Introduction
Lymphoma is the fifth most common type of cancer;
~90% of cases of which are non-Hodgkin lymphoma (NHL) and the
remaining 10% are Hodgkin lymphoma. Lymphoma is currently one of
the fastest-growing cancers, with an annual increase in rate of
4–5% (1,2). NHL consists of a large group of
immune system neoplasms, and represents a heterogeneous group of
diseases that are characterized by the monoclonal expansion of B or
T lymphocytes (3,4). The classification of NHL is diverse,
and includes diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma (FL), extranodal lymphoma of mucosa-associated lymphoid
tissue (MALT) and mantle cell lymphoma (5,6).
Despite marked efforts to develop novel
therapeutics, and recently observed improvements in overall
survival rate, which are likely due to the routine incorporation of
monoclonal antibody therapy, NHL remains predominantly incurable
with standard therapeutic approaches (7,8).
Previous studies have demonstrated that adhesion to cultured
stromal cells or ligand-coated surfaces is able to protect
malignant B cells from chemotherapy-induced apoptosis; this process
is known as cell adhesion-mediated drug resistance (CAM-DR)
(9,10). Bone marrow stroma has long been
known as a 'sanctuary site' for lymphoma cells during traditional
chemotherapy (8). Two modes of
CAM-DR have been described: i) Cell interaction, known as the
stromal model, in which cells interact with a stromal cell
monolayer, establishing CAM-DR by heterocellular cell interaction;
ii) substrate interaction, known as the fibronectin (FN) model, in
which CAM-DR is mediated by cell-substrate interaction. Although
the initial type of interaction markedly differs between these
models, the subsequently activated intracellular molecular
mechanisms are often similar, or even identical (11).
Human far upstream element (FUSE) binding protein 1
(FBP1) was initially recognized as a factor that binds to the FUSE
DNA sequence, which is located upstream of the c-myc proto-oncogene
promoter (12). It has previously
been reported that the c-myc oncogene is associated with apoptosis,
growth and proliferation, and that FBP and c-myc share the same
expression pattern (13).
Furthermore, FBP and c-myc are expressed in proliferating cells,
but not in quiescent or differentiated cells (13). FBP1 has been reported to be a
potential c-myc regulator in renal cancer, but not in prostate and
bladder cancer (12). In addition,
several proteins, including FBP1, have been shown to be modified in
Jurkat T cells during apoptosis (14). A previous study demonstrated that
knockdown of FBP1 in hepatocellular carcinoma (HCC) cells resulted
in increased sensitivity to apoptotic stimuli and reduced cell
proliferation (15). Undoubtedly,
FBP1 is a multifunctional protein; however, its function in
lymphoma remains unknown.
The present study investigated the expression of
FBP1 in various histological types of human B-cell NHL, and
determined its prognostic role in NHL. Since stromal cell-mediated
drug resistance is a common feature of chronic B-cell malignancies,
including multiple myeloma and chronic lymphocytic leukemia
(16,17), the present study also investigated
the role of FBP1 in CAM-DR in NHL. The results may provide a novel
perspective for a better understanding of the mechanism underlying
drug resistance in NHL.
Materials and methods
Pathological samples
The present study collected 99 B-cell lymphoma and
19 reactive lymphadenopathy (RL) biopsy samples, which were
histopathologically and clinically diagnosed at the Affiliated
Cancer Hospital of Nantong University (Nantong, China), between
January 1, 1993 and April 1, 2005. Diagnoses were made according to
the World Health Organization criteria (18). Written informed consent was
obtained from all patients prior to obtaining specimens for the
present study. All of the tissues were fixed with formalin and were
embedded in paraffin and sectioned (3–4 mm) for histopathological
diagnosis and immunohistochemical study. The study was approved by
the ethics committee of the Affiliated Cancer Hospital of Nantong
University.
Immunohistochemistry
Immunohistochemical staining was performed with a
Dako Autostainer (Dako Denmark A/S, Glostrup, Denmark) using a
polymer detection system. Briefly, sections were deparaffinized in
xylene and were rehydrated in a graded series of ethanol. Hydrogen
peroxide (0.3%) was used to block endogenous peroxide activity for
10 min. Slides were then incubated with anti-FBP1 (1:900; cat. no.
sc-271241; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 4 h
at room temperature. The tissue sections were then counterstained
with hematoxylin, dehydrated and mounted.
For assessment of FBP1, >2,000 cells from five
high-power fields in each specimen were selected randomly, and the
staining was examined by light microscopy to determine the mean
percentage. Tumor cell proportion was scored as follows: 0, no
positive tumor cells; 1, 1–24% positive tumor cells; 2, 25–49%
positive tumor cells; and 3, 50–100% positive tumor cells. Staining
intensity was graded according to the following criteria: 0, no
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining (19). The product of
staining intensity and the proportion of positive tumor cells was
used to calculate the staining index (SI) (20). Using this method, FBP1 expression
was examined in lymph node tissues by calculating the SI (scores,
0, 1, 2, 3, 4, 6 or 9). With the SI, an optimal cutoff value was
identified: SI score ≥4 was used to define tumors with high FBP1
expression, and SI score ≤3 was used to indicate low FBP1
expression.
Cell lines, co-culture and adhesion
assay
The Daudi and OCI-LY8 human malignant lymphoma cell
lines were cultured in RPMI-1640 supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 2 mM L-glutamine at 37°C in an atmosphere containing 5%
CO2. The HS-5 human bone mesenchymal stem cell line was
obtained from Shanghai Bioleaf Biotech Co., Ltd. (Shanghai, China),
and was cultured in RPMI F12 (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). Six-well culture dishes were coated overnight
at 37°C with a monolayer of HS-5 cells or 5
µg/cm2 human FN (Sigma-Aldrich; Merck Millipore).
Lymphoma cell lines were then allowed to adhere to the
pre-established monolayer of HS-5 cells or FN, or were maintained
in suspension for 12–24 h at 37°C. Subsequently, lymphoma cells
were carefully removed, and the monolayer of HS-5 cells remained
intact.
The Huh-7 human HCC and L02 normal liver cell lines
were purchased from the Cell Library of the Chinese Academy of
Science (Beijing, China). Cells were maintained in Dulbecco's
modified Eagle's medium (Sigma-Aldrich; Merck Millipore)
supplemented with 10% FBS, 100 U/ml penicillin, and 100
µg/ml streptomycin prior to use in western blot analysis to
examine FBP1 expression.
