Introduction
Gastric cancer is a malignant disease of the
digestive system; it has a high incidence rate and is the second
leading cause of cancer-associated mortality worldwide (1). The clinical symptoms in the early
stage are not obvious, and predominantly include upper abdominal
pain, heartburn, nausea and loss of appetite (2,3).
Currently, radical gastrectomy is the predominant treatment method
for patients with early stage disease. The curative effects are
satisfactory, with a 5-year survival rate of >90% (4,5).
However, the majority of patients are diagnosed at an advantage
stage, which limits the opportunities for radical surgery, and the
overall 5-year survival rate is only ~30% (6,7).
Later symptoms include yellow skin, vomiting, weight loss and the
presence of blood in the stools. Several studies have found that
gastric cancer is a multistage pathological state, and
environmental factors, particularly improper dietary habits, are
considered to be important in the development of gastric cancer
(8–12). At present, the pathogenesis of
gastric cancer remains to be fully elucidated. Therefore, it is
important for gastric cancer prevention to investigate the
pathogenesis of gastric cancer, and identify novel tumor markers
for early diagnosis, prediction and prognosis of recurrence.
Following further developments in the molecular
biology of gastric cancer, novel targets, which are associated with
tumor cell growth, apoptosis, cell cycle, and the invasion and
infiltration of gastric cancer have become the focus of interest in
the investigation of gastric cancer. Cell cycle abnormalities i are
usually found in tumorigenesis and tumor progression. The cell
cycle is tightly regulated by cyclin-dependent kinases (CDKs),
cyclines and CDK inhibitors (13).
The degradation of these regulatory proteins is mediated and
regulated by the ubiquitin-proteasome system. S-phase
kinase-associated protein 2 (Skp2) is the substrate of E3 ligases
and is involved in the recruiting component of the SCFSkp2 complex.
Skp2 is important for inducing the degradation of CDK inhibitors,
including p21cip1, p27kip1 and
p57kip2 (14–16). It is commonly observed in numerous
types of human cancer, and is important in tumor progression and
metastasis (17,18). In salivary malignancies,
significant correlations have been found between survival rates and
the expression levels of Skp2, p27 and p53, demonstrating that Skp2
is important in the pathogenesis of salivary cancer (19). In patients with advanced prostate
cancer, Skp2 has been found to regulate androgen receptors through
ubiquitin-mediated degradation, independent of Akt/mammalian target
of rapamycin pathways in prostate cancer cells (20). Davidovich et al (21) found that the overexpression of Skp2
was associated with resistance to chemotherapeutic drugs. In
patients with locally advanced breast cancer, Skp2 has been used as
a diagnostic marker for predicting the response to
doxorubicin-based preoperative chemotherapy and clinical outcome.
The inhibition of Skp2 offers a potential strategy to suppress
tumorigenesis in cases where tumor suppressor genes, including
retinoblastoma and tumor protein P53 are mutated (22,23).
However, the molecular mechanism underlying the
expression of Skp2 during the progression of gastric cancer remains
to be fully elucidated. In the present study, the role of Skp2 in
patients with gastric cancer was further clarified, and the
molecular mechanisms were examined using in vitro and in
vivo experiments. These investigations may provide novel clues
for the clinical treatment of human gastric cancer.
Materials and methods
Patients
A total of 66 tissue specimens were collected from
the Third Xiangya Hospital (Changsha, China) in the present study,
including 47 gastric cancer tissue specimens (21 female and 26
male) and 19 normal gastric tissue specimens (8 female and 11
male). The median age of the patients was 48.67 years (range, 26–84
years), and the samples were collected between September 2013 and
September 2015. The specimens were stored at −80°C and used for
western blot analysis. The human investigations in the present
study were performed in compliance with the Declaration of
Helsinki. Approval for the study was obtained from the Ethics
Committee of Third Xiangya Hospital. The patients were well
informed of the details and signed relevant consent forms prior to
commencement of the investigation The experiments using mice in the
present study were performed in accordance with Animal Ethical Care
(24,25).
Cell lines and reagents
The BGC-823 and MKN-45 gastric cancer cell lines
were obtained from Shanghai Institute of Materia Medica, Chinese
Academy of Tumor Cell Bank (Shanghai, China). The cells were
cultured at 37°C and 5% CO2 in Dulbecco's modified
Eagle's medium (DMEM) with 10% fetal bovine serum (GE Healthcare
Life Sciences, Logan, UT, USA), 1% penicillin and 1% streptomycin.
Skp2 inhibitor C1 (SKPin C1) was purchased from MedChem Express
(Princeton, NJ, USA). A purified recombinant protein of human p45
Skp2, transcript variant 1 with an N-terminal His tag, expressed in
Escherichia coli (50 µg), was obtained from OriGene
Technologies (Beijing, China). MTT reagent was obtained from
Sigma-Aldrich (St. Louis, MO, USA). The human Skp2 short hairpin
(sh)RNA, four unique 29-mer shRNA constructs in the retroviral
green fluorescent protein (GFP) vector, and the non-effective
29-mer scrambled shRNA cassette in the pGFP-V-RS vector were
obtained from OriGene Technologies. Bioinformatics analysis was
conducted using the BioGPS gene annotation portal (http://biogps.org/).
Western blot analysis
The cells were lysed with radioimmunoprecipitation
assay lysis buffer (P0013B), which was obtained from Beyotime
Institute of Biotechnology (Haimen, China). The proteins in the
lysates were separated by 10% polyacrylamide gel electrophoresis,
which was prepared in house including a stacking gel and a
separating gel. The proteins were loaded on a polyvinylidene
difluoride membrane. The antibodies used in the experiments were as
follows: Rabbit polyclonal anti-Skp2 antibody (1:1,000; cat. no.
15010-1-AP) was obtained from ProteinTech Group, Inc. (Wuhan,
China). Rabbit polyclonal anti-p27kip1 antibody
(1:1,000; cat. no. ab137736) was purchased from Abcam (Cambridge,
MA, USA). Rabbit polyclonal anti-β-actin antibody (1:1,000; cat.
no. sc-7210) and horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (sc-2030) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The blots were visualized
using the GelDoc XR system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and Quantity One 1-D analysis software, version 4.6.9
(Bio-Rad Laboratories, Inc.).
MTT assay
The cell survival rate and proliferation were
determined using an MTT assay, as described (26,27).
