Introduction
The temporomandibular joint (TMJ) is a complex
structure that consists of the condyle, fibrocartilaginous disc and
glenoid fossa, which is essential for jaw movement and is only
found in mammals (1,2). Osteoarthritis (OA) is a TMJ disorder
that affects 8–16% of the human population, and can lead to chewing
difficulties and chronic myofacial pains (3). Degradation of the condyle cartilage
is one of the main factors that lead to TMJ OA (4). Condyle structure and function are
affected extracellular matrix (ECM) components, including
collagens, glycosaminoglycans (GAGs) and proteoglycans (PGS)
(5,6). Investigation of the alterations that
may occur in condyle composition is important for understanding the
basis of the pathological process of TMJ OA (7).
The ECM components, which are critical for
resistance to compressive forces and for maintaining tensile
properties, are altered in several cartilage-related pathologies
and at different stages of the same pathological process (8). Additionally, cartilage degradation
results from an imbalance between anabolic and catabolic cytokine
signaling pathways, which can lead to an increase in
matrix-degrading proteases and a decrease in matrix synthesis
(9,10). These alterations are characterized
by the significant upregulation of matrix metalloproteinases
(MMPs), which are responsible for the significant degradation of
cartilage ECM proteins (11). MMPs
function to cleave aggrecan and collagens, the two most abundant
ECM components of skeletal tissue (12). MMP9, an MMP subtype implicated in
the degradation of cartilage ECM proteins, has been observed to
play a central role in connective tissue remodeling and basement
membrane turnover (2,5). MMP13, a member of the MMP family of
neutral endopeptidases, is highly overexpressed in chondrocytes and
synovial cells during OA (13,14).
The short stature homeobox 2 (Shox2) gene is
important for the development of all long bones that undergoes
endochondral ossification (15,16).
A previous study has demonstrated that mice expressing human
SHOX (Shox2SHOX KI/KI mice) do
not exhibit TMJ dysplasia and ankylosis at birth, but display a
postnatal, prematurely eroded articular disc (2). This suggests that, although human
SHOX can exert similar functions to mouse Shox2 in
the regulation of early TMJ development, the human gene has a
distinct function in regulating TMJ growth in postnatal mice
(2). Therefore, the cellular and
molecular alterations that contribute to the congenital OA-like
disease of the TMJ in Shox2SHOX KI/KI
mice were investigated in the present study.
Materials and methods
Mouse details
Animal procedures used in the present study were
approved by the Institutional Animal Care and Use Committee of the
Fujian University of Traditional Chinese Medicine (Fuzhou, China).
The generation of Shox2SHOX KI/KI mice
from the lab of Dr Yiping Chen (Department of Cell and Molecular
Biology, Tulane University, New Orleans, LA, USA) was conducted as
described previously (2,17). A total of 96 mice (48 male and 48
female; 5/cage) were bred on a C57BL/6 J background (42 wild-type
Shox2+/+ mice, 12
Shox2SHOX KI/+ mice and 42
Shox2SHOX KI/KI mice). All mice were
exposed to a 12-h light/dark cycle, in a temperature (22±1°C) and
humidity (56±5%)-controlled environment and maintained on a 0.3%
sodium diet. Mice were sacrificed using carbon dioxide
(CO2) and the heads from postnatal day 0 (P0), P7, P14
and P21 mice were fixed and decalcified in Surgipath's Decalcifier
I® (Leica Biosystems GmbH, Wetzlar, Germany) for a
variable time-period (2–7 days) that was dependent on the age of
the mice (2,5).
Histological analyses
A total of 36 mouse heads were dehydrated using a
graded ethanol series, cleared with xylene (Sigma-Aldrich, St.
Louis, MO, USA), paraffin-embedded and sectioned at 10 µm
using a microtome. Histological analysis of TMJ sections was
performed as previously described using standard hematoxylin-eosin
(HE) staining (18), Safranin
O-Fast Green staining (19) and
azon red/anilin blue (Sigma-Aldrich) staining (2,16).
Bone staining
A total of 6 P21 wild-type
Shox2+/+ and Shox2SHOX
KI/KI mice were fixed in 90% ethanol for a minimum
of 1 week. Prior to fixation, the skins, adipose tissues, eyeballs
and thoracoabdominal organs were removed. Specimens were placed in
85% ethanol containing 1.5% potassium hydroxide (KOH) and 1%
Alizarin Red S (Sigma-Aldrich) for 7 days. The specimens were
macerated in 1% aqueous KOH and cleared in a 50% glycerin
solution.
Immunohistochemical analyses
A total of 54 mouse heads were dehydrated using a
graded ethanol series, cleared with xylene, paraffin-embedded and
sectioned at 8 µm for immunohistochemical analysis.
