Introduction
Lung cancer is a common malignancy and has the
highest rate of cancer-associated mortality worldwide (1). Metastasis is the predominant cause of
mortality in patients with cancer and accounts for approximately
90% of cases of cancer associated-mortality (2). In patients with lung cancer, the
cancer cells frequently metastasize to the brain, bone, liver,
adrenal glands and other organs (3). The clonal growth of cancer cells in
these organs considerably impairs organ function and thus is
life-threatening. The metastatic cancer cells exhibit biological
characteristics different from cancer cells in the primary lesion
and tend to have higher rates of malignancy (4). At present, the therapeutic methods
used for metastatic cancer including chemotherapy, radiotherapy,
targeted therapy, biological therapy and combination therapy remain
based on the characteristics of cancer cells in the primary lesion.
It is therefore difficult to use these methods to effectively treat
metastatic cancers due to the lack of specificity. The prognosis is
often much poorer in patients with metastatic cancer than in those
with non-metastatic cancer. Therefore, it has important clinical
significance to further investigate the key cell regulatory
proteins in metastatic cancers.
TAR [human immunodeficiency virus (HIV)-1] RNA
binding protein 2 (TARBP2) is a double-stranded RNA-binding protein
and serves an important role in the physiological functions of
microRNAs. The discovery of this protein is attributable to its
ability to bind with TAR, a RNA regulatory element regulating gene
expression in the HIV genome, in addition to TAT protein, a
trans-activating factor of HIV (5). A previous study identified that
TARBP2 had a tendency to bind with guanine-cytosine-rich
double-stranded RNAs (6). Taking
these binding characteristics into account, the intracellular
TARBP2 has been suggested to bind with microRNA hairpin precursors
and serve a role in the maturation process. In addition, it has
been reported that the Dicer complex can be recruited into Ago2
complexes to implement miRNA-mediated gene silencing (7). By comparing the differences in RNA
expression profiles between metastatic and non-metastatic breast
cancer cells, Goodarzi et al (8) demonstrated that TARBP2, as a
regulatory factor for mRNA stability, was overexpressed in
metastatic breast cancer cells. The results of the current study
indicated that TARBP2 was able to bind with the mRNA hairpin
structure to mediate the expression of amyloid β (A4) precursor
protein (APP), zinc finger protein 395 (ZNF395) and additional
metastasis-suppressor proteins, in addition to down-regulating the
expression of pro-metastatic interleukin (IL)-1β, IL-8,
cyclooxygenase (COX)-2 and associated proteins. Due to the fact
that the roles of TARBP2 in metastatic cancers remain unclear, the
present study was designed to investigate the roles of TARBP2 in
metastatic non-small cell lung cancer, identifiying a basis of the
mechanisms of action at a molecular level.
Materials and methods
Reagents and instruments
The human lung cancer cell line NCI-H1299 was
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). The viral vectors and
Lipofectamine 2000 Thermo Fisher Scientific, Inc. (Waltham, MA,
USA) for small hairpin RNA (shRNA)-mediated silencing were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
(MTS) and Reverse Transcription-Quantitative Polymerase Chain
Reaction (RT-qPCR) kits were purchased from Promega Corporation
(Madison, WI, USA). The in vitro invasion test kit was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The
monoclonal antibodies were purchased from Abcam (Cambridge, MA,
USA). The Enhanced Chemiluminescence (ECL) Immunoblotting Substrate
kit was purchased from EMD Millipore (Billerica, MA, USA). The
Multiskan FC microplate reader and Arktik Thermal Cycler for PCR
were purchased from Thermo Fisher Scientific, Inc..
Cell culture and induction of the highly
metastatic cell line
The cells were maintained in RPMI-1640 medium
supplemented with 10% fetal calf serum (Corning Incorporated,
Manassas, VA, USA) and were cultured in a 37°C incubator under 5%
CO2 and saturated humidity. The cells were digested with
0.25% trypsin-EDTA (Beyotime Institute of Biotechnology, Shanghai,
China) for passaging. Cells in the logarithmic growth phase were
used in all experiments. In order to screen out the highly
metastatic cell line, H1299 cells were cultured in the upper
chamber of Transwell (Corning Incorporated, Corning, NY, USA)
coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for
24 h. Subsequently, the cells migrating to the lower chamber were
collected for clone culture. The above procedures were repeated 10
times to screen out the highly metastatic cell clones.
shRNAs silences TARBP2
Two different shRNAs, termed sh1 and sh2, were used
to achieve different TARBP2 silencing effects. The Sigma-Aldrich
clone numbers for sh1 and sh2 were TRCN0000330642 and
TRCN000019339, respectively, and they were incorporated into the
lentivirus vector (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
using Invitrogen Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.). Subsequent to culture to the logarithmic growth phase, these
cells were transfected in accordance with the manufacturer's
instructions (Sigma-Aldrich), then the successfully transfected
cells were screened out.
MTS cell proliferation assay
Cells in the logarithmic growth phase
(5×104 cells/ml) were seeded into 96-well microplates
(100 µl/well) and cultured overnight to allow cell adhesion.
Subsequent to continued culture for 48 h at 4°C, the medium was
removed and MTS was added in accordance with manufacturer's
instructions, with continued culture for 4 h at 4°C. Finally, the
optical density value was determined to measure the cell counts at
a wavelength of 490 nm with the microplate reader.
