Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality worldwide, with 1,000,000 new cases
annually, in terms of incidence and mortality rates (1). It accounted for 13% (1,600,000 cases)
of the total cases of cancer and 18% (1,800,000 cases) of
cancer-associated mortality in 2008 (2). Tobacco use is considered to be the
primary cause of lung cancer, accounting for 80–90% of cases
(3,4), among which cigarette smoking totals
~95% of tobacco use (5).
Non-small-cell lung cancer (NSCLC) accounts for ~80% of all cases
of lung cancer (6). The management
of patients with NSCLC is based on systemic chemotherapy. However,
patient survival rates remains low. Additionally, although
chemotherapy can prolong survival rates among patients with
advanced disease, clinically significant adverse effects reduce its
efficacy as excessive toxicity is often reported (7).
Traditional chemotherapy remains an important
therapeutic strategy for human cancer. However, the majority of
chemotherapeutic drugs for the treatment of NSCLC are cytotoxic
agents with a high risk of side effects, including adriamycin,
cisplatin, 5-fluorouracil and doxorubicin (8,9).
Furthermore, chemoresistance can develop in patients with NSCLC,
which presents a major obstacle to the long-term efficacy of
chemotherapeutic treatments (10,11).
Therefore, alternative treatments require development to improve
the efficiency of NSCLC therapy.
Traditional Chinese medicine (TCM) is valued for its
5,000-year-old history and retains an important position in primary
healthcare in China. TCM can complement Western medicine using
modern techniques, thus, TCMs have increased in interest in Western
countries. Sophora flavescens Ait is a TCM widely used for
several diseases, including viral hepatitis, cardiac arrhythmia,
lung cancer and skin inflammation, in China (12). The active components of Sophora
flavescens are various alkaloids, among which matrine has been
characterized as the major bioactive component (13).
As an alkaloid, matrine has favorable medical
effects. Its antiviral activity is promising in the treatment of
chronic hepatitis B (14). It has
been reported that intramuscular injection of matrine improves the
clinical symptoms of patients with chronic hepatitis B, recovers
liver function and alters serum conversion from positive to
negative hepatitis B virus DNA (15). It has also been shown that matrine
exerts antifibrotic activity, which inhibits the activities of
platelet-derived growth factor and transforming growth factor-β in
hepatic stellate cells (16).
Previous studies have shown that matrine is effective at inhibiting
cell growth and inducing differentiation in human leukemia K562
cells (13,17,18).
Matrine is also a differentiation inducer in SMMC-7721 cells
(19). In human multiple myeloma
cells and MKN45 gastric cancer cells, matrine can induce tumor cell
apoptosis by interrupting cell-cell adhesion and inhibiting cancer
metastasis (20,21), and matrine can also prevent tumor
invasion (22). Consequently,
matrine may be a promising alternative anticancer drug for the
treatment of NSCLC. In China, matrine has been used for the
treatment of NSCLC in mice (23),
however, the antitumor therapeutic efficacy and the underlying
molecular mechanisms of matrine, with respect to the physiological
and pharmacological effects on human NSCLC, remain to be fully
elucidated.
In the present study, the therapeutic effects and
the underlying molecular mechanisms of matrine on the A549 human
NSCLC cell line were investigated. This included investigation of
its inhibitory effect on cell proliferation, alterations of cell
morphology and induction of cell apoptosis, and the expression of
microRNA (miR)-126 and its target gene, vascular endothelial
growth factor (VEGF), as miR-126 is known to be
downregulated in NSCLC cell lines, including A549 (24). Matrine may serve as a promising TCM
for NSCLC therapy.
Materials and methods
Cell line and matrine treatment
The A549 human NSCLC cell line was purchased from
the Cancer Research Institute of China Medical University
(Shenyang, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% (m/v) fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin on culture plates at 37°C in a 5% CO2
atmosphere with stable humidity. The density of cells was 1×105
cells/ml prior to culture. Matrine was obtained from Xian Botany
Garden (Shanxi, China), and its purity was >99% as assessed by
high performance liquid chromatography. The matrine stock solution
was prepared in ddH2O at 10 mg/ml. Log-phase growing cells were
seeded at a density of 1×105 cells/ml and exposed to matrine at
concentrations, 0 (negative control), 0.2, 0.5, 0.8 and 1.0 mg/ml
for 48 h at 37°C.