In brief, the BGC-823 cells and MKN-45 cells were plated into
48-well plates at 1,000 cells/well, and the cells were transfected
with Skp2-specific shRNA or negative control (N.C.) shRNA using
Lipofectamine 2000 for 24, 48, 72 and 96 h, respectively, at room
temperature. At 4 h prior to analysis, 5 mg/ml of MTT was added
into the medium. Finally, the purple crystals was dissolved with
DMSO, and the data were analyzed at a test wavelength of 490
nm.
For Skp2 inhibition, the human gastric cancer cells
were plated into 96-well plates at 1,000 cells/well at 37°C with 5%
CO2. After 6 h, the BGC-823 cells and MKN-45 cells were
treated with SKPin C1 at concentrations of 1, 5 and 10 µM
for 48 h, and with 5 µM SKPin C1 for 24, 48 and 72 h. The
other steps were as described above.
Animal groups and tumor challenge
A total of 30 (10/group) C57BL/6 male nude mice (6–8
week-old; 18–20 g) were obtained from the Si Lai Ke Jing Da
Laboratory Animal Co., Ltd. (Changsha, China). The mice were
randomly divided into three groups and maintained in specific
pathogen-free conditions at 22°C under a 12/12 h light/dark cycle
with free access to food and water. The mice were challenged
subcutaneously in the flank area with 5×105 BGC-823
tumor cells, BGC-823 cells transfected with Skp2 shRNA or BGC-823
cells transfected with N.C. shRNA. The tumor volumes were recorded
every 3 days. At 30 days post-tumor injection, the mice were
sacrificed by cervical dislocation and the tumor were weighed. Each
group contained >10 mice.
Statistical analysis
The data in the present study were analyzed using
SPSS software (SPSS, Inc., Chicago, IL, USA). Student's
t-tests were used to evaluate statistical significance. Data
are expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
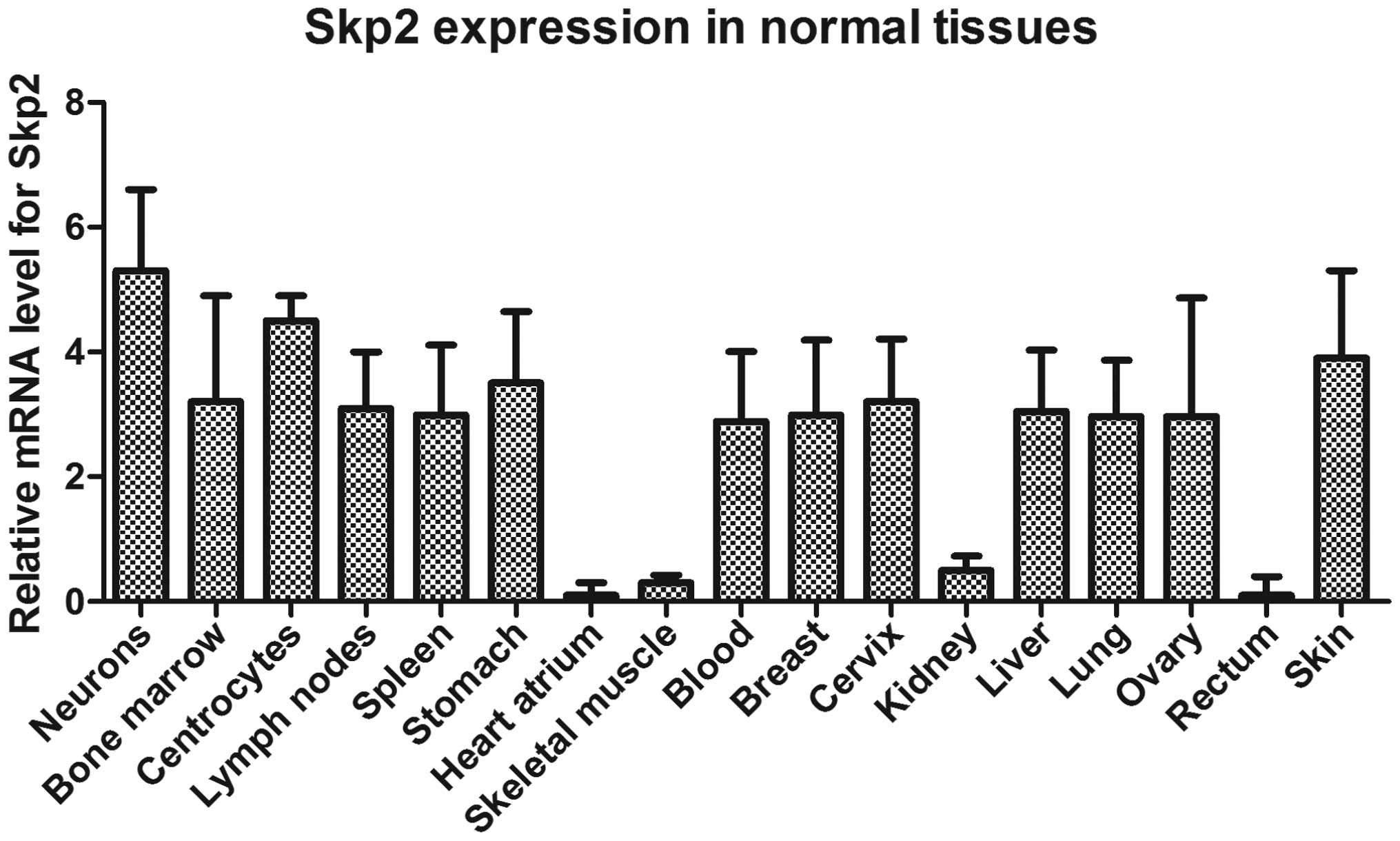
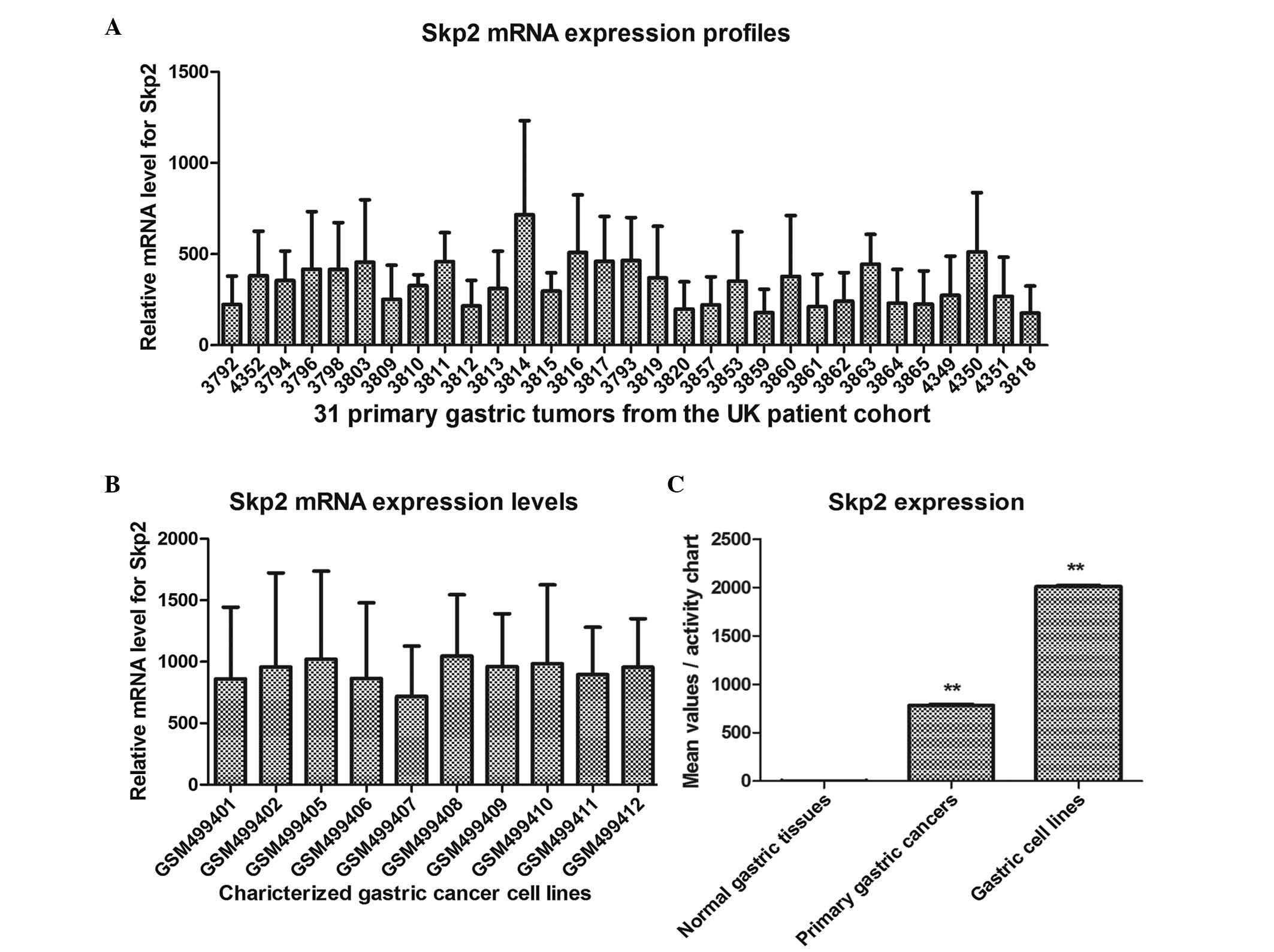
Expression levels of Skp2 in normal
tissues, primary gastric cancer tissues and gastric cancer cell
lines, determined using the BioGPS platform
In order to determine the association between the
expression of Skp2 and the progression of human gastric cancer, the
presents study used bioinformatics methods to analyze the
expression of Skp2 in the gastric tissues. BioGPS is a centralized
gene portal for aggregating distributed gene annotation resources
(28). The expression levels of
Skp2 in the normal tissues, primary gastric cancer tissues and
