Introduction
Acute kidney injury (AKI) is a multifactorial and
multiphasic clinical syndrome, characterized by an abrupt (hours to
days) reduction in renal function, together with an accumulation of
metabolic waste and toxins, such as serum creatinine (Scr) and
blood urea nitrogen (BUN), and/or decreased urine output (1,2). It
is estimated that AKI affects 1.9% of all hospital inpatients,
including >60% of patients in the intensive care unit (2,3). The
physiopathological mechanism of AKI is characterized by renal
tubular damage, vascular injury and inflammation (1,4).
Injury and death of tubular cells contributes to the pathogenesis
of AKI and apoptosis appears to be crucial during this process
(1). Cisplatin (CP), an effective
antineoplastic drug, induces a loss of renal function in 25–35% of
patients following a single administration (5). Acute tubular damage caused by CP
occurs primarily in the renal proximal tubular cells, particularly
in the S3 segment, due to CP accumulation in the area (5,6). The
pathophysiology of CP-induced acute renal tubular injury is
associated with inflammation, oxidative stress and apoptosis
(5–7). Via these mechanisms, apoptosis of the
renal tubular cells is a key mode of cell death (8).
The extrinsic apoptotic pathway may contribute to
tubular cell loss in AKI (1). TNF
receptor 1 (TNFR1) and Fas (also known as CD95 or APO-1) are
important transmembrane components of the tumor necrosis factor
(TNF) family of receptors (9–11),
whose ligands are TNF-α and Fas-ligand (Fas-L), respectively
(5,11). Following the interaction between
TNF family ligands and receptors, the conserved death domains
located in the cytoplasmic tails allow recruitment of downstream
adaptors, such as TNFR1-associated death domain protein and
FAS-associated death domain protein (FADD). The subsequent
interaction of FADD with Fas or TNFR1 activates the recruitment of
caspase-8 (6,12,13).
Caspase-8 directly activates the downstream effector, caspase-3, or
cleaves BH3 interacting domain death agonist (BID), a
death-inducing member of the B cell lymphoma 2 (Bcl-2) family
(14,15). BID is cleaved to form truncated BID
(tBID), which is translocated to the mitochondria and promotes a
mitochondrial-dependent apoptotic pathway involving Bcl-2
associated X (Bax) and Bcl-2 (14,16).
Previous studies have demonstrated that TNFR1 knockout mice are
resistant to CP-induced AKI (6,12).
However, studies that have investigated the role of TNFR1 in
apoptosis in renal tubular cells are limited and preliminary
(13). In addition, whether
Fas/Fas-L induces apoptosis in renal tubular cells still remains
controversial (10). However, high
Fas expression levels have been observed in renal tubular cells
following acute and chronic renal failure (17,18).
Resveratrol (trans-3,4′,5-trihydroxystilbene; RSV)
is a polyphenolic phytoalexin present in numerous edible plants,
including mulberries, peanuts and grapes (19–21).
RSV has been studied in vivo and in vitro (19,21)
and has been demonstrated to possess a wide range of
pharmacological effects, including cardioprotective (22), neuroprotective (23), nephroprotective (24), antineoplastic (25) and antidiabetic (26) effects, as a result of its
anti-inflammatory, antioxidant and cytoprotective properties
(19). Previous studies have
demonstrated the benefits of RSV towards several types of kidney
disease, including diabetic nephropathy (27), drug-induced renal injury (28,24),
and ischemia-reperfusion and sepsis-induced kidney injuries
(29,30). The present study, therefore, aimed
to determine whether RSV attenuates CP-induced AKI in a rat model
and to investigate the potential mechanisms of attenuation.
Materials and methods
Reagents
CP (CAS no. 15663-27-1) and RSV (CAS no. 501-36-0)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary
antibodies were supplied as follows: Mouse anti-Fas-L (cat. no.
sc-19988), mouse anti-Bcl-2 (cat. no. sc-7382), rabbit-anti-Bax
(cat. no. sc-6236), rabbit anti-BID (cat. no. sc-11423) from Santa
Cruz Biotechnology, Inc. (Santa Cruz, Dallas, TX, USA); rabbit
anti-TNF-α (cat. no. ab9755) and rabbit anti-caspase-8 (cat. no.
ab181580) from Abcam (Cambridge, UK); and rabbit anti-β-actin from
Merck Millipore (Darmstadt, Germany). Horseradish peroxidase
(HRP)-conjugated anti-mouse IgG (cat. no. 12-349) and anti-rabbit
IgG secondary antibodies (cat. no. 12-348) were obtained from Merck
Millipore. The terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL) in situ cell death detection kit was
purchased from Roche Diagnostics GmbH (Mannheim, Germany).
Animals
A total of 28 adult male Wistar rats (weighing
180–200 g; 6–8 weeks old) were obtained from Shandong University
Laboratory Animal Center (Jinan, China). Rats were acclimated to
laboratory conditions for one week prior to experiments, and were
consistently maintained in polycarbonate cages under standard
conditions of temperature (20–23°C) and humidity (50–70%) with 12 h
light-dark cycles. All rats had unrestricted access to food and
water. All experiments involving rats were performed in accordance
with the Guidelines for Animal Experiments of Qilu Hospital,
Shandong University (Jinan, China) and all experimental procedures
were approved by the Institutional Ethics Committee for Laboratory
Animal Care of Qilu Hospital, Shandong University.