The cell adhesive ability was assessed by staining
lymphoma cells with calcein (Santa Cruz Biotechnology, Inc.),
according to the manufacturer's protocol, for 30 min. The cells
were then incubated in 96-well plates with a FN-coated surface or
pre-established monolayer of HS-5 cells in the recommended media
containing 10% FBS. After 2 h of culture, the non-adherent cells
were washed off twice with 1 ml phosphate-buffered saline (PBS) and
the number of adherent cells was measured using a fluorometer
(CytoFluor; Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Preparation of small interfering (si)RNA
and transient transfection
Daudi and OCI-LY8 cells (Jiangsu Institute of
Hematology, Suzhou, China) were cultured in FBS-free RPMI 1640
medium (Sigma-Aldrich; Merck Millipore) without antibiotics.
Control and FBP-siRNA (#1, 5′-GGTGCTGACAAACCTCTT-3′; #2,
5′-CCCATATAAAGTTCAACA-3′; and #3, 5′-GCTGCTTATTACGCTCAC-3′) were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and
transfection of cells with duplex synthetic siRNA was performed
with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. After 48 h, cells
were resuspended in normal medium at 106/ml and
processed for further experiments.
Immunoblot analysis
The OCI-LY8 and Daudi cells, and lymph node tissues
were homogenized in lysis buffer [1% NP-40, 50 mmol/l Tris (pH
7.5), 5 mmol/l EDTA, 1% sodium dodecyl sulfate (SDS), 1% sodium
deoxycholate, 1% Triton X-100, 1 mmol/l phenylmethylsulfonyl
fluoride, 10 g/ml aprotinin and 1 g/ml leupeptin] and were cleared
by centrifugation at 9,388 × g for 20 min in a
microcentrifuge at 4°C (21). A
nuclei acid analyzerwas used to determine the protein concentration
and equal amounts of total protein (100 µg) were separated
by 10% SDS-polyacrylamide gel electrophoresis and were
electrophoretically transferred to polyvinylidene fluoride
membranes (EMD Millipore, Bedford, MA, USA). The membranes were
then blocked with 5% nonfat milk and were incubated with anti-FBP1
(1:1,000); anti-cyclin-dependent kinase 2 (CDK2; 1:500; cat. no.
sc-163); anti-cyclin A (1:500; cat. no. sc-751;); anti-cyclin D1
(1:500; cat. no. sc-753); anti-proliferating cell nuclear antigen
(PCNA; 1:1,000; cat. no. sc-790); anti-c-myc (1:1,000; cat. no.
sc-764); anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
1:1,000; cat. no. sc-32233), all obtained from Santa Cruz
Biotechnology, Inc.; and anti-cleaved caspase (1:2,000; cat. no.
C8487; Sigma-Aldrich; Merck Millipore) antibodies overnight at 4°C.
Following three washes with Tris-buffered saline-0.1% Tween 20, the
blots were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:10,000; cat. no. PV-6000-D; OriGene
Technologies, Inc., Beijing, China) for 2 h at room temperature.
The signals were visualized using an enhanced chemiluminescence
detection system. Protein levels were analyzed using Image J
(version 1.49; imagej.nih.gov/ij/) and normalized with GAPDH levels.
The results were derived from at least three independent
experiments.
Cell cycle analysis and viability
assay
OCI-LY8 and Daudi cells were fixed in 75% ethanol at
−20°C overnight. Subsequently, the cells were incubated with 1
mg/ml RNase A for 30 min, and were stained with propidium iodide
(PI; 50 µg/ml; BD Biosciences, San Jose, CA, USA) in PBS
containing 0.5% Tween-20. Cells were analyzed using a BD FACScan
flow cytometer (BD Biosciences).
Cell proliferation and viability were assessed using
the standard Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Rockville, MD, USA), according to the
manufacturer's protocol. The viability of lymphoma cells following
treatment with 1 µM doxorubicin (Sigma-Aldrich; Merck
Millipore) for 48 or 72 h was determined by CCK-8 assay.
Statistical analysis
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform statistical analysis. Differences between two
groups were compared using χ2 test (Table I). Multivariate analysis was
performed using Cox's proportional hazards model (Table II). The risk ratio and its 95%
confidence interval were recorded for each marker. Other
statistical analyses were performed using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment consisted of at least three replicates
per condition.
 | Table IFBP1 expression and
clinicopathological parameters in 99 non-Hodgkin lymphoma
specimens. |
Table I
FBP1 expression and
clinicopathological parameters in 99 non-Hodgkin lymphoma
specimens.
| Parameter | Total | FBP1 expression
| P-value |
|---|
| Low | High |
|---|
| Age (years) | | | | <0.001a |
| ≤60 | 59 | 36 | 23 | |
| >60 | 40 | 11 | 29 | |
| Gender | | | | 0.743 |
| Male | 56 | 30 | 26 | |
| Female | 43 | 17 | 26 | |
| B symptoms | | | | 0.967 |
| Absent | 20 | 11 | 9 | |
| Present | 79 | 36 | 43 | |
| Extranodal
sites | | | | 0.025b |
| <2 | 64 | 35 | 29 | |
| ≥2 | 35 | 12 | 23 | |
| Lactate
dehydrogenaseb | | | | 0.557 |
| Normal | 30 | 17 | 13 | |
| Elevated | 33 | 11 | 22 | |
| Treatment | | | | 0.224 |
| CHOP | 55 | 24 | 31 | |
| Other | 44 | 22 | 22 | |
| Ki-67
expression | | | | <0.001b |
| <70% | 42 | 33 | 9 | |
| ≥70% | 57 | 11 | 46 | |
| Histological
type | | | | 0.482 |
| Indolent | 63 | 39 | 24 | |
| Invasive | 36 | 13 | 23 | |
 | Table IIMultivariate analysis with Cox
regression model of 99 non-Hodgkin lymphoma specimens. |
Table II
Multivariate analysis with Cox
regression model of 99 non-Hodgkin lymphoma specimens.
| Parameter | Relative ratio | 95% confidence
interval | P-value |
|---|
| Age (years) | | | <0.01a |
| >60 | 1.114 | 0.434–0.717 | |
| Gender | | | 0.615 |
| Female | 0.842 | 0.504–0.615 | |
| B symptoms | | | 0.019a |
| Present | 0.723 | 0.048–0.419 | |
| Extranodal
sites | | | <0.01a |
| ≥2 | 0.967 | 0.157–0.514 | |
| Lactate
dehydrogenaseb | | | 0.045a |
| Elevated | 0.924 | 0.011–0.404 | |
| Treatment | | | 0.598 |
| CHOP | 0.979 | 0.135–0.247 | |
| Ki-67
expression | | | <0.01a |
| ≥70% | 1.156 | 0.420–0.717 | |
| Histological
type | | | 0.006a |
| Invasive | 1.949 | 0.096–0.447 | |
| FBP1
expression | | | <0.01a |
| High | 1.138 | 0.521–0.766 | |
Results
FBP1 is expressed in RL and human B-cell
NHL tissues
FBP1 is highly expressed in several solid neoplasms,
including basal-like breast cancer, renal cancer, HCC, colon cancer
and non-small cell lung cancer (12,22–25);
however, whether this is true for hematological malignancies
remains to be elucidated. Therefore, immunohistochemical analysis
was performed to investigate the in vivo expression of FBP1
in clinical specimens of RL and lymphoma tissues, including FL,
MALT and DLBCL (Fig. 1). HCC
tissue was used as a positive control, whereas adjacent normal
tissue was used as a negative control (15) (Fig. 1A
and B). In RL tissues, FBP1 was predominantly expressed in
proliferating germinal centers (Fig.
