Introduction
Mesenchymal stem cells (MSCs) are a promising cell
source for regenerative medicine due to their potential for
self-renewal and multilineage differentiation into bone, cartilage,
muscle, ligament, tendon, adipose, and endothelial cells (1). The isolation of MSCs from human
donors or patients is relatively easy, and MSC cultures are able to
expand rapidly for ≥30 population doublings. In addition, the
differentiation of MSCs into various phenotypes is simple in
lineage-specific culture conditions. These properties make MSCs
attractive candidates for the treatment of various diseases, and
>500 clinical trials are being conducted using MSCs to overcome
a diverse range of diseases (2).
Despite their beneficial effects, the application of
MSCs is limited due to pathophysiological environmental conditions,
including oxidative stress, inflammation, low oxygen levels and
restricted nutrient supply (3).
Various stress conditions trigger reduced proliferation and loss of
stemness, and are able to induce senescence, resulting in >99%
cell death during the first few days following MSC transplantation
(4–7). Therefore, protection against several
stressors and optimization of MSC culture conditions are required
to produce functional MSCs with high therapeutic efficiency. To
address this issue, preconditioning, hypoxic culture, pretreatment
and genetic manipulation have been suggested to increase the
survival of MSCs (3).
Cirsium setidens is a wild perennial herb
that possesses various bioactivities, including antitumor,
antioxidant and hepatoprotective effects (8–10).
C. setidens, which is a bioactive flavonoid, has previously
been used to treat hemostasis, hematemesis, hematuria and
hypertension (11). Although C.
setidens exerts various biological activities, there is no
evidence regarding the protective effects of C. setidens
against oxidative stress in MSCs. The present study aimed to assess
the effects of C. setidens on ROS-induced oxidative stress
in MSCs, and to elucidate the mechanism underlying its
anti-apoptotic effects against oxidative stress.
Materials and methods
MSCs culture conditions
Human adipose tissue-derived MSCs were obtained from
the American Type Culture Collection (Manassas, VA, USA), and were
confirmed to be pathogen- and mycoplasma-free. The supplier
certified that the MSCs expressed specific cell surface markers
[cluster of differentiation (CD)73 and CD105, but not CD31], and
had adipogenic and osteogenic differentiation potential when
cultured with specific differentiation media. MSCs were cultured in
α-minimum essential medium (α-MEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin. MSC cultures were
grown in a 5% CO2 humidified incubator at 37°C.
Preparation of C. setidens water
extract
C. setidens was collected from the
Jeongseon-Gondre Farming Association Corporation (Jeongseon-gun,
South Korea). C. setidens was extracted by suspending 100 g
into 5 L of hot distilled water for 3 h. The water extract was
filtered to remove plant particles through filter paper and was
then concentrated in a vacuum under reduced pressure and
lyophilized using a freeze dryer for three days in order to fully
dry the sample. In total, 19.2 g C. setidens extracted
powder was recovered and was maintained at −20°C. The powder was
then dissolved in phosphate buffer (pH 7.4, 20 mg/ml) and was
stored at −80°C.
High-performance liquid chromatography
(HPLC)
A Shimadzu LC-20A (Shimadzu Corporation, Kyoto,
Japan), consisting of two LC-20 AD Pumps, a DGU-20A3 vacuum
degasser, a SPD-M20A photodiode array detector (PDA) and a CTO 20A
Autosampler, was employed. The chromatographic analysis for
determination of pectolinarin was carried out using a Luna 5 u C18
100A column (250×4.6 mm; Phenomenex, Inc., Torrance, CA, USA). The
mobile phase consisted of solvent A (0.1% formic acid in water) and
100% acetonitrile (solvent B). The solvent gradient elution
conditions were 5% (B) for 0–5 min, 5–60% (B) for 5–22 min, 60–60%
(B) for 22–24 min and 60-5% (B) for 24–30 min at a flow-rate of 1.0
ml/min, and the column oven was operated at 40°C throughout the
study. Detection was conducted with different wavelengths of 273
nm. Injection volume of the sample solutions was 10 µl. Peak
identity was confirmed by comparison of spectra obtained from the
PDA detector. A spectrum of pectolinarin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and C. setidens water extract
were compared and analyzed using PDA, at a wavelength of 200–400
nm. The data of chromatogram and spectrum were stored and displayed
on a computer.
Chemical treatment of MSCs
MSCs were washed twice with phosphate-buffered
saline, and the medium was replaced with fresh α-MEM supplemented
with 10% FBS. To assess cell viability, MSCs were pretreated with
C. setidens (100 µg/ml or 200 µg/ml) at 37°C
for 30 min, and were then treated with hydrogen peroxide
(H2O2; 200 µM) for the indicated
durations (0, 4, 6 and 8 h). To investigate various cell signaling
pathways, MSCs were treated with H2O2 (200
µM) for the indicated duration (0, 15, 30, 60 and 120 min).
MSCs were treated with C. setidens (100 µg/ml) for 30
min and were then treated with H2O2 (200
µM) for 120 min.
Cell viability assay
Subconfluent, exponentially growing MSCs were
incubated in a 96-well plate with C. setidens for various
durations. Cell viability was determined using a modified
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay,
which is based on the conversion of the tetrazolium salt
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-tetrazolium
to formazan by mitochondrial NAD(P)H-dependent oxidoreductase
enzymes. After 4 h formazan levels were quantified by measuring the
absorbance at 575 nm using a microplate reader (Tecan Group AG,
Männedorf, Switzerland).
