Introduction
Papillary thyroid carcinoma (PTC) is the most common
type of thyroid gland cancer, and it is commonly well
differentiated with abilities for iodine uptake, thyroglobulin
secretion and responsivity to thyroid-stimulating hormone (TSH)
(1). Rearranged during
transfection (RET)/PTC rearrangement and the BRAF V600E point
mutation are the two most common genetic alterations associated
with PTC (2). PTC is frequently
associated with a RET gene rearrangement that generates a RET/PTC
oncogene. This fusion results in a constitutively active
mitogen-activated protein kinase (MAPK) pathway, which serves a key
role in PTC development. PTC accounts for approximately 80% of
thyroid malignancies (3,4), and ionizing radiation has been
described as an important etiological factor in PTC development
(5), with it widely known that
following the Chernobyl disaster, 1,000s of people developed
thyroid cancer (6,7). PTC is characterized by gene
rearrangements affecting the RET proto-oncogene located on
chromosome 10q11.2 and coding for a cell membrane tyrosine kinase
receptor (8). RET serves a
regulatory role in cell survival, growth, differentiation and
migration (9). In PTC, RET fuses
with different ubiquitous genes on the same or alternate
chromosomes to yield various RET/PTC fusion rearrangements, leading
to the abnormal expression of a chimeric RET protein that is
constitutively activated in thyroid follicular cells (10). Among the 13 fusion patterns of RET
with 12 different genes reported at present (11), RET/PTC1 and RET/PTC3 are the major
variants, while the others are rare and hypothesized to be of
little clinical significance.
CCR7 is expressed on all naïve T-cells and on
certain memory T-cells, B-cells and mature dendritic cells
(12). Chemokine (C-C motif)
ligand 21 (CCL21)/C-C chemokine receptor type 7 (CCR7) interaction
drives cell cycle progression involving the G2/M phase.
CCR7 is highly expressed in PTC, non-small cell lung cancer, breast
cancer and head and neck squamous cell carcinoma, and it mediates
metastasis in certain cancer cell lines (13,14).
Iodine serves an important role in the normal
thyroid follicular cell, regulating differentiation and
proliferation, whereas excess iodine serves an anti-oncogenic role
during thyroid oncogenic activation. RET/PTC3 fusion is primarily
associated with radiation-associated PTC. Epidemiological studies
have reported a lower incidence of PTC in radiation-exposed regions
that are associated with an iodine-rich diet (6). At present, the association between
iodine concentrations and CCL21/CCR7 interaction and the cell cycle
in PTC remain to be fully elucidated.
In the current study, it was demonstrated that
CCL21/CCR7 interaction contributes to the time-dependent
proliferation of PTC cells by upregulating cyclin A, cyclin B1 and
cyclin-dependent kinase 1 (CDK1) expression via the extracellular
signal-regulated kinase (ERK) pathway associated with iodine
[10−5 M sodium iodide (NaI)]. The current study aimed to
provide insight into the mechanisms of survival of
CCL21/CCR7-mediated cancer cells in association with iodine and
elucidate the implications for treatment targets in PTC.
Materials and methods
Cell culture
Primary cultures of thyrocytes derived from PTC
thyroid tissue from six patients aged 25–57 years (two men and four
women) from Jinshan Hospital, Fudan University, Shanghai, China
between March 2013 and May 2013, obtained immediately subsequent to
thyroidectomy, were tested in accordance with a previously
described method (15). Patient
consent was obtained, and the institutional review board had
approved the project. Thyroid cells were dissociated using a
discontinuous trypsin-ethylene
glycol-O-O′-bis(2-amino-ethyl)-N,N,N′,N′-tetra-acetic acid (EGTA)
treatment. Aliquots of freshly isolated cell suspension (5 ml;
3×106 cells per ml) in Eagle's minimum essential medium
(pH 7.4), containing 10% (v/v) foetal calf serum and 1 mU/ml TSH,
were seeded onto polystyrene flasks treated for tissue culture
(Nunc®; Nalge Nunc International, Copenhagen, Denmark)
and incubated at 37°C in a 95% air/5% CO2,
water-saturated atmosphere. Under these conditions, cells organized
themselves into follicle-like structures adhering to the
plastic-treated surface. Thyroid cells were observed using
phase-contrast microscopy (Olympus IMT-2; Olympus Corp., Tokyo,
Japan). Subsequent to dissociation, the primary culture cells were
maintained in Roswell Park Memorial Institute (RPMI) 1640 medium
supplemented with TSH. Fibroblast contamination was controlled by
pathologists; potential fibroblast contamination was detected with
a rabbit anti-vimentin monoclonal antibody (cat. no. ab92547;
1:200; Abcam, Cambridge, UK), and no immunostaining was observed.