In vitro invasion and migration test
The CultreCoat® 96-Well BME-Coated Cell
Invasion Optimization Assay kit supplied by R&D Systems, Inc.
was used to evaluate the in vitro invasive ability of cancer
cells. Subsequent to starvation in serum-free RPMI-1640 for 16 h,
the cells were collected and seeded into the upper chamber of the
Transwell system at a density of 2.5 × 104 cells/well.
Following continued culture for 48 h, the number of invasive cells
was assessed in accordance with the manufacturer's instructions.
The in vitro migrative ability of cancer cells was evaluated
with the wound-healing assay by culturing the cells in 6-well
plates until monolayer fusion occurred. Subsequent to starvation in
serum-free medium overnight, 200-µl pipette tips were used
to score the cell layer. Subsequent to continued culture for 48 h,
the distance between the scorings was observed and measured under
an SZX16 stereo microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
Using β-actin (1:5,000; ab8227; Abcam) as an
internal control, the expression levels of the following proteins
were detected by western blotting with the following antibodies
from Abcam: Mouse monoclonal TARBP2 (1:2,000; ab129325), IL-1β
(1:2,000; ab8320) and IL-8 (1:2,000; ab18672); rabbit polyclonal
APP (1:2,000; ab59592), ZNF395 (1:2,000; ab75727), phosphorylated
c-Jun N-terminal kinase (p-JNK; 1:1,000; ab4821), matrix
metalloproteinase 2 (MMP2; 1:2,000; ab37150) and MMP9 (1:2,000;
ab38898); goat polyclonal COX-2 (1:2,000; ab35995); mouse
monoclonal signal transducer and activator of transcription 3
(STAT3; 1:2,000; ab119352); and rabbit monoclonal JNK (1:1,000;
ab76125), p-STAT3 (1:1,000; ab76315), protein kinase B (AKT;
1:2,000; ab32505), and p-AKT (1:1,000; ab81283). The cell lysates
were collected by centrifugation at 13,000 × g for 30 min at 4°C
prior to extraction of the proteins with a protein extraction kit
(Beyotime Institute of Biotechnology) and separation using 12%
sodium dodecyl sulfate-polyacrylimide gel electrophoresis (Beyotime
Institute of Biotechnology). The proteins were then transferred
onto a polyvinylidene difluoride membrane to detect the target
proteins incubating with the antibodies at 4°C overnight.
Subsequent to washing with PBS with 5% Tween 80 (Beyotime Institute
of Biotechnology), the goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:2,500; W4011; Promega
Corporation) was added with incubation for 1 h at 37°C. Following
several washes, the ECL kit was used to develop the immunoreactive
bands.
RT-qPCR
Subsequent to extraction of the total RNA in each
group using Invitrogen TRIzol (Thermo Fisher Scientific, Inc.),
reverse transcription was conducted using the RT-qPCR kit (Takara
Biotechnology, Co., Ltd., Dalian, China) to obtain the cDNA (1
µl). mRNA expression levels of TARBP2, APP, ZNF395, IL-1β,
IL-8 and COX-2 were then detected. The primer sequences used were
as follows: TARBP2, forward 5′-CAG GAG TAT GGG ACC AGA ATA GG-3′
and reverse 5′-ACC CGG AAG GTG AAA TTA GGC -3′; APP, 5′-AAC CAC CGT
GGA GCT CCT T-3′ and reverse 5′-ATG CCA CGG CTG GAG ATC -3′;
ZNF395, forward 5′-TCA TGG CTT TGA GAC CGA TCC -3′ and reverse
5′-CCA CAA TGG AGC GCA GAA CT-3′; IL-1β, forward 5′-GCA CGA TGC ACC
TGT ACG AT-3′ and reverse 5′-CAC CAA GCT TTT TTG CTG TGA GT-3′;
IL-8, forward 5′-CAA GAG CCA GGA AGA AAC CA-3′ and reverse 5′-GTC
CAC TCT CAA TCA CTC TCAG-3′; COX-2, forward 5′-CAG CCA TAC AGC AAA
TCC T-3′ and reverse 5′-TCT CCA TAG AAT CCT GTCCG-3′; MMP2, forward
5′-TGA TCT TGA CCA GAA TAC CAT CGA-3′ and reverse 5′-GGC TTG CGA
GGG AAG AAG TT-3′; MMP9, forward 5′-GTG CTG GGC TGC TGC TTT GCTG-3′
and reverse 5′-GTC GCC CTC AAA GGT TTG GAA T-3′; GAP DH, forward
5′-CTT AGA TTT GGT CGT ATT GG-3′ and reverse 5′-GAA GAT GGT GAT GGG
ATT -3′. PCR was conducted using an ABI7500 Real-Time PCR system
(Thermo Fisher Scientific, Inc.) with 2 µg cDNA and the
following cycling conditions: Annealing at 54°C for 60 sec, and 40
cycles of 95°C for 2 min, 94°C for 10 sec, 68°C for 1 min. The
2−ΔΔCq method was used for quantification (9).
Statistical analysis
The experimental data were presented as the mean ±
standard deviation and were analyzed using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
was used for comparison and P<0.05 was considered to indicate a
statistically significant difference.