Animal treament
Rats were randomly divided into four treatment
groups, with seven animals per group: i) Control group (NS)
received intraperitoneal (ip) injections of 0.9% saline (10 ml/kg)
on day 1 and day 3; ii) RSV group received ip injections of 10
mg/kg RSV (2 mg/ml, dissolved in 0.9% saline) on day 1 and day 3;
iii) CP group received an ip injection of 8 mg/kg CP (1 mg/ml,
dissolved in 0.9% saline) on day 1 and an ip injection of 0.9%
saline (10 ml/kg) on day 3; iv) CP+RSV group received ip injections
of 8 mg/kg CP (1 mg/ml, dissolved in 0.9% saline) followed 30 min
later by 10 mg/kg RSV (2 mg/ml, dissolved in 0.9% saline) on day 1,
and an ip injection of 10 mg/kg RSV (2 mg/ml, dissolved in 0.9%
saline) on day 3.
On day 5 of treatment, rats were first weighed
before they were anesthetized by intraperitoneal injection of 10%
chloral hydrate (0.4 ml/100 g body weight; CAS no. 302-17-0; Damao
Chemical Reagent Factory, Tianjin, China), and sacrificed by
bloodletting from the left ventricle. Serum was separated from the
blood by centrifugation (1,500 × g at 4°C for 15 min) and
immediately stored at −20°C prior to analysis. Rat kidneys were
perfused in situ through the left ventricle with 0.9%
saline, then excised, weighed and cut in half by coronal position.
Half of each kidney was immediately stored at −80°C, and half
immersed in 4% paraformaldehyde buffered with phosphate-buffered
saline (PBS) at 4°C, then fixed for 24 h and embedded in
paraffin.
Assessment of renal function
Measurements of Scr and BUN were conducted in Qilu
Hospital of Shandong University by a Cobas® 8000 modular
analyzer (Roche Diagnostics GmbH). The renal index (RI) was
calculated as follows: Both kidney weights (g) / animal weight (g)
× 1,000.
Histopathological observation
Paraffinized kidneys were cut into 3–5
µm-thick sections, before they were deparaffinized and
stained with hematoxylin and eosin (H&E; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Sections were immersed
in 0.5% eosin for 1 min and 0.1% hematoxylin for 5 min. The tubular
damage score assessment was performed in 10 fields of 5 sections
per group using the following index of renal tubular necrosis:
Score of 0 (absence of damage); score of 1 (<25% damage); score
of 2 (25–50% damage); score of 3 (50–75% damage); and score of 4
(>75% damage).
Immunohistochemistry
Tissue sections underwent deparaffinization and
rehydration with xylene and 100, 95, 90 and 80% ethanol gradients.
Following 3 washes with PBS, slices were placed in 0.01% sodium
citrate buffer (pH 6.0), and heated by microwave (10 min at
93–95°C) for antigen retrieval. Tissue sections were then cooled,
washed with PBS 3 times and immersed in 0.1% Triton X-100 for 15
min. Sections were incubated with 3% hydrogen peroxide for 10 min
at 15–25°C in the dark, to block endogenous peroxidase activity,
followed by incubation with 10% goat serum (Nanjing Jiancheng
Bioengineering Institute) for 45 min at 37°C, and then with mouse
anti-Fas-L (dilution, 1:200) rabbit anti-Bax (dilution, 1:200) and
mouse anti-Bcl-2 (dilution, 1:200) primary antibodies at 4°C
overnight. The negative control sections were treated with PBS.
Sections were subsequently washed and incubated with polymer helper
for 20 min at 37°C, together with HRP-labeled anti-rabbit IgG
polymer (cat. no. PV-9001) or HRP-labeled anti-mouse IgG polymer
(cat. no. PV-9002) from the Polink-2 plus® Polymer HRP
Detection System (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China). After washing 3 times with PBS, tissue
slices were stained with 3,3′-diaminobenzidine solution (DAB; cat.
no. ZLI-9017; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd). Tissue slices were subsequently stained with 0.1% hematoxylin
for 5 min. The stained slides were observed under a light
microscope, and brown areas were deemed as positively stained. High
power fields (n=10 per section) were randomly selected and 2
sections/group were scored. The intensity of dye color was graded
as follows: Score 0, no color; score 1 light yellow; score 2, light
brown; and score 3, brown. The percentage of positive areas was
graded as follows: Score 0, <5%; score 1, 5–25%; score 2,
25–50%; score 3, 50–75%; and score 4, >75%. The two parameters
were added to produce a final score.
TUNEL assay
The in situ cell death detection kit was used
to detect cell apoptosis. TUNEL assay was performed according to
the manufacturer's instructions. Briefly, deparaffinized tissue
sections were incubated with 3% hydrogen peroxide in methanol (10
min at 15–25°C in the dark), washed 3 times with PBS, then
incubated with 0.1% Triton X-100 in freshly prepared 0.01% sodium
citrate (8 min at 25°C). Tissue sections were incubated with enzyme
and labeling solutions (1:9; 60 min at 37°C). Following 3 washes in
PBS, slices were stained with DAB and hematoxylin. Negative
controls were incubated with labeling solution only. TUNEL positive
nuclei were counted in 10 random, non-overlapping high power fields
of 2 tissue sections.
