Introduction
Renal osteodystrophy is used to describe abnormal
changes in bone structure and function, including bone loss and
increased bone fragility, in patients with chronic kidney disease
(CKD), and is also a measure of CKD-mineral and bone disorder
syndrome (1–3). Renal osteodystrophy is found in
almost all patients with dialysis-requiring CKD and in the majority
of patients with CKD of stages 3–5 (4–6), and
contributes to increased risk of fracture. In addition, fracture in
patients with end-stage kidney disease often causes increased
mortality rates (7–10).
The prevalence of CKD in the US adult population was
11% in 2003, as revealed by the Third National Health and Nutrition
Examination Survey (11). In
particular, of those aged ≥70 years, only 26% had normal kidney
function (11). Almost 6% of
patients with CKD suffer from fractures in 5-year-follow up, and
this rate is higher in elderly patients (12). Patients with end-stage renal
disease in their 40s have an 80-fold higher risk of hip fracture
compared with that of age and gender-matched controls (13). In patients with stage 4 CKD, the
risk of hip fracture has been reported to be almost 4-fold that of
the general population without CKD (14). Thus, the healthcare costs
associated with renal osteodystrophy and secondary fracture are
substantial (15).
The treatment options for renal osteodystrophy
include phosphate binders for lowering high serum phosphorus and
maintaining serum calcium, calcitriol or vitamin D analogues for
lowering serum parathyroid hormone levels, growth hormone,
bisphosphonates and other osteoporosis medications (2,3).
However, the effectiveness and feasibility of these treatments are
limited due to significantly reduced kidney function. In addition,
extraskeletal calcification may be exacerbated by certain
therapies, which are used to correct changes in mineral and bone in
patients with CKD.
Osthole (7-methoxy-8-isopentenoxycoumarin), a
derivative of the Chinese herbal medicine, Fructus Cnidii,
has been demonstrated to be capable of significantly reversing bone
loss and improving the mechanical properties of long bones in
ovariectomized rats (16,17). Thus, the present study hypothesized
that osthole may also have a protective effect against bone loss in
an animal model of renal osteodystrophy. Thus, osthole may be a
useful treatment method of CKD-induced bone loss. To confirm this
hypothesis and reveal the underlying mechanism, a 5/6 nephrectomy
mouse model was established to induce bone loss in mice, and
osthole was injected intraperitoneally 1 month following
nephrectomy, which had been established for 2 months. Subsequent
histomorphlogical analysis was performed to examine bone structure,
and in vitro experiments were performed to investigate
osthole-regulated osteoclastogenesis.
Materials and methods
Establishment of the 5/6 nephrectomy
mouse model and grouping
The use of animals in the present study was approved
by the Shanghai Laboratory Animal Use Committee (Shanghai, China).
A total of 40 2-month-old male C57/BL6 mice were obtained from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and
were allowed to acclimatize for 1 week. The mice were housed in
environmentally controlled animal facilities at 22°C with a 12-h
light/dark cycle. The animals were had access to a commercial diet
and distilled water ad libitum. CKD was achieved following
two surgical procedures. The mice were anesthetized by
intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg).
For the first surgical procedure, a 2-cm right flank incision was
made and the left kidney was exposed. The perirenal fat was
separated from the kidney, and the upper third and lower third of
the kidney were removed using an electrotome. Following compression
hemostasis with gelatin for 5 min, the subcutaneous tissues and the
skin were sutured. The second surgical procedure was performed 1
week later. The right kidney was exposed using the same procedure
as that described above, following which the hilum was ligated and
the kidney was excised. The incisions were closed, as described
above (18). A total of 30 mice
underwent 5/6 nephrectomy, and 12 subsequently succumbed. At 1
month following the second surgical procedure, the remaining 18
mice were randomly divided into two groups. In one group, the mice
received daily intraperitoneal injections of 0.15 ml 1mg/ml osthole
(dissolved in corn oil; 5 mg/kg/day) for 2 months, and in the
second group, the mice received corn oil (0.15 ml) instead of the
osthole. Mice in the sham group (n=10) underwent the two surgical
procedures with incisions to expose the kidney only and were
treated with 0.15 ml corn oil.
Detection of blood urea nitrogen (BUN)
and serum creatinine (Scr)
The mice were sacrificed by cervical dislocation 1
month following the second surgical procedure following
intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg),
and blood was collected by eyeball removal and transferred into
heparinized tubes. The serum was separated by centrifugation at
1,000 × g for 10 min at 4°C. The levels of BUN and Scr were
measured using a two-site immunoradiometric assay with commercially
available kits (Immutopics, Inc., San Clemente, CA, USA).
Three-dimensional (3D) reconstruction
analyses
The lumbar 4 (L4) vertebrae from the mice in all
groups were dissected 2 months following treatment and fixed in 4%
paraformaldehyde for 24 h at 4°C, followed by washing in flowing
water for 2 h. Subsequently, 3D reconstruction analyses were
performed using a Micro-CT 80 scan machine (Scanco Medical AG,
Bassersdorf, Switzerland). The vertebrae underwent fine scanning in
400–500 slices with 10 µm slice increments. The X-ray source
voltage was 55 kVp, the source current was 72 µA and the
integration time was 400 msec. A reconstruction of the bitmap data
set was used to construct the 3D images of the spongy bone using
the built-in software (Scanco Holding AG, Brüttisellen,
Switzerland.
Histological evaluation
The L4 vertebrae were subjected to histological
analysis in order to reveal the pathological structure. The
vertebrae were fixed in 4% paraformaldehyde, decalcified,
dehydrated and embedded in paraffin. Serial mid-sagittal sections
(4-µm thick) of the vertebrae were cut and stained with
hematoxylin and eosin (H&E). Morphometric analysis was
performed using a light microscope (Olympus BX50; Olympus
Corporation, Tokyo, Japan) with a camera (Olympus DP71; Olympus
Corporation) and Image Pro Plus 6.0 software (Media Cybernetics,
Inc. (Rockville, MD, USA).
Immunohistochemical (IHC) staining
The L4 vertebrae were collected and fixed in 4%
paraformaldehyde, decalcified, dehydrated and embedded in paraffin.
Serial mid-sagittal sections (4-µm thick) were cut.