1C and D). Furthermore, in lymphoma tissues other than MALT,
overexpression of FBP1 was detected. FBP1 immunoreactivity was
primarily localized in the follicular mantle zones of FL (Fig. 1E and F), whereas in DLBCL tissues
FBP1 was diffusely expressed (Fig. 1I
and J). Compared with FL and DLBCL tissues, the expression of
FBP1 in MALT tissues was much weaker (Fig. 1G and H). Furthermore, the positive
rate of FBP1 expression was evaluated among the subtypes of
lymphoma. FBP1 was highly expressed in several lymphoma tissues,
with 63.88% positivity in DLBCL (23/36), 35.29% positivity in FL
(6/17), and 40.74% positivity in MALT (11/27). Generally speaking,
the FBP1 expression pattern varied among the various lymphoma
types; however, there were no quantifiable differences between
expression of FBP1 between tumor types, or between malignant and
non-malignant specimens (P=0.482).
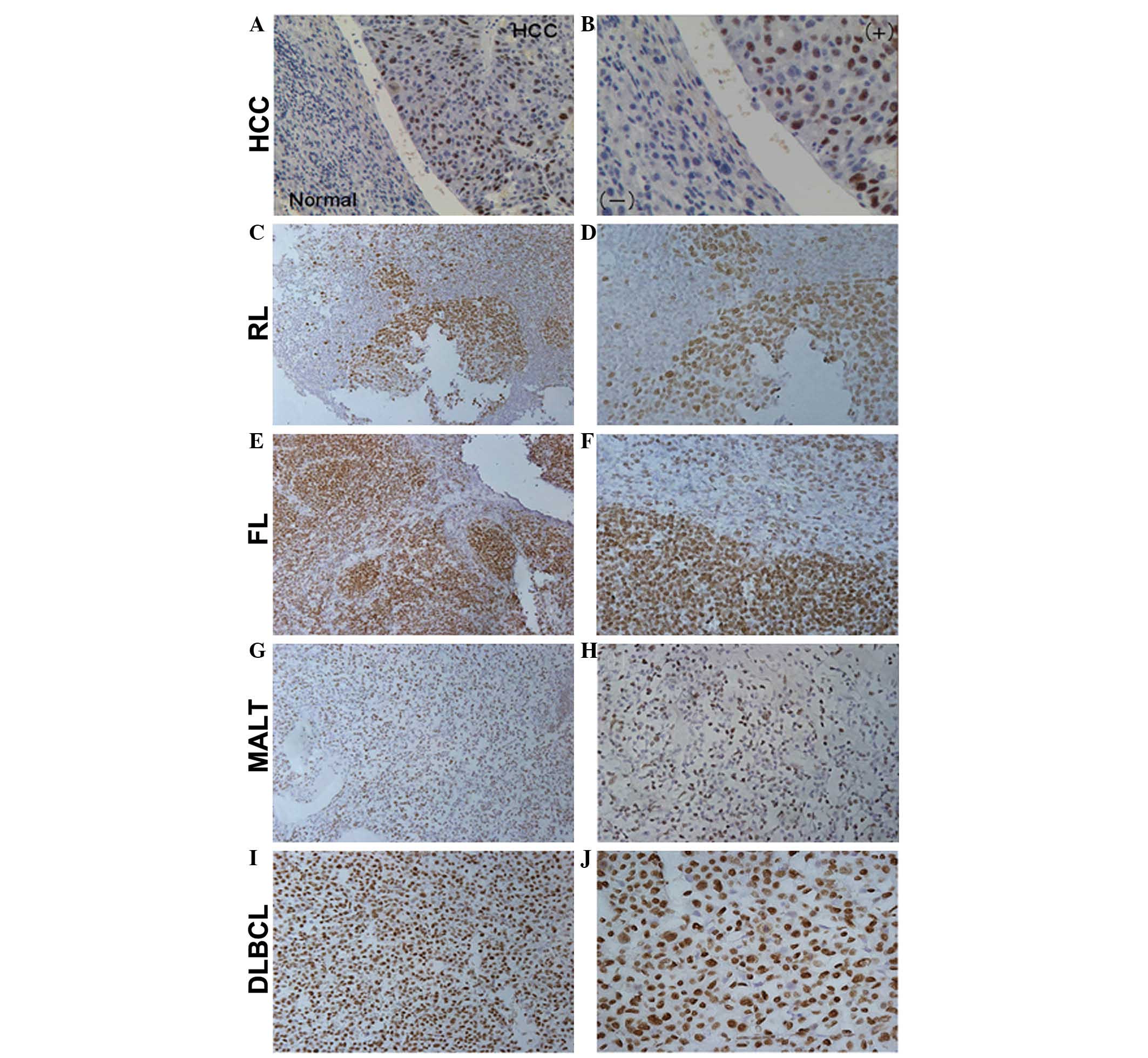 | Figure 1Immunohistochemical staining (IHC)
results for far upstream element binding protein 1 (FBP1)
expression in reactive lymphadenopathy (RL) and human B-cell
non-Hodgkin lymphoma tissues. IHC was performed to detect FBP1
expression in (A and B) hepatocellular carcinoma (HCC) and adjacent
normal tissues, as the positive and negative controls (A, x20; B,
x40); (C and D) RL (C, x20; D, x40); (E and F) follicular lymphoma
(FL; E, x20; F, x40); (G and H) extranodal lymphoma of
mucosa-associated lymphoid tissue (MALT; G, x20; H, x40) and (I and
J) diffuse large B-cell lymphoma (DLBCL; I, x20; J, x40). The FBP1
expression pattern varied between the different lymphoma types
(DLBCL vs. FL vs. MALT=63.88% vs. 35.29% vs. 40.74%). |
FBP1 expression is associated with
high-risk clinical parameters in NHL
Various clinicopathological parameters were compared
between patients with high or low FBP1 expression (Table I). A significant positive
correlation was detected between FBP1 expression and Ki-67
(P<0.001), which is a proliferative marker. There were also
significant correlations between high levels of FBP1 expression and
two other adverse prognostic factors: Advanced age (P<0.001) and
multiple extranodal sites (P<0.05). However, high levels of FBP1
expression were not significantly correlated with gender, serum
lactate dehydrogenase (LDH) levels, histological type, chemotherapy
or clinical symptoms. Survival analysis was performed on 99
patients who had follow-up data until mortality. After all
variables were compared separately with survival status, FBP1
(P<0.01), Ki-67 (P<0.01), age (P<0.01), B symptoms
(P=0.019), extranodal sites (P<0.01), LDH (P=0.045) and invasive
histological type (P=0.006) significantly influenced survival
(Table II).