Western blot
Perfused kidney tissue was snap frozen in liquid
nitrogen, and 30 mg of kidney tissue was homogenized with
radioimmunoprecipitation assay buffer. The supernatant was
collected for protein quantification following centrifugation at
15,000 × g for 30 min at 4°C. The concentration of protein
extracted from rat kidney tissue samples was quantified using a
Bicinchoninic Acid Protein assay kit (Beyotime Institute of
Biotechnology, Jiangsu, China). A total of 30 µg protein
from each sample was separated on 12% gels by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
electroblotted onto polyvinylidene difluoride membranes. Membranes
were incubated with blocking buffer (5% skimmed milk) at room
temperature for 60 min and then incubated with primary antibodies
(mouse anti-Fas-L, 1:200; rabbit anti-BID, 1:200; rabbit anti-Bax,
1:200; mouse anti-Bcl-2, 1:200; rabbit anti-TNF-α, 1:1,000; rabbit
anti-caspase-8, 1:1,000; and rabbit anti-β-actin, 1:500) at 4°C
overnight. Following washing in Tris-buffered saline with 0.1%
Tween 20 (TBST), membranes were incubated with anti-mouse or
anti-rabbit secondary antibodies (dilution, 1:5,000) at room
temperature for 60 min. Membranes were then washed in TBST and the
blots were detected using the Immobilon™ Western Chemiluminescent
HRP Substrate (EMD Millipore, Billerica, MA, USA) followed by
autoradiography. Protein density for quantification was determined
using Image J software (version 1.45; National Institutes of
Health, Bethesda, MD, USA). β-actin was used as a loading control
and relative quantities of all proteins were expressed as a ratio
to that of the NS group.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Analyses were performed using one-way analysis of variance with
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). The
least-significant difference post-hoc test was used
to assess differences between two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of RSV on renal function in
CP-induced AKI
It is known that typical AKI is characterized by a
significant increase in Scr levels (2). As presented in Table I, CP led to significant increases
in the levels of Scr, BUN and RI compared with the control group
(P<0.001). However, co-treatment of RSV and CP attenuated the
increase of Scr, BUN and RI compared with the CP group
(P<0.001).
 | Table IEffects of RSV on levels of Scr, BUN
and RI. |
Table I
Effects of RSV on levels of Scr, BUN
and RI.
| Group | n | Scr
(µmol/l) | BUN (mmol/l) | RI |
|---|
| NS | 7 | 31.14±6.26 | 7.42±1.21 | 7.99±0.12 |
| RSV | 7 | 28.71±5.28 | 6.13±0.61 | 8.00±0.16 |
| CP | 7 |
192.29±11.44a | 43.39±2.20a | 9.36±0.11a |
| CP+RSV | 7 | 63.86±8.71b | 19.04±1.94b | 8.81±0.17b |
Renoprotective and anti-apoptotic effects
of RSV in CP-induced AKI
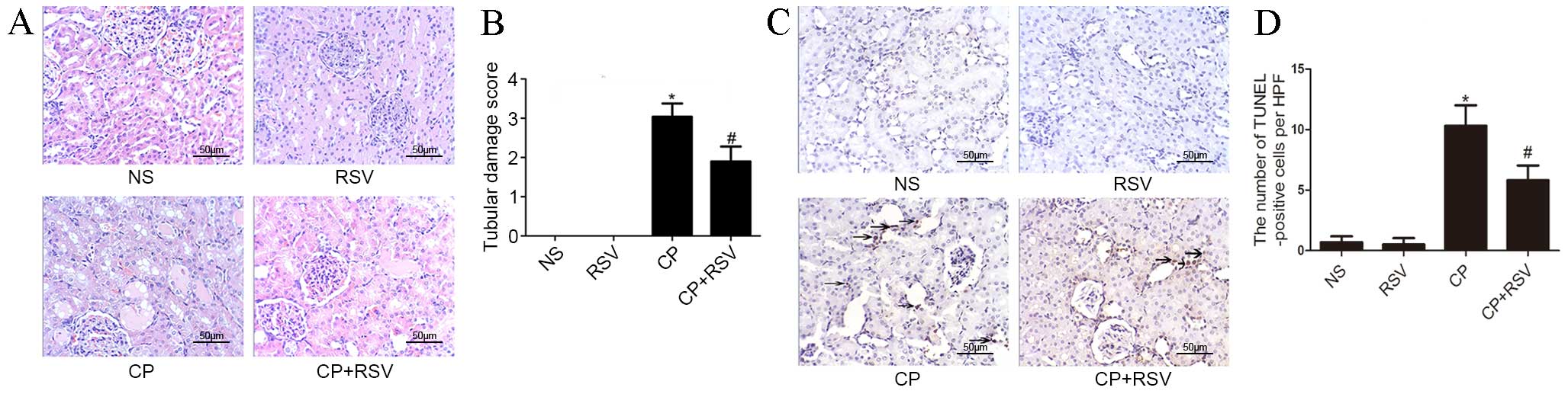
H&E staining of kidney tissues was used to
evaluate the histological changes in renal tubular epithelium. As
presented in Fig. 1A, histological
examination revealed that the kidneys from the control and RSV
groups maintained normal tubular morphology, whereas the kidneys
from the CP group displayed significant features of AKI, including
brush border loss, epithelial cell vacuolation and cast formation.
Semi-quantitative analysis of the extent of the histological damage
in the CP group was scored as 3.04, in comparison with no damage in
the control group (P<0.001; Fig.
1B). By contrast, the extent of tubular damage was
significantly reduced in the CP+RSV group compared with the CP
group, with a histological damage score of 1.9 (P<0.001;
Fig. 1A and B).
Tubular cell apoptosis was detected in the
CP-induced AKI model by TUNEL assay, as presented in Fig. 1C. The NS and RSV groups exhibited
limited apoptosis (Fig. 1D),
whereas the number of TUNEL-positive nuclei, indicating apoptosed
cells, was significantly increased in the CP group compared with
the NS group (P<0.001; Fig.