Endogenous peroxidase was blocked in 3% hydrogen peroxide/methanol
for 15 min at room temperature, followed by antigen retrieval in 20
µg/ml proteinase K for 10 min at 37°C. Following blocking in
5% bovine serum albumin (from IHC kit, Wuhan Boster Biological
Technology, Co., Ltd., Wuhan, China) at room temperature for 30
min, the slices were incubated with primary antibody diluted in 2%
goat serum (Wuhan Boster Biological Technology, Co., Ltd.),
including rabbit polyclonal anti-cathepsin K (1:100; Abcam,
Cambridge, MA, USA; ab19027), rabbit polyclonal anti-NFATc1 (1:100;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-13033)
and rabbit anti-c-Fos (1:100 dilution; Santa Cruz Biotechnology,
Inc.; cat. no. sc-253), at 4°C overnight. The slices were then
incubated in 1:200 biotinylated goat anti-rabbit secondary antibody
(from IHC kit, Wuhan Boster Biological Technology, Co., Ltd.) for
15 min at 37°C, followed by incubation in 1:250 horseradish
peroxidase-streptavidin for 10 min at 37°C. Following staining with
3,3′-Diaminobenzidine solution, the slices were counterstained in
hematoxylin, dehydrated, cleared and mounted. Negative control
slices were incubated in 2% goat serum instead of the primary
antibodies. The images were captured using a light microscope
(Olympus BX50) with a camera (Olympus DP71) and Image Pro Plus 6.0
software.
Calvarial osteoblast isolation and
treatment
Mouse pups (n=8; age, 3 days) were obtained from
Shanghai SLAC Laboratory Animal Co., Ltd. and sacrificed by
cervical dislocation on arrival at the laboratory. The soft tissue
and periosteal layers were carefully removed and placed in 70%
alcohol. The calvarial tissue was digested in 1 mg/ml collagenase
A/α-minimum essential media (α-MEM; BioSera, Nauille, France) for
45 min in a 37°C water bath, and the enzyme solution was replaced
every 15 min. Following the third digestion, the enzyme and cell
mixture were filtered and collected. The remaining calvarial tissue
was digested in enzyme solution for another 15 min, and the cells
were collected. Following three repetitions, the collected cells
were mixed, centrifuged at 300 × g for 5 min at room temperature
and resuspended in complete α-MEM with 50 µg/ml ascorbic
acid. Calvarial osteoblasts were plated into 12-well culture plates
at a density of 1×105 cells/well. For osthole treatment,
the calvarial osteoblasts were cultured in α-MEM without fetal
bovine serum (FBS; BioSera) overnight at 37°C and treated with 0.1,
1 and 10 µM osthole/dimethyl sulphoxide (DMSO) in 10%
FBS/Dulbecco's modified Eagle's medium (BioSera), respectively, for
2 days at 37°C. DMSO without osthole was used in the control
group.
In vitro osteoclast differentiation
assay
Bone marrow cells were isolated from the femurs and
tibias of 4 male 1-month-old wild-type mice (Shanghai SLAC
Laboratory Animal Co., Ltd.). The mice were sacrificed by cervical
dislocation on arrival at the laboratory following anesthesia with
2% sodium pentobarbital (40 mg/kg), and were plated into 24-well
culture plates at a density of 4×105 cells/well with
α-MEM supplemented with 10% FBS. The cells were treated with
macrophage colony-stimulating factor (M-CSF; 20 ng/ml) at 37°C for
3 days, and were then switched to differentiation medium with 10
ng/ml M-CSF and 50 ng/ml receptor activator for nuclear factor-κ B
ligand (RANKL) at 37°C for a further 7 days. For osthole treatment,
the bone marrow cells were cultured in α-MEM without FBS overnight
at 37°C and treated with 0.1, 1 and 10 µM osthole/DMSO in
10% FBS/α-MEM at 37°C, respectively, for 7 days. DMSO without
osthole was used in the control group. Tartrate-resistant acid
phosphatase (TRAP) staining was performed using a TRAP assay kit
(Sigma-Aldrich; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the L4 vertebrae using
TRIzol reagent, and from the primary cultured cells using an RNeasy
mini kit (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse
transcription was performed using an iScript cDNA Synthesis kit
(Qiagen, Suzhou, China), and cDNA was amplified by PCR using a
total volume of 20 µl reaction system containing 10
µl SYBR Green Master Mix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), 1 µl of the diluted (1:5) cDNA and 10 pM
of the forward and reverse primers specific for the genes (Table I). The PCR thermocycling conditions
were as follows: 95°C for 10 min; 40 cycles of 95°C for 10 sec,
58°C for 15 sec, and 72°C for 20 sec. The Cq values were recorded
and used to calculate relative quantities in the groups as
previously described (19).
 | Table INames and sequences of primers used
for polymerase chain reaction analysis. |
Table I
Names and sequences of primers used
for polymerase chain reaction analysis.
