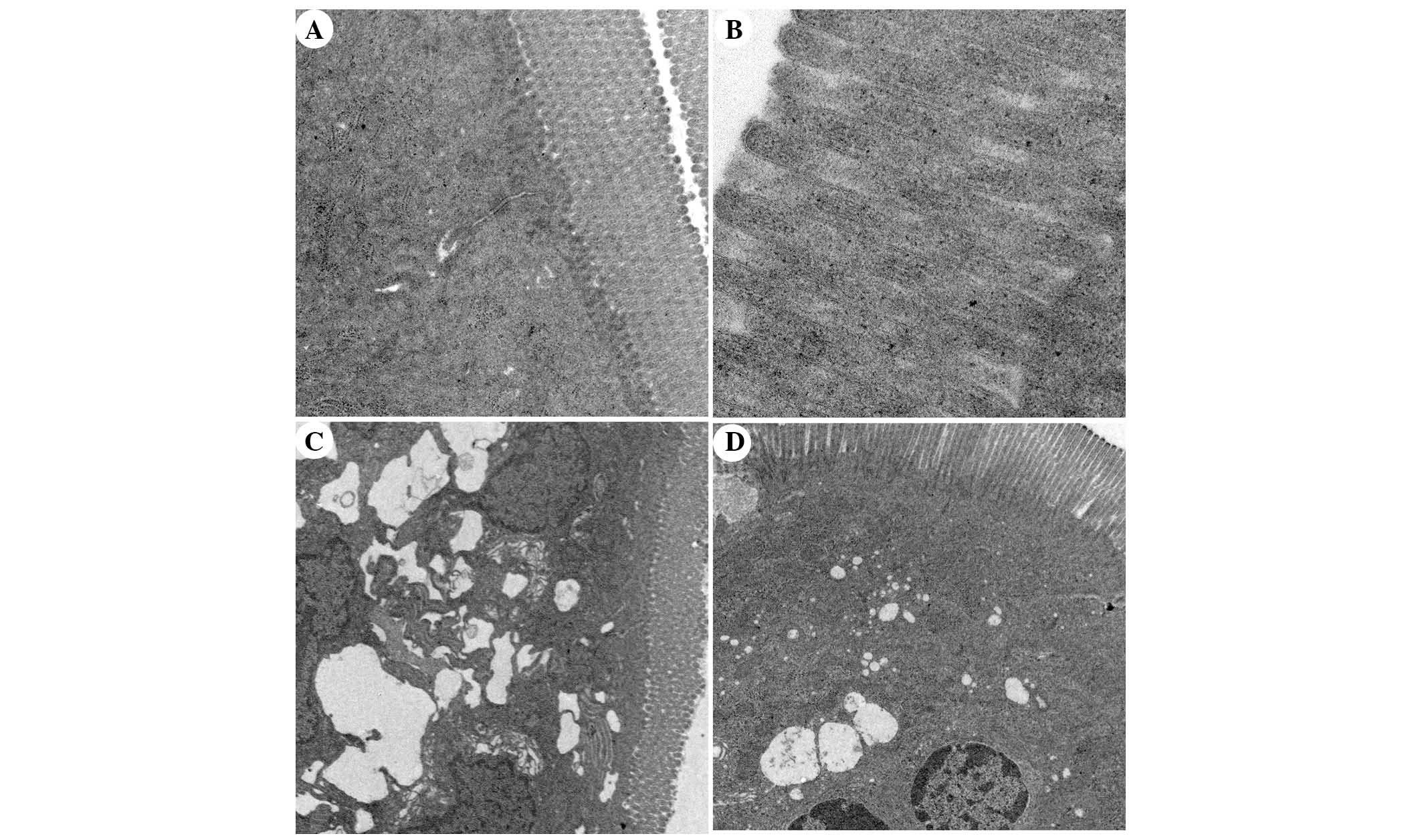 | Figure 10Transmission electron microscopic
comparison of intestinal epithelial architecture. (A) Intestinal
epithelium of the sham operation (SO) group exhibited columnar
intestinal epithelial cells (IECs) and long microvilli. Cytoplasmic
organelles were normal (magnification, ×5,000). (B) The integrity
of microvilli in the SO group was maintained and microvilli were
long (magnification, ×20,000). (C) In the severe acute pancreatitis
(SAP) group, IECs and microvilli were not intact, microvilli were
absent and became shorter in various locations. The mitochondria
and endoplasmic reticulum were grossly expanded and ribosome
numbers were significantly increased. The nuclear double membrane
appeared irregular in shape (magnification, ×2,500). (D) In the
apocynin group, IECs were intact and microvilli became longer than
in the SAP group. Enlarged mitochondria, endoplasmic reticulum and
ribosomes were significantly decreased compared with the SAP group
(magnification, ×2,500). |
Discussion
NOX is a ROS-generating transmembrane flavoprotein
enzyme, which has a key role in innate immunity, mitogenic
signaling, hormone synthesis, apoptosis, angiogenesis, and
oxidative modification of the extracellular matrix and
extracellular proteins (8). The
involvement of NOX in the pathophysiology of cerulean-induced
pancreatitis has been indicated by increased NOX activity in AR42 J
cells 15 min after induction of experimental pancreatitis,
accompanied by NF-κB activation and IL-6 expression (10). In addition, the involvement of NOX
has been studied in several models of acute pancreatitis and has
been recognized to contribute to its progression (7,16–18).
Pancreatitis-induced release of inflammatory factors, cytokines and
growth factors may be responsible for local and distant organ NOX
activation (19), thus resulting
in further development of acute pancreatitis. It has previously
been reported that apocynin treatment reduces serum levels of
TNF-α, IL-1β and NF-κB, and intercellular adhesion molecule 1
expression, and decreases the severity of acute lung inflammation
(20). Apocynin has also been used
in several experimental studies, including those associated with
liver injury (21), lung injury
(22), kidney injury (23), brain injury (24) and ischemic reperfusion injury