gastric cancer cell lines were readily and directly compared with
each other. As shown in Figs. 1
and 2, the mean relative
expression of Skp2 in the normal gastric tissues was 3.50, however,
the mean values were 772 and 2,001.8 in the 31 primary gastric
tumor tissues from the UK patient cohort and 10 gastric cancer cell
lines of the side population, respectively. These data demonstrated
that Skp2 was significantly upregulated in the primary gastric
cancer tissues and gastric cancer lines, and that Skp2 may be an
oncogene in the progression of gastric cancer.
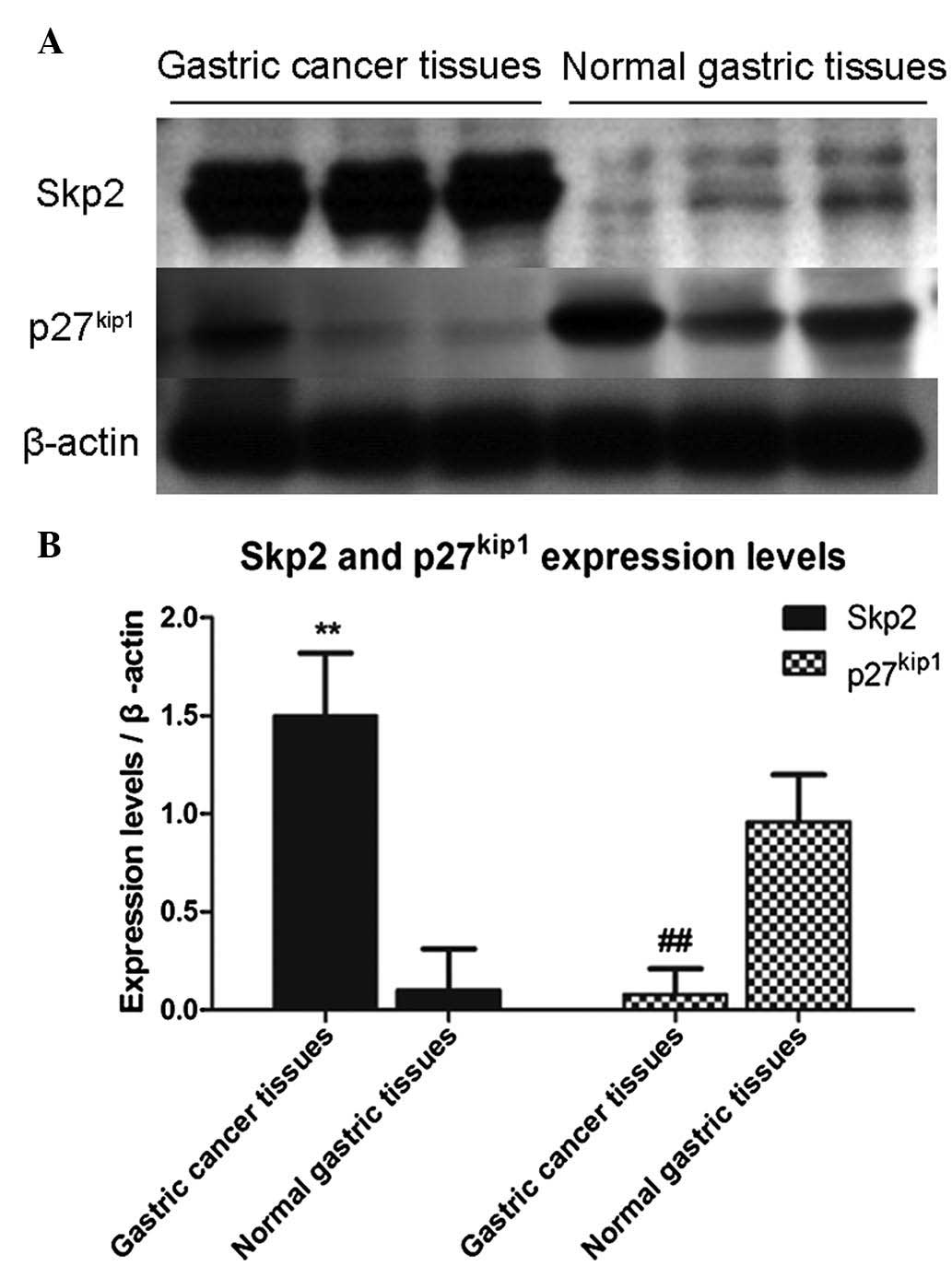
Higher expression of Skp2 correlates with
lower expression of p27kip1 in gastric cancer
tissues
In order to confirm the results of the
bioinformatics analysis, tissue specimens from patients with
gastric cancer and the specimens of normal gastric tissues were
collected. Western blot analysis was used to determine the
expression levels of Skp2 and p27kip1, as degradation of
the tumor suppressor, p27kip1, is mediated by Skp2. In
the present study, the data were normalized to the level of
β-actin, followed by analysis using Student's t-test and
presentation of data as the mean ± standard deviation. As shown in
Fig. 3, three independent samples
were used to detect the expression levels of Skp2 and
p27kip1, and the results demonstrated that the
expression levels of Skp2 in the gastric cancer tissues were
significantly increased, compared with those in the normal gastric
tissues. However, the expression levels of p27kip1 were
inversely correlated with the levels of Skp2.
As shown in Table
I, the expression levels of Skp2 in tissue specimens from 47
patients with gastric cancer and 19 normal gastric tissue specimens
were assessed and analyzed using western blot analysis. Positive
expression of Skp2 was observed in 41 specimens (87.2%) of the
gastric cancer samples, whereas the positive rate of expression was
5.6% in the normal gastric tissue samples (P<0.01). The results
were consistent with those of the bioinformatics analysis.
 | Table IProtein expression of Skp2 in gastric
carcinoma tissues and normal gastric tissues. |
Table I
Protein expression of Skp2 in gastric
carcinoma tissues and normal gastric tissues.
| Tissue | n | Skp2
| Positive rate
(%) |
|---|
| + | − |
|---|
| Normal gastric | 19 | 1 | 18 | 5.6 |
| Gastric cancer | 47 | 41 | 6 | 87.2a |