Results
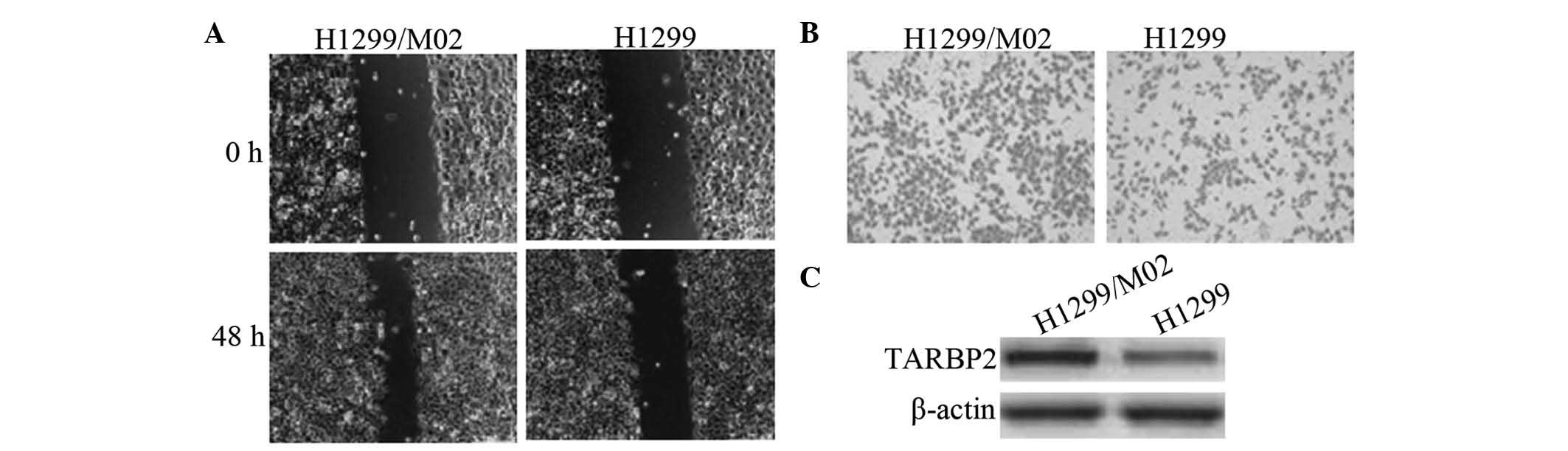
H1299/M02 is highly metastatic and
overexpresses TARBP2
Subsequent to in vitro screening, highly
metastatic cell clones were successfully obtained. One of the
clones was named as H1299/M02, and was identified by the in
vitro invasion assay and the wound-healing assay to exhibit
greater invasive ability than that of the parent cell line H1299.
In addition, western blot analysis indicated that this clone
overexpressed TARBP2 (Fig. 1).
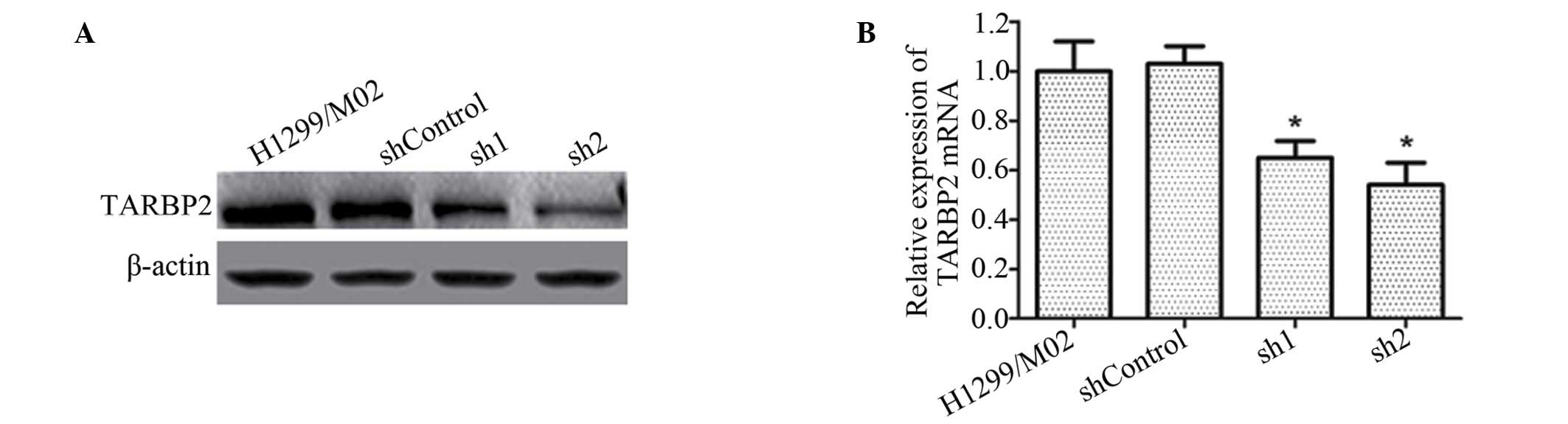
sh1 and sh2 significantly inhibit TARBP2
expression
The shRNA-transfected cell clones were successfully
obtained. Western blotting and RT-qPCR analysis indicated that sh1
and sh2 silencing resulted in a significant reduction in the
expression levels of TARBP2 in H1299/M02 cells, to ~45% and 23% of
the shControl group level in protein levels, respectively. No
significant differences were observed between the shControl and
H1299/M02 groups (Fig. 2).