Intracellular reactive oxygen species
(ROS) assay
CM-H2DCFDA (DCF-DA), which acts as
H2O2-sensitive fluorophore, was used to
detect the H2O2-induced production of ROS.
DCF-DA (10 µM) was added to the cells and incubated for 30
min at room temperature in the dark. The cells were subsequently
observed under a laser confocal microscope (magnification, ×600;
Fluoview 1000; Olympus Corporation, Tokyo, Japan) with excitation
and emission wavelengths of 488 and 515–540 nm, respectively.
Western blot analysis
Total protein was extracted using RIPA Lysis Buffer
(Thermo Fisher Scientific, Inc.). Bicinchoninic acid assay kit
(Thermo Fisher Scientific, Inc.) was used to quantify proteins. The
cell lysate (50 µg protein) was separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, and the
proteins were transferred to nitrocellulose membranes. The
membranes were then blocked with 5% skim milk for 1 h at room
temperature, and were incubated with the primary antibodies at the
dilutions recommended by the supplier. Ataxia telangiectasia
mutated (ATM; cat. no. sc-377293), phosphorylated (p)-ATM (cat. no.
sc-47739), p38 (cat. no. sc-81621), p-p38 (cat. no. sc-101758),
c-Jun N-terminal kinase (JNK; cat. no. sc-7345), p-JNK (cat. no.
sc-6254), p53(cat. no. sc-126) and p-p53 (cat. no. sc-101762),
B-cell lymphoma 2 (BCL-2;cat. no. sc-7382), BCL-2-associated X
protein (BAX; cat. no. sc-6236), cleaved poly (ADP ribose)
polymerase-1 (PARP-1; cat. no. sc-56196), cleaved caspase-3 (cat.
no. sc-7272) and β-actin (cat. no. sc-47778) primary antibodies
were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). All primary antibodies were diluted 1:1,000 in 5% skim milk
and incubated with membranes overnight at 4°C, the membranes were
washed, and the primary antibodies were detected following
incubation with secondary antibodies horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; cat.
no. sc-2004; 1:10,000) or goat anti-mouse IgG (cat. no. sc-2005;
1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. The
bands were visualized using enhanced chemiluminescence reagents
(Amersham; GE Healthcare Life Sciences, Little Chalfont, UK). The
semi-quantification of western blotting bands were used Image J
version 1.47 (National Institutes of Health, Bethesda, MD, USA) and
compared to β-actin.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) assay
A TUNEL assay was performed using the TdT
Fluorescein In Situ Apoptosis Detection kit (Trevigen,
Gaithersburg, MD, USA). The MSCs were pretreated with C.
setidens for 30 min, and were then treated with
H2O2 for 8 h. MSCs were labeled according to
the manufacturer's instructions. Stained MSCs were visualized under
a fluorescent microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. All experiments were repeated 5 times and were
analyzed by one-way analysis of variance, followed by a comparison
of the treatment and control groups using the Bonferroni-Dunn test
using SPSS version 19 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
C. setidens exerts protective effects
against H2O2-induced cell death in MSCs
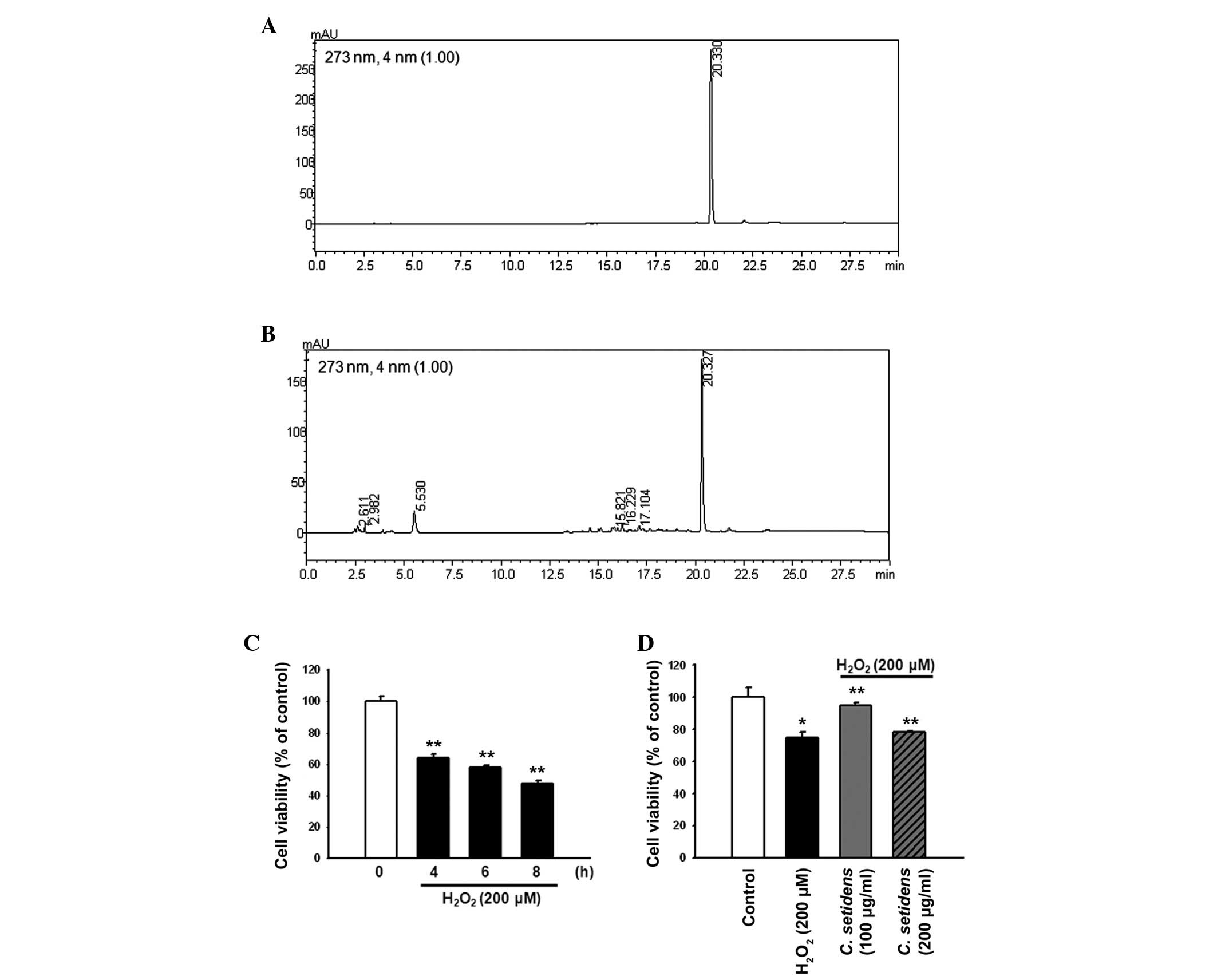
Examination of the HPLC chromatograms of C.