Defective regulation of Skp2 or the
presence of Skp2 inhibitor contribute to decreased proliferation in
human gastric cancer cell lines
In order to determine whether inhibiting the
expression of Skp2 affects the proliferation of human gastric
cancer cells, the present study used Skp2-specific shRNA to
interfere with the endogenous expression of Skp2. As shown in
Fig. 4A, the human gastric cancer
cells were transfected with Skp2 shRNA and cultured for 24, 48, 72
and 96 h, respectively. The subsequent MTT assay showed that the
Skp2 shRNA-transfected cells had significantly lower survival
rates, compared with the cells transfected with the N.C. shRNA. By
contrast, the administration of recombinant Skp2 protein at a
concentration of 0.1 µg/ml promoted the proliferation of the
BGC-823 cells and MKN-45 cells, although no statistically
significant difference was found between the recombinant
Skp2-treated cells and the untreated gastric cancer cells.
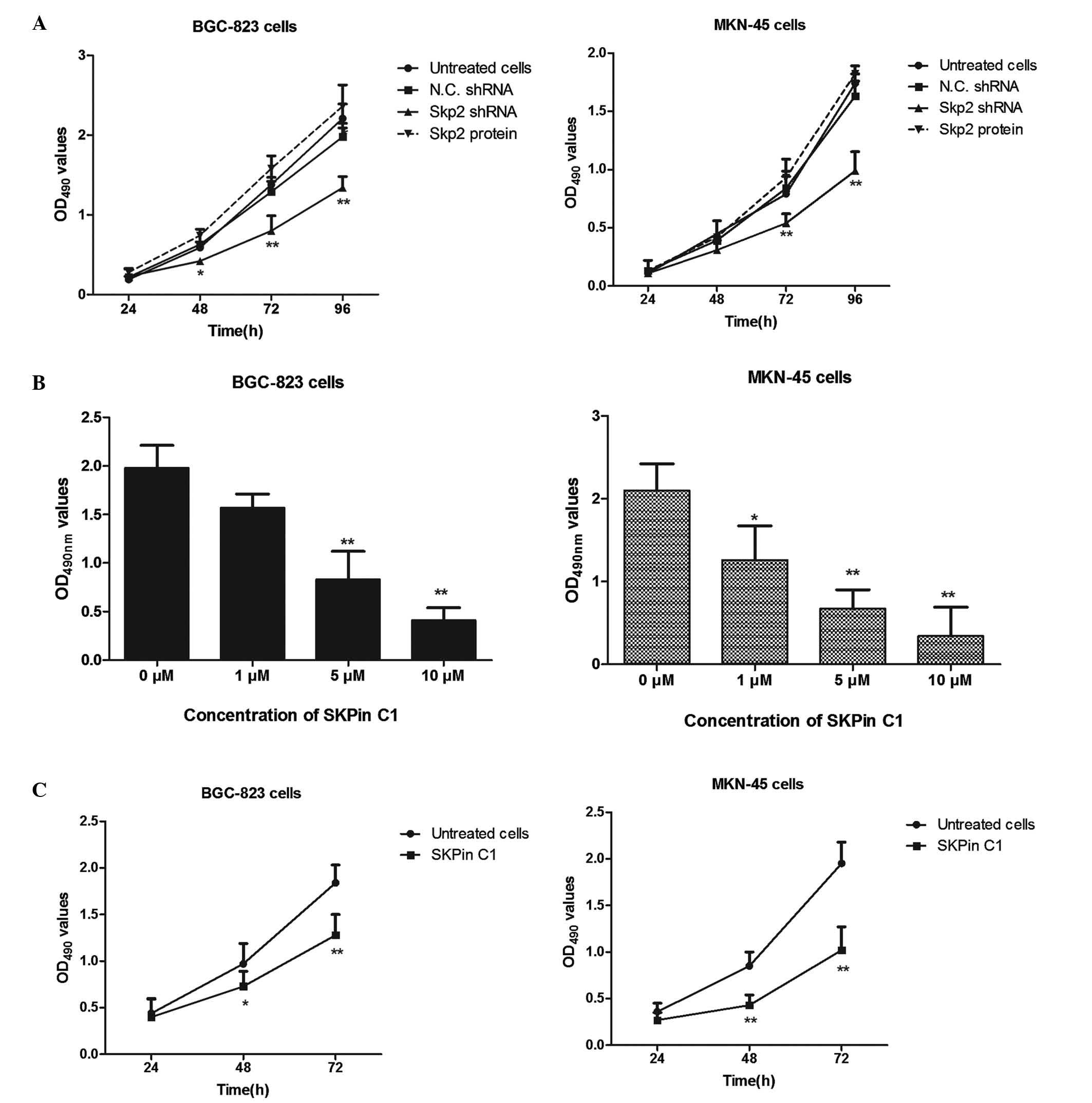 | Figure 4Defective regulation of Skp2 or Skp2
inhibition contributes to lower proliferation of human gastric
cancer cell lines. (A) Interference with endogenous Skp2 inhibited
human gastric cancer cells. BGC-823 and MKN-45 cells were
transfected with Skp2 shRNA or N.C. shRNA, or administered with
recombinant Skp2 protein for the indicated periods of time. An MTT
assay was used to detect the proliferation of the BGC-823 and
MKN-45 cells (*P<0.05 and **P<0.01).
(B) Skp2 inhibitor inhibited the proliferation of gastric cancer
cells in a dose-dependent manner. The BGC-823 and MKN-45 human
gastric cancer cells were treated with increasing concentrations of
SKPin C1 (1, 5 and 10 µM) for 48 h, and the survival rates
were determined using an MTT assay. (C) Treatment with the Skp2
inhibitor contributed to the lower survival rates of the gastric
cancer cells in a time-dependent manner. The gastric cancer cells
were treated with 5 µM SKPin C1 for 24, 48 and 72 h,
respectively. The cell proliferation was determined using an MTT
assay (*P<0.05 and **P<0.01, compared
with the untreated cells). Data are expressed as the mean ±
standard deviation. Skp2, S-phase kinase-associated protein 2;
SKPin C1, Skp2 inhibitor C1; shRNA, short hairpin RNA; N.C.,
negative control; OD, optical density. |
Skp2 inhibitor inhibits the proliferation
of gastric cancer cells in a dose-dependent manner