Immunohistochemical staining was performed as previously described
(2). Paraffin sections were
de-paraffinized and rehydrated in a series of alcohol dilutions,
incubated for 20 min at 100°C in 10 mM sodium citrate buffer (pH
6.0) for antigen retrieval and then cooled to room temperature.
Sections were blocked using goat serum (1:10, Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and incubated for 15 min
at room temperature. This was followed by overnight incubation at
4°C with polyclonal antibodies against runt-related transcription
factor 2 (1:1,000; ab76956), sex determining region Y-box 9 (1:500;
ab26414), collagen type I (col I; 1:500; ab34710), collagen type II
(col II; 1:200; ab53047), aggrecan (1:500; ab36861), MMP9 (1:300;
ab38898), MMP13 (1:50; ab75606) and Indian hedgehog (Ihh; 1:200;
ab39634). Antibodies were obtained from Abcam (Cambridge, MA, USA).
Slides were then washed 3 times using phosphate-buffered saline
(PBS), followed by incubation with a biotinylated horseradish
peroxidase goat anti-rabbit secondary antibody (1:1,000; Thermo
Fisher Scientific, Inc.) for 20 min at 37°C. Slides were then
washed 3 times using PBS. Immunolabeled samples were visualized by
incubating in 0.05% diaminobenzidine (Invitrogen; Thermo Fisher
Scientific, Inc.) diluted in PBS for 5 min at room temperature,
followed by rinsing for 10 min under a running water tap.