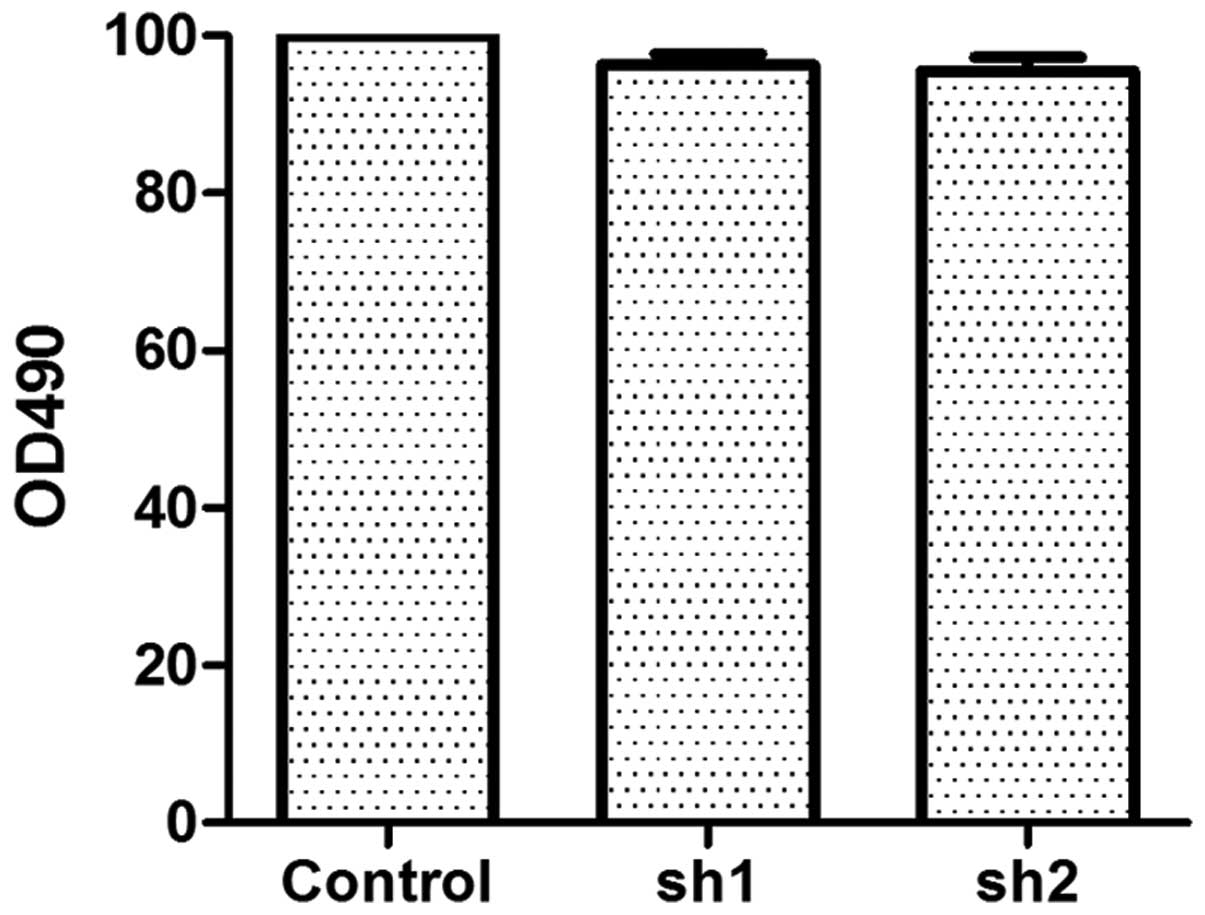
TARBP2 silencing does not significantly
affect cell growth
As indicated by the results of the MTS cell
proliferation assay, although sh1 and sh2 downregulated TARBP2
expression in H1299/M02 cells, the cell growth was not affected
significantly (Fig. 3).
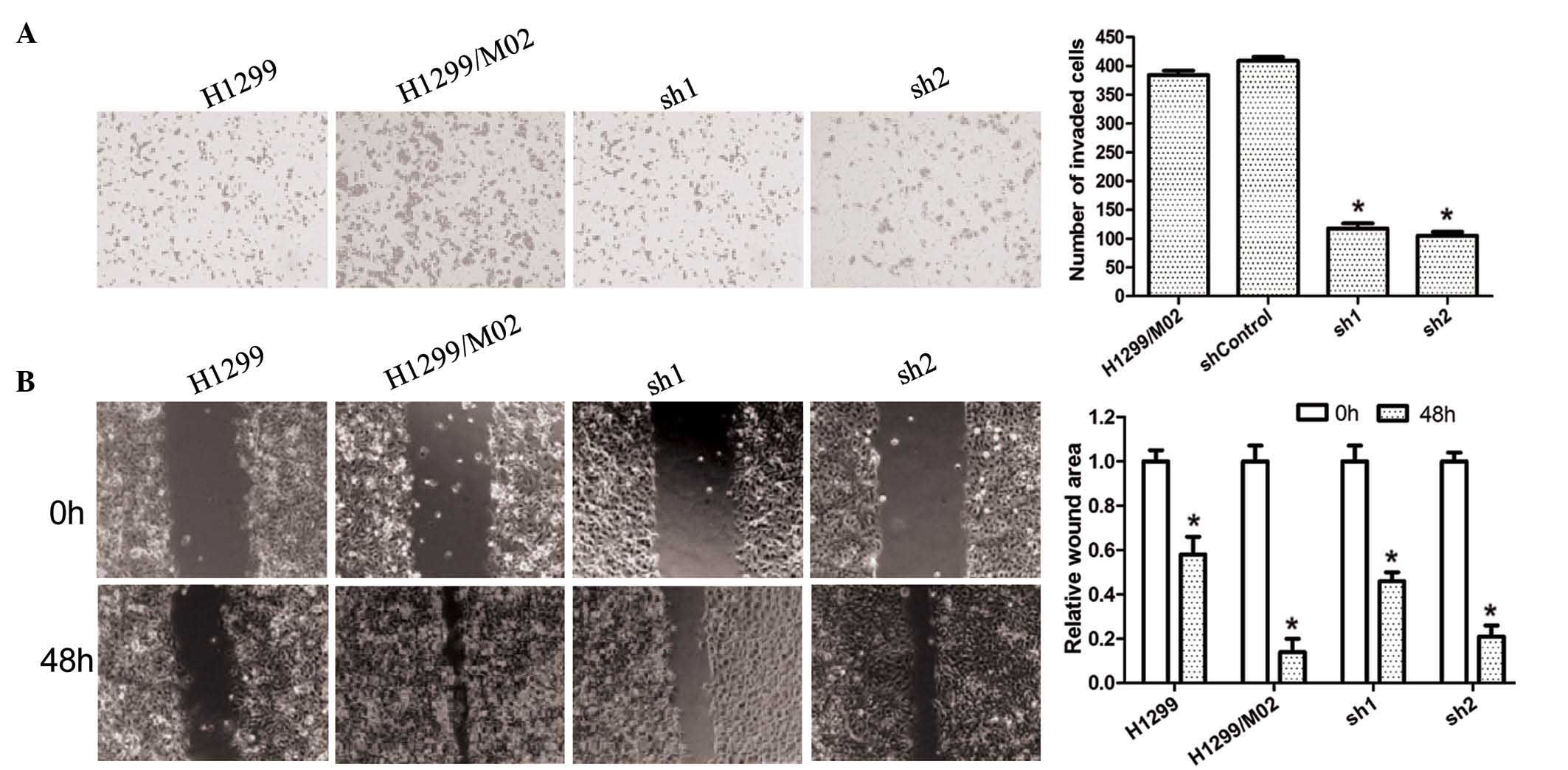
TARBP2 silencing inhibits in vitro
invasion and migration
As demonstrated by culture in Transwell for 24 h,
invasive cells were clearly observed in the H1299/M02 group when
compared with the H1299 group; whereas the number of invasive cells
was significantly reduced in the sh1 and sh2 groups when compared
with the H1299/M02 group. The wound-healing assay also indicated
that 48 h subsequent to scoring, no significant alterations in the
wound widths were observed in the H1299 group. Wound width was the
smallest in the H1299/M02 group while the widths in the sh1 and sh2
groups were significantly reduced compared with that of 0 h
(Fig. 4). These assay results
indicated that silencing of TARBP2 was able to inhibit the in
vitro invasion and migration of cancer cells, which was
associated with the extent of TARBP2 silencing.
TARBP2 silencing upregulates the
expression of APP and ZNF395, and downregulates the expression
levels of IL-1β, IL-8, COX-2, MMP2 and MMP9 in H1299/M02 cells
Western blot analysis results indicated that APP and