Subsequently, all cultured cells were immunostained using
antibodies against a mouse mesothelioma monoclonal antibody
(HBME-1; cat. no. ab101139, 1:100; Abcam) and/or a mouse
cytokeratin 19 monoclonal antibody (1:200; cat. no. ab7754, Abcam).
The PTC1 and PTC3 cell lines derived from the six primary PTC cell
cultures were cultured in RPMI-1640 or Dulbecco's modified Eagle's
medium-F12 supplemented with 10% fetal bovine serum (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) in an atmosphere of 5%
CO2 at 37°C. Cells were grown in culture flasks and
harvested in trypsin-ethylenediaminetetraacetic acid solution
during the logarithmic growth phase. The antibodies used were:
Anti-cyclin A2 (rabbit monoclonal antibody; cat. no. ab32498,
1:500, Abcam), anti-cyclin B1 (mouse monoclonal antibody, cat. no.
ab72, 1:800, Abcam), anti-CDK1 (rabbit monoclonal antibody; cat.
no. ab32384, 1:2,000, Abcam), anti-phosphorylated ERK (P-ERK;
(rabbit monoclonal antibody; cat. no. ab76299, 1:8,000, Abcam),
anti-ERK (rabbit monoclonal antibody; cat. no. ab184699, 1:10,000,
Abcam), anti-immunoglobulin G (IgG; rabbit, cat. no. sc-2027,
dilution 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and anti-β-actin [rabbit monoclonal antibody (13E5); cat. no.
#4970, 1:1,000, Cell Signalling Technology, Inc., Beverly, MA,
USA). Recombinant human CCL21 and Cell Counting Kit-8 (CCK-8) were
obtained from the State Key Laboratory of Molecular Oncology,
Chinese Academy of Medical Sciences (Beijing, China).
For the incubations, all cultured cells were washed
with phosphate-buffered saline (PBS; pH 7.0) three times and
blocked with 10% bovine serum albumin (BSA; Sigma Chemical Co., St.
Louis, MO, USA) for 30 min at room temperature; subsequently, the
cells were incubated overnight at 4℃ with the first antibodies.
After washing with PBS, cells were incubated with the secondary
antibody for 1 h at room temperature. Finally, cells were stained
with 3,3′-diaminoben-zidine.
Cell proliferation assay
Cell proliferative activities were examined using
CCK-8. PTC1 and PTC3 cells were seeded in 96-well plates and
treated with CCL21 (100 ng/ml) for 0, 24, 48 or 72 h. Following
treatment, CCK-8 was added to each well according to the
manufacturer's instructions and incubated for 4 h at 37°C. The
optical density was measured using a microplate reader (Guava
Technologies, Inc., Hayward, CA, USA) at 450 nm.
Cell cycle analysis
Subsequent to 24-h treatment with CCL21, cells were
harvested and washed twice with PBS and fixed in 75% ethanol for 2
h at 4°C. The fixed cells were washed twice with 500 µl cold
PBS, and then were stained with 500 µl propidium iodide
staining solution for 30 min at room temperature in the dark. A
total of 10,000 events per sample were acquired using a FACScan
flow cytometer (Guava Technologies, Inc.), and the percentage of
cells in the G0/G1, S and G2/M
phases of the cell cycle were determined using ModFit LT software,
version 3.0 (Guava Technologies, Inc.).
Western blot analysis
Western blot analyses were performed as previously
described (16). Briefly, proteins
were electrophoresed on 12% polyacrylamide gels and transferred to
Hybond-P polyvinylidene difluoride membranes (GE Healthcare Life
Sciences, Chalfont, UK). Western blot analysis was conducted with
specific primary antibodies (against cyclin A2, cyclin B1, CDK1,
P-ERK and ERK; see the previous section for further details)
diluted in 1% BSA (Sigma Chemical Co.) in Tris-buffered saline with
Tween-20 (TBST), followed by the peroxidase-conjugated secondary
antibody [goat anti-mouse polyclonal antibody; cat. no. L3032,
dilution 1:6,000; Signalway Antibody (SAB), College Park, MD, USA].