FBP1 expression promotes proliferation of
NHL cell lines
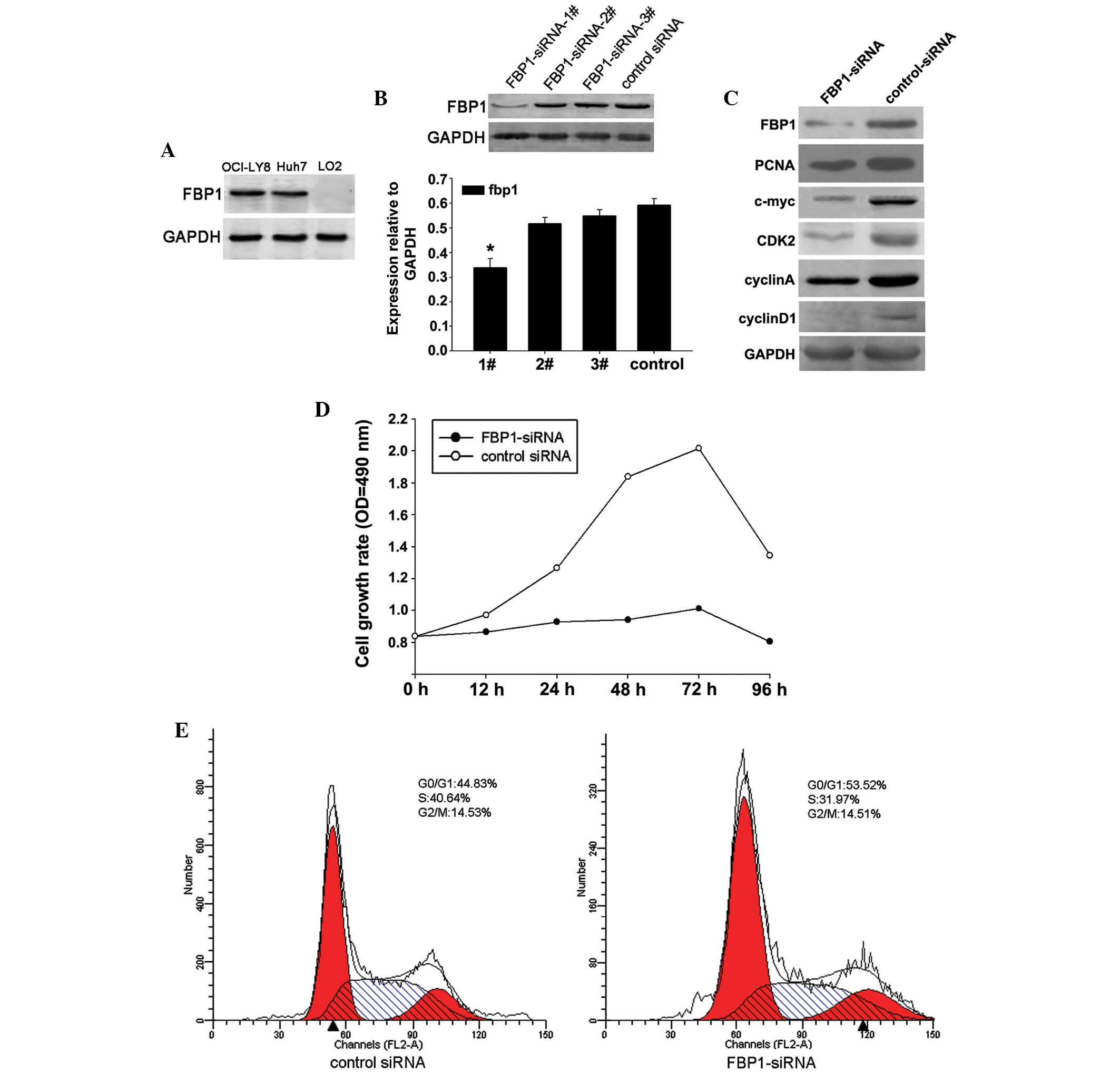
According to a previous study (23), FBP1 can be detected in HCC cell
lines, including Huh-7; however, it is undetectable in the L02
normal liver cell line. Therefore, the present study assessed the
protein expression levels of FBP1 in three cell lines: OCI-LY8, and
the positive and negative control cells, Huh-7 and L02 (Fig. 2A). To further investigate the
potential effects of FBP1 on NHL cell proliferation, OCI-LY8 cells
were transiently transfected with FBP1-siRNA for 48 h, and the
efficacy of FBP1 siRNA-mediated downregulation was confirmed by
western blot analysis (Fig. 2B).
Of the three siRNAs used FBP1-siRNA#1 exhibited the highest
efficacy (Fig. 2B). Therefore,
FBP1-siRNA#1 was chosen for subsequent assays. OCI-LY8 cells were
transfected with FBP1-siRNA#1, and the expression levels of FBP1,
c-myc, PCNA, CDK2, cyclin A and cyclin D1 were measured by western
blotting (Fig. 2C). Previous
studies have suggested that FBP1 is associated with carcinogenesis
via c-myc dependent or independent pathways (15,26).
The present study demonstrated that the expression levels of c-myc
were higher when FBP1 expression was not suppressed. Knockdown of
FBP1 resulted in a marked decrease in the expression of PCNA, which
is a marker of cell proliferation. Furthermore, knockdown of FBP1
was correlated with the downregulation of several key cell cycle
regulators, including CDK2, cyclin A and cyclin D1. Subsequently, a
CCK8 assay was performed to determine cell viability of cells
transfected with FBP1-siRNA (Fig.