Immunohistochemically stained mouse TMJs were visualized using an
Olympus BH-2 light microscope (Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Experiments were repeated a minimum of 3 times and
presented as the mean ± standard deviation. Statistical analysis of
the data was achieved using one-way analysis of variance with SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
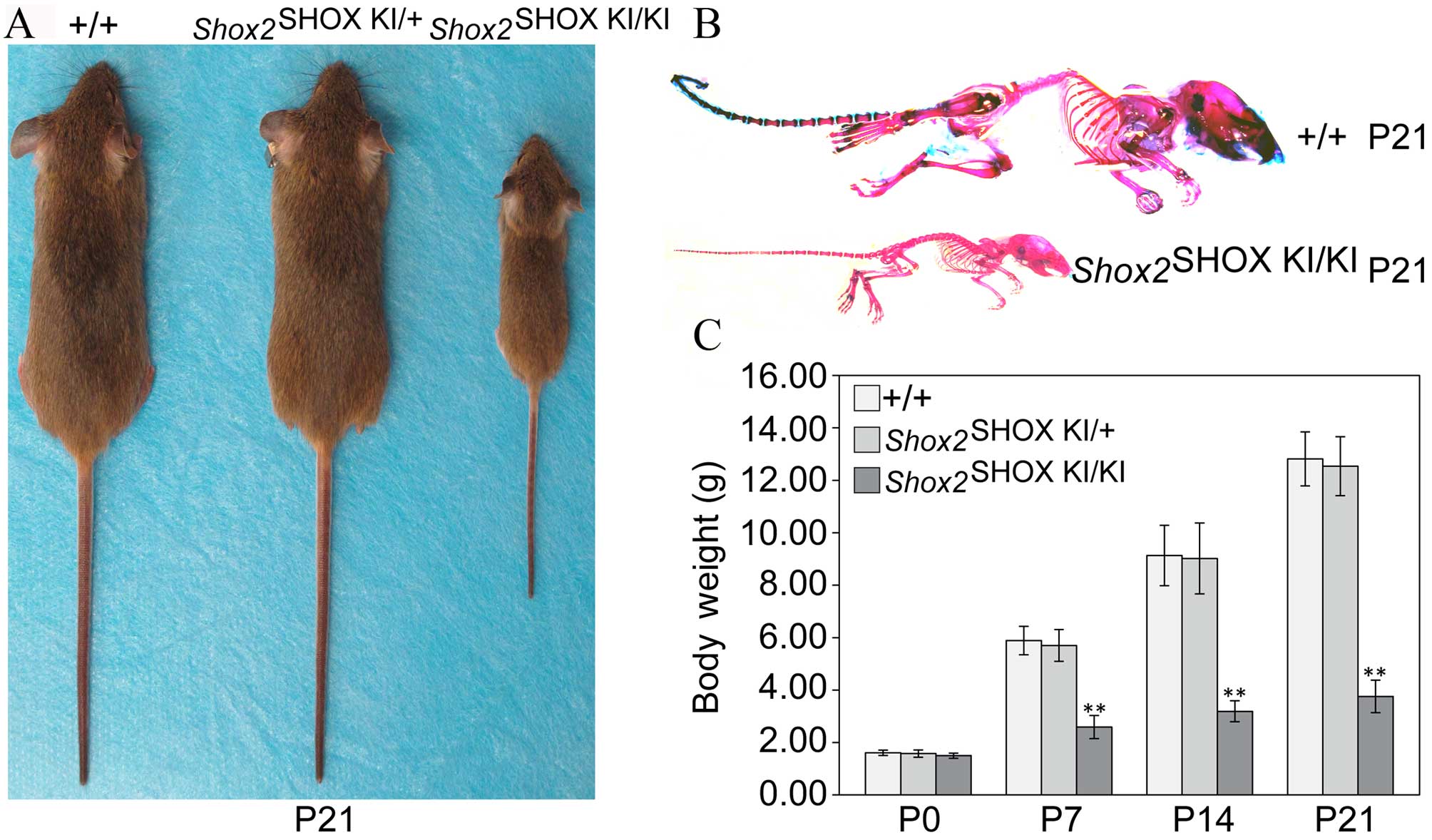
Shox2SHOX KI/KI
mice develop a severe muscle wasting syndrome
The majority of Shox2SHOX
KI/KI mice survived beyond 7 days after birth but
gradually developed a severe muscle wasting syndrome (Fig. 1). At P21, 5/20 (25%)
Shox2SHOX KI/KI mice survived
following weaning, but were notably reduced in size compared with
Shox2SHOX KI/+ and wild-type
Shox2SHOX+/+ mice (Fig. 1A and B). Body weight analysis at
P0, P7, P14, and P21 demonstrated that wild-type
Shox2SHOX+/+, Shox2SHOX
KI/+ and Shox2SHOX
KI/KI mice displayed a similar body weight at
birth (P0); however, Shox2SHOX KI/KI
mice displayed a significant reduction in body weight at P7
(P<0.0001), P14 (P<0.0001) and P21 (P<0.0001) compared
with wild-type Shox2SHOX+/+ mice
(Fig. 1C).
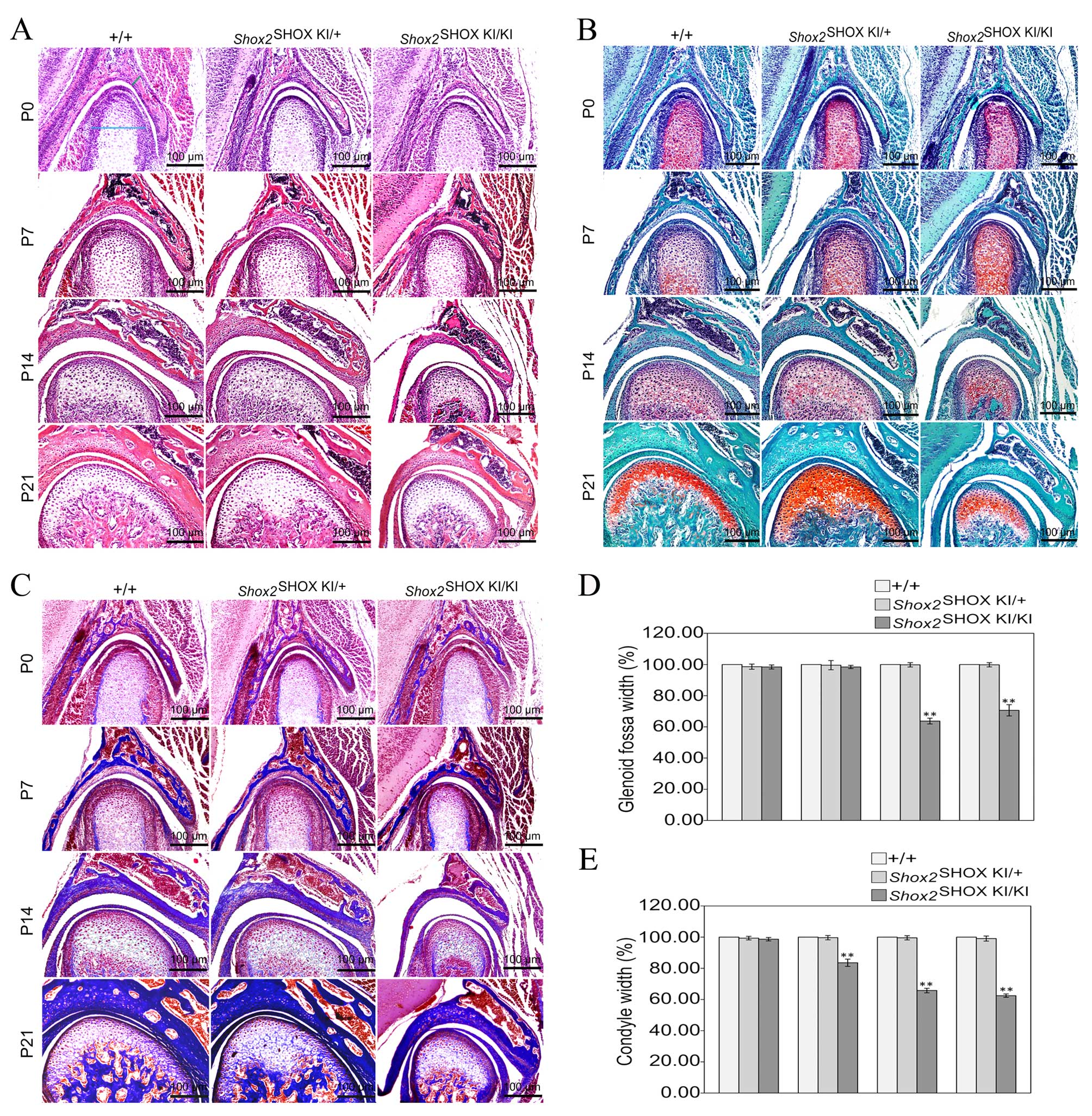
Shox2SHOX KI/KI
mice exhibit an abnormal TMJ
Histological analysis of TMJ development in
postnatal mice using HE staining (Fig.