(25,26). The results of the present study
further supported the beneficial effects of treatment with the NOX
antagonist apocynin, since the severity of pancreatitis-associated
intestinal barrier dysfunction was ameliorated following apocynin
treatment.
Pancreatitis-associated gut barrier dysfunction is
characterized by the passage of enteric bacteria through the
mucosal barrier to extraintestinal sites (bacterial translocation),
impaired intestinal motility, and increased intestinal permeability
(27). Destruction of the
intestinal mucosal mechanical barrier also results in the release
of a large amount of DAO enzymes and active substances (28). Previous studies have reported that
intestinal barrier injury may lead to SIRS and MODS (29,30).
DAO is an effective biomarker, which reflects the integrity and
mucosal function of the small intestine. The concentration of DAO
in the serum and mucosa of the small intestine may be determined as
a measure of small intestine barrier function. In the present
study, TEM demonstrated that microvilli of the intestinal mucosa
exhibited reduced width and height, tight junctions were damaged
and DAO levels were increased in rats in the SAP group. These
parameters were significantly reduced following apocynin
treatment.
The present study demonstrated that SAP was
successfully induced in the rats after 12 h of modeling, as
characterized by increased serum levels of AMY and LIP, pancreatic
tissue injury, and pathological alterations. The serum levels of
DAO combined with negative morphological alterations to the ileum
indicated the presence of obvious intestinal barrier dysfunction
and tissue injury during the progression of SAP. The results
demonstrated that pretreatment with apocynin attenuated the
following: i) Serum AMY and LIP levels; ii) serum DAO levels; iii)
morphological alterations to the pancreas and intestine; iv)
proinflammatory cytokine production; v) MDA and SOD content; vi)
NOX2, NF-κB and p38 MAPK expression; and vii) ultrastructural
alterations to the intestinal mucosa. All of these observations
suggested that NOX may participate in the process of intestinal
barrier damage in SAP, and treatment with a NOX inhibitor exerts
potent anti-inflammatory and antioxidative effects, and ameliorates
the degree of SAP and associated intestinal barrier injury in
rats.