setidens indicated that the extract contained pectolinarin
(Fig. 1A and B). To explore the
protective effects of C. setidens against
H2O2-induced oxidative stress, MSCs were
treated with H2O2 (200 µM) for the
indicated time periods (0, 4, 6 and 8 h). The maximal effect of
H2O2 on MSC cell death was observed following
8 h of treatment (Fig. 1C). MSCs
were pretreated with C. setidens (0, 100 or 200
µg/ml) for 30 min and were then treated with
H2O2 for 8 h. Pretreatment with C.
setidens significantly increased the viability of MSCs,
particularly when used at a concentration of 100 µg/ml
(Fig. 1D).
C. setidens mediates the inhibition of
H2O2-induced ROS generation and
phosphorylation of stress-associated mitogen-activated protein
kinases (MAPKs)
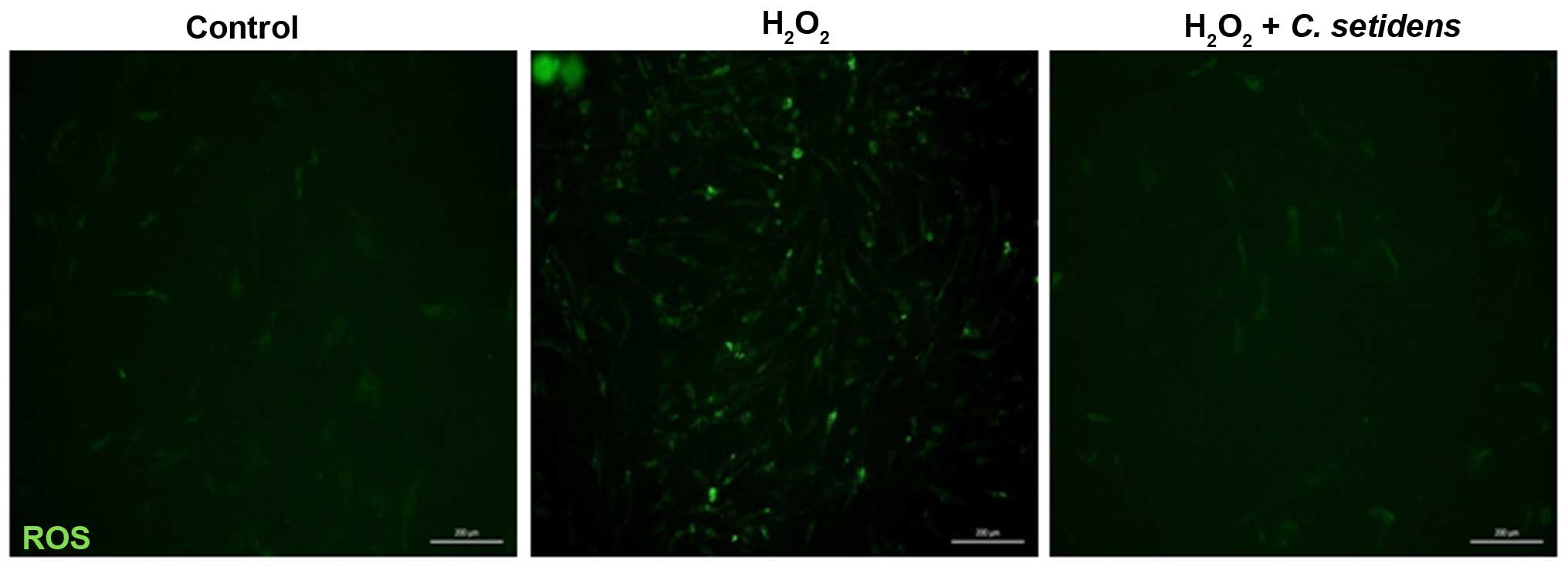
To investigate the inhibitory effects of C.