ZNF395 protein expression was significantly reduced in the
H1299/M02 cells when compared with that of the H1299 cells. The APP
and ZNF395 expression levels were then signifiantly increased when
TARBP2 was silenced by sh1 and sh2. In contrast, IL-1β, IL-8,
COX-2, MMP2 and MMP9 expression levels were significantly increased
in H1299/M02 cells, whereas expression of these proteins was
significantly reduced in cells in the sh1 and sh2 groups. RT-qPCR
results indicated that the regulation of expression of these
proteins occurred at the transcriptional level (Fig. 5).
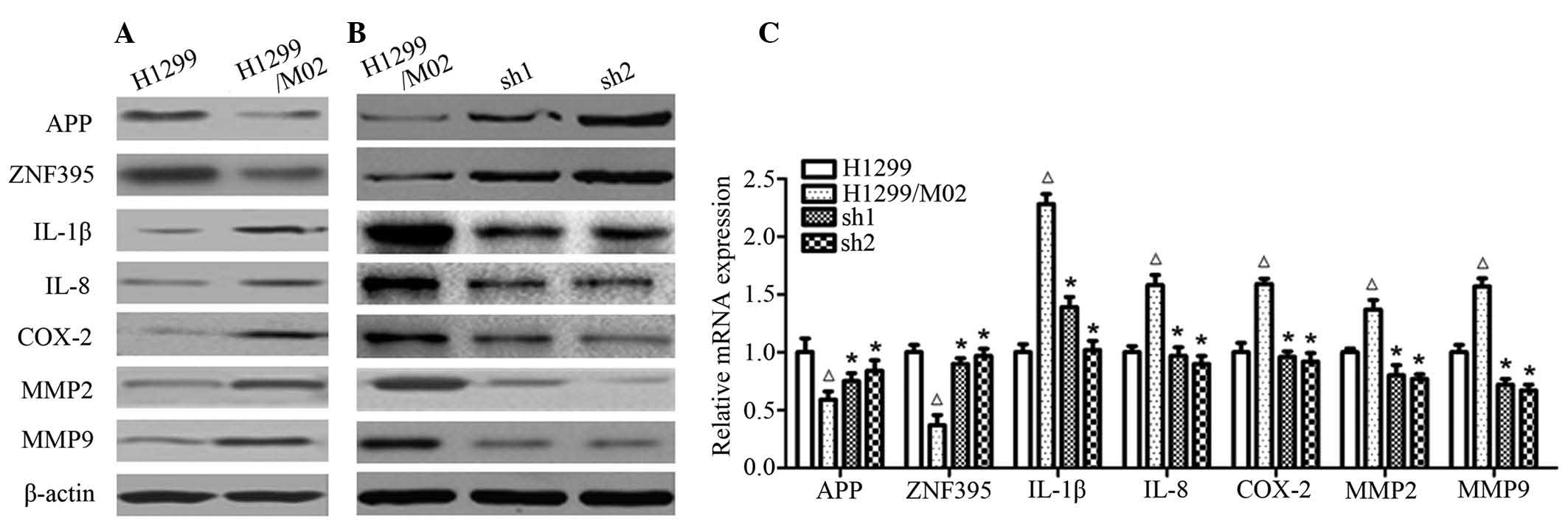 | Figure 5The effect of TARBP2 silencing on the
expression of tumor-associated genes in lung cancer cell lines. (A)
Immunoblotting for the proteins associated with migration and
invasion in H1299/M02 and H1299 cell lines. Cell lysates were
prepared and subjected to enhanced chemiluminescence-western
blotting using β-actin as the internal control. Results are
representative of three independent experiments. (B) H1299/M02 cell
lines were transfected with TARBP2 shRNA1 (sh1) and shRNA2 (sh2) or
negative control, then the proteins associated with migration and
invasion were analyzed by immunoblotting, with β-actin as the
internal control. (C) mRNA expression levels of the assessed genes
were analyzed by RT-qPCR assay. Glyceraldehyde 3-phosphate
dehydrogenase was used as the internal control for RT-qPCR.