| Gene | Sequence |
|---|
| β-actin | F:
5′-GGAGATTACTGCCCTGGCTCCTA-3′ |
| R:
5′-GACTCATCGTACTC CTGCTTGCTG-3′ |
| Trap | F:
5′-TTGCGACCATTGTTAGCCACATA-3′ |
| R:
5′-TCAGATCCATAGTGAAA CCGCAAG-3′ |
| Mmp9 | F:
5′-CCATGCACTGGGCTTAGATCA-3′ |
| R:
5′-GGCCTTGGGTCAGGCTTAGA-3′ |
| Opg | F: 5′-CAGAGCGAAACAC
AGTTTG-3′ |
| R:
5′-CACACAGGGTGACATCTATTC-3′ |
| Rankl | F:
5′-CAGGTTTGCAGGACTCGAC-3′ |
| R:
5′-AGCAGGGAAGGGTTGGACA-3′ |
Statistical analysis
Data are presented as the mean with 95% confidence
intervals. Statistical analyses were performed using one-way
analysis of variance followed by Dunnett's test. For experiments
involving two groups, Student's t-test (unpaired) was
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Evaluation of renal insufficiency
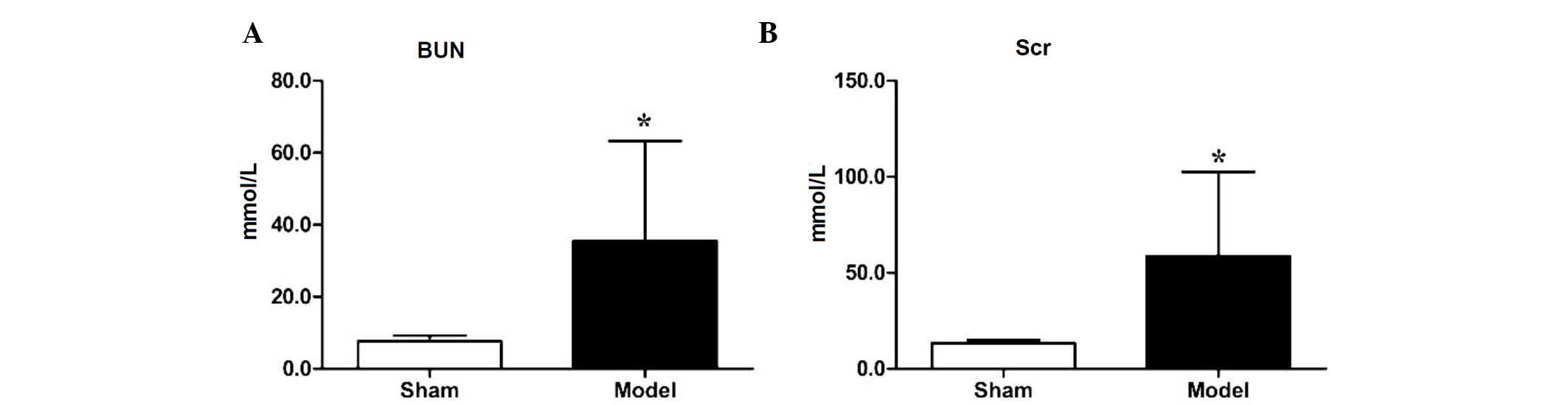
To verify the establishment of the 5/6 nephrectomy
model, the levels of BUN and Scr in the sham group and nephrectomy
group were measured 1 month following the second surgical
procedure. Compared with the sham group, the level of BUN in the
nephrectomy model group was increased significantly (7.64±1.60, vs.
35.52±27.76 mmol/l, respectively; P<0.05), as was the level of
Scr (13.33±1.65, vs. 58.73±43.80 µmol/l, respectively
(P<0.05), as shown in Fig. 1A and
B, indicating that the 5/6 nephrectomy model had been
established in the mice.
Bone loss is rescued in 5/6 nephrectomy
mice treated with osthole
To observe the overall effects of osthole on bone
loss in the 5/6 nephrectomy mice, L4 vetrebrae were harvested 2
months following osthole treatment, and micro CT scanning and
reconstruction were performed (Fig.
2A). The micro CT analysis showed a reduction in BMD in the L4
vertebrae of the 5/6 nephrectomy group, compared with the sham
group (732.766±92.240, vs. 948.974±86.823, respectively; P<0.01;
Fig. 2B) and the same was true for
bone volume/total volume (BV/TV; 0.100±0.044, vs. 0.201±0.008;
P<0.01; Fig. 2C). In addition,
trabecular number (Tb.N; 4.140±0.376, vs. 4.860±0.166; P<0.01;
Fig. 2D) and trabecular thickness
(Tb.Th; 0.030±0.006, vs. 0.0464±0.004; P<0.01; Fig. 2E) were decreased in the 5/6
nephrectomy group, compared with the sham group, whereas trabecular
separation (Tb.Sp; 0.245±0.022, vs. 0.208±0.012; P<0.05;
Fig. 2F) was significantly
increased, compared with the sham group. Following treatment with
osthole for 2 months, the BMD (923.356±11.916, vs. 732.766±92.240;
P<0.05; Fig. 2B) and BV/TV
(0.192±0.012, vs. 0.100±0.044; P<0.01; Fig. 2C) of the L4 vertebrae were
increased, compared with the 5/6 nephrectomy group. A significant
increase in Tb.Th was also observed (0.046±0.007, vs. 0.030±0.006;
P<0.01; Fig. 2E). However, no
significant changes were found in Tb.N (4.313±0.235, vs.
4.140±0.376; P>0.05; Fig. 2D)
or Tb.Sp (0.220±0.014, vs. 0.245±0.022, P>0.05; Fig. 2F). Taken together, these data
suggested that osthole partially rescued the bone loss induced by
5/6 nephrectomy in mice.
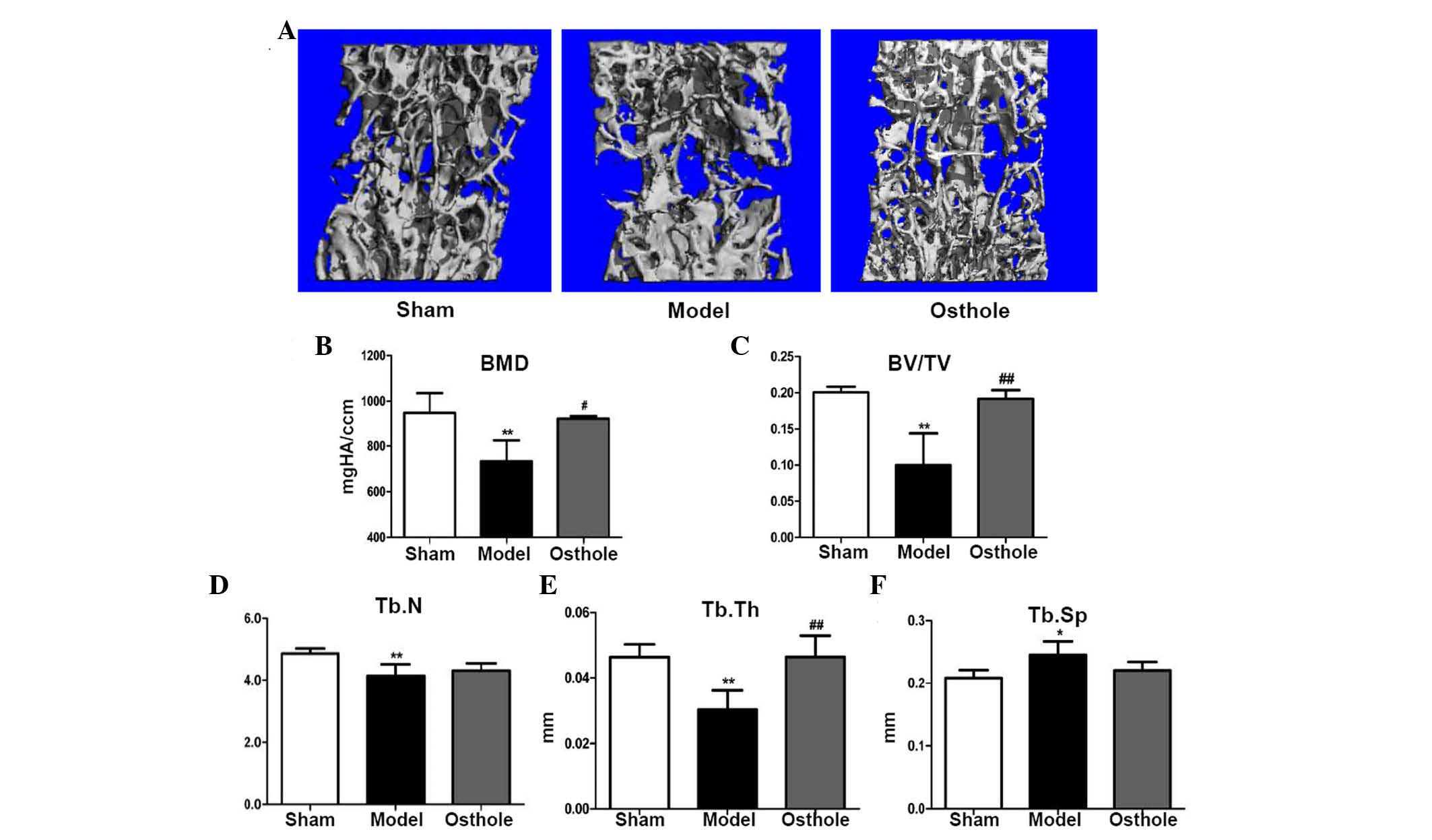 | Figure 2Bone loss in 5/6 nephrectomy mice is
rescued by treatment with osthole. The L4 vertebrae of the mice in
the sham, model and osthole group were harvested 2 months following