2A), Safranin O-Fast green staining (Fig. 2B) and azon red/anilin blue staining
(Fig. 2C), demonstrated that
Shox2SHOX KI/KI mice displayed
congenital cartilage degradation from P7. To investigate the
Shox2SHOX KI/KI TMJ phenotype further,
changes in condyle and glenoid fossa width, starting from the
region that displayed the most significant change, was investigated
at P0, P7, P14 and P21 time points. The average glenoid fossa and
condyle width in wild-type
Shox2SHOX+/+ mice at each time point
was defined as 100%. The glenoid fossa and condyle width was
similar in wild-type Shox2SHOX+/+,
Shox2SHOX KI/+ and
Shox2SHOX KI/KI mice at P0 (Fig. 2D and E). However, the glenoid fossa
and condyle width in Shox2SHOX KI/KI
mice was significantly reduced when compared with wild-type
Shox2SHOX+/+ and Shox2SHOX
KI/+ mice at P14 (P<0.0001) and P21
(P<0.0001) stages (Fig. 2D and
E). Notably, these developmental alterations of the TMJ, led to
restrained jaw movement and eating and drinking difficulties, which
is clinically defined as TMJ OA.
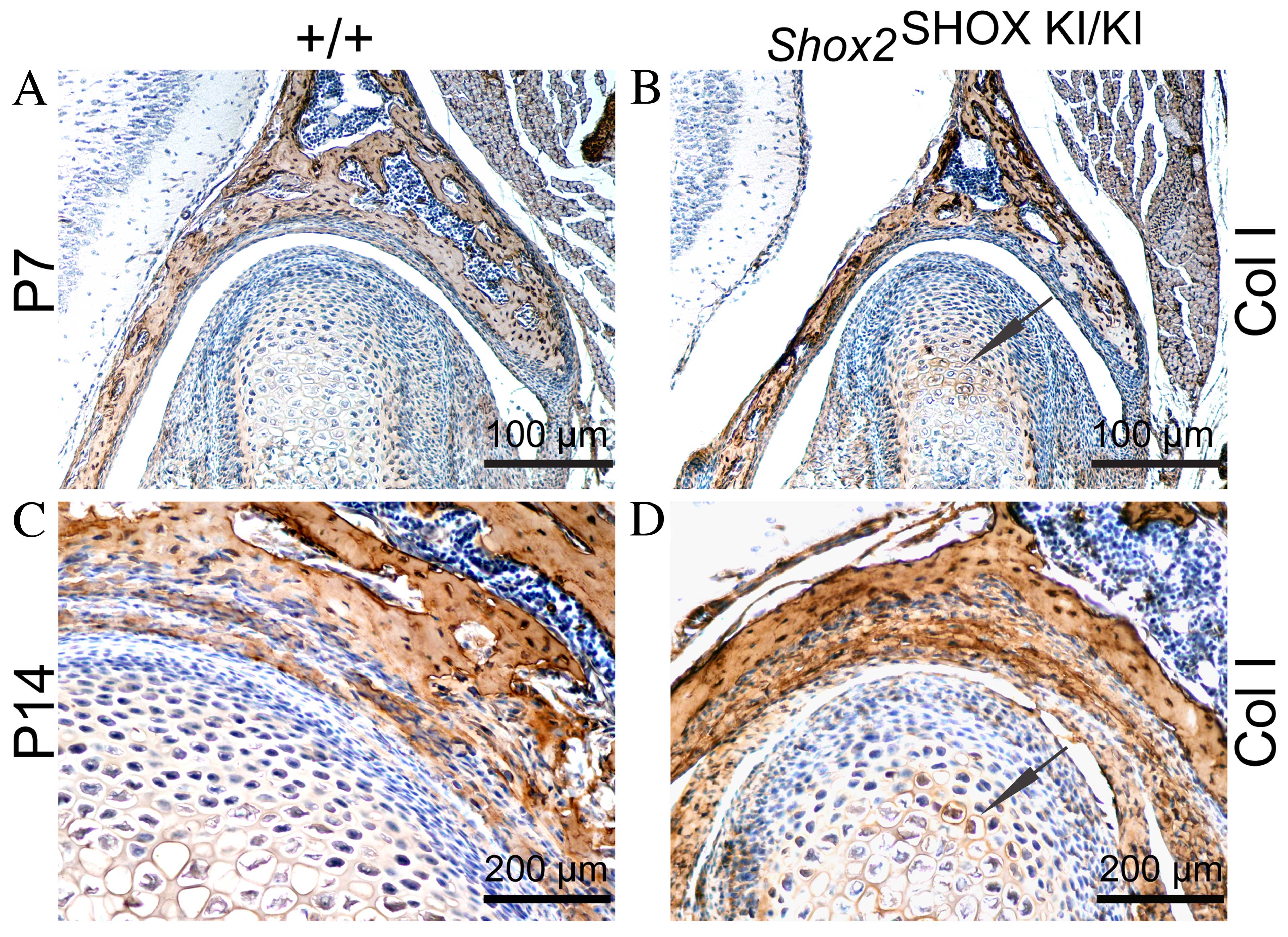
Upregulation of col I and downregulation
of col II and Ihh in the condyle of the Shox2SHOX
KI/KI mice
The ECM of the condyle is composed of collagens,
PGS, GAGs and aggrecan (20). ECM
components serve an important role in maintaining tensile
properties and resistance to compressive forces. Alterations in ECM
components are associated with TMJ cartilage degradation (8). Therefore, the expression levels of
the matrix proteins col I, col II, aggrecan and Ihh were
investigated in the present study, in order to determine an
association between the observed TMJ growth alterations in
postnatal Shox2SHOX KI/KI mice and
changes in ECM components. Immunohistochemical analysis
demonstrated an increase in col I expression levels in the condyles