2D). Knockdown of FBP1 resulted in a marked inhibition of cell
growth rate. To explore the mechanism underlying FBP1-siRNA-induced
decreased cell growth, cell cycle distribution was determined
following transfection with FBP1-siRNA or control siRNA by flow
cytometry. The percentage of cells in S phase was markedly
decreased in the FBP1-siRNA group compared with in the control
siRNA group (Fig. 2E), thus
suggesting that FBP1 may promote G0/G1-S
transition and accelerate cell growth. Similar results were
detected in the experiments that used the Daudi cell line (data not
shown).
Adhesion to FN or bone marrow stromal
cells induces FBP1 protein expression, which in turn facilitates
cell adhesion
It has previously been demonstrated that adhesion of
hematological malignant cells to the extracellular matrix component
FN reverses cell cycle arrest (27). The present study conducted an
adhesion assay where lymphoma cells were adhered to stromal cells
or FN in an attempt to elucidate the correlation between cell
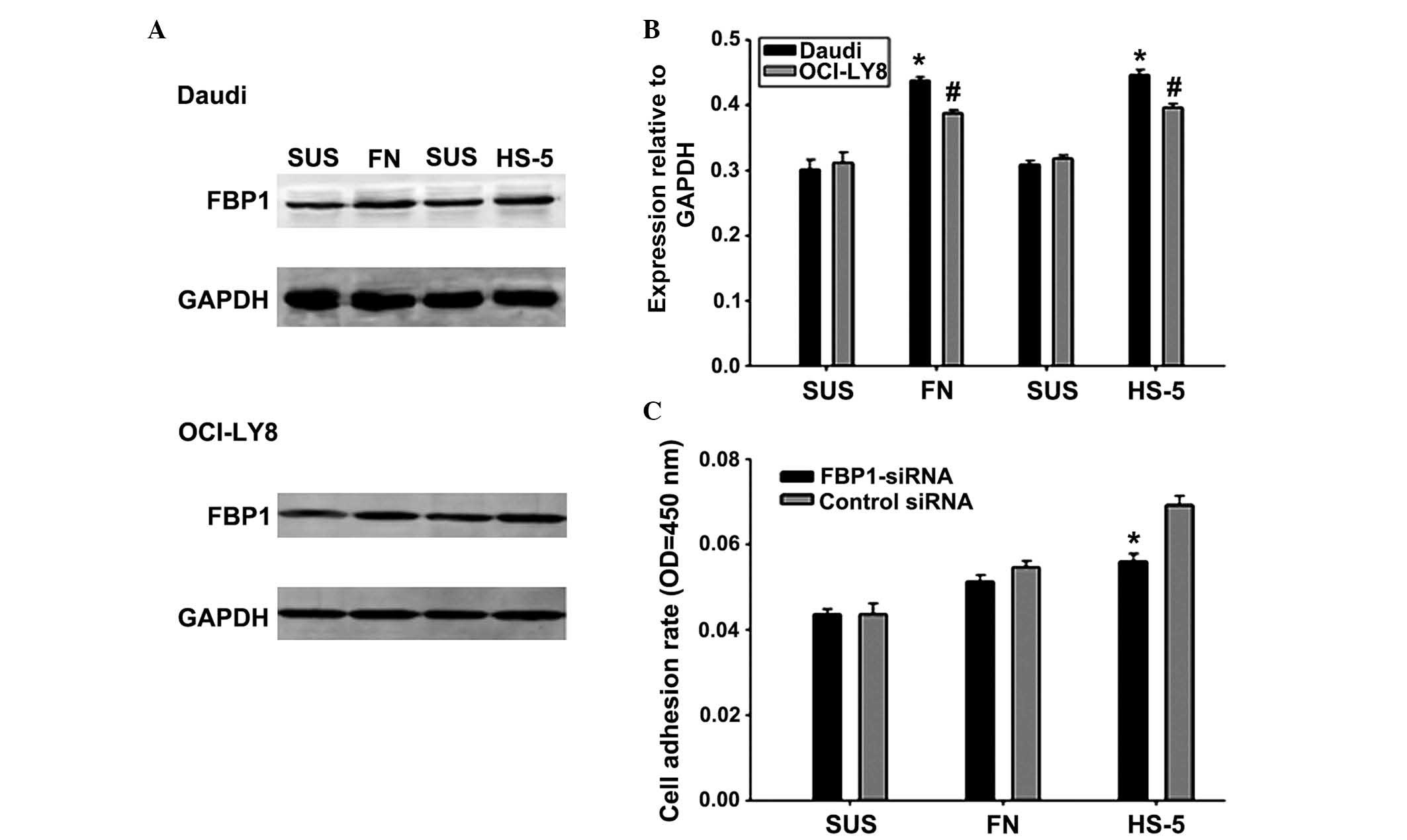
adhesion and the expression of FBP1. A western blot analysis was
conducted to evaluate the expression of FBP1 in Daudi and OCI-LY8
cells following 24 h of adhesion to stromal cells or FN, or in
suspension. FBP1 protein expression was markedly increased when
lymphoma cells adhered to FN or stromal cells compared with cells
in suspension (Fig. 3A and B).
Subsequently, the role of FBP1 in promoting adhesion of lymphoma
cells to FN or HS-5 cells was investigated using FBP1-siRNA. When
cells were cultured in the presence of FN or HS-5, the adhesion
rates of Daudi cells were more significantly increased compared
with OCI-LY8 cells. Therefore, Daudi cells were transiently
transfected with FBP1-siRNA or control siRNA for 48 h and were
stained with calcein for 30 min. Subsequently, they were plated on
a FN-coated surface or pre-established monolayers of HS-5 and were
cultured for a further 2 h. Cell adhesion assay revealed that the
cell adhesion rate was significantly reduced following knockdown of
FBP1 in the HS-5 cell adhesion group (Fig. 3C). Taken together, the expression
of FBP1 may promote the adhesion of Daudi cells to FN or HS-5 cell,
which may lead to CAM-DR.