treatment, and micro CT scanning and reconstruction was performed.
(A) Micro CT reconstruction of L4 vertebrae. Decreased (B) BMD, (C)
BV/TV, (D) Tb.N and (E) Tb.Th, and increased (F) Tb.Sp of the L4
vertebrae were shown in the 5/6 nephrectomy group, compared with
the sham group. Following treatment with osthole for 2 months, (B)
BMD, (C) BV/TV and (E) Tb.Th of the L4 vertebrae were significantly
increased, compared with the 5/6 nephrectomy group. No significant
changes in (D) Tb.N or (F) Tb.Sp were found. *P<0.05
and **P<0.01, compared with the sham group;
#P<0.05 and ##P<0.01, compared with the
model group (n=6). BMD, bone mineral density; BV/TV, bone
volume/total volume; Tb.N, trabecular number; Tb.Th, trabecular
thickness; Tb.Sp, trabecular separation. |
Osteoclast numbers are reduced in 5/6
nephrectomy mice treated with osthole
Changes in the microstructure of the L4 vertebrae
were revealed using H&E staining. The trabecular bone of the L4
vertebrae from the 5/6 nephrectomy was reduced in thickness and
quantity at 3 months post-5/6 nephrectomy, confirming that bone
loss was induced (Fig. 3A). By
contrast, the structure of the trabecular bone of the L4 vertebrae
in the osthole-treated 5/6 nephrectomy mice was markedly improved,
as shown by the histological data (Fig. 3A), which was consistent with the
results of the micro CT analysis (Fig.
2A). To determine whether osteoclast formation was altered,
TRAP staining was performed using sections from 5/6 nephrectomy
mice and osthole-treated 5/6 nephrectomy mice. In the L4 vertebrae
of the sham mice, only a few osteoclasts were found to be
TRAP-positive on the surface of the trabecular bone in the 5/6
nephrectomy mice; however, the number of osteoclasts were
significantly increased, compared with the sham group. In the
osthole-treated 5/6 nephrectomy mice, there were only a few
osteoclasts, similar to the sham group (Fig. 3B).
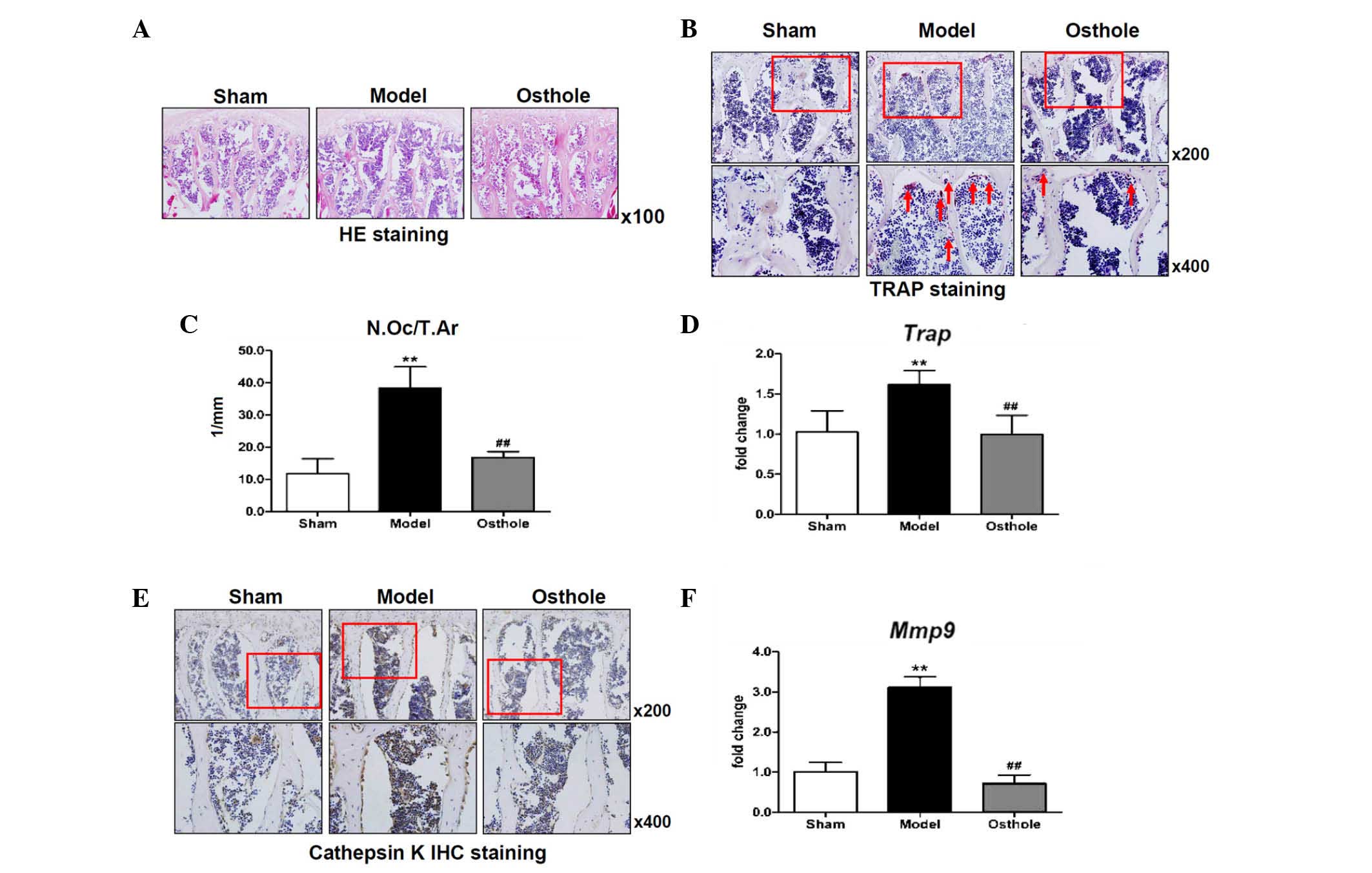 | Figure 3Osteoclast number is reduced in 5/6
nephrectomy mice treated with osthole. (A) HE staining showed that
the trabecular bone of the L4 vertebrae was reduced in thickness
and quantity in the 5/6 nephrectomy mice, which was improved by
osthole treatment (magnification, ×100). (B) TRAP staining. (C)
Quantification of N.Oc/T.Ar and reverse transcription-quantitative
polymerase chain reaction analysis for (D) Trap showed
increased osteoclast numbers in the L4 vertebrae of the 5/6
nephrectomy mice, compared with the sham mice, and this was
decreased in the osthole-treated 5/6 nephrectomy mice, compared
with the 5/6 nephrectomy mice. (E) cathepsin K and (F) Mmp9
in the L4 vertebrae were expressed at high levels following 5/6
nephrectomy, and these increases were partially inhibited by
osthole. **P<0.01, compared with the sham group;
##P<0.01, compared with the model group. Red arrows
indicate TRAP-positive osteoclasts and red boxes show area
magnifiied in the image below. HE, hematoxylin and eosin;
N.Oc/T.Ar, osteoclast number/trabecular bone area; IHC,
immunohistochemical; TRAP, tartrate-resistant acid phosphatase;
Mmp9, matrix metalloproteinase 9. |
Quantification of the TRAP staining also showed an
increase in the number of osteoclasts/trabecular bone area in the