In order to confirm the role of Skp2 in the
proliferation of human gastric cancer cells, an Skp2 inhibitor was
to treat the BGC-823 cells and MKN-45 cells. SKPin C1 is a potent
inhibitor of Skp2, and selectively inhibits Skp2-mediated
p27kip1 degradation by targeting the SCF-Skp2
protein-protein interface. As shown in Fig. 4B, the BGC-823 cells and MKN-45
cells were treated with increasing concentrations of SKPin C1 for
48 h. The subsequent MTT assay results demonstrated that, as the
concentrations of the Skp2 inhibitor increased, the survival rate
of the human gastric cancer cells decreased significantly, compared
with that of the untreated cells.
Treatment with Skp2 inhibitor contributes
to lower survival rates of gastric cancer cells in a time-dependent
manner
To examine the effects of Skp2 over time, 5
µM was selected as an appropriate middle-range concentration
for treatment of the human gastric cancer cells. As shown in
Fig. 4C, the BGC-823 and MKN-45
cells were treated with 5 µM of SKPin C1 for 24, 48 and 72
h, respectively. The results revealed that the proliferation of the
BGC-823 cells and MKN-45 cells were significantly suppressed,
compared with the untreated cells, in a time-dependent manner.
Transfection with Skp2 shRNA or treatment
with SKPin C1 contributes to the accumulation of p27kip1
in human gastric cancer cells
The present study also investigated the association
between the levels of Skp2 and p27kip1 in human gastric
cancer cells. RNA interference technology and the specific
inhibitor of Skp2 were used to interrupt the effect of Skp2 in the
BGC-823 cells. As shown in Fig.
5A, the BGC-823 cells were transfected with Skp2 shRNA, to
interfere with the expression of endogenous Skp2, for 48 and 72 h.
The Skp2-depleted BGC-823 cells showed marked accumulation of
p27kip1, compared with the N.C. shRNA-transfected
BGC-823 cells. Consistent with the RNA interference experiment, the
present study used increasing concentrations of Skp2 inhibitors to
treat the BGC-823 cells for 48 h, The results revealed that the
endogenous expression of Skp2 was not affected significantly by
SKPin C1, however, the accumulation of p27kip1 in the
BGC-823 cells was significantly upregulated as the concentration of
SKPin C1 increased. These data suggested that the Skp2-mediated
degradation of p27kip1 was inhibited by Skp2 shRNA
transfection and Skp2 inhibition.