It has been demonstrated that ROS generated by NOX
have prominent roles in the pathogenesis of acute pancreatitis
(31). Under physiological
conditions, tissues contain various endogenous antioxidant enzymes,
including glutathione and SOD, which scavenge ROS and prevent lipid
peroxidation (32). In the process
of SAP, ROS are overproduced, thus inducing an imbalance between
ROS and endogenous antioxidants or antioxidant enzymes. The
overproduced ROS are able to directly or indirectly damage
intestinal tissues, resulting in intestinal barrier dysfunction and
histological alterations. The results of the present study
demonstrated that SOD activity was significantly increased
following apocynin treatment of SAP rats. Conversely, MDA levels
were decreased. These results, combined with the intestinal
morphological alterations detected, indicated that apocynin may
reduce ROS levels by inhibiting NOX, further ameliorating
intestinal oxidative damage.
NOX is a major contributor during inflammation, and
has a role as an inflammatory stimulator, activating the leukocyte
system, which can then produce and release various secondary
inflammatory mediators. It has previously been demonstrated that
apocynin significantly inhibits the expression levels of TNF-α and
IL-1β, which are potent triggers associated with leukocyte
migration, and that suppression of NOX activity by apocynin results
in attenuation of ROS-mediated signal transduction and inducible
nitric oxide synthase expression in lipopolysaccharide + interferon
γ-stimulated microvascular endothelial cells (20). In addition, evidence suggests that
inflammatory cell infiltration, including infiltration of TNF-α,
IL-1β and IL-6, is an early and vital event in acute pancreatitis,
which leads to local and systemic complications (19). Data from the present study
demonstrated that these cytokines were increased following
induction of SAP; however, their serum levels were reduced in rats
treated with apocynin. In addition, in the SAP group, intestinal
barrier dysfunction was correlated with increased intestinal
injury, as confirmed using histological analysis. In the APO group,
milder swelling, necrosis and loss of mucosal structure, and
reduced inflammatory cell infiltration was detected compared with
in the SAP group. Pathological grading of intestinal injury was
significantly decreased in the SAP group pretreated with APO.
ROS are able to induce cytokine expression,
apoptosis, and NF-κB, MAPK and Janus kinase/signal transducer and
activator of transcription pathway activation, thus regulating
inflammation and apoptosis in pancreatic acinar cells (19). Consequently, NOX, NF-κB and MAPK
may be involved in the pathogenesis of acute pancreatitis (33). NF-κB is a transcription factor that
is necessary for the transcription of numerous proinflammatory
mediators, including cytokines, chemokines, and oxygen-derived free
radicals. In quiescent cells, NF-κB is present in the cytosol where
it is complexed with IκB. The phosphorylation of IκB on serines
within the amino-terminal domain results in the dissociation of
NF-κB and its subsequent translocation to the nucleus, consequently
initiating gene transcription (34). Translocation of NF-κB to the
nucleus mediates the expression of cytokines, which has a major
role in the pathogenesis of pancreatitis (33). It has previously been reported that
inhibition of NF-κB activation efficiently suppresses IL-6
expression and attenuates the severity of cerulean-induced
pancreatitis in AR42J cells (10).
The present study demonstrated that the translocation of activated
NF-κB into the nucleus was significantly increased in the
intestinal tissues of SAP rat. However, following treatment with
apocynin, the nuclear expression of NF-κB was decreased. These
results suggested that the activation of NF-κB could be inhibited
by apocynin via NOX suppression.
P38 MAPK is serine-threonine kinase, which mediates
intracellular signaling associated with several cellular
activities, including cell proliferation, differentiation,
survival, death and transformation (35). Recently, it has been revealed that
p38 MAPK may be involved in the NOX signal transduction pathway.