of Shox2SHOX KI/KI mice compared with
wild-type Shox2SHOX+/+ mice at P7 and
P14, whereas col II expression levels were reduced (Fig. 3A–H). However, no difference in the
expression levels of aggrecan in the condyles of
Shox2SHOX KI/KI mice and wild-type
Shox2SHOX+/+ mice were observed
(Fig. 3I–L). The expression levels
of Ihh were determined using immunohistochemical analysis at P7.
The results demonstrated that Ihh expression levels in
Shox2SHOX KI/KI mouse condyles were
significantly downregulated compared with wild-type
Shox2SHOX+/+ mice (Fig. 3M and N). Therefore, upregulation of
col I and downregulation of col II and Ihh in the condyles may be
responsible for the observed congenital cartilage degradation in
Shox2SHOX KI/KI mice.
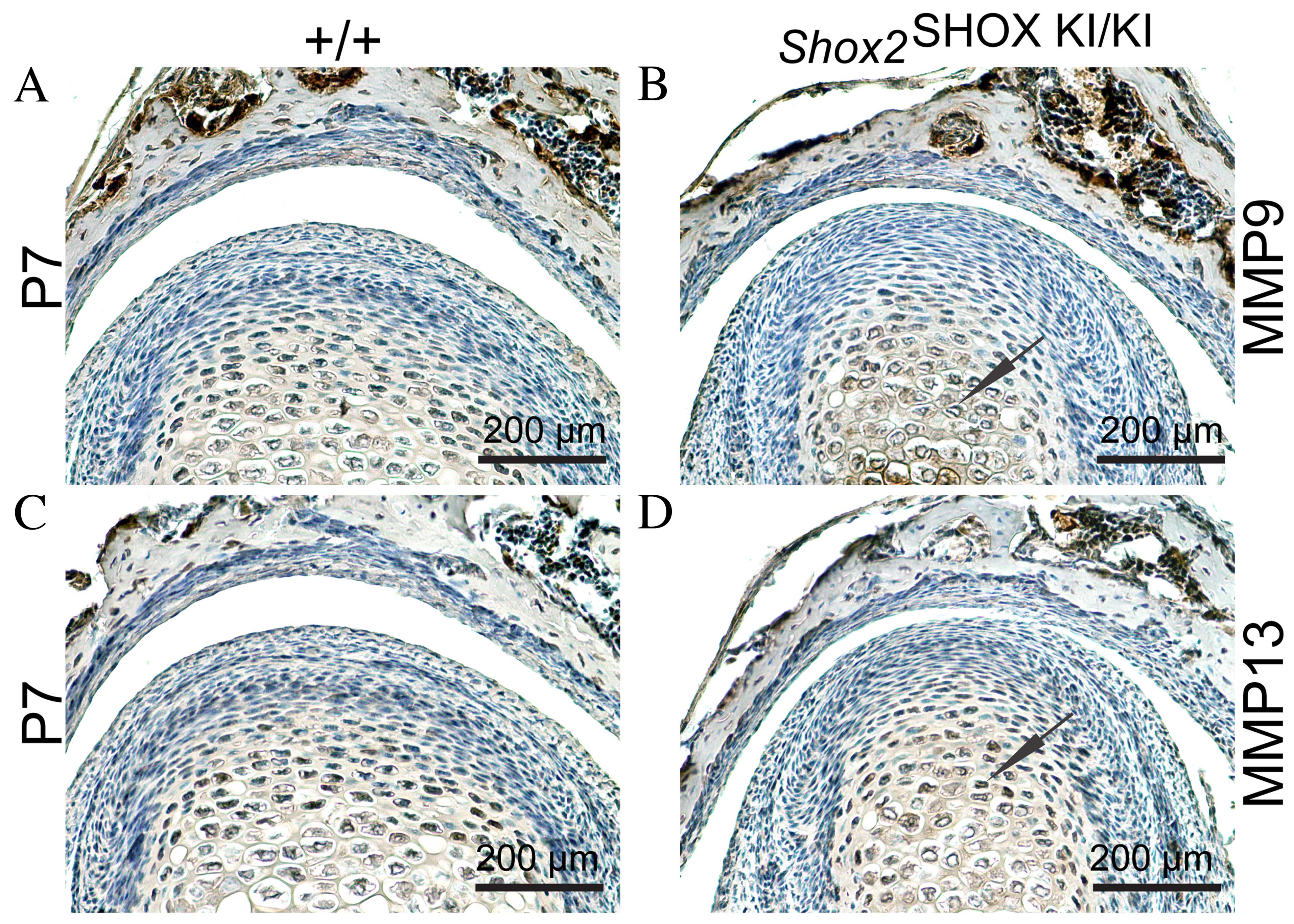
Upregulation of MMP9 and MMP13 in the
condyle of the Shox2SHOX KI/KI mice
MMP9 and MMP13 are zinc-dependent endopeptidases
that function to degrade ECM collagens, and have been observed to
be elevated in TMJ OA (21).
Therefore, the next aim of the present study was to investigate
whether the observed reduction in col II expression levels in
Shox2SHOX KI/KI mouse TMJs was caused
by increased MMP9 and MMP13 activity. To test this hypothesis,
immunohistochemical analysis was conducted to determine MMP9 and
MMP13 protein expression levels in Shox2SHOX
KI/KI and wild-type Shox2SHOX
+/+ mouse TMJs. As presented in Fig. 4D, MMP9 and MMP13 exhibited
increased expression levels in Shox2SHOX
KI/KI mouse TMJs at P7 compared with the wild-type
Shox2SHOX+/+ mice. These results
indicate that increased MMP9 and MMP13 activity may be responsible
for the observed reduction in col II expression levels and the
congenital cartilage degradation in Shox2SHOX
KI/KI mouse TMJs.
Discussion
Conditional inactivation of Shox2 has been