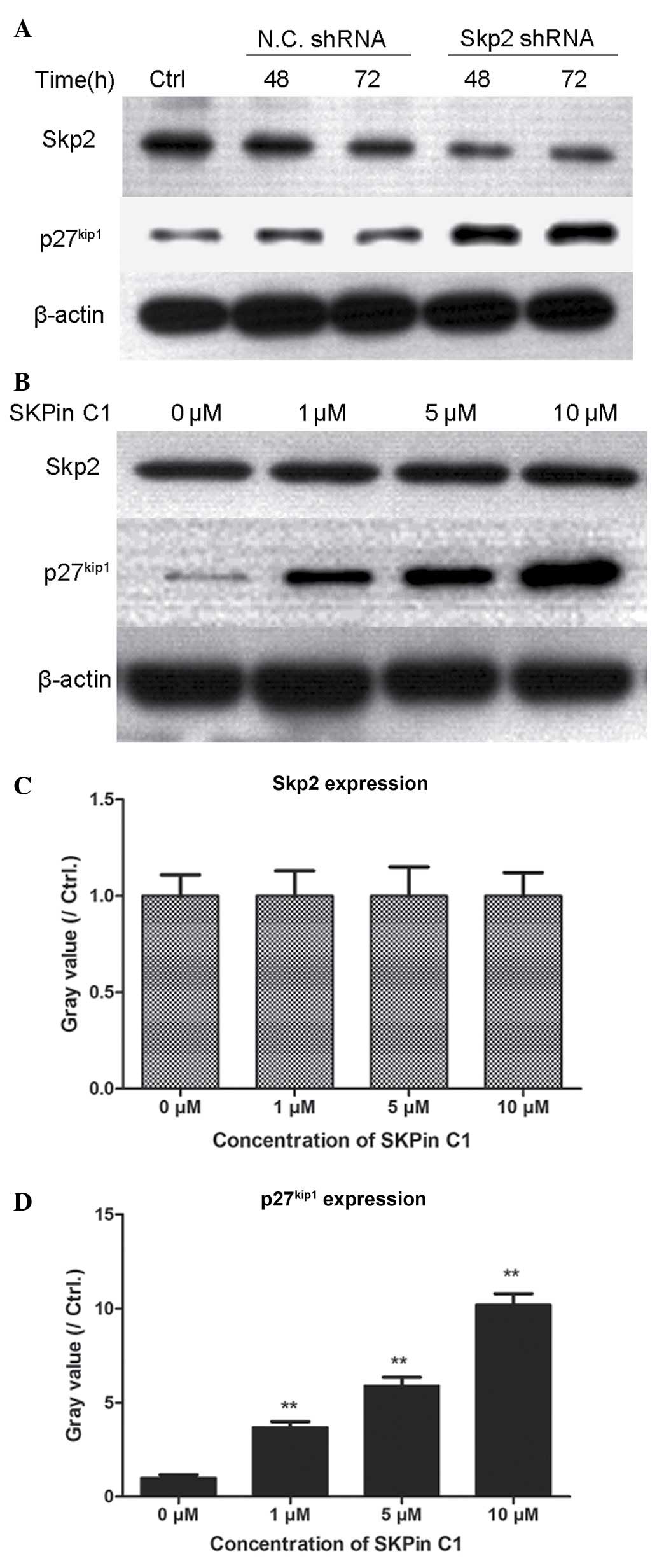 | Figure 5Transfection with Skp2 shRNA or
treatment with SKPin C1 contributes to the accumulation of p27kip1
in human gastric cancer cells. (A) BGC-823 cells (5×105
cells/well) were plated into 48 well plates. Following culture for
6 h, the cells were transfected with Skp2 shRNA and N.C. shRNA. The
cells were then cultured for 48 and 72 h, and cell lysates were
prepared. The expression levels of Skp2 and p27kip1 were
determined using western blot analysis. (B) BGC-823 human gastric
cancer cells (3×105 cells/well) were plated into 48-well
plates. After 6 h, the cells were treated with SKPin C1 at
concentrations of 1, 5 and 10 µM for 48 h. The expression
levels of Skp2 and p27kip1 were detected using western
blot analysis and grey values were calculated, as presented in (C)
(Skp2) and (D) (p27kip1). **P<0.01 vs.
control. Skp2, S-phase kinase-associated protein 2; SKPin C1, Skp2
inhibitor C1; shRNA, short hairpin RNA; N.C., negative control;
Ctrl, control. |
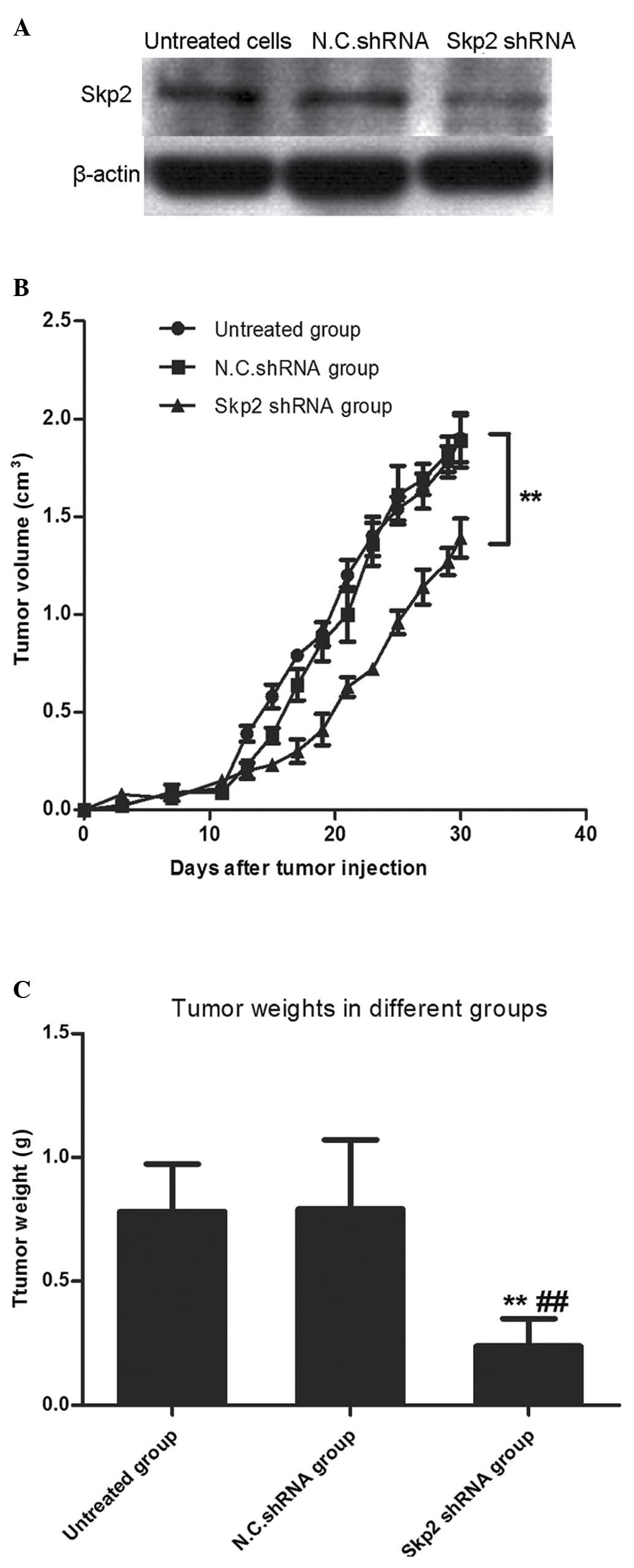
Interference with the endogenous
expression of Skp2 inhibits tumor cell growth in nude mice
To further identify the inhibitory efficacy of Skp2
deletion in human gastric cancer cells, tumorigenicity experiments
were performed in nude mice. The nude mice were randomly divided
into three groups (>10 mice per group), as follows: Skp2 shRNA
group, N.C. shRNA group and untreated group. The stably transfected
BGC-823 cells were subcutaneously injected into the mice. As shown
in Fig. 6, the tumor weight in the
Skp2 shRNA group was significantly lower, compared with that in the
N.C. shRNA group (P<0.01) and untreated group (P<0.01). This
was consistent with the results obtained in the analysis of tumor
volume in the tumorigenicity experiments (P<0.01, vs. N.C. shRNA
group).