1D). However, the number of TUNEL-positive cells in the CP+RSV
group was significantly reduced compared with the CP group
(P<0.001), indicating reduced apoptosis in this group.
Effect of RSV on vital protein
expressions in the process of CP-induced AKI
Death receptor-mediated apoptotic pathways have been
implicated in CP-induced AKI. Thus, the expression of certain vital
proteins in this signaling pathway, including Fas-L, TNF-α and
caspase-8, were examined. The function of caspase-8 in the
intrinsic mitochondrial apoptotic pathway was also investigated by
examining the expression levels of BID, Bax and Bcl-2.
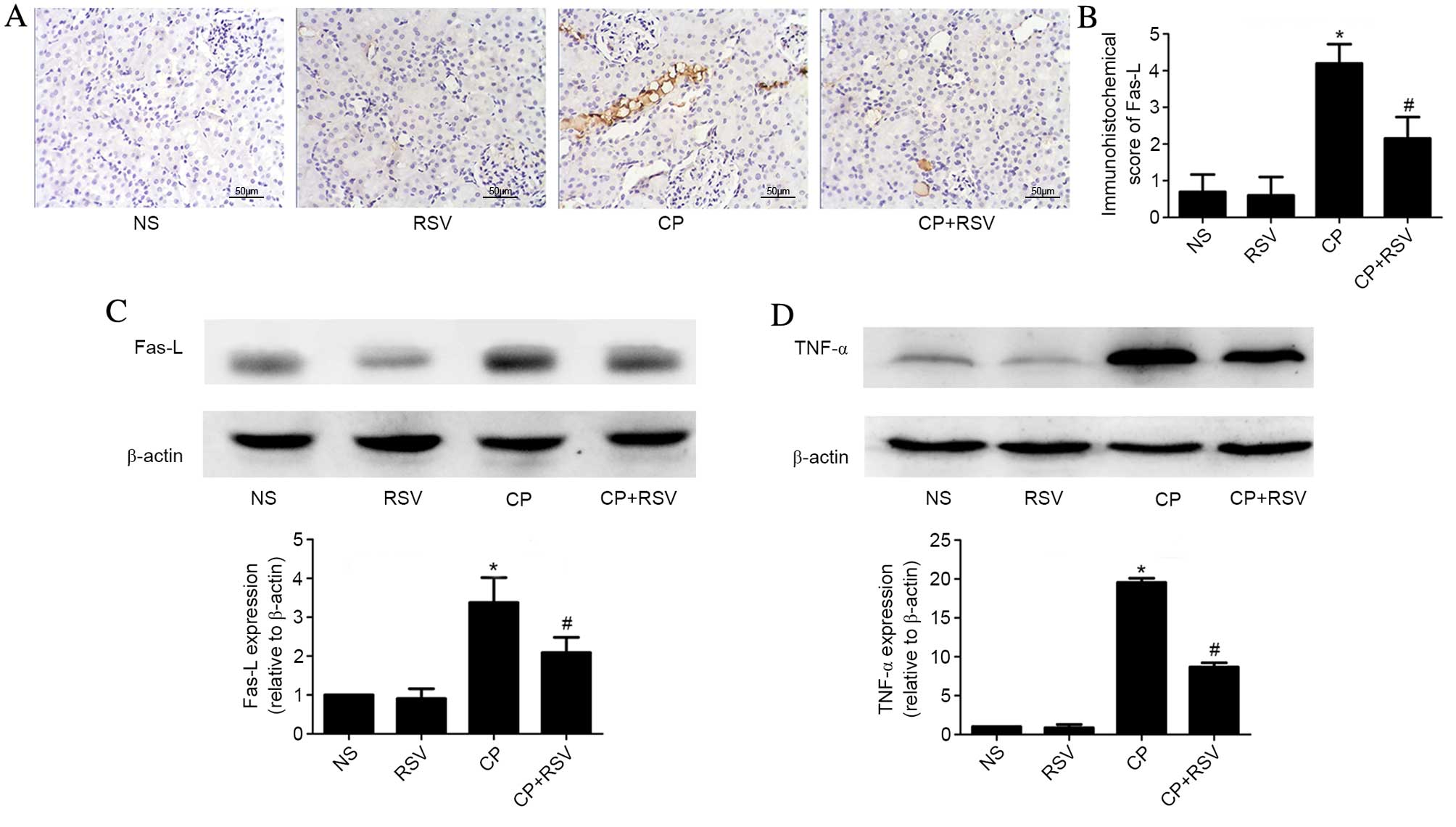
As presented in Fig. 2A
and B, Fas-L staining score in the damaged tubules was
significantly higher in the CP group compared with the NS group
(P<0.001), and significantly reduced in the CP+RSV group
compared with the CP group (P<0.001). Western blot analysis of
Fas-L protein expression was consistent with the
immunohistochemical observations (NS vs. CP, P<0.001; CP vs.
CP+RSV, P=0.004; Fig. 2C).
Similarly, TNF-α expression was significantly higher in the CP
group compared with the NS group (P<0.001), whereas the CP+RSV
group demonstrated significantly less expression of TNF-α compared
with the CP group (P<0.001; Fig.
2D).