After blocking with 5% non-fat milk in TBST for 1 h at room
temperature, Western blot analysis was performed with the specific
primary antibodies in 1% BSA in TBST for an incubation overnight at
4℃, followed by an incubation with the peroxidase-conjugated
secondary antibody for 1 h at room temperature. TBST was used for
the washing steps. Target proteins were observed using the enhanced
chemiluminescence detection system (GE Healthcare Life Sciences)
and autoradiography on Fuji Super RX film (Fujifilm Corporation,
Tokyo, Japan), with 1–2 min exposure.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. PCR was used to
quantify mRNA expression, and phosphoglycerate kinase (PGK-1) was
used as the internal control. For mRNA, RT-PCR was performed using
a One-Step RT-PCR kit (cat. no. RR057A; Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol. Aliquots of 20
µl cDNA (150 ng) were used for the amplification reaction.
The primer sequences for CCR7, RET/PTC1, RET/PTC3 and PGK-1 used
were as described previously (11,17).
The cycling conditions were as follows: denaturation at 95°C for 15
sec, followed by 40 cycles of annealing at 56°C for 60 sec and
extension at 72°C for 60 sec
Co-immunoprecipitation
Cells were extracted with lysis buffer and
homogenized for 30 min at 4°C subsequent to 24-h treatment with
CCL21. The extracts were centrifuged at 12,000 × g for 15 min at
4°C, and the supernatants containing total protein were harvested.
Equal amounts of protein were exposed to the antibodies against
cyclin A, cyclin B1, CDK1, P-ERK or IgG. The beads were washed
extensively with lysis buffer, boiled, and microcentrifuged at
3,000 rpm (1,007 g) for 3 min at 4°C following a 3 h incubation at
4°C. Proteins were detected with antibodies against cyclin A,
cyclin B1, CDK1, P-ERK or IgG by western blotting.
Statistical analysis
Differences between the groups were analyzed by
one-way analysis of variance using SPSS software, version 13.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate statistically significant difference.
Results
NaI (10−5 M) abolishes the
effects of CCL21/CCR7 interaction on proliferation
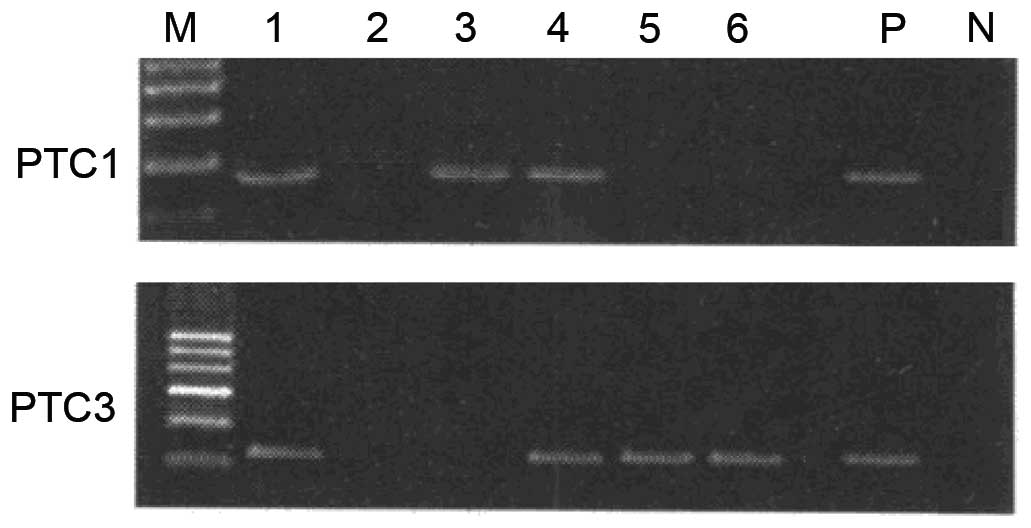
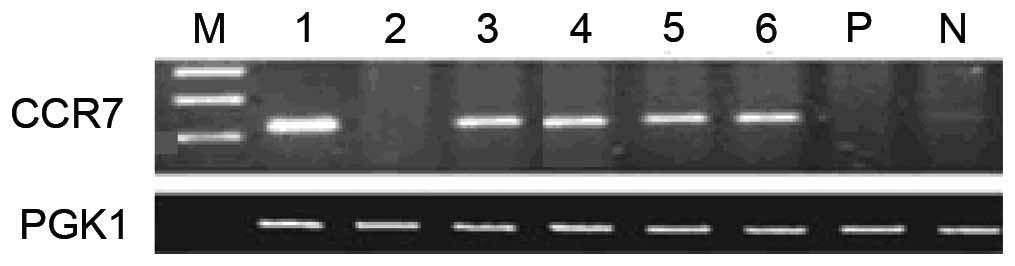
The current study identified two cell lines from six
primary PTC cultures, RET/PTC1 and RET/PTC3, which both have high
CCR7 expression (Figs. 1 and
2). According to the results of a
previous study (18), 100 ng/ml
CCL21 markedly promoted cell proliferation as compared with 50
ng/ml CCL21, while there was no clear difference observed between
the effects of 100 and 200 ng/ml CCL21 on cell proliferation.