L4 vertebrae of the 5/6 nephrectomy mice, compared with the sham
mice. Decreases in osteoclast number/trabecular bone area were
observed in the L4 vertebrae of the osthole-treated mice, compared
with the 5/6 nephrectomy mice (Fig.
3C). Similarly, the expression of Trap was increased in
the L4 vertebrae of the 5/6 nephrectomy mice, and was reversed to a
level similar to that in the sham group in the osthole-treated mice
(Fig. 3D). For further
confirmation, changes in the expression levels of cathepsin K and
Mmp9, markers of osteoclast formation, were revealed using
IHC staining and RT-qPCR analysis, respectively. The expression
levels of cathepsin K and Mmp9 in the L4 vertebrae were
higher following 5/6 nephrectomy, and these increases were
partially inhibited by treatment of the 5/6 nephrectomy mice with
osthole (Fig. 3E and F).
Osthole inhibits osteoclast formation
through regulation of the expression of OPG/RANKL in
osteoblasts
To confirm the effect of osthole on osteoclast
formation, primary bone marrow cells were cultured for osteoclast
formation and treated with osthole for 7 days. Osteoclast formation
was shown using TRAP staining. Osteoclast formation was markedly
inhibited by osthole treatment in a dose-dependent manner (Fig. 4A). To determine the mechanisms
underlying the regulation of osteoclast formation in the 5/6
nephrectomy mice and osthole treated 5/6 nephrectomy mice, RT-qPCR
analysis was performed to determine the expression levels of
Opg and Rankl in the L4 vertebrae. The expression of
Opg in the L4 vertebrae of the 5/6 nephrectomy mice was
markedly decreased, compared with that of the sham group, however,
no significant alteration in the expression of Rankl was
observed. Therefore, the Opg/Rankl ratio was
decreased significantly, which induced osteoclast formation in the
5/6 nephrectomy mice. In the L4 vertebrae of the osthole-treated
5/6 nephrectomy mice, the expression of Opg was increased
and the expression of Rankl was decreased, compared with the
5/6 nephrectomy mice. Therefore, increased Opg/Rankl
may be responsible for reduced osteoclast formation following
osthole treatment (Fig. 4B–D). To
confirm the changes in Opg and Rankl in vitro,
primary calvarial osteoblasts were cultured and treated with
osthole for 2 days. The expression levels of Opg and
Rankl were detected in osteoblasts, respectively. Similar to
the in vivo results, the expression of Opg was
upregulated and the expression of Rankl was downregulated by
treatment with osthole in a dose-dependent manner. The
Opg/Rankl ratio was also upregulated (Fig. 4E–G). These results suggested that
osthole treatment increased the expression of Opg in
osteoblasts, therefore osteoblasts could secrete more OPG protein
into the surrounding microenvironment. NFATc-1 and c-Fos are the
key regulators of osteoclast differentiation upon RANKL induction.
The IHC staining for the expression of NFATc-1 and c-Fos in the L4
vertebrae showed increased expression levels of NFATc-1 and c-Fos
in the 5/6 nephrectomy mice, and inhibition of the induced
expression levels of NFATc-1 and c-Fos following treatment with
osthole (Fig. 4H and I).