observed to result in dysplasia of the condyle and glenoid fossa
and articular disc ankylosis (16). Additionally, Shox2SHOX
KI/KI mice exhibit premature erosion of the
articular disc (2). These results
support the putative functional role of Shox2 in TMJ
formation and development. In the present study, alterations in TMJ
growth in postnatal Shox2SHOX KI/KI
mice were observed. The results demonstrate that the downregulation
of col II and Ihh, and upregulation of col I, MMP9 and MMP13 may
contribute to the congenital OA-like phenotype observed in
postnatal Shox2SHOX KI/KI mouse
TMJs.
To further investigate the mechanism of the
congenital OA-like disease, the present study focused on the
changes in ECM composition, Ihh and MMPs in the postnatal
Shox2SHOX KI/KI mouse TMJs.
Understanding the alterations that occur in the ECM composition of
TMJ are necessary to elucidate the pathological process of TMJ OA
(6). Cartilage destruction,
mediated by the loss of col II and PGS, is a characteristic feature
of OA (22,23). The initial loss of col II occurs in
the superficial and upper-middle zones of the TMJ and extends into
the deeper zones, with increasing destruction accompanied by the
pathological process of OA (24).
In normal hyaline cartilage, the predominant collagen type is col
II, which is produced by chondrocytes. However, col I is also
involved in the pathogenesis of OA; the relative concentrations of
carboxyterminal telopeptides of col I and II may therefore act as a
marker of cartilage degradation (25). The cleavage of fibrillar collagens
by MMPs contributes to the substantial degradation of tissues
during OA. MMP9 and MMP13 demonstrate a preference for degrading
fibrillar collagen substrates, such as col II, however, also
possess the ability to degrade the large hydrodynamic cartilage
proteoglycan aggregate (26,27).