Therefore, 100 ng/ml CCL21 was selected for use in the current
study.
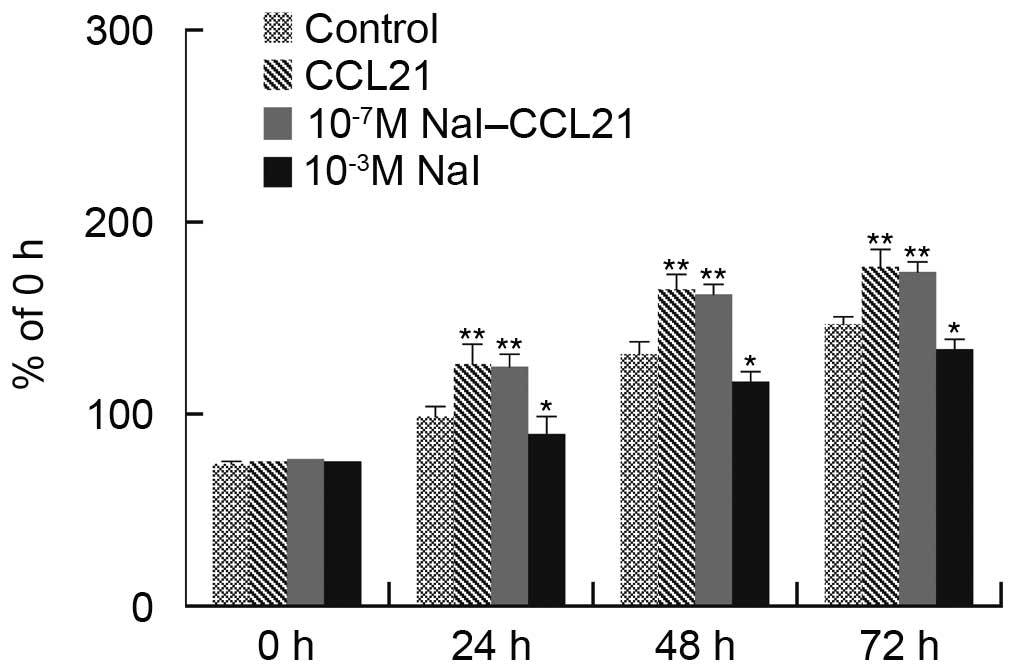
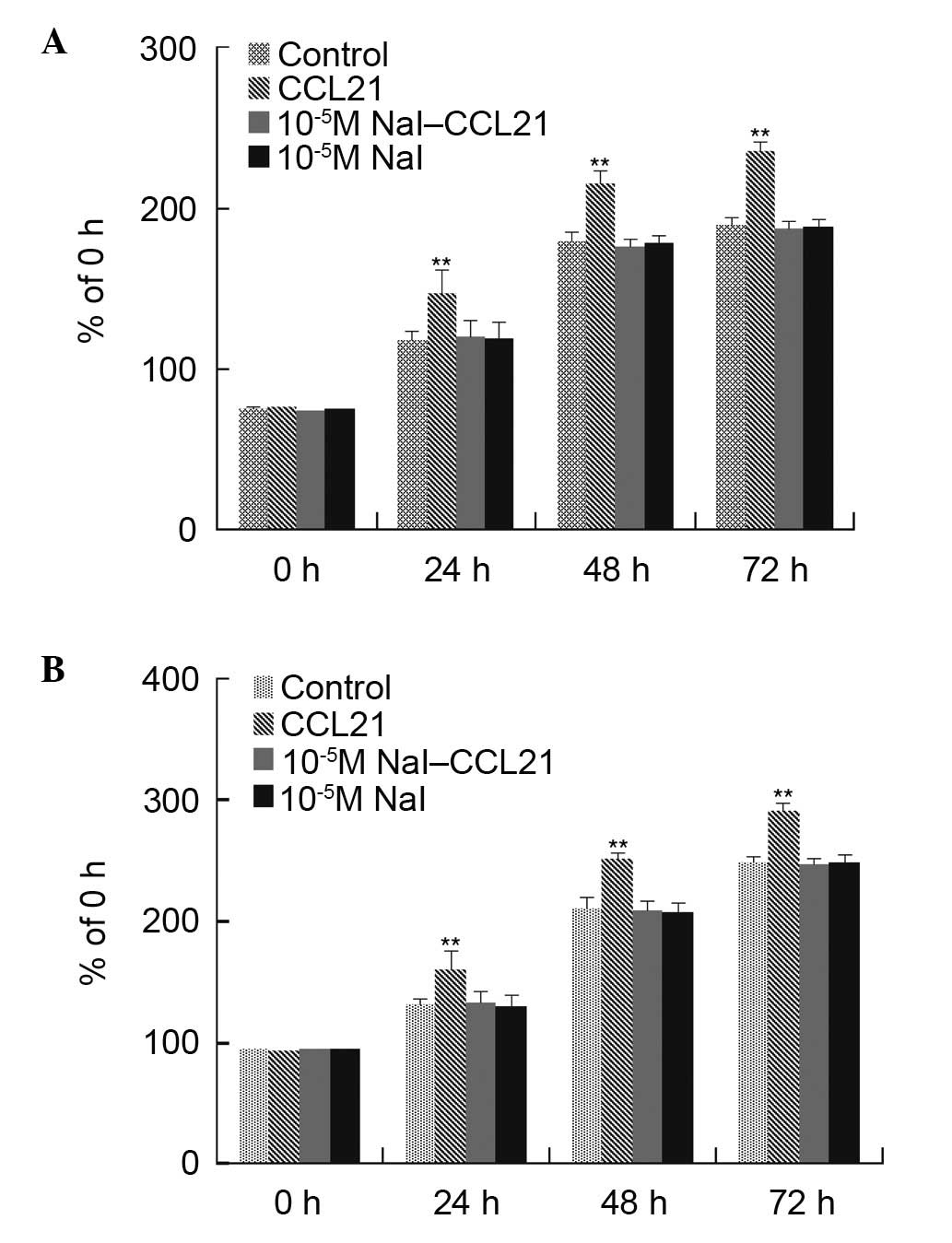
The experiments determined that 10−5 M
NaI significantly abrogated the effects of CCL21/CCR7 interaction
on cell proliferation; the intermediate concentration of
10−5 M NaI alone did not affect cell proliferation
significantly, and the physiological concentration of
10−7 M NaI could not abrogate the effects of CCL21/CCR7
interaction on cell proliferation. The high concentration of
10−3 M NaI alone had significant effects on cell
proliferation. The 10−5 M NaI concentration was used to
verify whether it could affect the effects of CCL21/CCR7
interaction on PTC1 and PTC3 cell proliferation. Cell viability and
cell cycle distribution were examined using the CCK-8 assay and
flow cytometry, respectively. It was identified that
10−5 M NaI significantly abrogated the effects of
CCL21/CCR7 interaction on cell proliferation (Figs. 3 and 4).
CCL21/CCR7 interaction augments the
proportion of cells in G2/M
Cell cycle analysis was performed using flow