ΔP<0.05 vs. H1299 group; *P<0.05 vs.
H1299/M02 group. TARBP2, TAR (human immunodeficiency virus 1) RNA
binding protein 2; sh, small hairpin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; APP, amyloid
β (A4) precursor protein; ZNF395, zinc finger protein 395; IL,
interleukin; COX-2, cyclooxygenase 2; MMP, matrix
metalloproteinase. |
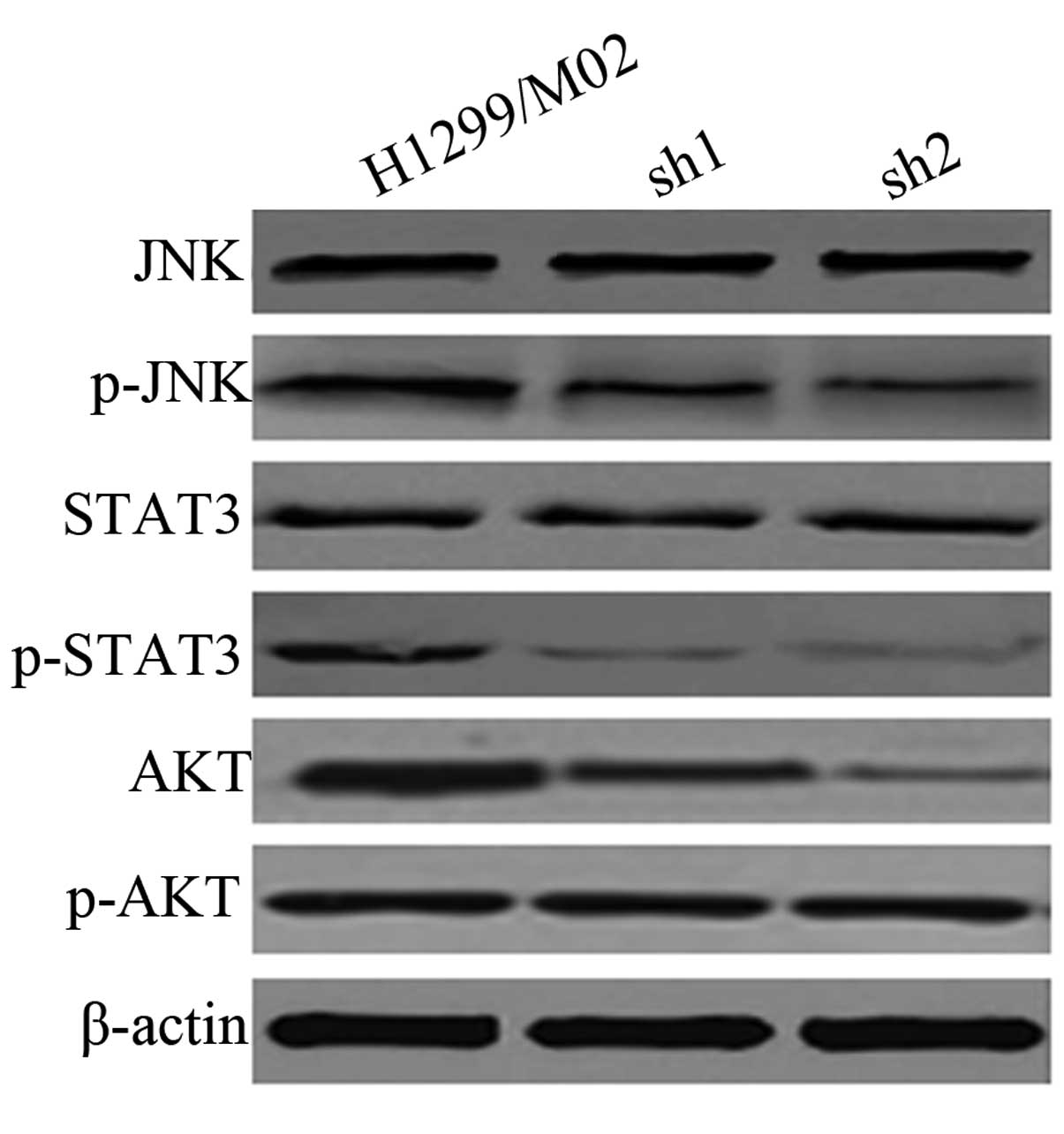
TARBP2 inhibits the phosphorylation of
JNK. STAT3 and AKT
Western blot analysis indicated that JNK and STAT3
expression levels were not significantly affected by TARBP2
silencing. As presented in Fig. 6,
the phosphorylation of JNK, STAT3 and AKT was reduced when TARBP2
was silenced in highly metastatic cancer cells, indicating that
activity of the JNK/STAT3/AKT signaling pathway was inhibited.