MTT assay
The effects of matrine on cell viability were
assessed busing an MTT assay, as described previously (25). Specifically, the cells were plated
at a density of 3,000 cells per well into 96-well plates. At the
end of treatment, the supernatant was removed, following which 20
µl of the tetrazolium compound, MTT, and 270 ml fresh Iscove's
modified Dulbecco's medium (NewTopBio Co., Shenzhen, China) were
added. Following incubation for 4 h at 37°C, 120 µl of DMSO was
added to each well to dissolve the tetrazolium crystals. Finally,
the absorbance at a wavelength of 570 nm was recorded using a
multi-well plate reader (Tecan Schweiz AG, Maennedorf,
Switzerland). Each experiment was performed four times. The results
are expressed as the percentage growth inhibition with respect to
the untreated cells.
Microscopic examination
The cells were digested by trypsin-EDTA, washed and
resuspended in serum-free medium, counted and then fixed overnight
in 75% ethanol at 4°C. The cells were washed and resuspended in
phosphate-buffered saline (PBS; pH 7.4). The digested cell culture
(3×105 cells/ml) was added to a 24-well plate (0.9 ml in each well)
and incubated for 12 h at 37°C. Subsequently, 0.1 ml/well of
matrine at a low (0.2 mg/ml) or high (1.0 mg/ml) concentration was
added. The cells were incubated for 48 h at 37°C prior to
observation. The cells were examined using an Olympus IX70 inverted
microscope (Olympus Corporation, Tokyo, Japan), a DVC1310 digital
video camera (DVC Co., Austin, TX, USA) and a QED camera (Media
Cybernetics, Inc., Rockville, MD, USA) with Standalone 145 software
(Media Cybernetics, Inc.).
Flow cytometry (FCM)
A549 cells in the log phase were collected at a
final concentration of 2×105 cells/ml, and were incubated in a
6-well plate for 12 h at 37°C (2.7 ml in each well). Subsequently,
matrine at a low (0.2 mg/ml) or high (1.0 mg/ml) concentration (0.3
ml per well) was added to induce the cells for 48 h.
Simultaneously, 0.3 ml of cell culture, as a negative control, was
cultured for 48 h at 37°C, collected, washed with PBS, and fixed
with 70% ethanol, in sequence. The cells were centrifuged at 5,300
× g for 5 min at 4°C to eliminate ethanol, washed with PBS and
stained with propidium iodide in the dark for 30 min prior to
analysis using FCM. Finally, a BD FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) was used to detect cell
cycle. The cells were sampled using CellQuest 3.0 sampling software
(BD Biosciences). The proportion of cells in the different phases
were quantified using ModFitLT 3.0 (26). Each experiment was performed four
times.
Measurement of apoptosis
The cells (1×106) were treated with 0, 0.2, 0.5, 0.8
and 1.0 mg/ml matrine for 48 h at 37°C, and then collected by
centrifugation at 5,300 × g for 5 min at 4°C. The pellets were
lysed in DNA lysis buffer, containing 10 mM Tris (pH 7.5), 400 mM
EDTA and 1% Triton X-100, and then centrifuged at 5,300 × g for 5
min at 4°C. The supernatant obtained was incubated overnight at
37°C with proteinase K (0.1 mg/ml) and then with RNase (0.2 mg/ml)
for 2 h at 37°C. Following extraction with phenol chloroform (1:1),
DNA was separated on 2% agarose gel and visualized under UV
following staining with ethidium bromide. The quantitative
assessment of apoptotic cells was assessed using the terminal
deoxynucleotidyl transferase-mediated deoxyuridine triphosphate
nick-end labeling (TUNEL) method, which was performed to examine
DNA-strand breaks during apoptosis using a BD ApoAlert DNA
Fragmentation Assay kit (BD Biosciences).
Immunohistochemical (IHC)
staining
IHC staining was performed using the specific
affinity-purified polyclonal anti-VEGF antibody. As a negative
control, nonimmune serum was used instead of the primary antibody.