cytometry to verify whether the effect of CCL21/CCR7 interaction on
PTC1 and PTC3 cell proliferation is associated with an alteration
in cell cycle distribution. CCL21/CCR7 interaction significantly
enhanced the proportion of cells in G2/M, however had no
significant effect on the proportion of cells in the
G0/G1 or S phases as compared with control
cells. NaI (10−5 M) significantly abolished this effect
of CCL21, however did not have a significant effect on cell cycle
distribution (Tables I and
II).
 | Table IEffect of CCL21 and NaI on cell cycle
distribution in PTC1 cells. |
Table I
Effect of CCL21 and NaI on cell cycle
distribution in PTC1 cells.
| Group |
G0/G1 phase (%) | S phase (%) | G2/M
phase(%) |
|---|
| Control | 73.35±9.43 | 21.35±11.28 | 2.13±2.76 |
| CCL21 | 65.72±1.23 | 19.60±2.91 | 11.25±1.54a |
| 10−5 M
NaI-CCL21 | 64.08±9.08 | 22.90±8.28 | 6.50±3.49 |
| 10−5 M
NaI | 71.73±4.32 | 17.09±3.33 | 7.19±3.46 |
 | Table IIEffect of CCL21 and NaI on cell cycle
distribution in PTC3 cells. |
Table II
Effect of CCL21 and NaI on cell cycle
distribution in PTC3 cells.