Discussion
In the current study, the highly metastatic and
TARBP2-overexpressing non-small cell lung cancer cell line
H1299/M02 was obtained, and TARBP2-silencing cell clones were
obtained using the shRNA transfection technique. Results of the
Transwell assay and wound-healing assay indicated significant
reductions of cell invasive and migratory abilities subsequent to
TARBP2 silencing. By contrast, the MTS assay indicated that
silencing TARBP2 had no significant effect on cell growth,
suggesting that TARBP2-mediated cell invasion and migration was not
correlated with cell growth. Accordingly, the mechanisms by which
TARBP2 inhibited H1299/M02 cell metastasis were further
investigated.
APP is first a membrane protein and later forms a
soluble product subsequent to digestion by proteases, upon which it
serves a key role in Alzheimer's disease (10). Previous studies have identified
that APP additionally served an important role in cancer. It was
reported that TARBP2 overexpression in metastatic breast cancer
resulted in downregulation of APP expression, resulting in a
reduction of its ability to suppress cancer cell metastasis
(11). Fan et al (12) identified in ovarian cancer that
miR-20a was able to target and downregulate APP expression to
promote cancer cell proliferation and invasion. However, additional
studies on prostate cancer (13,14)
suggested that APP exerted the opposite effect, promoting prostate
cancer cell growth and invasion, suggesting that the role of APP in
cancer may be associated with specific disease and cell types. In
the current study, it was observed that APP expression was
upregulated with the silencing of TARBP2 and the suppression of
H1299/M02 metastasis, indicating that APP may serve a tumor
suppressor role in these cancer cells. ZNF395 is associated with
Huntington's disease and is involved in Huntington gene expression
(15). Similar to that of APP,
previous studies have indicated that ZNF395 is involved in the
pathological progression of cancer. The report showed that TARBP2
over-expression in metastatic breast cancer was able to
downregulate ZNF385 expression, suggesting a tumor suppressor role
of TARBP2 (9). Pang et al
(16) identified that miR-525-3p
promoted the metastasis and invasion of liver cancer cells by
downregulating ZNF395 expression. In the current study,
downregulation of ZNF395 expression in highly metastatic H1299/M03
cells and upregulation of ZNF395 expression subsequent to TARBP2
silencing-mediated supression of H1299/M03 metastasis was observed.
This suggested that ZNF395 additionally exerted inhibitory effects
on metastasis in these cells. It was demonstrated that ZNF395 was
able to inhibit the expression of IL-1β, IL-8 and COX-2, which was
observed in the H1299/M02 cells in the present study. Previous
studies have indicated that IL-1β and IL-8 serve important roles in
lung cancer metastasis (17–21).
MMPs are important in the promotion of angiogenesis, tumor invasion
and tumor metastasis. MMP2 and MMP9, which can degrade collagen IV,
the major extracellular membrane component of the basement
membrane, have been suggested to be critical for the invasive and
metastatic potential in lung carcinoma, activating JNK/STAT3/AKT
and additional signaling pathways via autocrine or paracrine
mechanisms (22–24). It has been previously observed that
MMP2 and MMP9 were able to promote the growth and metastasis of
tumor cells, in addition to microenvironment remodeling and
angiogenesis (25,26). AKT, as an important downstream
effector of STAT3, serves a key role in tumor-associated signaling
pathways, in order to regulate the expression and activity of
tumor-associated genes and transcription factors, respectively. In
the current study, ZNF395 was observed to inhibit the activity of
the JNK/STAT3/AKT signaling pathway.
In conclusion, the present study demonstrated that
TARBP2 serves a role in the highly metastatic H1299/M02 cell line;
silencing TARBP2 can inhibit the in vitro invasion and
migration of H1299/M02 cells; and its mechanisms of action may be
associated with the regulation of APP, ZNF395 and COX-2 expression
and the JNK/STAT3/AKT signaling pathway.
Acknowledgments
The present study was supported by Tianjin Medical
University Cancer Institute and Hospital Level Program (grant no.
Y1302).
References
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: epidemiology, etiology and prevention. Clin Chest Med.
32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spano JP, Chouahnia K and Morère JF:
Cyclooxygenase 2 inhibitors and lung carcinoma. Bull Cancer.
91(Suppl 2): S109–S112. 2004.In French.
|
|
3
|
D'Addario G, Früh M, Reck M, Baumann P,
Klepetko W and Felip E; ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 21(Suppl 5):
v116–v119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gatignol A, Buckler-White A, Berkhout B
and Jeang KT: Characterization of a human TAR RNA-binding protein
that activates the HIV-1 LTR. Science. 251:1597–1600. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gatignol A, Buckler C and Jeang KT:
Relatedness of an RNA-binding motif in human immunodeficiency virus
type 1 TAR RNA-binding protein TRBP to human P1/dsI kinase and
Drosophila staufen. Mol Cell Biol. 13:2193–2202. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chendrimada TP, Gregory RI, Kumaraswamy E,
Norman J, Cooch N, Nishikura K and Shiekhattar R: TRBP recruits the
Dicer complex to Ago2 for microRNA processing and gene silencing.