Discussion
Gastric cancer is a common malignant disease, which
represents a serious health problem worldwide (29,30).
Although there have been substantial advances in the treatment of
gastric cancer, the prognosis of metastatic gastric cancer remains
poor. There are two ways to improve this, one is to identify novel
potential biomarkers of prognostic significance in the early stage
of gastric cancer, and the other is to improve current
understanding of the molecular biology of the progression of
gastric cancer, which offers the potential to improve treatment
options for curing the disease. In the present study, the
expression of Skp2 in gastric cancer tissues was analyzed and
compared with that in normal gastric tissues using bioinformatics
methods. As expected, the expression levels of Skp2 were
significantly higher in the gastric cancer tissues and gastric
cancer cell lines, compared with the normal gastric tissues, which
was confirmed and consistent with the results obtained from western
blot analysis. Additionally, the positive rate of Skp2 was 87.2% in
the gastric cancer tissue samples, which was significantly higher,
compared with the positive rate of 5.6% in the normal gastric
samples (P<0.01). The above data confirmed that Skp2 worked as
an oncogene during the progression of gastric cancer.
The ubiquitin protein ligase, SCFSkp2, is
important in the degradation of tumor suppressor genes, including
p27kip1 and p53, and is composed of Skp1, cullin 1,
regulator of cullins 1/RING box protein 1 and the F-box protein,
Skp2 (31–33). Skp2 is the substrate-recognition
subunit in the SCF ubiquitin-protein ligase complex (34,35).
In the present study, the endogenous expression of Skp2 and
p27kip1 were detected in tissue specimens from patients
with gastric cancer, and the results showed that the expression of
Skp2 was negatively correlated with the expression of
p27kip1 in the patients with gastric cancer.
Furthermore, interference of the endogenous expression of Skp2 was
induced using shRNA specific to human Skp2, and SKPin C1, the Skp2
inhibitor, was used to inhibit the involvement and function of Skp2
in human gastric cancer cells. The results demonstrated that
inhibiting the involvement and function of Skp2 in BGC-823 and
MKN-45 cells significantly inhibited the proliferation of human
gastric cancer cells.
The above results were confirmed by performing in
vivo experiments. The stably transfected gastric cancer cells
were subcutaneously injected into nude mice. The tumor weights and
tumor volumes in Skp2 shRNA group were significantly lower,
compared with those in the N.C. shRNA group (P<0.01) and
untreated group (P<0.01). This was consistent with the results
obtained in the Skp2 interference experiments, suggesting that the
antitumor activity was partly due to the accumulation of the tumor
inhibitor protein, p27kip1, which was induced by
inhibiting or interfering with, the function of Skp2. Thus, Skp2
was shown to be a promising and effective drug target for the
treatment of human gastric cancer.
References
|
1
|
Fu DG: Epigenetic alterations in gastric
cancer (Review). Mol Med Rep. 12:3223–3230. 2015.PubMed/NCBI
|
|
2
|
Afuwape OO, Irabor DO, Ladipo JK and
Ayandipo B: A review of the current profile of gastric cancer
presentation in the university college hospital Ibadan, a tertiary
health care institution in the tropics. J Gastrointest Cancer.
43:177–180. 2012. View Article : Google Scholar
|
|
3
|
Tey J, Choo BA, Leong CN, Loy EY, Wong LC,
Lim K, Lu JJ and Koh WY: Clinical outcome of palliative
radiotherapy for locally advanced symptomatic gastric cancer in the
modern era. Medicine (Baltimore). 93:e1182014. View Article : Google Scholar
|
|
4
|
Katai H: Function-preserving surgery for
gastric cancer. Int J Clin Oncol. 11:357–366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikeguchi M, Oka S, Gomyo Y, Tsujitani S,
Maeta M and Kaibara N: Prognostic benefit of extended radical
lymphadenectomy for patients with gastric cancer. Anticancer Res.
20:1285–1289. 2000.PubMed/NCBI
|
|
6
|
Jiang S, Ge W, Zheng L and Chen G:
Analysis of clinicopathological features and prognosis in 30 cases
with multifocal gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi.
18:135–138. 2015.In Chinese. PubMed/NCBI
|
|
7
|
Cui H, Deng J, Liang H, Zhang R, Ding X,
Pan Y, Wang B and Wu W: Advantage of D2+ lymph node dissection for
distal advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi.
18:127–130. 2015.In Chinese. PubMed/NCBI
|
|
8
|
Almubarak MM, Laé M, Cacheux W, de Cremoux
P, Pierga JY, Reyal F, Bennett SP, Falcou MC, Salmon RJ, Baranger B
and Mariani P: Gastric metastasis of breast cancer: A single centre
retrospective study. Dig Liver Dis. 43:823–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karagulle M, Fidan E, Kavgaci H and
Ozdemir F: The effects of environmental and dietary factors on the
development of gastric cancer. BUON. 19:1076–1082. 2014.
|
|
10
|
Lee YY and Derakhshan MH: Environmental
and lifestyle risk factors of gastric cancer. Arch Iran Med.