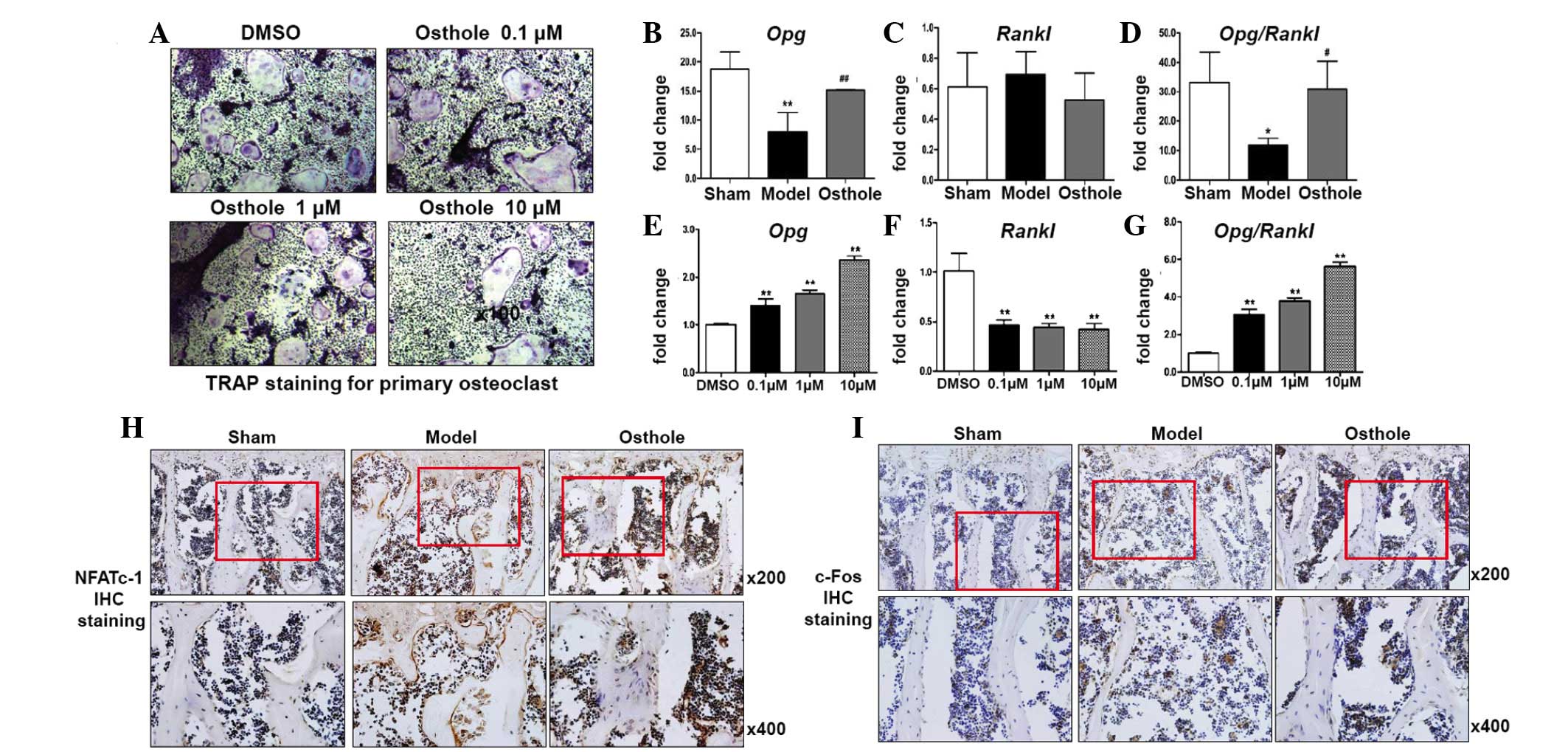 | Figure 4Osthole inhibits osteoclast formation
by regulating the expression of OPG/RANKL in osteoblasts. (A)
Primary bone marrow cells were cultured for osteoclast formation
and treated with osthole for 7 days. Osteoclast formation was shown
using TRAP staining and was decreased by osthole in a
dose-dependent manner. Reverse transcription-quantitative
polymerase chain reaction analysis showed a significant decrease in
the expression of (B) Opg in the L4 vertebrae of 5/6
nephrectomy mice, compared with the sham group. (C) Rankl
did not change significantly. The decreased Opg and (D)
Opg/Rankl were partially inhibited by osthole.
Primary calvarial osteoblasts were cultured and treated with
osthole for 2 days. (E) Expression of Opg was upregulated
and (F) Rankl was downregulated by osthole. (G)
Opg/Rankl was also upregulated by osthole in a
dose-dependent manner. IHC staining showed increased (H) NFATc1 and
(I) c-Fos in the L4 vertebrae of the 5/6 nephrectomy mice, compared
with sham group, and this was inhibited by osthole.
*P<0.05 and **P<0.01, compared with the
sham group; #P<0.05 and ##P<0.01,
compared with the model group. Red boxes indicate the area was
magnified for the image below. TRAP, tartrate-resistant acid
phosphatase; opg, osteoprotegerin; rankl, receptor
activator for nuclear factor-κB ligand; NFATc-1, nuclear factor of
activated T-cells, cytoplasmic-1; IHC, immunohistochemical. |
Discussion
Osthole is a coumarin derivative present in several
plants, including Angelica pubescens and Cnidium
monnieri (20,21). These plants have been used for the
treatment of primary and secondary osteoporosis in traditional
Chinese medicine. Previous studies have confirmed that osthole is
capable of significantly reversing ovariectomy-induced bone loss in
rats (16,17). In the present study, osthole was
found to be effective in the treatment of bone loss in 5/6
nephrectomy mice, and the mechanism were correlated with osteoclast
inhibition.
Bone resorption is abnormally high in the late stage
of CKD (22,23). Bone loss (24) and marked bone resorption have also
been observed in a 5/6 nephrectomy model (25,26),
which is consistent with the results of the present study. In the
present study, osthole treatment partially reversed the bone loss
induced by 5/6 nephrectomy in the mice. Histological analysis
revealed that the increase of osteoclast formation in the 5/6
nephrectomy mice was rescued by osthole treatment, shown by
decreased expression levels of Trap, cathepsin K and
Mmp9, suggesting that the enhanced bone resorption induced
by nephrectomy in the mice was prohibited by osthole. The in
vitro experiments also confirmed that the osteoclast formation
of primary bone marrow cells was reduced when the cells were
cultured in the presence of osthole.