setidens on ROS generation in MSCs, DCF-DA was used as an
indicator of ROS production, and alterations to intracellular
peroxide levels were assessed. Following treatment of MSCs with
H2O2 (200 µM), the intracellular ROS
levels were markedly increased compared with in the untreated
cells. Conversely, pretreatment of MSCs with C. setidens
(100 µg/ml) markedly suppressed intracellular ROS levels
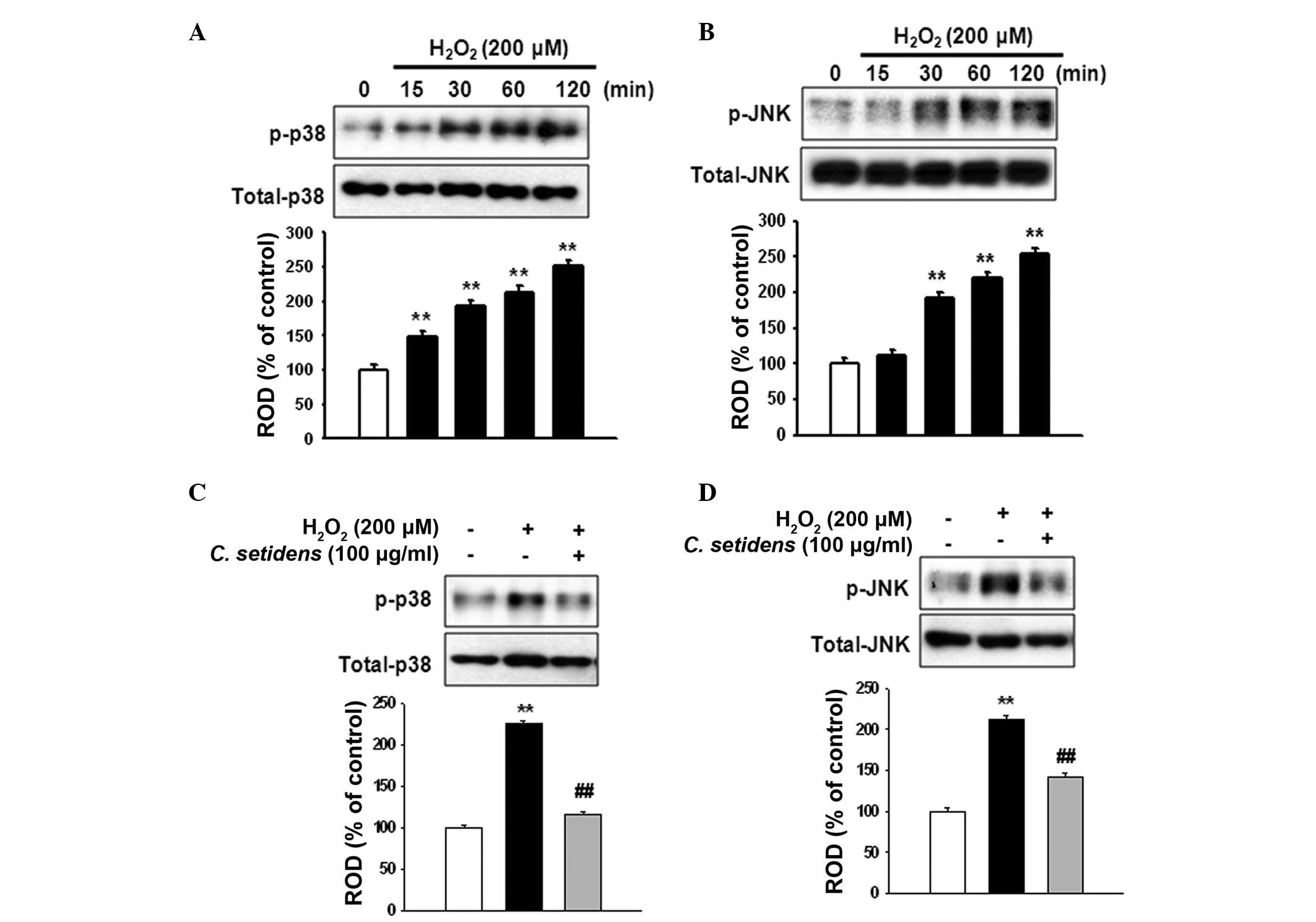
(Fig. 2). To determine the
regulation of stress-associated MAPKs in ROS-induced cell death,
MSCs were treated with H2O2 for the indicated
time periods (0, 15, 30, 60 and 120 min).
H2O2 increased the phosphorylation of p38 and
JNK MAPKs in a time-dependent manner (Fig. 3A and B), whereas the
phosphorylation of p38 and JNK was significantly decreased
following pretreatment with C. setidens (Fig. 3C and D).