16:358–365. 2013.PubMed/NCBI
|
|
11
|
Zuk K, Peczek L, Stec-Michalska K, Medrek
M and Nawrot B: SATB1 expression in gastric mucosa in relation to
Helicobacter pylori infection and family history of gastric cancer.
Adv Med Sci. 57:237–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Q, Wang Y, Cao Y, Chen A, Ren M, Ge
Y, Yu Z, Wan S, Hu A, Bo Q, et al: Potential health risks of heavy
metals in cultivated topsoil and grain, including correlations with
human primary liver, lung and gastric cancer, in Anhui province,
Eastern China. Sci Total Environ. 470–471:340–347. 2014. View Article : Google Scholar
|
|
13
|
Shin JY, Kim HS, Lee KS, Kim J, Park JB,
Won MH, Chae SW, Choi YH, Choi KC, Park YE and Lee JY: Mutation and
expression of the p27KIP1 and p57KIP2 genes in human gastric
cancer. Exp Mol Med. 32:79–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bretones G, Acosta JC, Caraballo JM,
Ferrándiz N, Gómez-Casares MT, Albajar M, Blanco R, Ruiz P, Hung
WC, Albero MP, et al: SKP2 oncogene is a direct MYC target gene and
MYC down-regulates p27 (KIP1) through SKP2 in human leukemia cells.
J Biol Chem. 286:9815–9825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cen B, Mahajan S, Zemskova M, Beharry Z,
Lin YW, Cramer SD, Lilly MB and Kraft AS: Regulation of Skp2 levels
by the Pim-1 protein kinase. J Biol Chem. 285:29128–29137. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Ji LH, Liu W, Zhao G and Wu ZY:
Skp2-RNAi suppresses proliferation and migration of gallbladder
carcinoma cells by enhancing p27 expression. World J Gastroenterol.
19:4917–4924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai YS, Lai CL, Lai CH, Chang KH, Wu K,
Tseng SF, Fazli L, Gleave M, Xiao G, Gandee L, et al: The role of
homeostatic regulation between tumor suppressor DAB2IP and
oncogenic Skp2 in prostate cancer growth. Oncotarget. 5:6425–6436.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang H, Song Y, Wu Y, Guo N, Ma Y and
Qian L: Erbin loss promotes cancer cell proliferation through
feedback activation of Akt-Skp2-p27 signaling. Biochem Biophys Res
Commun. 463:370–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ben-Izhak O, Akrish S, Gan S and Nagler
RM: Skp2 and salivary cancer. Cancer Biol Ther. 8:153–158. 2009.
View Article : Google Scholar
|
|
20
|
Li B, Lu W, Yang Q, Yu X, Matusik RJ and
Chen Z: Skp2 regulates androgen receptor through ubiquitin-mediated
degradation independent of Akt/mTOR pathways in prostate cancer.
Prostate. 74:421–432. 2014. View Article : Google Scholar
|
|
21
|
Davidovich S, Ben-Izhak O, Shapira M,
Futerman B and Hershko DD: Over-expression of Skp2 is associated
with resistance to preoperative doxorubicin-based chemotherapy in
primary breast cancer. Breast Cancer Res. 10:R632008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao Z and Huang S: E3 ubiquitin ligase
Skp2 as an attractive target in cancer therapy. Front Biosci
(Landmark Ed). 20:474–490. 2015. View
Article : Google Scholar
|
|
23
|
Chan CH, Morrow JK, Zhang S and Lin HK:
Skp2: A dream target in the coming age of cancer therapy. Cell
Cycle. 13:679–680. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orlans FB: Case studies of ethical
dilemmas: Animal Care and Use Committee. Lab Anim Sci. 7(Spec No):
59–64. 1987.
|
|
25
|
Rowan AN: Animals, science, and ethics -
Section IV. Ethical review and the animal care and use committee.
Hastings Cent Rep. 20:S19–24. 1990.PubMed/NCBI
|
|
26
|
Stockert JC, Blázquez-Castro A, Cañete M,
Horobin RW and Villanueva A: MTT assay for cell viability:
Intracellular localization of the formazan product is in lipid
droplets. Acta Histochem. 114:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yilmaz Z, Dogan AL, Ozdemir O and Serper
A: Evaluation of the cytotoxicity of different root canal sealers
on L929 cell line by MTT assay. Dent Mater J. 31:1028–1032. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu C, Macleod I and Su AI: BioGPS and
MyGene.info: Organizing online, gene-centric information. Nucleic
Acids Res. 41(Database Issue): D561–D565. 2013. View Article : Google Scholar :
|
|
29
|
Popiela T, Kulig J, Kolodziejczyk P and
Sierzega M; Polish Gastric cancer study group: Long-term results of
surgery for early gastric cancer. Br J Surg. 89:1035–1042. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fejzo MS, Anderson L, Chen HW, Anghel A,
Zhuo J, Anchoori R, Roden R and Slamon DJ: ADRM1-amplified
metastasis gene in gastric cancer. Genes Chromosomes Cancer.
2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L,
Zhang Q, Wu Y, Jiang H and Sun X: STAT3 interacts with Skp2/p27/p21
pathway to regulate the motility and invasion of gastric cancer
cells. Cellular Signal. 25:931–938. 2013. View Article : Google Scholar
|
|
32
|
Yoon JH, Seo HS, Choi WS, Kim O, Nam SW,
Lee JY and Park WS: Gastrokine 1 induces senescence and apoptosis
through regulating telomere length in gastric cancer. Oncotarget.
5:11695–11708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pavlides SC, Huang KT, Reid DA, Wu L,
Blank SV, Mittal K, Guo L, Rothenberg E, Rueda B, Cardozo T and
Gold LI: Inhibitors of SCF-Skp2/Cks1 E3 ligase block
estrogen-induced growth stimulation and degradation of nuclear
p27kip1: Therapeutic potential for endometrial cancer.
Endocrinology. 154:4030–4045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pascal LE and Wang Z: Virtual drug design:
Skp1-Skp2 inhibition targets cancer stem cells. Asian J Androl.
15:717–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan CH, Morrow JK, Li CF, Gao Y, Jin G,
Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al: Pharmacological
inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem
cell traits and cancer progression. Cell. 154:556–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|