Ihh, a regulator of chondrocyte and chondroprogenitor proliferation
as well as chondrocyte maturation, has been observed to be
essential for embryonic and postnatal TMJ development (28–30).
Therefore, in order to investigate the mechanisms of congenital
OA-like disease development in Shox2SHOX
KI/KI mice in the present study, the composition
of ECM proteins (col I, col II and aggrecan), Ihh, MMP9 and MMP13
protein expression levels in postnatal Shox2SHOX
KI/KI mouse TMJs were determined. Using
immunohistochemical staining, the results demonstrated decreased
expression of col II and Ihh, and increased expression of col I,
MMP9 and MMP13 in Shox2SHOX KI/KI
condyles compared with wild-type
Shox2+/+ mice at stages P7 and P14.
This provides additional evidence that OA-like disease occurred in
Shox2SHOX KI/KI mouse TMJs. It is
currently unknown whether the observed reduction in Ihh expression
levels confers elevated MMP activity and loss of col II in
postnatal Shox2SHOX KI/KI mouse TMJs.
However, the Ihh signaling pathway serves an important role in
regulating tissue structure homeostasis during TMJ development and
requires further investigation.
In conclusion, postnatal Shox2SHOX
KI/KI mouse TMJs were observed to develop
congenital OA-like disease, which provides a novel in vivo
model for studying the molecular and cellular mechanisms of TMJ
OA.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81202645
and 81230087), the Program for New Century Excellent Talents in
Fujian Province University (grant no. JA14150), and the Fujian
University of Traditional Chinese Medicine Tube Project (grant no.
X2015021). The authors appreciate the assistance of Dr Yiping Chen
(Department of Cell and Molecular Biology, Tulane University,
USA).
References
|
1
|
Wang Y, Liu C, Rohr J, Liu H, He F, Yu J,
Sun C, Li L, Gu S and Chen Y: Tissue interaction is required for
glenoid fossa development during temporomandibular joint formation.
Dev Dyn. 240:2466–2473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Liu H, Gu S, Liu C, Sun C, Zheng Y
and Chen Y: Replacing Shox2 with human SHOX leads to congenital
disc degeneration of the temporomandibular joint in mice. Cell
Tissue Res. 355:345–354. 2014. View Article : Google Scholar :
|
|
3
|
Wang XD, Zhang JN, Gan YH and Zhou YH:
Current understanding of pathogenesis and treatment of TMJ
osteoarthritis. J Dent Res. 94:666–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Embree MC, Iwaoka GM, Kong D, Martin BN,
Patel RK, Lee AH, Nathan JM, Eisig SB, Safarov A, Koslovsky DA, et
al: Soft tissue ossification and condylar cartilage degeneration
following TMJ disc perforation in a rabbit pilot study.
Osteoarthritis Cartilage. 23:629–639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Liang W, Ye H, Weng X, Liu F and Liu
X: Overexpression of Shox2 leads to congenital dysplasia of the
temporomandibular joint in mice. Int J Mol Sci. 15:13135–13150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su SC, Tanimoto K, Tanne Y, Kunimatsu R,
Hirose N, Mitsuyoshi T, Okamoto Y and Tanne K: Celecoxib exerts
protective effects on extracellular matrix metabolism of mandibular
condylar chondrocytes under excessive mechanical stress.
Osteoarthritis Cartilage. 22:845–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilusz RE, Zauscher S and Guilak F:
Micromechanical mapping of early osteoarthritic changes in the
pericellular matrix of human articular cartilage. Osteoarthritis
Cartilage. 21:1895–1903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garnero P, Rousseau JC and Delmas PD:
Molecular basis and clinical use of biochemical markers of bone,
cartilage, and synovium in joint diseases. Arthritis Rheum.
43:953–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polur I, Lee PL, Servais JM, Xu L and Li
Y: Role of HTRA1, a serine protease, in the progression of
articular cartilage degeneration. Histol Histopathol. 25:599–608.
2010.PubMed/NCBI
|
|
10
|
Hill A, Duran J and Purcell P: Lubricin
protects the temporomandibular joint surfaces from degeneration.
PLoS One. 9:e1064972014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asakawa-Tanne Y, Su S, Kunimatsu R, Hirose
N, Mitsuyoshi T, Okamoto Y, Tanaka E, Tanne K and Tanimoto K:
Effects of enzymatic degradation after loading in temporomandibular
joint. J Dent Res. 94:337–343. 2015. View Article : Google Scholar :
|
|
12
|
Gepstein A, Arbel G, Blumenfeld I, Peled M
and Livne E: Association of metalloproteinases, tissue inhibitors
of matrix metalloproteinases, and proteoglycans with development,
aging, and osteoarthritis processes in mouse temporomandibular
joint. Histochem Cell Biol. 120:23–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu L, Flahiff CM, Waldman BA, Wu D, Olsen
BR, Setton LA and Li Y: Osteoarthritis-like changes and decreased
mechanical function of articular cartilage in the joints of mice
with the chondrodysplasia gene (cho). Arthritis Rheum.