Effects of C. setidens on apoptosis
regulation in MSCs
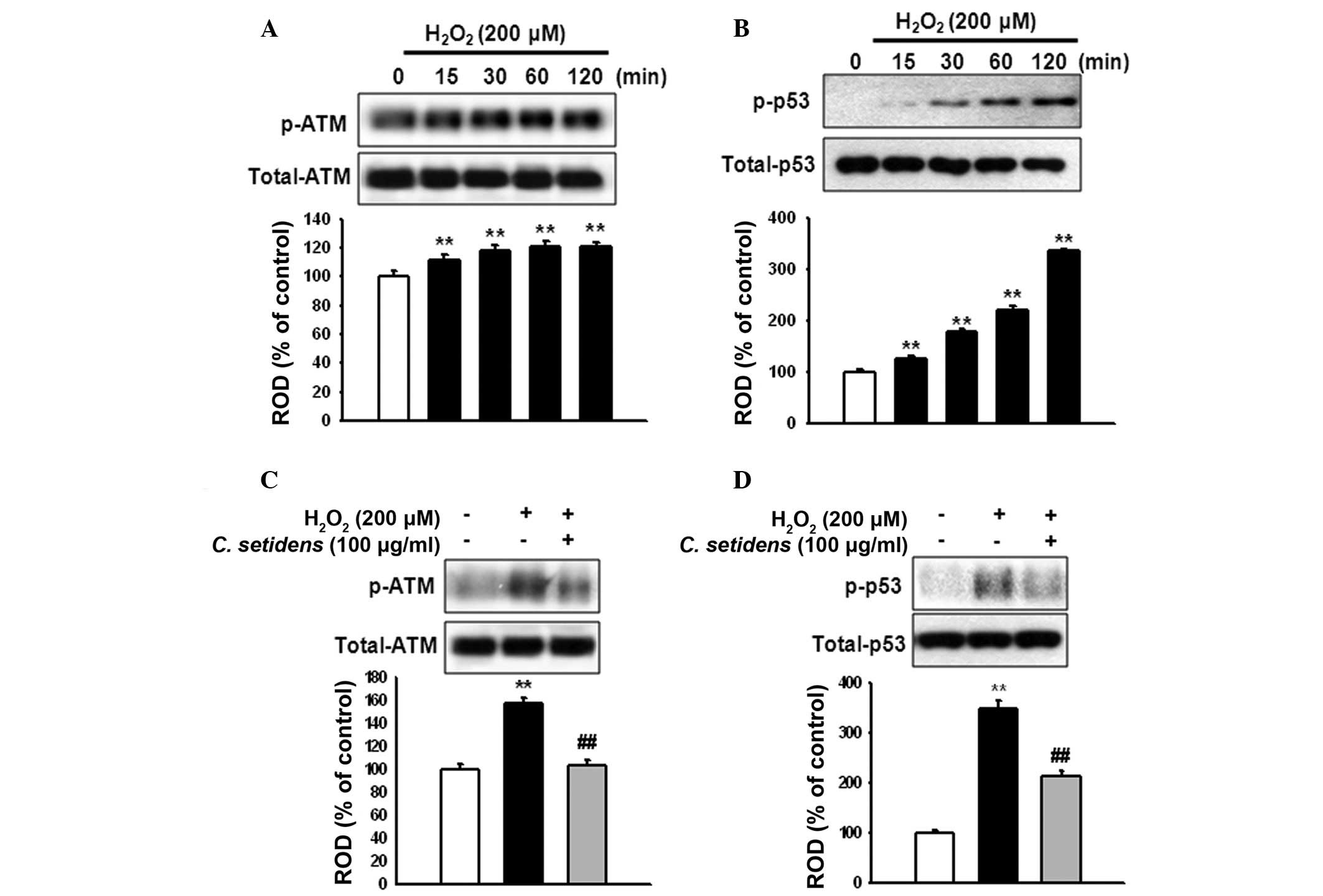
To elucidate the involvement of ATM and p53
activation in ROS-induced apoptosis, MSCs were treated with
H2O2 for the indicated time periods (0, 15,
30, 60 and 120 min). H2O2 increased the
phosphorylation of ATM and p53 in a time-dependent manner (Fig. 4A and B). However, the activation of
ATM and p53 was significantly reduced following pretreatment with
C. setidens (Fig. 4C and
D).
Anti-apoptotic effects of C. setidens on
the regulation of apoptosis-associated proteins in MSCs
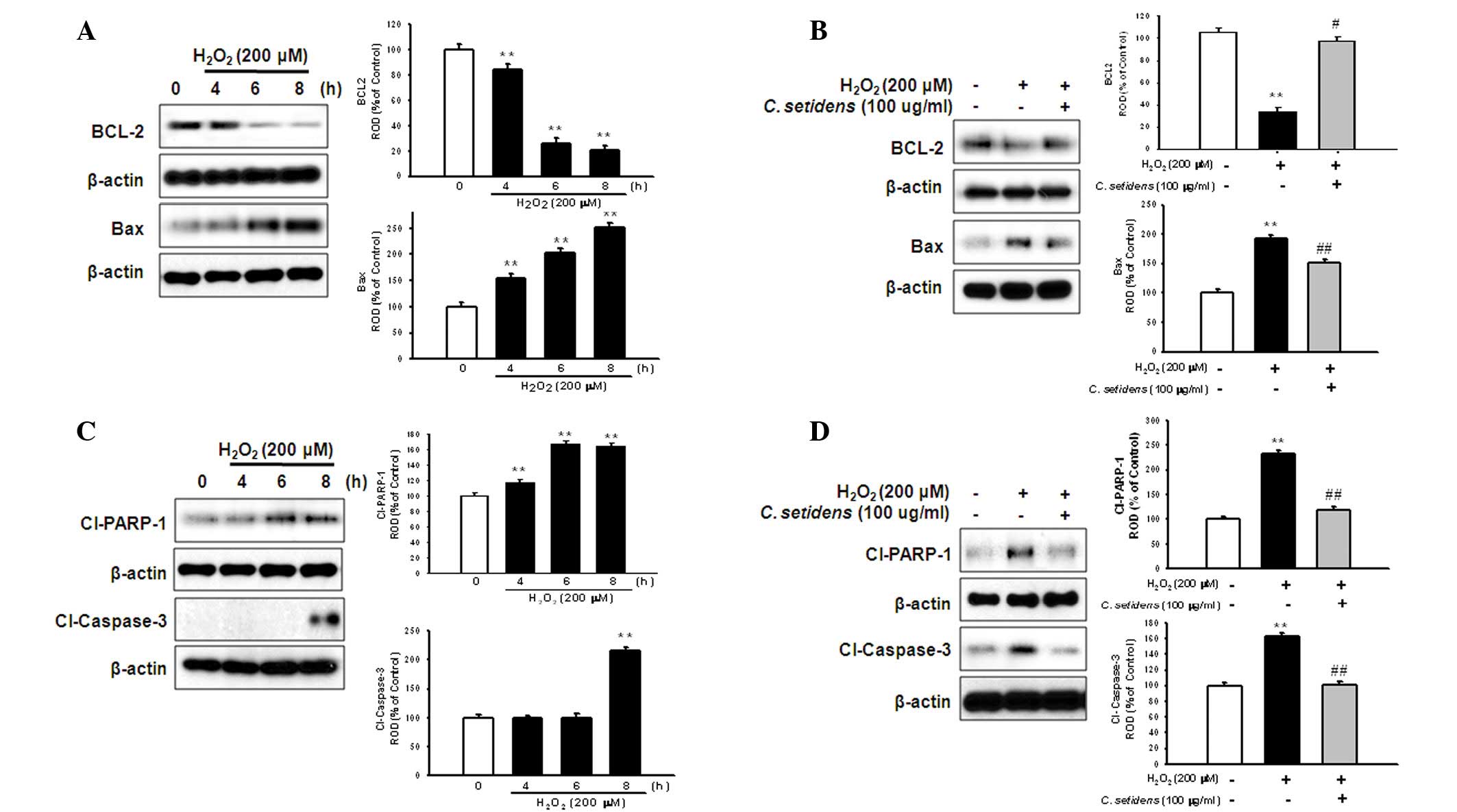
To determine whether pretreatment with C.