Nature. 436:740–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goodarzi H, Zhang S, Buss CG, Fish L,
Tavazoie S and Tavazoie SF: Metastasis-suppressor transcript
destabilization through TARBP2 binding of mRNA hairpins. Nature.
513:256–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Zhang X, Qi L, Cai Y, Yang P, Xuan
G and Jiang Y: HULC long noncoding RNA silencing suppresses
angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling
pathway in human gliomas. Oncotarget. 12:14429–14440. 2016.
|
|
10
|
Kuhn PH, Wang H, Dislich B, Colombo A,
Zeitschel U, Ellwart JW, Kremmer E, Rossner S and Lichtenthaler SF:
ADAM10 is the physiologically relevant, constitutive
alpha-secretase of the amyloid precursor protein in primary
neurons. EMBO J. 29:3020–3032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pastuszak-Lewandoska D, Domańska D,
Czarnecka KH, Kordiak J, Migdalska-Sęk M, Nawrot E, Kiszałkiewicz
J, Antczak A, Górski P and Brzeziańska E: Expression of STAT5,
COX-2 and PIAS3 in correlation with NSCLC histhopathological
features. PLoS One. 9:e1042652014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan X, Liu Y, Jiang J, Ma Z, Wu H, Liu T,
Liu M, Li X and Tang H: miR-20a promotes proliferation and invasion
by targeting APP in human ovarian cancer cells. Acta Biochim
Biophys Sin (Shanghai). 42:318–324. 2010. View Article : Google Scholar
|
|
13
|
Gough M, Blanthorn-Hazell S, Delury C and
Parkin E: The E1 copper binding domain of full-length amyloid
precursor protein mitigates copper-induced growth inhibition in
brain metastatic prostate cancer DU145 cells. Biochem Biophys Res
Commun. 453:741–747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyazaki T, Ikeda K, Horie-Inoue K and
Inoue S: Amyloid precursor protein regulates migration and
metalloproteinase gene expression in prostate cancer cells. Biochem
Biophys Res Commun. 452:828–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka K, Shouguchi-Miyata J, Miyamoto N
and Ikeda JE: Novel nuclear shuttle proteins, HDBP1 and HDBP2, bind
to neuronal cell-specific cis-regulatory element in the promoter
for the human Huntington's disease gene. J Biol Chem.
279:7275–7286. 2004. View Article : Google Scholar
|
|
16
|
Pang F, Zha R, Zhao Y, Wang Q, Chen D,
Zhang Z, Chen T, Yao M, Gu J and He X: MiR-525-3p enhances the
migration and invasion of liver cancer cells by downregulating
ZNF395. PLoS One. 9:e908672014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yano S, Nokihara H, Yamamoto A, Goto H,
Ogawa H, Kanematsu T, Miki T, Uehara H, Saijo Y, Nukiwa T and Sone
S: Multifunctional interleukin-1beta promotes metastasis of human
lung cancer cells in SCID mice via enhanced expression of
adhesion-, invasion- and angiogenesis-related molecules. Cancer
Sci. 94:244–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng D, Kong H and Li Y: Prognostic
values of VEGF and IL-8 in malignant pleural effusion in patients
with lung cancer. Biomarkers. 18:386–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng CY, Hsieh HL, Sun CC, Lin CC, Luo SF
and Yang CM: IL-1 beta induces urokinase-plasminogen activator
expression and cell migration through PKC alpha, JNK1/2 and
NF-kappaB in A549 cells. J Cell Physiol. 219:183–193. 2009.
View Article : Google Scholar
|
|
20
|
Carmi Y, Rinott G, Dotan S, Elkabets M,
Rider P, Voronov E and Apte RN: Microenvironment-derived IL-1 and
IL-17 interact in the control of lung metastasis. J Immunol.
186:3462–3471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryan BM, Pine SR, Chaturvedi AK, Caporaso
N and Harris CC: A combined prognostic serum interleukin-8 and
interleukin-6 classifier for stage 1 lung cancer in the prostate,
lung, colorectal, and ovarian cancer screening trial. J Thorac
Oncol. 10:1494–1503. 2014. View Article : Google Scholar
|
|
22
|
Iizasa T, Fujisawa T, Suzuki M, Motohashi
S, Yasufuku K, Yasukawa T, Baba M and Shiba M: Elevated levels of
circulating plasma matrix metalloproteinase 9 in non-small cell
lung cancer patients. Clin Cancer Res. 5:149–153. 1999.PubMed/NCBI
|
|
23
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiraga M1, Yano S, Yamamoto A, Ogawa H,
Goto H, Miki T, Miki K, Zhang H and Sone S: Organ heterogeneity of
host-derived matrix metalloproteinase expression and its
involvement in multiple-organ metastasis by lung cancer cell lines.
Cancer Res. 62:5967–5973. 2002.PubMed/NCBI
|
|
25
|
Jacobs EJ, Hsing AW, Bain EB, Stevens VL,
Wang Y, Chen J, Chanock SJ, Zheng SL, Xu J and Thun MJ:
Polymorphisms in angiogenesis-related genes and prostate cancer.
Cancer Epidemiol Biomarkers Prev. 17:972–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiao Y, Sun KK, Zhao L, Xu JY, Wang LL and
Fan SJ: Suppression of human lung cancer cell proliferation and
metastasis in vitro by the transducer of ErbB-2.1 (TOB1). Acta
Pharmacol Sin. 33:250–260. 2012. View Article : Google Scholar
|