FBP1 knockdown reverses adhesion-mediated
drug resistance
CAM-DR is considered a major mechanism by which
tumor cells escape the cytotoxic effects of therapeutic agents
(28,29). The present study demonstrated that
FBP1 expression promotes adhesion of lymphoma cells. However, the
specific association of FBP1 with drug resistance has yet to be
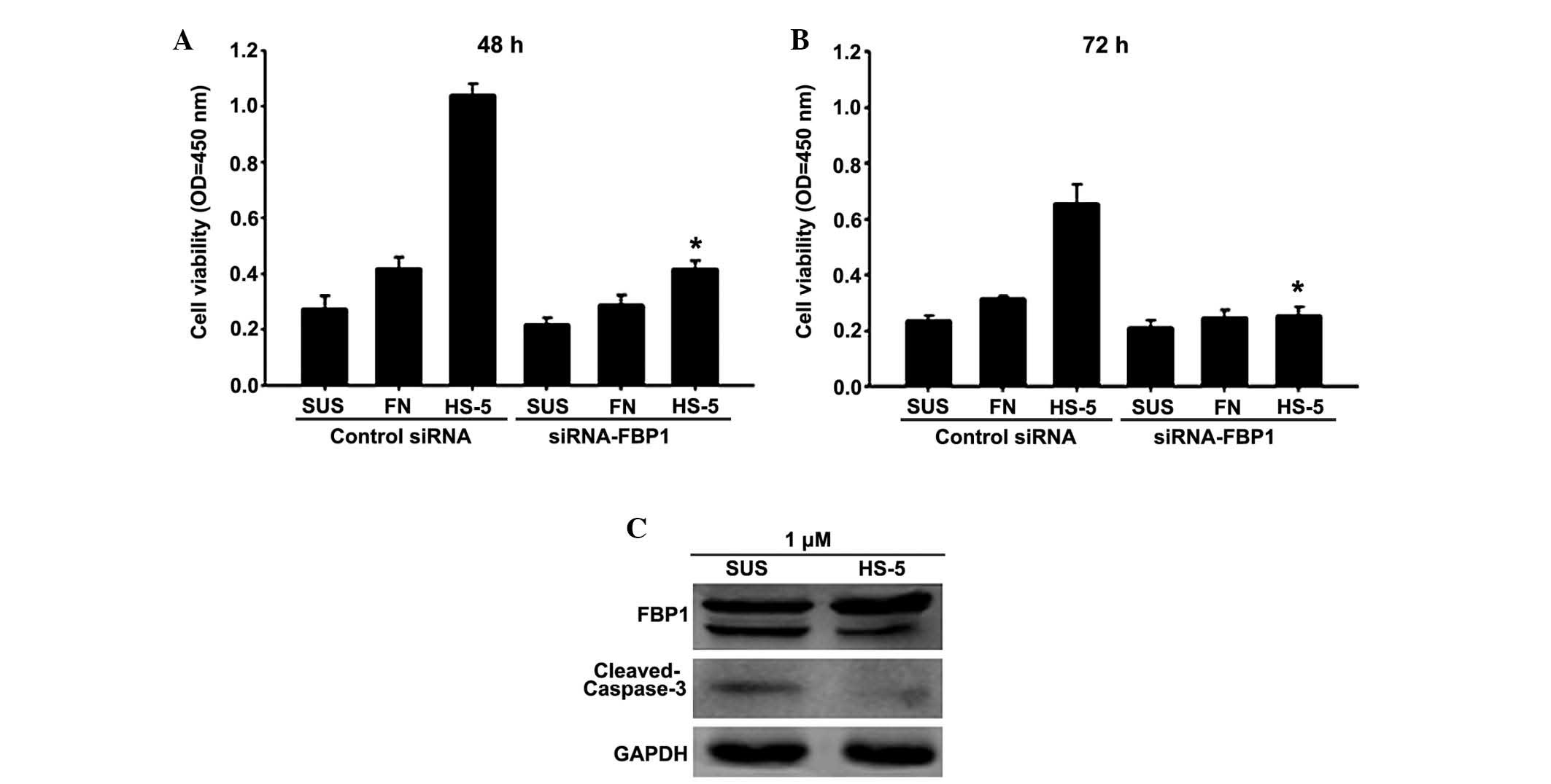
elucidated. Therefore, FBP1-siRNA was used to knock down FBP1
expression in Daudi lymphoma cells cultured in three different
conditions: Adhesion to FN, adhesion to bone marrow stromal cells,
or in suspension. Subsequently, in order to evaluate the effects of
FBP1 downregulation on drug resistance, a CCK8 assay was conducted
following treatment with doxorubicin for 48 or 72 h (Fig. 4A and B). In a preliminary
experiment, the appropriate drug concentration for treatment of
lymphoma cells was determined to be 1 µM; this was the
concentration at which cells were shown to be sensitive to
drug-induced apoptosis. The results of the present study
demonstrated that cell adhesion to HS-5 cells significantly
protected Daudi lymphoma cells from the cytotoxicity of
doxorubicin, as compared with cells in suspension. Conversely, this
effect was partially abrogated following knockdown of FBP1.
Adhesion to FN only resulted in weak drug resistance, and there was
no marked downregulation of drug resistance in FN-adhered cells
following FBP1-siRNA transfection. These results support a role for
FBP1 in conferring drug resistance through cell-adhesion
mechanisms.
It has previously been reported that FBP1 can be
cleaved in the process of chemotherapy-induced apoptosis (13). Therefore, cleavage of FBP1, along
with caspase-3 was evaluated by western blot analysis (Fig. 4C). Exposure of Daudi lymphoma cells
to 1 µM doxorubicin resulted in cleavage of FBP1 and
caspase-3. Cell adhesion to HS-5 cells led to decreased cleavage of
FBP1 and caspase-3, thus suggesting that stromal cell adhesion
inhibited drug-induced apoptosis. Furthermore, compared with cells
in suspension, adhesion to HS-5 cells increased FBP1 expression,
which was consistent with previous results. These findings suggest
that cell-cell interactions can substitute for cell-substrate
interactions in conferring apoptotic resistance in NHL cells. FBP1
has an important role in stromal/lymphoma cell interactions and may
be considered a novel therapeutic target for residual resistant
lymphoma following chemotherapy.
Discussion
NHL is a disease that demonstrates a high
proliferative rate and eventually becomes resistant to chemotherapy
(30). Lymphoma cells are present
in the bone marrow or secondary lymphoid organs, thus suggesting
that the microenvironment may provide necessary components for
growth and survival of tumor cells (31). FBP1, which acts as an activator of
transcription for the proto-oncogene c-myc, contributes to
decreased cell sensitivity to apoptotic stimuli and increased cell
proliferation (23). FBP1 is a
potential target of malignant cell transformation. It has been
found to be upregulated in several types of cancer, including
basal-like breast cancer, renal cancer, HCC, colon cancer and
non-small cell lung cancer (15,22–26).
These findings have implicated FBP1 expression in the progression
of solid cancers; however, whether FBP1 is involved in
hematological malignancies remains largely unknown. The present
study detected FBP1 expression in RL tissues and several types of
B-cell lymphoma tissue, including FL and DLBCL, by
immunohistochemical analysis. In addition, FBP1 expression was
shown to be associated with high-risk clinical parameters, and was
identified as an independent prognostic factor for NHL in
multivariate analysis. Therefore, evaluation of FBP1 expression may
be considered an important factor in identifying poor prognosis in
patients with NHL.