ROS mediate activation of the p38 MAPK pathway and promote protein
phosphorylation in pancreatic acinar cells (19). A previous study reported that p38
MAPK is associated with the severity of pancreatitis and in the
respiratory distress syndrome associated with acute pancreatitis
(34). Another study using
pancreatic fragments stimulated by the cholecystokinin analog,
cerulein, demonstrated that proinflammatory mediator production was
increased in parallel with the activation of p38 MAPK, and that
these alterations were attenuated by treatment with chemical
inhibitors of p38 MAPK (36).
During the progression of SAP, the p38 MAPK
signaling pathway is involved in the regulation of NF-κB
activation, which has a crucial role in the inflammatory cascade
(37,38). Activation of p38 MAPK results in
phosphorylation of MAPK-activated protein kinase 2, a downstream
protein kinase of p38 MAPK, which triggers NF-κB and exaggerates
inflammation (39). The present
study demonstrated that intestinal p38 and p-p38 expression in the
SAP group was markedly increased. Conversely, p38 expression was
suppressed by apocynin pretreatment, thus suggesting that p38 MAPK
may be involved in the pathogenesis of intestinal injury in SAP
rats. These results suggested that the NOX inhibitor apocynin may
inhibit the expressions of the inflammatory cytokines by
suppressing NF-κB activation and p38 expression. The results also
indicated that intestinal cells constitutively expressed the NOX
subunit NOX2.
In conclusion, SAP-associated intestinal barrier
dysfunction was aggravated in the SAP group. Treatment with
apocynin, a NOX inhibitor, reduced the severity of
pancreatitis-associated intestinal dysfunction, which was
associated with a reduction in the systemic concentration of
cytokines, reduced oxidative stress and downregulated NF-κB and p38
MAPK expression. Thus, apocynin treatment may ameliorate
SAP-induced intestinal barrier injury and these findings may
provide a basis for further clinical studies of apocynin as a novel
and adjuvant therapy to treat intestinal barrier injury with
SAP.
Acknowledgments
The present study was supported by the NSFC (grant
no. 81360081/H0320), the Natural Science Foundation of Hubei
Province (grant no. 2013CFB459), and the Independent Research
Project of Wuhan University (grant no. 2042015KF0090).
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ammori BJ: Role of the gut in the course
of severe acute pancreatitis. Pancreas. 26:122–129. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahman SH, Ammori BJ, Holmfield J, Larvin
M and McMahon MJ: Intestinal hypoperfusion contributes to gut
barrier failure in severe acute pancreatitis. J Gastrointest Surg.
7:26–35; discussion 35–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capurso G, Zerboni G, Signoretti M,
Valente R, Stigliano S, Piciucchi M and Delle Fave G: Role of the
gut barrier in acute pancreatitis. J Clin Gastroenterol.
46:S46–S51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XP, Zhang J, Song QL and Chen HQ:
Mechanism of acute pancreatitis complicated with injury of
intestinal mucosa barrier. J Zhejiang Univ Sci B. 8:888–895. 2007.
View Article : Google Scholar
|
|
6
|
Tian R, Tan JT, Wang RL, Xie H, Qian YB
and Yu KL: The role of intestinal mucosa oxidative stress in gut
barrier dysfunction of severe acute pancreatitis. Eur Rev Med
Pharmacol Sci. 17:349–355. 2013.PubMed/NCBI
|
|
7
|
Masamune A, Watanabe T, Kikuta K, Satoh K
and Shimosegawa T: NADPH oxidase plays a crucial role in the
activation of pancreatic stellate cells. Am J Physiol Gastrointest
Liver Physiol. 294:G99–G108. 2008. View Article : Google Scholar
|
|
8
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hancock JT, Desikan R and Neill SJ:
Hydrogen peroxide and nitric oxide in plant defence: Revealing
potential targets for oxidative stress tolerance? Biofactors.