| Group |
G0/G1 phase (%) | S phase (%) | G2/M
phase(%) |
|---|
| Control | 59.50±3.70 | 33.33±4.41 | 7.22±2.63 |
| CCL21 | 61.38±6.35 | 28.08±4.16 | 13.54±2.95a |
| 10−5 M
NaI-CCL21 | 68.25±4.44 | 20.40±3.11 | 6.02±2.77 |
| 10−5 M
NaI | 62.57±2.11 | 27.00±9.66 | 4.43±1.10 |
NaI (10−5 M) significantly
abrogates the upregulatory effects of CCL21/CCR7 interaction on
cyclin A, cyclin B1, CDK1, and P-ERK expression (Fig. 5)
To determine the possible mechanism by which
CCL21/CCR7 interaction influences G2/M distribution in
PTC cells, cyclin A, cyclin B1 and CDK1 expression were assessed
using western blotting. Compared with the control cells, cells with
CCL21/CCR7 interaction exhibited significantly upregulated protein
levels of cyclin A, cyclin B1 and CDK1 (Fig. 5A). It was identified that
10−5 M NaI significantly abrogated the effects of CCL21;
however, it had no significant effect on cyclin A, cyclin B1 or
CDK1 expression. To verify whether the CCL21/CCR7 interaction may
additionally enhance P-ERK expression in PTC cells, ERK and P-ERK
expression levels were measured using western blotting. CCL21/CCR7
interaction upregulated P-ERK expression significantly, however,
had no significant effect on ERK expression. Following treatment
with 10−5 M NaI, the significant upregulation of P-ERK
expression by CCL21/CCR7 interaction was abrogated (Fig. 5B).
Induced by CCL21/CCR7 interaction, P-ERK
interacts with cyclin A, cyclin B1 and CDK1
Co-immunoprecipitation was used to determine whether
there is interaction between P-ERK and cyclin A, cyclin B1 or CDK1.
PTC3 cells were incubated for 24 h with or without CCL21, then were
immunoprecipitated with antibodies against P-ERK or IgG, followed
by western blotting for cyclin A, cyclin B1 and CDK1. There was a
pronounced, specific interaction between P-ERK and cyclin A, cyclin
B1 and CDK1, particularly subsequent to 24-h treatment with CCL21.
Reciprocal immunoprecipitation was assessed with antibodies against
cyclin A, cyclin B1, CDK1 or IgG using western blotting for P-ERK.
The resulst were consistent with those above mentioned, with the
interaction between P-ERK and cyclin A, cyclin B1 or CDK1 observed
to be clear, particularly in the presence of CCL21. PTC3 cells
incubated for 1 h with or without 10−5 M NaI were
immunoprecipitated with antibodies against P-ERK or IgG, followed
by western blotting for cyclin A, cyclin B1 and CDK1. There was a
reduced interaction between P-ERK and cyclin A, cyclin B1 and CDK1
in response to the 10−5 M NaI exposure (Fig. 6).
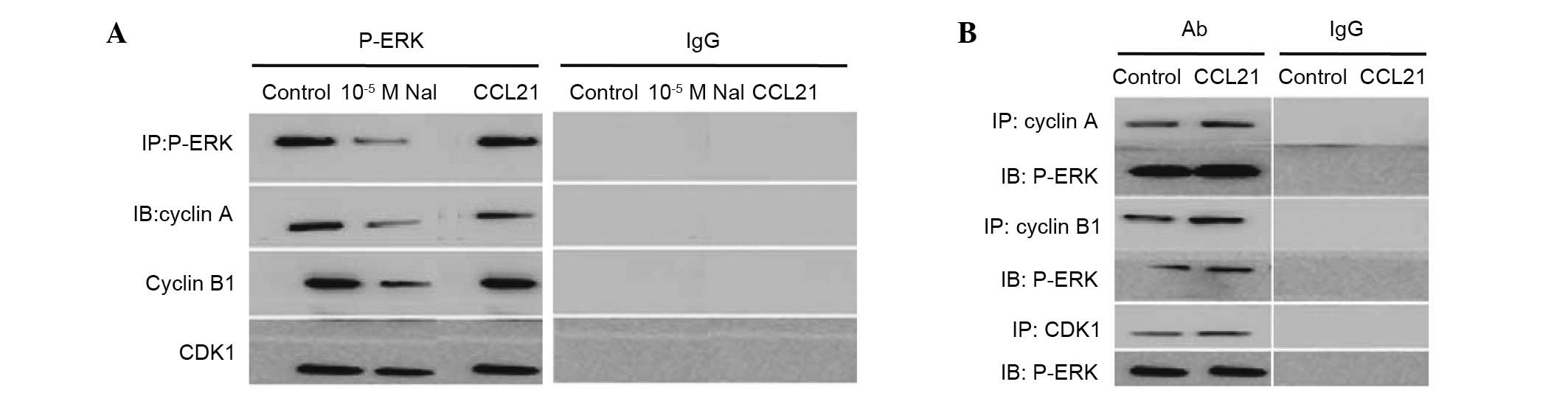 | Figure 6Interaction between P-ERK and cyclin
A, cyclin B1 or CDK1 with or without CCL21 or 10−5 M
NaI. (A) Western blot analysis for cyclin A, cyclin B1 and CDK1 in
PTC3 cells incubated with or without CCL21 (100 ng/ml) or
10−5 M NaI for 24 h. (B) Western blot analysis for P-ERK
was used to analyze reciprocal immunoprecipitation with antibodies
against cyclin A, cyclin B1, CDK1 or IgG. P-, phosphorylated; ERK,
extracellular signal-related kinase; CDK1, cyclin-dependent kinase
1; CCL21, chemokine (C-C motif) ligand 21; PTC, papillary thyroid
carcinoma; IgG, immunoglobulin G; IP, immunoprecipitation; IB,
immunoblotting; Ab, antibody. |
Discussion
Iodine serves an important role in regulating
differentiation and proliferation in the normal thyroid follicular
cell, whereas during thyroid oncogenic activation, excess iodine
serves an anti-oncogenic role (5).