To explore the mechanisms underlying the effects of
FBP1 on the promotion of NHL cell proliferation, knockdown of FBP1
via FBP1-siRNA transfection was performed in OCI-LY8 and Daudi
cells. Downregulation of FBP1 expression significantly decreased
the protein expression levels of cyclin A, cyclin D1 and CDK2, and
suppressed the cell cycle progression of OCI-LY8 cells. It is
well-known that G1/S phase transition is a major
checkpoint for cell cycle progression, and two complexes, cyclin
D1-CDK4 and cyclin E-CDK2, function as critical positive regulators
during this transition (32).
Accordingly, the results of the present study indicated that the
expression levels of cyclin D1 and CDK2 were decreased in
FBP1-siRNA-transfected OCI-LY8 cells. These results suggested that
FBP1 may be considered a promising novel target for the treatment
of NHL therapy.
A better understanding regarding the biology of
B-cell malignancies is required for the development of potential
therapeutic agents that target specific intracellular pathways and
the interaction between malignant B cells and their
microenvironment (33). The 'tumor
microenvironment' is a critical determinant for tumor initiation,
progression, response to therapy, and drug resistance. Previous
studies, including results from our own laboratory, have reported
that cell adhesion of hematopoietic tumor cell lines to stromal
cells confers a multidrug-resistant phenotype, and that disruption
of cell adhesion-mediated signaling may increase the efficacy of
currently used cytotoxic agents (34–36).
The present study investigated the effects of adhesion between
stromal cells and B-cell lymphoma cells on the survival of NHL cell
lines. The results indicated that FBP1 may have an important role
in the adhesion and survival of NHL cells. Downregulation of FBP1
expression attenuated the observed CAM-DR. Therefore, a better
understanding regarding the molecular mechanisms underlying the
effects of FBP1 on stromal/lymphoma cell interactions may lead to
the generation of novel therapeutic approaches for the treatment of
residual resistant lymphoma following chemotherapy. However, in the
process of CAM-DR, it remains unclear as to which signal pathway or
targets are affected by FBP1 expression; therefore, this mechanism
may warrant further investigation.
In conclusion, the present study demonstrated that
FBP1 has a critical role in NHL cell proliferation, adhesion and
drug resistance. These results may lead to the generation of a
novel therapeutic approach that targets FBP1.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81172879,
81201858 and 81372537); the Natural Scientific Foundation of
Jiangsu Province Grant (grant no. BK2012231); the National Funds
for Distinguished Young Scientists of Nantong City (grant no.
WQ2016057); and a Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Lwin T, Hazlehurst LA, Li Z, Dessureault
S, Sotomayor E, Moscinski LC, Dalton WS and Tao J: Bone marrow
stromal cells prevent apoptosis of lymphoma cells by upregulation
of anti-apoptotic proteins associated with activation of NF-kappaB
(RelB/p52) in non-Hodgkin's lymphoma cells. Leukemia. 21:1521–1531.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding BB, Yu JJ, Yu RY, Mendez LM,
Shaknovich R, Zhang Y, Cattoretti G and Ye BH: Constitutively
activated STAT3 promotes cell proliferation and survival in the
activated B-cell subtype of diffuse large B-cell lymphomas. Blood.
111:1515–1523. 2008. View Article : Google Scholar
|
|
3
|
Wang K, Jiang Y, Zheng W, Liu Z, Li H, Lou
J, Gu M and Wang X: Silencing of human
phosphatidylethanolamine-binding protein 4 enhances
rituximab-induced death and chemosensitization in B-cell lymphoma.
PLoS One. 8:e568292013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Fei M, Cheng C, Zhang D, Lu J, He
S, Zhao Y, Wang Y and Shen A: Jun activation domain-binding protein
1 negatively regulate p27 kip1 in non-Hodgkin's lymphomas. Cancer
Biol Ther. 7:460–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hang Q, Fei M, Hou S, Ni Q, Lu C, Zhang G,
Gong P, Guan C, Huang X and He S: Expression of Spy1 protein in
human non-Hodgkin's lymphomas is correlated with phosphorylation of
p27 Kip1 on Thr187 and cell proliferation. Med Oncol. 29:3504–3514.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Fei M, Wang Y, Lu M, Cheng C and
Shen A: Expression of Foxo3a in non-Hodgkin's lymphomas is
correlated with cell cycle inhibitor p27. Eur J Haematol. 81:83–93.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lwin T, Lin J, Choi YS, Zhang X, Moscinski
LC, Wright KL, Sotomayor EM, Dalton WS and Tao J: Follicular
dendritic cell-dependent drug resistance of non-Hodgkin lymphoma
involves cell adhesion-mediated Bim down-regulation through
induction of microRNA-181a. Blood. 116:5228–5236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lwin T, Crespo LA, Wu A, Dessureault S,
Shu HB, Moscinski LC, Sotomayor E, Dalton WS and Tao J: Lymphoma
cell adhesion-induced expression of B cell-activating factor of the
TNF family in bone marrow stromal cells protects non-Hodgkin's B
lymphoma cells from apoptosis. Leukemia. 23:170–177. 2009.
View Article : Google Scholar
|
|
9
|
Mraz M, Zent CS, Church AK, Jelinek DF, Wu
X, Pospisilova S, Ansell SM, Novak AJ, Kay NE, Witzig TE and
Nowakowski GS: Bone marrow stromal cells protect lymphoma B-cells
from rituximab-induced apoptosis and targeting integrin alpha-4-β-1
(VLA-4) with natalizumab can overcome this resistance. Br J
Haematol. 155:53–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hazlehurst LA, Argilagos RF, Emmons M,
Boulware D, Beam CA, Sullivan DM and Dalton WS: Cell adhesion to
fibronectin (CAM-DR) influences acquired mitoxantrone resistance in
U937 cells. Cancer Res. 66:2338–2345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westhoff MA, Zhou S, Bachem MG, Debatin KM
and Fulda S: Identification of a novel switch in the dominant forms
of cell adhesion-mediated drug resistance in glioblastoma cells.