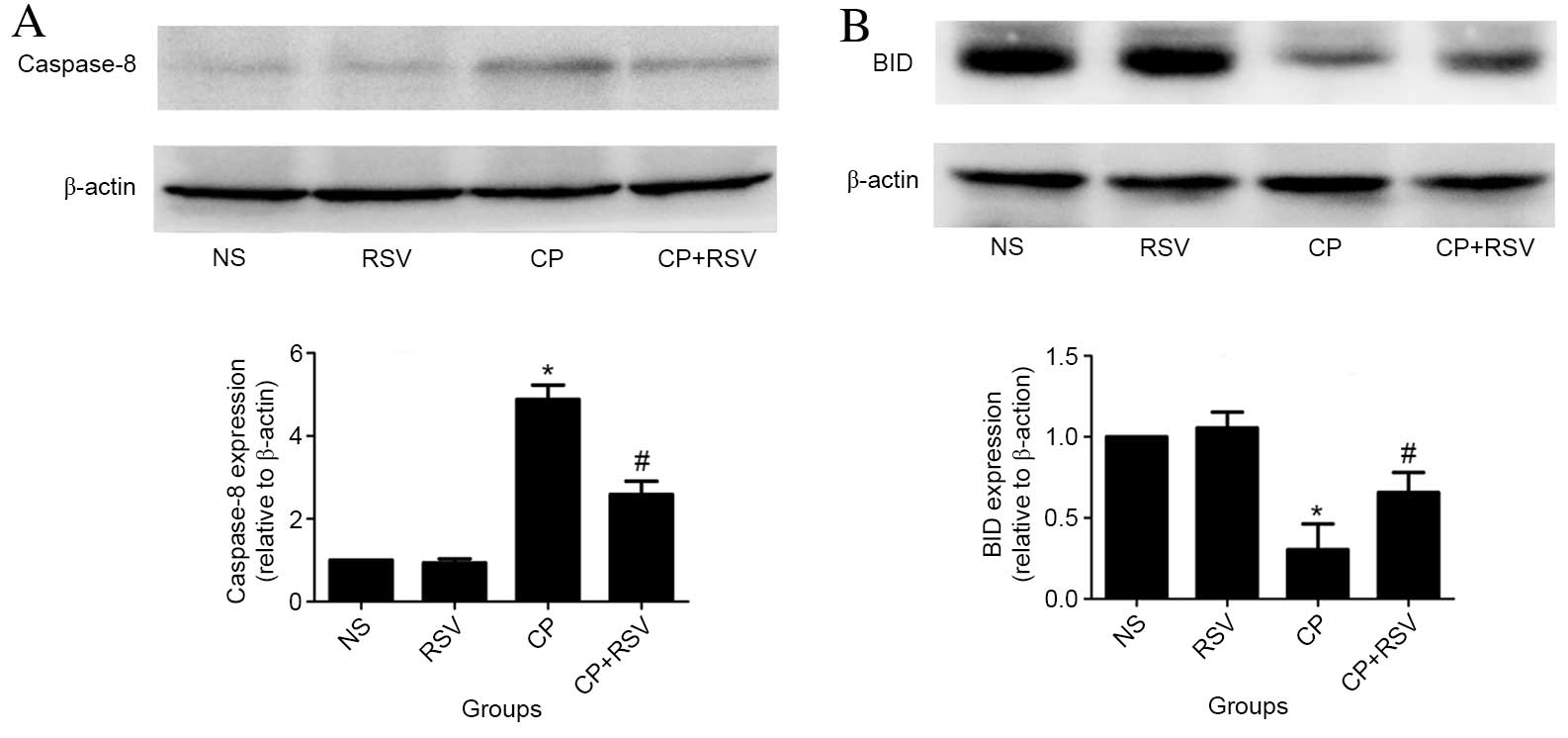
In addition, the expression of caspase-8
significantly increased in the CP group compared with the NS group
(P<0.001; Fig. 3A), while the
CP+RSV group exhibited significantly less expression than the CP
group (P<0.001). Activated caspase-8 is known to cleave BID, and
thus, may cause the significantly reduced level of BID detected by
western blot analysis in the CP group compared with the NS group
(P<0.001; Fig. 3B). By
contrast, treatment with RSV suppressed this downregulation of BID
protein levels in the CP+RSV group compared with the CP group
(P=0.005; Fig. 3B).