Previous studies have reported that CCR7 activation mediates
survival in certain cancer cell lines by promoting cell migration
and proliferation or by inhibiting apoptosis (17,18).
At present, the association between iodine concentrations and
CCL21/CCR7 interaction and the cell cycle in RET/PTC remains to be
fully elucidated. In the current study, CCL21/CCR7 interaction
significantly enhanced human RET/PTC cell proliferation in a
time-dependent manner, and involved cyclin A, cyclin B1 and CDK1
upregulation, possibly via the ERK pathway. In addition,
10−5 M NaI regulates G2/M progression induced
by CCL21/CCR7 interaction in primary cultures of PTC1 and PTC3
cells.
CCR7 activation has been previously reported to
increase P-ERK levels (18,19).
ERK belongs to the MAPK family; activated by mitogenic stimuli, the
ERK cascade is critical for cell proliferation and survival
(20) and is required for normal
progression to mitosis (18). The
cell cycle is regulated by cyclins and CDKs: cyclin A is essential
for progression through the S phase (21,22).
Cyclin A and cyclin B1 associate with CDK1 to promote entry into
mitosis (18,21,22).
In the current study, both protein and mRNA levels of cyclin A,
cyclin B1 and CDK1 in PTC1 and PTC3 cells were observed to be
significantly upregulated when cells had been treated with CCL21
for 24 h, indicating that CCL21/CCR7 interaction accelerates
G2/M progression to promote cell proliferation. This
observation demonstrates that CCL21/CCR7 interaction drives cell
cycle progression involving the G2/M phase in PTC1 and
PTC3 cells.
As CCL21/CCR7 interaction increases P-ERK
expression, it was determined whether there was an interaction
between P-ERK and cyclin A, cyclin B1 or CDK1.
Co-immunoprecipitation and reciprocal immunoprecipitation strongly
suggested interaction between P-ERK and cyclin A, cyclin B1 and
CDK1, particularly in the presence of CCL21; inhibiting ERK with
10−5 M NaI weakened this interaction. And
10−5 M NaI abolished the effect of CCL21/CCR7
interaction on PTC1 and PTC3 cell proliferation and G2/M
progression and downregulated P-ERK, cyclin A, cyclin B1 and CDK1
expression. These results demonstrate the effect of CCL21/CCR7
interaction on cell proliferation and that cyclin A, cyclin B1 and
CDK1 upregulation may occur via the ERK pathway in association with
10−5 M NaI in PTC cells.
The present study suggests that activating CCR7 with
CCL21 significantly promotes PTC1 and PTC3 cell proliferation in a
time-dependent manner and involves cyclin A, cyclin B1 and CDK1,
potentially via the ERK pathway. In addition, it was identified
that iodine regulates G2/M progression induced by
CCL21/CCR7 interaction in primary cultures of PTC cells with
RET/PTC expression. This information may aid in clarifying the
mechanisms of cancer cell survival and identify potential targets
for PTC treatment.
Acknowledgments
The current study was supported by a grant from the
Shanghai Municipal Health Bureau Scientific Foundation of China
(grant no. 2010–51).
References
|
1
|
Mousavi Z, Dourandish L, Rokni H, Sadeghi
R and Rasoul Zakavi S: Effects of short-term metformin therapy
associated with levothyroxine dose decrement on TSH and thyroid
hormone levels in patients with thyroid cancer. Minerva Endocrinol.