Oncogene. 27:5169–5181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber A, Kristiansen I, Johannsen M,
Oelrich B, Scholmann K, Gunia S, May M, Meyer HA, Behnke S, Moch H
and Kristiansen G: The FUSE binding proteins FBP1 and FBP3 are
potential c-myc regulators in renal, but not in prostate and
bladder cancer. BMC Cancer. 8:3692008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang M, Park BC, Kang S, Chi SW, Cho S,
Chung SJ, Lee SC, Bae KH and Park SG: Far upstream element-binding
protein-1, a novel caspase substrate, acts as a cross-talker
between apoptosis and the c-myc oncogene. Oncogene. 28:1529–1536.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiede B, Dimmler C, Siejak F and Rudel T:
Predominant identification of RNA-binding proteins in Fas-induced
apoptosis by proteome analysis. J Biol Chem. 276:26044–26050. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rabenhorst U, Beinoraviciute-Kellner R,
Brezniceanu ML, Joos S, Devens F, Lichter P, Rieker RJ, Trojan J,
Chung HJ, Levens DL and Zörnig M: Overexpression of the far
upstream element binding protein 1 in hepatocellular carcinoma is
required for tumor growth. Hepatology. 50:1121–1129. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hazlehurst LA, Damiano JS, Buyuksal I,
Pledger WJ and Dalton WS: Adhesion to fibronectin via beta1
integrins regulates p27kip1 levels and contributes to cell adhesion
mediated drug resistance (CAM-DR). Oncogene. 19:4319–4327. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shain KH, Yarde DN, Meads MB, Huang M,
Jove R, Hazlehurst LA and Dalton WS: Beta1 integrin adhesion
enhances IL-6-mediated STAT3 signaling in myeloma cells:
Implications for microenvironment influence on tumor survival and
proliferation. Cancer Res. 69:1009–1015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naz E, Mirza T, Aziz S, Danish F, Siddiqui
ST and Ali A: Frequency and clinicopathologic correlation of
different types of non Hodgkin's lymphoma according to WHO
classification. J Pak Med Assoc. 61:260–263. 2011.PubMed/NCBI
|
|
19
|
Filtenborg-Barnkob BE and Bzorek M:
Expression of anaplastic lymphoma kinase in Merkel cell carcinomas.
Hum Pathol. 44:1656–1664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Yang X, Shi H, Ren H, Chen X, Zhang
S, Zhu J and Zhang J: Downregulated expression of the
cyclase-associated protein 1 (CAP1) reduces migration in esophageal
squamous cell carcinoma. Jpn J Clin Oncol. 43:856–864. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wang Y, Cheng C, Chen Y, Shi S, Qin
J, Xiao F, Zhou D, Lu M, Lu Q and Shen A: A relationship between
p27(kip1) and Skp2 after adult brain injury: Implications for glial
proliferation. J Neurotrauma. 27:361–371. 2010. View Article : Google Scholar
|
|
22
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malz M, Weber A, Singer S, Riehmer V,
Bissinger M, Riener MO, Longerich T, Soll C, Vogel A, Angel P, et
al: Overexpression of far upstream element binding proteins: A
mechanism regulating proliferation and migration in liver cancer
cells. Hepatology. 50:1130–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen M, Zhang J, Li N, Qian Z, Zhu M, Li
Q, Zheng J, Wang X and Shi G: Promoter hypermethylation mediated
downregulation of FBP1 in human hepatocellular carcinoma and colon
cancer. PLoS One. 6:e255642011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singer S, Malz M, Herpel E, Warth A,
Bissinger M, Keith M, Muley T, Meister M, Hoffmann H, Penzel R, et
al: Coordinated expression of stathmin family members by far
upstream sequence element-binding protein-1 increases motility in
non-small cell lung cancer. Cancer Res. 69:2234–2243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding Z, Liu X, Liu Y, Zhang J, Huang X,
Yang X, Yao L, Cui G and Wang D: Expression of far upstream element
(FUSE) binding protein 1 in human glioma is correlated with c-Myc
and cell proliferation. Mol Carcinog. 54:405–415. 2015. View Article : Google Scholar
|
|
27
|
Burger JA, Ghia P, Rosenwald A and
Caligaris-Cappio F: The microenvironment in mature B-cell
malignancies: A target for new treatment strategies. Blood.
114:3367–3375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fei M, Hang Q, Hou S, He S and Ruan C:
Adhesion to fibronectin induces p27(kip1) nuclear accumulation
through down-regulation of Jab1 and contributes to cell
adhesion-mediated drug resistance (CAM-DR) in RPMI 8,226 cells. Mol
Cell Biochem. 386:177–187. 2014. View Article : Google Scholar
|
|
29
|
Fei M, Hang Q, Hou S and Ruan C: Cell
adhesion to fibronectin down-regulates the expression of Spy1 and
contributes to drug resistance in multiple myeloma cells. Int J
Hematol. 98:446–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yagi K, Yamamoto K, Umeda S, Abe S, Suzuki
S, Onishi I, Kirimura S, Fukayama M, Arai A, Kitagawa M and Kurata
M: Expression of multidrug resistance 1 gene in B-cell lymphomas:
Association with follicular dendritic cells. Histopathology.
62:414–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurtova AV, Tamayo AT, Ford RJ and Burger
JA: Mantle cell lymphoma cells express high levels of CXCR4, CXCR5,
and VLA-4 (CD49d): Importance for interactions with the stromal
microenvironment and specific targeting. Blood. 113:4604–4613.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan C, Shi H, Wang H, Zhang J, Ni W, Chen
B, Hou S, Yang X, Shen A and Ni R: CtBP2 contributes to malignant
development of human esophageal squamous cell carcinoma by
regulation of p16INK4A. J Cell Biochem. 114:1343–1354. 2013.
View Article : Google Scholar
|
|
33
|
Tjin EP, Groen RW, Vogelzang I, Derksen
PW, Klok MD, Meijer HP, van Eeden S, Pals ST and Spaargaren M:
Functional analysis of HGF/MET signaling and aberrant HGF-activator
expression in diffuse large B-cell lymphoma. Blood. 107:760–768.
2006. View Article : Google Scholar
|
|
34
|
Fernandez-Vidal A, Ysebaert L, Didier C,
Betous R, De Toni F, Prade-Houdellier N, Demur C, Contour-Galcéra
MO, Prévost GP, Ducommun B, et al: Cell adhesion regulates CDC25A
expression and proliferation in acute myeloid leukemia. Cancer Res.
66:7128–7135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Huang Y, Xu X, Tang J, Huang X,
Zhu J, Liu J, Miao X, Wu Y, Yang F, et al: Expression of small
glutamine-rich TPR-containing protein A (SGTA) in Non-Hodgkin's
Lymphomas promotes tumor proliferation and reverses cell
adhesion-mediated drug resistance (CAM-DR). Leuk Res. 38:955–963.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He S, Huang Y, Wang Y, Tang J, Song Y, Yu
X, Ma J, Wang S, Yin H, Li Q, et al: Histamine-releasing
factor/translationally controlled tumor protein plays a role in
induced cell adhesion, apoptosis resistance and chemoresistance in
non-Hodgkin lymphomas. Leuk Lymphoma. 56:2153–2161. 2015.
View Article : Google Scholar
|