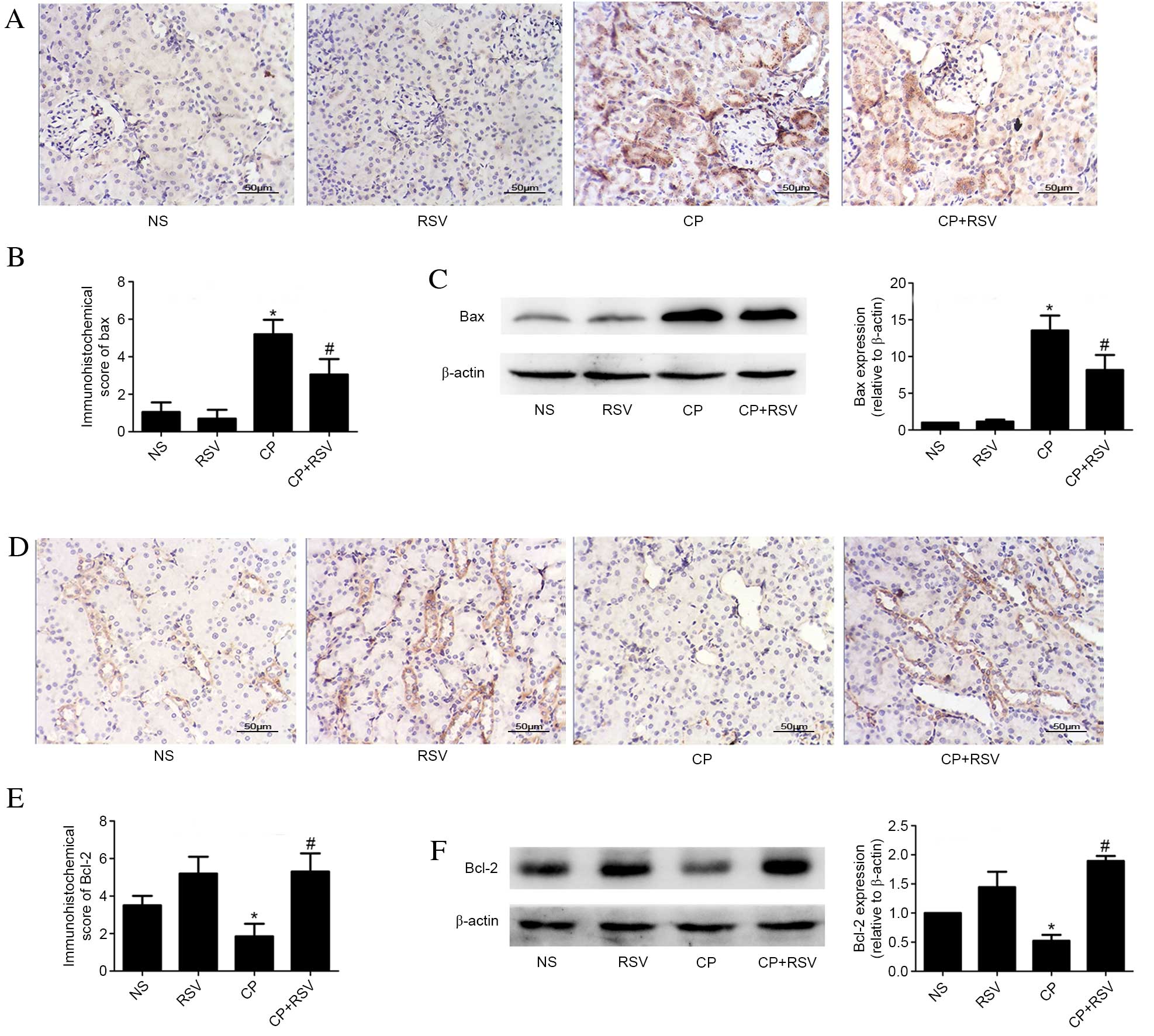
Furthermore, expression of the Bax apoptosis
regulator was measured by immunohistochemistry and western blotting
(Fig. 4). Significantly increased
levels of Bax protein expression were observed in the CP group
compared with the NS group by immunohistochemistry (P<0.001;
Fig. 4A and B) and western blot
(P<0.001; Fig 4C), while Bax
expression was significantly lower in the CP+RSV group than in the
CP group as demonstrated by both immunohistochemistry (P<0.001;
Fig. 4A and B) and western blot
(P=0.002; Fig 4C). Expression of
the anti-apoptosis regulator Bcl-2 was significantly reduced in the
CP group compared with the NS group as demonstrated by
immunohistochemistry (P<0.001; Fig.
4D and E) and western blot analysis (P=0.004; Fig. 4F). Expression of Bcl-2 detected in
kidneys from the CP+RSV group was significantly increased compared
with the CP group as demonstrated by immunohistochemistry
(P<0.001; Fig. 4D and E) and
western blot analysis (P<0.001; Fig. 4F).
Discussion
The present study sought to investigate whether RSV
exerted a renoprotective effect on CP-induced AKI in a rat model by
inhibiting death receptor-mediated apoptotic pathways. RSV was
revealed to facilitate a significant reduction in Scr, BUN and RI
levels, indicating significantly reduced renal injury and
dysfunction which was further verified by H&E staining and
apoptosis analysis of renal histology. In addition, RSV was
demonstrated to suppress the upregulation of Fas-L and TNF-α, and
to modulate the expression levels of the downstream signaling
effectors, caspase-8, BID, Bax and Bcl-2, to accomplish its
protective effect.
As a polyphenolic phytoalexin, RSV has been reported
to be beneficial for the prevention of numerous types of kidney
disease, including diabetic nephropathy (27), drug-induced renal injury (28,24),
and ischemia-reperfusion and sepsis-induced kidney injuries
(29,30). Consistent with these observations,
the results of the present study demonstrate the beneficial effects
of RSV in the prevention of renal tubular damage and dysfunction.
The protective effects of RSV were confirmed to be associated with
a reduction in Fas-L, TNF-α and caspase-8 expression levels, which
were augmented by CP. Previous studies have reported that RSV is
involved in mediating numerous signaling pathways in AKI (20,28,24),
however, no study conducted to date has demonstrated the role of
RSV in regulating extrinsic apoptotic signaling pathways.
In the present study, it remains unclear whether the
positive effects of RSV in preventing damage to renal tubular
cells, is directly associated with a reduction in the expression
levels of Fas-L, TNF-α and caspase-8. CP-induced tubular apoptosis
was initiated by Fas-L, which is expressed on renal tubular cells
and immune cells, and is capable of inducing adjacent tubule cell
death. Therefore, inhibiting Fas-L may reduce CP-induced tubular
apoptosis and completely restore the survival of mice treated with
a lethal CP dose (11). TNF-α
knockout mice exhibit reduced renal dysfunction, renal histological
injury and serum TNF-α levels (31). TNF-α receptor knockout mice also
consistently present less renal tubular cell death, further
supporting the involvement of death receptor-mediated pathways in
the pathogenesis of AKI induced by CP (11). And the present study demonstrated
that CP increased Fas-L, TNF-α and caspase-8 expression in an AKI
model, and that RSV suppressed the upregulation of these effectors.