setidens may affect ROS-induced expression of apoptotic
proteins in MSCs, MSCs were treated with H2O2
for the indicated time periods (0, 4, 6 and 8 h). Subsequently, the
expression levels of BCL-2 (anti-apoptotic protein) and Bax
(proapoptotic protein) were assessed using western blot analysis.
H2O2-induced ROS decreased the expression
levels of BCL-2 and increased the expression levels of Bax in a
time-dependent manner (Fig. 5A);
however, pretreatment with C. setidens significantly
increased BCL-2 expression and decreased Bax expression (Fig. 5B). To further investigate the
regulation of key signaling pathways against oxidative stress,
following pretreatment with C. setidens the expression
levels of proapoptotic regulators, cleaved PARP-1 and caspase-3,
were detected by western blot analysis.
H2O2-induced ROS activated cleaved PARP-1 and
caspase-3, whereas pretreatment with C. setidens
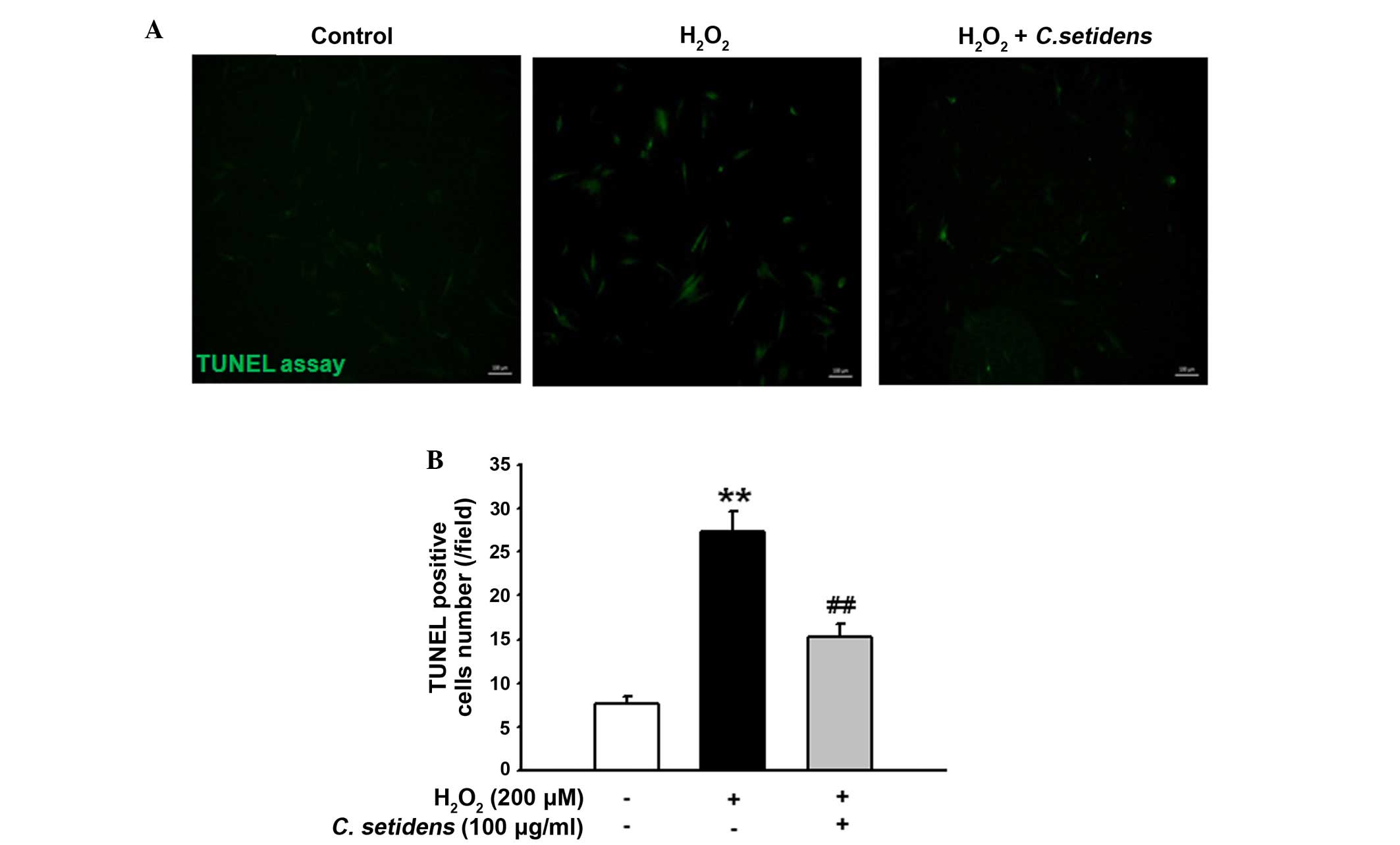
significantly inhibited their expression (Fig. 5C and D). In addition, the results
of a TUNEL assay indicated that pretreatment with C.
setidens significantly protected against oxidative
stress-induced apoptosis of MSCs (Fig.