48:2509–2518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inada M, Wang Y, Byrne MH, Rahman MU,
Miyaura C, López-Otín C and Krane SM: Critical roles for
collagenase-3 (Mmp13) in development of growth plate cartilage and
in endochondral ossification. Proc Natl Acad Sci USA.
101:17192–17197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cobb J, Dierich A, Huss-Garcia Y and
Duboule D: A mouse model for human short-stature syndromes
identifies Shox2 as an upstream regulator of Runx2 during long-bone
development. Proc Natl Acad Sci USA. 103:4511–4515. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu S, Wei N, Yu L, Fei J and Chen Y:
Shox2-deficiency leads to dysplasia and ankylosis of the
temporomandibular joint in mice. Mech Dev. 125:729–742. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Chen CH, Espinoza-Lewis RA, Jiao Z,
Sheu I, Hu X, Lin M, Zhang Y and Chen Y: Functional redundancy
between human SHOX and mouse Shox2 genes in the regulation of
sinoatrial node formation and pacemaking function. J Biol Chem.
286:17029–17038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YD, Liao LF, Zhang HY, Lu L, Jiao K,
Zhang M, Zhang J, He JJ, Wu YP, Chen D and Wang MQ: Reducing
dietary loading decreases mouse temporomandibular joint degradation
induced by anterior crossbite prosthesis. Osteoarthritis Cartilage.
22:302–312. 2014. View Article : Google Scholar :
|
|
19
|
Liang W, Li X, Gao B, Gan H, Lin X, Liao L
and Li C: Observing the development of the temporomandibular joint
in embryonic and post-natal mice using various staining methods.
Exp Ther Med. 11:481–489. 2016.PubMed/NCBI
|
|
20
|
Gu Z, Feng J, Shibata T, Hu J and Zhang Z:
Type II collagen and aggrecan mRNA expression by in situ
hybridization in rabbit temporomandibular joint posterior
attachment following disc displacement. Arch Oral Biol. 48:55–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jackson MT, Moradi B, Smith MM, Jackson CJ
and Little CB: Activation of matrix metalloproteinases 2, 9, and 13
by activated protein C in human osteoarthritic cartilage
chondrocytes. Arthritis Rheumatol. 66:1525–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lindhorst E, Wachsmuth L, Kimmig N, Raiss
R, Aigner T, Atley L and Eyre D: Increase in degraded collagen type
II in synovial fluid early in the rabbit meniscectomy model of
osteoarthritis. Osteoarthritis Cartilage. 13:139–145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Megías J, Guillén MI, Bru A, Gomar F and
Alcaraz MJ: The carbon monoxide-releasing molecule
tricarbonyldichlororuthenium(II) dimer protects human
osteoarthritic chondrocytes and cartilage from the catabolic
actions of interleukin-1beta. J Pharmacol Exp Ther. 325:56–61.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rogart JN, Barrach HJ and Chichester CO:
Articular collagen degradation in the Hulth-Telhag model of
osteoarthritis. Osteoarthritis Cartilage. 7:539–547. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miosge N, Hartmann M, Maelicke C and
Herken R: Expression of collagen type I and type II in consecutive
stages of human osteoarthritis. Histochem Cell Biol. 122:229–236.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak
JS, Lee G, Rhee J, Ryu JH, Chun CH and Chun JS: Regulation of the
catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis.
Cell. 156:730–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flannelly J, Chambers MG, Dudhia J, Hembry
RM, Murphy G, Mason RM and Bayliss MT: Metalloproteinase and tissue
inhibitor of metalloproteinase expression in the murine STR/ort
model of osteoarthritis. Osteoarthritis Cartilage. 10:722–733.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Purcell P, Joo BW, Hu JK, Tran PV,
Calicchio ML, O'Connell DJ, Maas RL and Tabin CJ: Temporomandibular
joint formation requires two distinct hedgehog-dependent steps.
Proc Natl Acad Sci USA. 106:18297–18302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ochiai T, Shibukawa Y, Nagayama M, Mundy
C, Yasuda T, Okabe T, Shimono K, Kanyama M, Hasegawa H, Maeda Y, et
al: Indian hedgehog roles in post-natal TMJ development and
organization. J Dent Res. 89:349–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shibukawa Y, Young B, Wu C, Yamada S, Long
F, Pacifici M and Koyama E: Temporomandibular joint formation and
condyle growth require Indian hedgehog signaling. Dev Dyn.
236:426–434. 2007. View Article : Google Scholar
|