High expression of Fas-L and TNF-α indicates the activation of the
extrinsic apoptotic pathway that can be caused by CP. With the
interaction between TNF family ligands and their receptors, the
downstream signaling molecule, caspase-8 is also activated, which
is a marker of the extrinsic apoptotic pathways (32). It has been demonstrated that RSV
can serve a protective role in CP-induced renal injury by reducing
free radicals (28) and activating
sirtuin 1 (24). The results of
the present study demonstrate that RSV can decrease Fas-L, TNF-α
and caspase-8 expression, which is similar to the effect of other
antioxidants on CP-induced renal injury, such as
epigallocatechin-3-gallate (10)
and dimethylthiourea (13).
Therefore, these findings indicate that the potential mechanism of
RSV in the prevention of CP-induced AKI involves extrinsic
apoptotic pathways.
The implementation of programmed cell death is
enforced by caspase-8 through two different pathways (14,33).
In pathway 1, the high caspase-8 concentration directly activates
the downstream effector, caspase-3. Caspase-3 is then cleaved and
stimulates apoptosis. In pathway 2, the low caspase-8 concentration
can cleave BID, a death-inducing member of the Bcl-2 family
(14,15), rather than directly activating
caspase-3 to execute programmed cell death. tBID translocates to
the mitochondria membrane (16,32,34).
Additionally, previous studies have demonstrated that tBID promotes
Bax activation and facilitates the insertion/oligomerization of Bax
into the mitochondrial outer membrane (35,36).
As a result, pores of the mitochondrial membranes are formed, and
apoptotic proteins residing in the intermembrane space are released
(36). As a member of the
anti-apoptotic Bcl-2 family, Bcl-2 can prevent mitochondrial
permeability transition of tBID and Bax, and is pivotal for the
inhibition of apoptosis (1,35).
Overexpression of Bcl-2 suppresses apoptosis (1,15),
thus, the cross talk between the mitochondrial and death
receptor-mediated apoptotic pathways occurs through caspase-8. In
the present study, CP has been suggested to increase the cleavage
of BID as the expression of BID in the CP group was significantly
lower than in the NS group, whereas compared with the CP group the
expression of BID increased upon treatment with RSV. Meanwhile, the
activation of the intrinsic mitochondrial pathway was examined
through examination of Bax and Bcl-2 expression. High level
expression of Bax and low level expression of Bcl-2 were observed
in the CP group, and the reverse phenomenon in the CP+RSV group.
Previous studies have demonstrated that caspase-8 (following
activation in the extrinsic pathway) can activate the intrinsic
apoptosis pathway through Bcl-2 family proteins, such as Bid
(16,32,34).
In the present study, CP is demonstrated to induce the apoptosis
pathway mediated by death receptors, which is similar to the
effects observed from dimethylthiourea on CP-induced renal injury
(13) and the loss of α(E)-catenin
on CP-challenged renal tubular epithelial cells (15). Finally, the expression of these
signal pathways leads to renal tubular cell apoptosis. Therefore,
RSV treatment may reduce Bax expression and upregulate BID and
Bcl-2 expression via the interaction between the extrinsic and
intrinsic signaling pathways through caspase-8, which could exert
an important protective role in the process of CP-induced AKI.
In conclusion, the present study indicated that RSV
may protect against CP-induced AKI, and the underlying mechanism is
associated with the suppression of apoptosis via death
receptor-mediated pathways. RSV may, therefore, have potential
value in the treatment of patients suffering from AKI and warrants
further investigation of its therapeutic activity in the
clinic.
Acknowledgments
The present study is supported by General Financial
Grant from China Postdoctoral Science Foundation (no. 2015M572048),
and Shandong Province Natural Science Foundation of China (grant
no. ZR2014HM037 and ZR2013HM100).
References
|
1
|
Linkermann A, Chen G, Dong G, Kunzendorf
U, Krautwald S and Dong Z: Regulated cell death in AKI. J Am Soc
Nephrol. 25:2689–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faubel S, Chawla LS, Chertow GM, Goldstein
SL, Jaber BL and Liu KD; Acute Kidney Injury Advisory Group of the
American Society of Nephrology: Ongoing clinical trials in AKI.
Clin J Am Soc Nephrol. 7:861–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lameire NH, Bagga A, Cruz D, De Maeseneer
J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W and
Vanholder R: Acute kidney injury: An increasing global concern.
Lancet. 382:170–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
dos Santos NA, Carvalho Rodrigues MA,
Martins NM and dos Santos AC: Cisplatin-induced nephrotoxicity and
targets of nephroprotection: An update. Arch Toxicol. 86:1233–1250.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sánchez-González PD, López-Hernández FJ,
López-Novoa JM and Morales AI: An integrative view of the
pathophysiological events leading to cisplatin nephrotoxicity. Crit
Rev Toxicol. 41:803–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karasawa T and Steyger PS: An integrated
view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol
Lett. 237:219–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou P, Song J, Jiang B, Pei F, Chen B,
Yang X, Liu G and Hu Z: Epigallocatechin-3-gallate protects against
cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int
J Clin Exp Pathol. 7:4607–4616. 2014.PubMed/NCBI
|
|
11
|
Linkermann A, Himmerkus N, Rölver L,
Keyser KA, Steen P, Bräsen JH, Bleich M, Kunzendorf U and Krautwald
S: Renal tubular Fas ligand mediates fratricide in
cisplatin-induced acute kidney failure. Kidney Int. 79:169–178.