39:59–65. 2014.PubMed/NCBI
|
|
2
|
Rossi M, Buratto M, Tagliati F, Rossi R,
Lupo S, Trasforini G, Lanza G, Franceschetti P, Bruni S, Degli
Uberti E and Zatelli MC: Relevance of BRAF(V600E) mutation testing
versus RAS point mutations and RET/PTC rearrangements evaluation in
the diagnosis of thyroid cancer. Thyroid. 25:221–228. 2015.
View Article : Google Scholar :
|
|
3
|
Knauf JA, Kuroda H, Basu S and Fagin JA:
RET/PTC-induced dedifferentiation of thyroid cells is mediated
through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene.
22:4406–4412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vivero M, Kraft S and Barletta JA: Risk
stratification of follicular variant of papillary thyroid
carcinoma. Thyroid. 23:273–279. 2013. View Article : Google Scholar
|
|
5
|
Fiore AP, Fuziwara CS and Kimura ET: High
iodine concentration attenuates RET/PTC3 oncogene activation in
thyroid follicular cells. Thyroid. 19:1249–1256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shakhtarin VV, Tsyb AF, Stepanenko VF,
Orlov MY, Kopecky KJ and Davis S: Iodine deficiency, radiation
dose, and the risk of thyroid cancer among children and adolescents
in the Bryansk region of Russia following the Chernobyl power
station accident. Int J Epidemiol. 32:584–591. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Z, Ciampi R, Nikiforova MN, Gandhi M
and Nikiforov YE: Prevalence of RET/PTC rearrangements in thyroid
papillary carcinomas: Effects of the detection methods and genetic
heterogeneity. J Clin Endocrinol Metab. 91:3603–3610. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marotta V, Guerra A, Sapio MR and Vitale
M: RET/PTC rearrangement in benign and malignant thyroid diseases:
A clinical standpoint. Eur J Endocrinol. 165:499–507. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santoro M, Melillo RM, Grieco M,
Berlingieri MT, Vecchio G and Fusco A: The TRK and RET tyrosine
kinase oncogenes cooperate with ras in the neoplastic
transformation of a rat thyroid epithelial cell line. Cell Growth
Differ. 4:77–84. 1993.PubMed/NCBI
|
|
10
|
Colato C, Vicentini C, Cantara S, Pedron
S, Brazzarola P, Marchetti I, Di Coscio G, Chilosi M, Brunelli M,
Pacini F and Ferdeghini M: Break-apart interphase fluorescence in
situ hybridization assay in papillary thyroid carcinoma: On the
road to optimizing the cut-off level for RET/PTC rearrangements.
Eur J Endocrinol. 172:571–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romei C and Elisei R: RET/PTC
translocations and clinicopathological features in human papillary
thyroid carcinoma. Front Endocrinol (Lausanne). 3:542012.
|
|
12
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: Correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Xi L, Hunt JL, Gooding W,
Whiteside TL, Chen Z, Godfrey TE and Ferris RL: Expression pattern
of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma
of the head and neck identifies a novel metastatic phenotype.
Cancer Research. 64:1861–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brest P, Lassalle S, Hofman V, Bordone O,
Gavric Tanga V, Bonnetaud C, Moreilhon C, Rios G, Santini J, Barbry
P, et al: MiR-129-5p is required for histone deacetylase
inhibitor-induced cell death in thyroid cancer cells. Endocr Relat
Cancer. 18:711–719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Liu ZB, Ren WM, Ye XG and Zhang
YY: The miR-200 family regulates the epithelial-mesenchymal
transition induced by EGF/EGFR in anaplastic thyroid cancer cells.
Int J Mol Med. 30:856–862. 2012.PubMed/NCBI
|
|
17
|
Sancho M, Vieira JM, Casalou C, Mesquita
M, Pereira T, Cavaco BM, Dias S and Leite V: Expression and
function of the chemokine receptor CCR7 in thyroid carcinomas. J
Endocrinol. 191:229–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Liu L, Qiu X, Jiang L, Huang B, Li
H, Li Z, Luo W and Wang E: CCL21/CCR7 promotes G2/M phase
progression via the ERK pathway in human non-small cell lung cancer
cells. PLoS One. 6:e211192011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nigg EA: Cyclin-dependent protein kinases:
Key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Girard F, Strausfeld U, Fernandez A and
Lamb NJ: Cyclin A is required for the onset of DNA replication in
mammalianfi broblasts. Cell. 67:1169–1179. 1991. View Article : Google Scholar : PubMed/NCBI
|