Multinucleated osteoclasts differentiate from
hematopoietic progenitors of the monocyte/macrophage lineage.
Osteoclast formation is predominantly regulated by the ratio of
OPG/RANKL. RANKL mediates the differentiation, activation and
survival of osteoclasts by binding to its receptor, RANK, which is
a member of the tumor necrosis factor receptor family. OPG disturbs
the binding between RANK and RANKL and exerts a function opposite
to that of RANKL (27–31). A previous study showed that
inhibiting RANKL with OPG-Fc reverses the high bone turnover and
low BMD induced by 5/6 nephrectomy, suggesting that RANKL may offer
potential as an important therapeutic target to protect bone loss
in patients with CKD (32).
Osthole may act like an estrogen (33) and regulate OPG/RANKL through
estrogen receptor α (34,35). Osthole has also been shown to
regulate the ratio of OPG/RANKL by upregulating the expression of
OPG and downregulating the expression of RANKL in vitro
(36), suggesting that osthole may
prohibit osteoclast formation through the regulation of OPG/RANKL
in the 5/6 nephrectomy mouse model. The results of the RT-qPCR
analysis in the present study of the expression levels of
Opg and Rankl in the L4 vertebrae suggested the in
vivo function of osthole on Opg/Rankl. The 5/6
nephrectomy decreased the expression of Opg in the vertebrae
of the mice, whereas the expression of Rankl remained
unchanged. Osthole reversed the reduction of Opg and reduced
the expression of Rankl, leading to reversal of the
reduction of Opg/Rankl and resulting in the
inhibition of osteoclast formation.
Osteoblast and bone marrow stromal cells are
important sources of OPG and RANKL. They are involved in osteoclast
differentiation in the bone marrow microenvironment through a
mechanism of cell-to-cell interaction with osteoclast progenitors
(27–29). The in vitro experiments in
the present study showed that osthole increased the expression of
OPG and decreased the expression of RANKL in primary osteoblasts.
NFATc1 is the key regulator of osteoclast differentiation. Upon
RANKL/RANK binding, NFATc1 can be induced through the TRAF6-NF-κB
and c-Fos pathways. c-Fos, together with continuously activated
calcium signaling, is also crucial for auto-amplification of the
NFATc1 signal (37). NFATc1
further regulates osteoclast-specific gene transcription in
cooperation with other transcription factors (38). In the present study, NFATc1 and
c-Fos were downregulated following treatment with osthole in
vivo and in vitro. Taken together, the results
demonstrated that osthole upregulated the expression of OPG and
downregulated the expression of RANKL in osteoblasts, and
prohibited the induction of NFATc1 signaling in hematopoietic
progenitors of the monocyte/macrophage lineage. The expression of
osteoclast-specific genes was downregulated and osteoclast
formation was prohibited.
In conclusion, the present study provided evidence
that osthole partially rescued bone loss of vertebrae induced by
5/6 nephrectomy in mice through the inhibition of osteoclast
formation. Mechanistically, osthole treatment upregulated OPG and
downregulated RANKL in osteoblasts and stromal cells, and further
inhibited the expression of NFATc1 and c-Fos in osteoclasts. Thus,
osthole may be a potential candidate therapeutic agent for high
turnover of bone loss induced by menopause, secondary
hyperparathyroidism and glucocorticoid intake.
Acknowledgments
This study was supported in part by the National
Natural Science Foundation of China (grant nos. 81403239 and
81503590), the Shanghai Natural Science Foundation (grant no.
12ZR1450400) and the Program for Innovative Research Team (grant
no. 2015RA4002). The authors would like to thank Professor Di Chen
(Rush University, Chicago, IL, USA) for assisting with the language
modification of this manuscript.
References
|
1
|
Moe S, Drüeke T, Cunningham J, Goodman W,
Martin K, Olgaard K, Ott S, Sprague S, Lameire N and Eknoyan G;
Kidney Disease: Improving Global Outcomes (KDIGO): Definition,
evaluation, and classification of renal osteodystrophy: A position
statement from Kidney Disease: Improving Global Outcomes (KDIGO).
Kidney Int. 69:1945–1953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uhlig K, Berns JS, Kestenbaum B, Kumar R,
Leonard MB, Martin KJ, Sprague SM and Goldfarb S: KDOQI US
commentary on the 2009 KDIGO clinical practice guideline for the
diagnosis, evaluation and treatment of CKD-Mineral and Bone
Disorder (CKD-MBD). Am J Kidney Dis. 55:773–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for
the diagnosis, evaluation, prevention, and treatment of Chronic
Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int.
Suppl(Suppl): S1–S130. 2009.
|
|
4
|
Sprague SM: The role of the bone biopsy in
the diagnosis of renal osteodystrophy. Semin Dial. 13:152–155.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barreto FC, Barreto DV, Moyses RM, Neves
CL, Jorgetti V, Draibe SA, Canziani ME and Carvalho AB:
Osteoporosis in hemodialysis patients revisited by bone
histomorphometry: A new insight into an old problem. Kidney Int.
69:1852–1857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malluche HH and Monier-Faugere MC: Renal
osteodystrophy: What's in a name? Presentation of a clinically
useful new model to interpret bone histologic findings. Clin
Nephrol. 65:235–242. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu CY: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Block GA, Hulbert-Shearon TE, Levin NW and
Port FK: Association of serum phosphorus and calcium × phosphate
product with mortality risk in chronic hemodialysis patients: A
national study. Am J Kidney Dis. 31:607–617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kestenbaum B, Sampson JN, Rudser KD,
Patterson DJ, Seliger SL, Young B, Sherrard DJ and Andress DL:
Serum phosphate levels and mortality risk among people with chronic
kidney disease. J Am Soc Nephrol. 16:520–528. 2005. View Article : Google Scholar
|
|
10
|
Keith DS, Nichols GA, Gullion CM, Brown JB
and Smith DH: Longitudinal follow-up and outcomes among a
population with chronic kidney disease in a large managed care
organization. Arch Intern Med. 164:659–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coresh J, Astor BC, Greene T, Eknoyan G
and Levey AS: Prevalence of chronic kidney disease and decreased
kidney function in the adult US population: Third National Health
and Nutrition Examination Survey. Am J Kidney Dis. 41:1–12. 2003.