6A and B).
Discussion
For applications such as preclinical and clinical
trials, the cell source for stem cell-based therapy should be
abundant, accessible, easy to deliver to the injured site, tested
and optimized in suitable animal models, and non-tumorigenic and
non-immunogenic (12). Among stem
cell sources, MSCs exhibit an extensive differentiation potential
and are easy to harvest from several tissues, including bone
marrow, adipose tissue, and skeletal muscle (1). In particular, adipose-derived MSCs
are an attractive cell source due to their abundance,
accessibility, multipotency and loss of immunogenicity (13). However, the survival rate and
differentiation of transplanted stem cells in injured sites is
limited due to apoptosis caused by the pathophysiological
environment (3). Therefore,
identification of a strategy for the protection of transplanted
MSCs is important for the development of MSCs-based therapy for
regenerative medicine. C. setidens is a phenolic and
flavonoid compound, which possesses anti-inflammatory, anticancer,
anti-mutation, anti-fungal, hepatoprotective and neuroprotective
activity, and is capable of immune enhancement (9,10,14–16).
In the present study, the HPLC chromatogram detected pectolinarin
in the C. setidens extract. Pectolinarin possess
anti-inflammatory effects through the inhibition of eicosanoid
formation (17). Despite these
attractive properties, the protective effects of C. setidens
on oxidative injury in MSCs have yet to be fully elucidated.
The present study demonstrated that pretreatment of
MSCs with C. setidens inhibited
H2O2-induced apoptosis, and reduced ROS
generation. Ischemic conditions induce intracellular ROS
production, resulting in the activation of inflammation and
apoptosis. In addition, oxidative stress activates MAPKs, which
contribute to modulation of the stress response (18). MAPK kinase kinases sense the degree
of stress-induced cell damage and regulate the MAPK cascade to
determine cell fate. Eventually, downstream MAPKs phosphorylate
several effectors, including p38 MAPK and JNK, to induce apoptosis
(19).
H2O2-induced ROS increased the
phosphorylation of p38 and JNK; however, pretreatment of MSCs with
C. setidens suppressed the phosphorylation of p38 and JNK.
These results suggested that pretreatment with C. setidens
may inhibit ROS-induced apoptosis through the regulation of
stress-associated MAPKs.
ATM is a pivotal regulatory molecule in ROS-induced
apoptosis (20), which has a key
role in the cellular response to DNA damage, particularly double
strand breaks (21). The
phosphorylation of ATM has previously been reported to differ
between oxidative stress-induced phosphorylation and double strand
break-dependent ATM-mediated phosphorylation (22). Treatment with
H2O2 induces the phosphorylation of ATM
(S1981) and its downstream target p53, but not histone 2AX, a
double strand break marker, thus suggesting that ROS-induced ATM
activation is independent of the double strand break process
(23). The results of the present
study revealed that treatment with C. setidens decreased the
phosphorylation of ATM and p53 in response to oxidative stress,
thus indicating that C. setidens may reduce oxidative
stress-induced cell death via the modulation of ATM and p53
activity, as well as MAPK activation.
Apoptosis occurs under pathophysiological conditions
via a cascade of cellular events, which involves various
apoptosis-associated genes (24).
Pathological apoptosis is induced by downregulation of
anti-apoptotic proteins, such as BCL-2, or by the expression of
proapoptotic proteins, such as Bax (25). In addition, the cleavage of
caspase-3 results in DNA fragmentation, cytoskeletal and nuclear
protein degradation, and the expression of ligands for phagocytic
cell receptors (25). The cleavage
of PARP-1 by caspases is one of the first biochemical markers of
apoptosis (26). The present study
indicated that H2O2 induced-ROS decreased the
BCL-2/Bax ratio, and increased the cleavage of caspase-3 and
PARP-1. Conversely, pretreatment of MSCs with C. setidens
restored the BCL-2/Bax ratio and reduced activation of caspase-3
and PARP-1. These results suggested that C. setidens may
inhibit ROS-induced apoptosis via regulation of the apoptotic
cascade.
The present study identified the cytoprotective
effects of C. setidens on MSCs exposed to oxidative stress.
Under oxidative stress conditions, C. setidens protected
MSCs from the harmful effects of intracellular ROS via regulation
of stress-associated MAPKs, ATM and p53 phosphorylation, and
apoptotic signal cascades. These findings suggested that C.
setidens may be developed as a cytoprotective agent for use
alongside MSCs-based therapy for the treatment of ischemic
diseases.
Acknowledgments
The present study was supported by the National
Research Foundation (NRF) grant funded by the Korean government
(MEST) (grant no. 2011-0009610) and the Ministry of Education
(grant. no. 2016R1D1A3B01007727). The funders had no role in study
design, data collection or analysis, the decision to publish, or
preparation of the manuscript.