15:99–101. 2001. View Article : Google Scholar
|
|
10
|
Yu JH, Lim JW, Kim H and Kim KH: NADPH
oxidase mediates interleukin-6 expression in cerulein-stimulated
pancreatic acinar cells. Int J Biochem Cell Biol. 37:1458–1469.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gukovskaya AS, Vaquero E, Zaninovic V,
Gorelick FS, Lusis AJ, Brennan ML, Holland S and Pandol SJ:
Neutrophils and NADPH oxidase mediate intrapancreatic trypsin
activation in murine experimental acute pancreatitis.
Gastroenterology. 122:974–984. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uysal A, Sahna E, Ozguler IM, Burma O and
Ilhan N: Effects of apocynin, an NADPH oxidase inhibitor, on levels
of ADMA, MPO, iNOS and TLR4 induced by myocardial ischemia
reperfusion. Perfusion. 30:472–477. 2015. View Article : Google Scholar
|
|
13
|
Zhang YS, He L, Liu B, Li NS, Luo XJ, Hu
CP, Ma QL, Zhang GG, Li YJ and Peng J: A novel pathway of NADPH
oxidase/vascular peroxidase 1 in mediating oxidative injury
following ischemia-reperfusion. Basic Res Cardiol. 107:2662012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohammed AM, Syeda K, Hadden T and Kowluru
A: Upregulation of phagocyte-like NADPH oxidase by cytokines in
pancreatic beta-cells: Attenuation of oxidative and nitrosative
stress by 2-bromopalmitate. Biochem Pharmacol. 85:109–114. 2013.
View Article : Google Scholar
|
|
17
|
Rebelato E, Mares-Guia TR, Graciano MF,
Labriola L, Britto LR, Garay-Malpartida HM, Curi R, Sogayar MC and
Carpinelli AR: Expression of NADPH oxidase in human pancreatic
islets. Life Sci. 91:244–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu R, Wang YL, Edderkaoui M, Lugea A, Apte
MV and Pandol SJ: Ethanol augments PDGF-induced NADPH oxidase
activity and proliferation in rat pancreatic stellate cells.
Pancreatology. 7:332–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao WL, Xiang XH, Chen K, Xu W and Xia SH:
Potential role of NADPH oxidase in pathogenesis of pancreatitis.
World J Gastrointest Pathophysiol. 5:169–177. 2014.PubMed/NCBI
|
|
20
|
Impellizzeri D, Esposito E, Mazzon E,
Paterniti I, Di Paola R, Bramanti P and Cuzzocrea S: Effect of
apocynin, a NADPH oxidase inhibitor, on acute lung inflammation.
Biochem Pharmacol. 81:636–648. 2011. View Article : Google Scholar
|
|
21
|
Kono H, Rusyn I, Uesugi T, Yamashina S,
Connor HD, Dikalova A, Mason RP and Thurman RG: Diphenyleneiodonium
sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced
liver injury in the rat. Am J Physiol Gastrointest Liver Physiol.
280:G1005–G1012. 2001.PubMed/NCBI
|
|
22
|
Dodd-O JM and Pearse DB: Effect of the
NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung
injury. Am J Physiol Heart Circ Physiol. 279:H303–H312.