View Article : Google Scholar
|
|
12
|
Chang NT, Lee YH, Hsu JC, Chan CL, Huang
GS, Renn JH and Yang NP: Epidemiological study of orthopedic
injuries in hemodialysis patients in Taiwan: A fixed cohort survey,
2004–2008. Clin Interv Aging. 8:301–308. 2013. View Article : Google Scholar :
|
|
13
|
Alem AM, Sherrard DJ, Gillen DL, Weiss NS,
Beresford SA, Heckbert SR, Wong C and Stehman-Breen C: Increased
risk of hip fracture among patients with end-stage renal disease.
Kidney Int. 58:396–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dooley AC, Weiss NS and Kestenbaum B:
Increased risk of hip fracture among men with CKD. Am J Kidney Dis.
51:38–44. 2008. View Article : Google Scholar
|
|
15
|
Schumock GT and Sprague SM: Clinical and
economic burden of fractures in patients with renal osteodystrophy.
Clin Nephrol. 67:201–208. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XX, Hara I and Matsumiya T: Effects of
osthole on postmenopausal osteoporosis using ovariectomized rats;
comparison to the effects of estradiol. Biol Pharm Bull.
25:738–742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang DZ, Hou W, Zhou Q, Zhang M, Holz J,
Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, et al: Osthole
stimulates osteoblast differentiation and bone formation by
activation of beta-catenin-BMP signaling. J Bone Miner Res.
25:1234–1245. 2000. View
Article : Google Scholar
|
|
18
|
Sangidorj O, Yang SH, Jang HR, Lee JP, Cha
RH, Kim SM, Lim CS and Kim YS: Bone marrow-derived endothelial
progenitor cells confer renal protection in a murine chronic renal
failure model. Am J Physiol Renal Physiol. 299:F325–F335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmittgen TD, Zakrajsek BA, Mills AG,
Gorn V, Singer MJ and Reed MW: Quantitative reverse
transcription-polymerase chain reaction to study mRNA decay:
Comparison of endpoint and real-time methods. Anal Biochem.
285:194–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YF, Tsai HY and Wu TS:
Anti-inflammatory and analgesic activities from roots of Angelica
pubescens. Planta Med. 61:2–8. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ko FN, Wu TS, Liou MJ, Huang TF and Teng
CM: Vasorelaxation of rat thoracic aorta caused by osthole isolated
from Angelica pubescens. Eur J Pharmacol. 219:29–34. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malluche HH, Ritz E, Lange HP, Kutschera
L, Hodgson M, Seiffert U and Schoeppe W: Bone histology in
incipient and advanced renal failure. Kidney Int. 9:355–362. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaye M, Zucker SW, Leclerc YG, Prichard S,
Hodsman AB and Barré PE: Osteoclast enlargement in endstage renal
disease. Kidney Int. 27:574–581. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomat A, Gamba CA, Mandalunis P, De Grandi
MC, Somoza J, Friedman S and Zeni S: Changes in bone volume and
bone resorption by olpadronate treatment in an experimental model
of uremic bone disease. J Musculoskelet Neuronal Interact.
5:174–181. 2005.PubMed/NCBI
|
|
25
|
Kawaguchi Y, Kawashima H, Ueno K, Izawa Y,
Makita T, Kurozumi S, Hashimoto Y, Yamamoto M, Kimura Y, Imamura N,
et al: Effect of 1 alpha-hydroxyvitamin D3 in rats with
experimental renal osteodystrophy. Endocrinol Jpn. 26(Suppl):
S73–S79. 1979. View Article : Google Scholar
|
|
26
|
Moscovici A, Bernheim J, Popovtzer MM and
Rubinger D: Renal osteodystrophy in rats with reduced renal mass.
Nephrol Dial Transplant. 11(Suppl 3): S146–S152. 1996. View Article : Google Scholar
|
|
27
|
Udagawa N, Takahashi N, Yasuda H, Mizuno
A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K
and Suda T: Osteoprotegerin produced by osteoblasts is an important
regulator in osteoclast development and function. Endocrinology.
141:3478–3484. 2000.PubMed/NCBI
|
|
28
|
Gori F, Hofbauer LC, Dunstan CR, Spelsberg
TC, Khosla S and Riggs BL: The expression of osteoprotegerin and
RANK ligand and the support of osteoclast formation by
stromal-osteoblast lineage cells is developmentally regulated.
Endocrinology. 141:4768–4776. 2000.PubMed/NCBI
|
|
29
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa
N, et al: A novel molecular mechanism modulating osteoclast
differentiation and function. Bone. 25:109–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakashima T, Hayashi M and Takayanagi H:
New insights into osteoclastogenic signaling mechanisms. Trends
Endocrinol Metab. 23:582–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Padagas J, Colloton M, Shalhoub V,
Kostenuik P, Morony S, Munyakazi L, Guo M, Gianneschi D, Shatzen E,
Geng Z, et al: The receptor activator of nuclear factor-kappaB
ligand inhibitor osteoprotegerin is a bone-protective agent in a
rat model of chronic renal insufficiency and hyperparathyroidism.
Calcif Tissue Int. 78:35–44. 2006. View Article : Google Scholar
|
|
33
|
Hsieh MT, Hsieh CL, Wang WH, Chen CS, Lin
CJ and Wu CR: Osthole improves aspects of spatial performance in
ovariectomized rats. Am J Chin Med. 32:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindberg MK, Erlandsson M, Alatalo SL,
Windahl S, Andersson G, Halleen JM, Carlsten H, Gustafsson JA and
Ohlsson C: Estrogen receptor alpha, but not estrogen receptor beta,
is involved in the regulation of the OPG/RANKL
(osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and
serum interleukin-6 in male mice. J Endocrinol. 171:425–433. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bord S, Ireland DC, Beavan SR and Compston
JE: The effects of estrogen on osteoprotegerin, RANKL, and estrogen
receptor expression in human osteoblasts. Bone. 32:136–141. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhai YK, Pan YL, Niu YB, Li CR, Wu XL, Fan
WT, Lu TL, Mei QB and Xian CJ: The importance of the prenyl group
in the activities of osthole in enhancing bone formation and
inhibiting bone resorption in vitro. Int J Endocrinol.
2014:9219542014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc-1
(NFAT2) integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Crabtree GR and Olson EN: NFAT signaling:
Choreographing the social lives of cells. Cell. 109(Suppl):
S67–S79. 2002. View Article : Google Scholar : PubMed/NCBI
|