2011. View Article : Google Scholar
|
|
12
|
Tsuruya K, Ninomiya T, Tokumoto M,
Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara
K, Hirakata H and Iida M: Direct involvement of the
receptor-mediated apoptotic pathways in cisplatin-induced renal
tubular cell death. Kidney Int. 63:72–82. 2003. View Article : Google Scholar
|
|
13
|
Tsuruya K, Tokumoto M, Ninomiya T,
Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Hirakata H
and Iida M: Antioxidant ameliorates cisplatin-induced renal tubular
cell death through inhibition of death receptor-mediated pathways.
Am J Physiol Renal Physiol. 285:F208–F218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scaffidi C, Fulda S, Srinivasan A, Friesen
C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME: Two
CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X and Parrish AR: Loss of
α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial
cells. Apoptosis. 20:921–929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nogae S, Miyazaki M, Kobayashi N, Saito T,
Abe K, Saito H, Nakane PK, Nakanishi Y and Koji T: Induction of
apoptosis in ischemia-reperfusion model of mouse kidney: Possible
involvement of Fas. J Am Soc Nephrol. 9:620–631. 1998.PubMed/NCBI
|
|
18
|
Schelling JR, Nkemere N, Kopp JB and
Cleveland RP: Fas-dependent fratricidal apoptosis is a mechanism of
tubular epithelial cell deletion in chronic renal failure. Lab
Invest. 78:813–824. 1998.PubMed/NCBI
|
|
19
|
Malhotra A, Bath S and Elbarbry F: An
organ system approach to explore the antioxidative,
anti-inflammatory and cytoprotective actions of resveratrol. Oxid
Med Cell Longev. 2015:8039712015. View Article : Google Scholar
|
|
20
|
Kitada M and Koya D: Renal protective
effects of resveratrol. Oxid Med Cell Longev. 2013:5680932013.
View Article : Google Scholar :
|
|
21
|
Catalgol B, Batirel S, Taga Y and Ozer NK:
Resveratrol: French paradox revisited. Front Pharmacol. 3:1412012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanamori H, Takemura G, Goto K, Tsujimoto
A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Morishita K,
Kawasaki M, et al: Resveratrol reverses remodeling in hearts with
large, old myocardial infarctions through enhanced
autophagy-activating AMP kinase pathway. Am J Pathol. 182:701–713.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turner RS, Thomas RG, Craft S, van Dyck
CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R and
Aisen PS; Alzheimer's Disease Cooperative Study: A randomized,
double-blind, placebo-controlled trial of resveratrol for Alzheimer
disease. Neurology. 85:1383–1391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP,
Lee S, Park SK, Han MK, Lee SY, Ramkumar KM, et al: SIRT1
activation by resveratrol ameliorates cisplatin-induced renal
injury through deacetylation of p53. Am J Physiol Renal Physiol.
301:F427–F435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oi N, Jeong CH, Nadas J, Cho YY, Pugliese
A, Bode AM and Dong Z: Resveratrol, a red wine polyphenol,
suppresses pancreatic cancer by inhibiting leukotriene
A4hydrolase. Cancer Res. 70:9755–9764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crandall JP, Oram V, Trandafirescu G, Reid
M, Kishore P, Hawkins M, Cohen HW and Barzilai N: Pilot study of
resveratrol in older adults with impaired glucose tolerance. J
Gerontol A Biol Sci Med Sci. 67:1307–1312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palsamy P and Subramanian S: Resveratrol
protects diabetic kidney by attenuating hyperglycemia-mediated
oxidative stress and renal inflammatory cytokines via Nrf2-Keap1
signaling. Biochim Biophys Acta. 1812:719–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Do Amaral CL, Francescato HD, Coimbra TM,
Costa RS, Darin JD, Antunes LM and Bianchi Mde L: Resveratrol
attenuates cisplatin-induced nephrotoxicity in rats. Arch Toxicol.
82:363–370. 2008. View Article : Google Scholar
|
|
29
|
Holthoff JH, Wang Z, Seely KA, Gokden N
and Mayeux PR: Resveratrol improves renal microcirculation,
protects the tubular epithelium, and prolongs survival in a mouse
model of sepsis-induced acute kidney injury. Kidney Int.
81:370–378. 2012. View Article : Google Scholar :
|
|
30
|
Liu FC, Tsai HI and Yu HP:
Organ-Protective effects of red wine extract, resveratrol, in
oxidative stress-mediated reperfusion injury. Oxid Med Cell Longev.
2015:5686342015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang B, Ramesh G, Norbury CC and Reeves
WB: Cisplatin-induced nephrotoxicity is mediated by tumor necrosis
factor-alpha produced by renal parenchymal cells. Kidney Int.
72:37–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz
FM and Gottlieb E: BID is cleaved by caspase-8 within a native
complex on the mitochondrial membrane. Cell Death Differ.
18:538–548. 2011. View Article : Google Scholar :
|
|
35
|
Ott M, Norberg E, Zhivotovsky B and
Orrenius S: Mitochondrial targeting of tBid/Bax: A role for the TOM
complex? Cell Death Differ. 16:1075–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Korsmeyer SJ, Wei MC, Saito M, Weiler S,
Oh KJ and Schlesinger PH: Pro-apoptotic cascade activates BID,
which oligomerizes BAK or BAX into pores that result in the release
of cytochrome c. Cell Death Differ. 7:1166–1173. 2000. View Article : Google Scholar
|