2000.PubMed/NCBI
|
|
23
|
Joshi S, Peck AB and Khan SR: NADPH
oxidase as a therapeutic target for oxalate induced injury in
kidneys. Oxid Med Cell Longev. 2013:4623612013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferreira AP, Rodrigues FS, Della-Pace ID,
Mota BC, Oliveira SM, Velho Gewehr Cde C, Bobinski F, de Oliveira
CV, Brum JS, Oliveira MS, et al: The effect of NADPH-oxidase
inhibitor apocynin on cognitive impairment induced by moderate
lateral fluid percussion injury: Role of inflammatory and oxidative
brain damage. Neurochem Int. 63:583–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Genovese T, Mazzon E, Paterniti I,
Esposito E, Bramanti P and Cuzzocrea S: Modulation of NADPH oxidase
activation in cerebral ischemia/reperfusion injury in rats. Brain
Res. 1372:92–102. 2011. View Article : Google Scholar
|
|
26
|
Liu PG, He SQ, Zhang YH and Wu J:
Protective effects of apocynin and allopurinol on
ischemia/reperfusion-induced liver injury in mice. World J
Gastroenterol. 14:2832–2837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leveau P, Wang X, Sun Z, Börjesson A,
Andersson E and Andersson R: Severity of pancreatitis-associated
gut barrier dysfunction is reduced following treatment with the PAF
inhibitor lexipafant. Biochem Pharmacol. 69:1325–1331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu
L, Wang YX, Wang GY and Xu CM: Therapeutic effect of Qingyi
decoction in severe acute pancreatitis-induced intestinal barrier
injury. World J Gastroenterol. 21:3537–3546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun JJ, Chu ZJ, Liu WF, Qi SF, Yang YH, Ge
PL, Zhang XH, Li WS, Yang C and Zhang YM: Perirenal space blocking
restores gastrointestinal function in patients with severe acute
pancreatitis. World J Gastroenterol. 19:8752–8757. 2013. View Article : Google Scholar :
|
|
30
|
Yue C, Wang W, Tian WL, Huang Q, Zhao RS,
Zhao YZ, Li QR and Li JS: Lipopolysaccharide-induced failure of the
gut barrier is site-specific and inhibitable by growth hormone.
Inflamm Res. 62:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung PS and Chan YC: Role of oxidative
stress in pancreatic inflammation. Antioxid Redox Signal.
11:135–165. 2009. View Article : Google Scholar
|
|
32
|
Ju KD, Lim JW, Kim KH and Kim H: Potential
role of NADPH oxidase-mediated activation of Jak2/Stat3 and
mitogen-activated protein kinases and expression of TGF-β1 in the
pathophysiology of acute pancreatitis. Inflamm Res. 60:791–800.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim H: Cerulein pancreatitis: Oxidative
stress, inflammation, and apoptosis. Gut Liver. 2:74–80. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williard DE, Twait E, Yuan Z, Carter AB
and Samuel I: Nuclear factor kappa B-dependent gene transcription
in cholecystokinin- and tumor necrosis factor-alpha-stimulated
isolated acinar cells is regulated by p38 mitogen-activated protein
kinase. Am J Surg. 200:283–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung
GH, Yoo BC and Cho JY: Functional roles of p38 mitogen-activated
protein kinase in macrophage-mediated inflammatory responses.
Mediators Inflamm. 2014:3523712014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao MH, Xu J, Cai HD, Lv ZW, Feng YJ, Li
K, Chen CQ and Li YY: p38 MAPK inhibition alleviates experimental
acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int.
14:101–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Papachristou DJ, Papadakou E, Basdra EK,
Baltopoulos P, Panagiotopoulos E and Papavassiliou AG: Involvement
of the p38 MAPK-NF-kappaB signal transduction pathway and COX-2 in
the pathobiology of meniscus degeneration in humans. Mol Med.
14:160–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu L, Song S, Pi Y, Yu Y, She W, Ye H, Su
Y and Hu Q: Cumulated Ca2+ spike duration underlies
Ca2+ oscillation frequency-regulated NFκB
transcriptional activity. J Cell Sci. 124:2591–2601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tudhope SJ, Finney-Hayward TK, Nicholson
AG, Mayer RJ, Barnette MS, Barnes PJ and Donnelly LE: Different
mitogen-activated protein kinase-dependent cytokine responses in
cells of the monocyte lineage. J Pharmacol Exp Ther. 324:306–312.
2008. View Article : Google Scholar
|