References
|
1
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marquez-Curtis LA, Janowska-Wieczorek A,
McGann LE and Elliott JA: Mesenchymal stromal cells derived from
various tissues: Biological, clinical and cryopreservation aspects.
Cryobiology. 71:181–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amiri F, Jahanian-Najafabadi A and
Roudkenar MH: In vitro augmentation of mesenchymal stem cells
viability in stressful microenvironments: In vitro augmentation of
mesenchymal stem cells viability. Cell Stress Chaperones.
20:237–251. 2015. View Article : Google Scholar :
|
|
4
|
Wei H, Li Z, Hu S, Chen X and Cong X:
Apoptosis of mesenchymal stem cells induced by hydrogen peroxide
concerns both endoplasmic reticulum stress and mitochondrial death
pathway through regulation of caspases, p38 and JNK. J Cell
Biochem. 111:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Q, Wang Y and Deng Z: Pre-conditioned
mesenchymal stem cells: A better way for cell-based therapy. Stem
Cell Res Ther. 4:632013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KA, Shim W, Paik MJ, Lee SC, Shin JY,
Ahn YH, Park K, Kim JH, Choi S and Lee G: Analysis of changes in
the viability and gene expression profiles of human mesenchymal
stromal cells over time. Cytotherapy. 11:688–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Qian J, Xie X, Lin L, Zou Y, Fu M,
Huang Z, Zhang G, Su Y and Ge J: High density lipoprotein protects
mesenchymal stem cells from oxidative stress-induced apoptosis via
activation of the PI3K/Akt pathway and suppression of reactive
oxygen species. Int J Mol Sci. 13:17104–17120. 2012. View Article : Google Scholar
|
|
8
|
Jeong DM, Jung HA and Choi JS: Comparative
antioxidant activity and HPLC profiles of some selected Korean
thistles. Arch Pharm Res. 31:28–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoo YM, Nam JH, Kim MY, Choi J and Park
HJ: Pectolinarin and pectolinarigenin of Cirsium setidens prevent
the hepatic injury in rats caused by d-galactosamine via an
antioxidant mechanism. Biol Pharm Bull. 31:760–764. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noh H, Lee H, Kim E, Mu L, Rhee YK, Cho CW
and Chung J: Inhibitory effect of a Cirsium setidens extract on
hepatic fat accumulation in mice fed a high-fat diet via the
induction of fatty acid β-oxidation. Biosci Biotechnol Biochem.
77:1424–1429. 2013. View Article : Google Scholar
|
|
11
|
Thao NT, Cuong TD, Hung TM, Lee JH, Na M,
Son JK, Jung HJ, Fang Z, Woo MH, Choi JS and Min BS: Simultaneous
determination of bioactive flavonoids in some selected Korean
thistles by high-performance liquid chromatography. Arch Pharm Res.
34:455–461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russo V, Young S, Hamilton A, Amsden BG
and Flynn LE: Mesenchymal stem cell delivery strategies to promote
cardiac regeneration following ischemic injury. Biomaterials.
35:3956–3974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cells for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee WB, Kwon HC, Cho OR, Lee KC, Choi SU,
Baek NI and Lee KR: Phytochemical constituents of Cirsium setidens
Nakai and their cytotoxicity against human cancer cell lines. Arch
Pharm Res. 25:628–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SH, Heo SI, Li L, Lee MJ and Wang MH:
Antioxidant and hepatoprotective activities of Cirsium setidens
Nakai against CCl4-induced liver damage. Am J Chin Med. 36:107–114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn MJ, Hur SJ, Kim EH, Lee SH, Shin JS,
Kim MK, Uchizono JA, Whang WK and Kim DS: Scopoletin from Cirsium
setidens increases melanin synthesis via CREB phosphorylation in
B16F10 cells. Korean J Physiol Pharmacol. 18:307–311. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim H, Son KH, Chang HW, Bae K, Kang SS
and Kim HP: Anti-inflammatory activity of pectolinarigenin and
pectolinarin isolated from Cirsium chanroenicum. Biol Pharm Bull.
31:2063–2067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuzawa A and Ichijo H: Redox control of
cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase
pathway in stress signaling. Biochim Biophys Acta. 1780:1325–1336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cuevas BD, Abell AN and Johnson GL: Role
of mitogen-activated protein kinase kinase kinases in signal
integration. Oncogene. 26:3159–3171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung YM, Park SH, Tsai WB, Wang SY, Ikeda
MA, Berek JS, Chen DJ and Hu MC: FOXO3 signalling links ATM to the
p53 apoptotic pathway following DNA damage. Nat Commun. 3:10002012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lavin MF, Gueven N, Bottle S and Gatti RA:
Current and potential therapeutic strategies for the treatment of
ataxia-telangiectasia. Br Med Bull. 81–82:129–147. 2007. View Article : Google Scholar
|
|
22
|
Guo Z, Kozlov S, Lavin MF, Person MD and
Paull TT: ATM activation by oxidative stress. Science. 330:517–521.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen BP, Li M and Asaithamby A: New
insights into the roles of ATM and DNA-PKcs in the cellular
response to oxidative stress. Cancer Lett. 327:103–110. 2012.
View Article : Google Scholar
|
|
24
|
Mollazadeh S, Fazly Bazzaz BS and
Kerachian MA: Role of apoptosis in pathogenesis and treatment of
bone-related diseases. J Orthop Surg Res. 10:152015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Virag L, Robaszkiewicz A, Rodriguez-Vargas
JM and Oliver FJ: Poly(ADP-ribose) signaling in cell death. Mol
Aspects Med. 34:1153–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|