Briefly, the cells were washed in PBS followed by preincubation
with 1.5% normal goat serum in phosphate buffer within a moist
chamber for 4 h at room temperature. These cells were then
incubated overnight at 4°C with rabbit polyclonal anti-VEGF
antibody (1:100; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany; cat. no. HPA027342) at a final concentration of 2 µg/ml.
Following being washed six times with PBS containing 0.02% Triton
X-100 for 15 min, the slides were processed for immunostaining
using the avidin-biotinylated peroxidase complex method (Vector
Laboratories, Inc., Burlingame, CA, USA), according to the
manufacturer's protocol. The cells were briefly counterstained with
Mayer's hematoxylin prior to mounting. The cultured cells were
grown on sterile coverslips in tissue culture dishes overnight,
fixed with 45% acetone/10% formaldehyde in 0.1 M phosphate buffer
for 5 min, and then processed for the IHC assay as described
previously (27).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR analysis
The expression of mature miR-126 in the A549
cells was assayed using a Taqman MicroRNA assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each sample was
analyzed in triplicate. Total RNA was extracted using TRIzol
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The RT reaction was performed on a 10 ng sample of total
RNA using looped primers. qPCR analysis was performed using the
standard Taqman MicroRNA assay protocol on a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The 20
µl PCR sample comprised 1.33 µl of the RT product, 1X Taqman
Universal PCR Master Mix without AmpErase UNG (cat. no. 4324018;
Applied Biosystems; Thermo Fisher Scientific, Inc.), 0.2 µmol/l
Taqman probe, 1.5 µmol/l forward primer and 0.7 µmol/l reverse
primer. The reactions were performed in a 96-well plate at 95°C for
10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. The primers used were as follows: Forward,
5′-TACCTCCACCATGCCAAGTG-3′, and reverse,
5′-ATGATTCTGCCCTCCTCCTTC-3′. The ∆∆Cq method for relative
quantitation of gene expression levels was used to determine the
miRNA expression levels. The ∆∆Cq was calculated by subtracting the
Cq value of U6 RNA from the Cq value of the miRNA of interest. The
∆∆Cq was calculated by subtracting the ∆Cq value of the reference
sample (untreated A549 cells) from the ∆Cq value of each sample.
The fold change was calculated using the 2-∆∆Cq equation. A pool of
three reference samples was used for standard curve calculation and
as a reference sample for the ∆∆Cq. The Taqman MicroRNA Assay for
U6 RNA was used to normalize the relative abundance of miRNA.
Western blot analysis
The cell lysates were prepared in RIPA buffer
containing 50 mmol/l Tris-HCl buffer (pH 7.4), 150 mmol/l NaCl, 1%
Triton X-100, 1% sodium deoxycholate and 0.1% sodium dodecyl
sulfate, supplemented with 1X Halt protease inhibitor cocktail and
1X Halt phosphatase inhibitor cocktail (Pierce; Thermo Fisher
Scientific, Inc.). A Bio-Rad protein assay (Bio-Rad Laboratories,
Inc.) was used to determine protein concentrations. The proteins
(~100 ng) were separated on 10–12% sodium dodecyl
sulfate-polyacrylamide gels by electrophoresis and transferred onto
PVDF membranes (Whatman, Boston, MA, USA). The membranes were first
hybridized with mouse monoclonal anti-VEGF antibody (1:800;
Sigma-Aldrich; Merck Millipore; cat. no. SAB1402390) overnight at
4°C and then with horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:3,000; Cell Signaling Technology, Inc., Danvers, MA,
USA; cat. no. 7072) at 25°C for 1 h. The protein bands were
visualized using a commercial Immobilon Western Chemiluminescent
HRP Substrate detection reagent (EMD Millipore, Billerica, MA,
USA). The chemiluminescence of proteins transferred onto PVDF
membranes was detected with ECL Plus (GE Healthcare, Piscataway,
NJ, USA). Relative protein expression values were quantitatively
determined via densitometry with ImageJ software (version 2.1.4.7;
imagej.nih.gov/).
Statistical analysis
All data were analyzed with the SPSS 16.0
statistical software package (SPSS, Inc., Chicago, IL, USA). The
results are expressed as the mean ± standard deviation. The
statistical significance of the results were determined using the
parametric unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
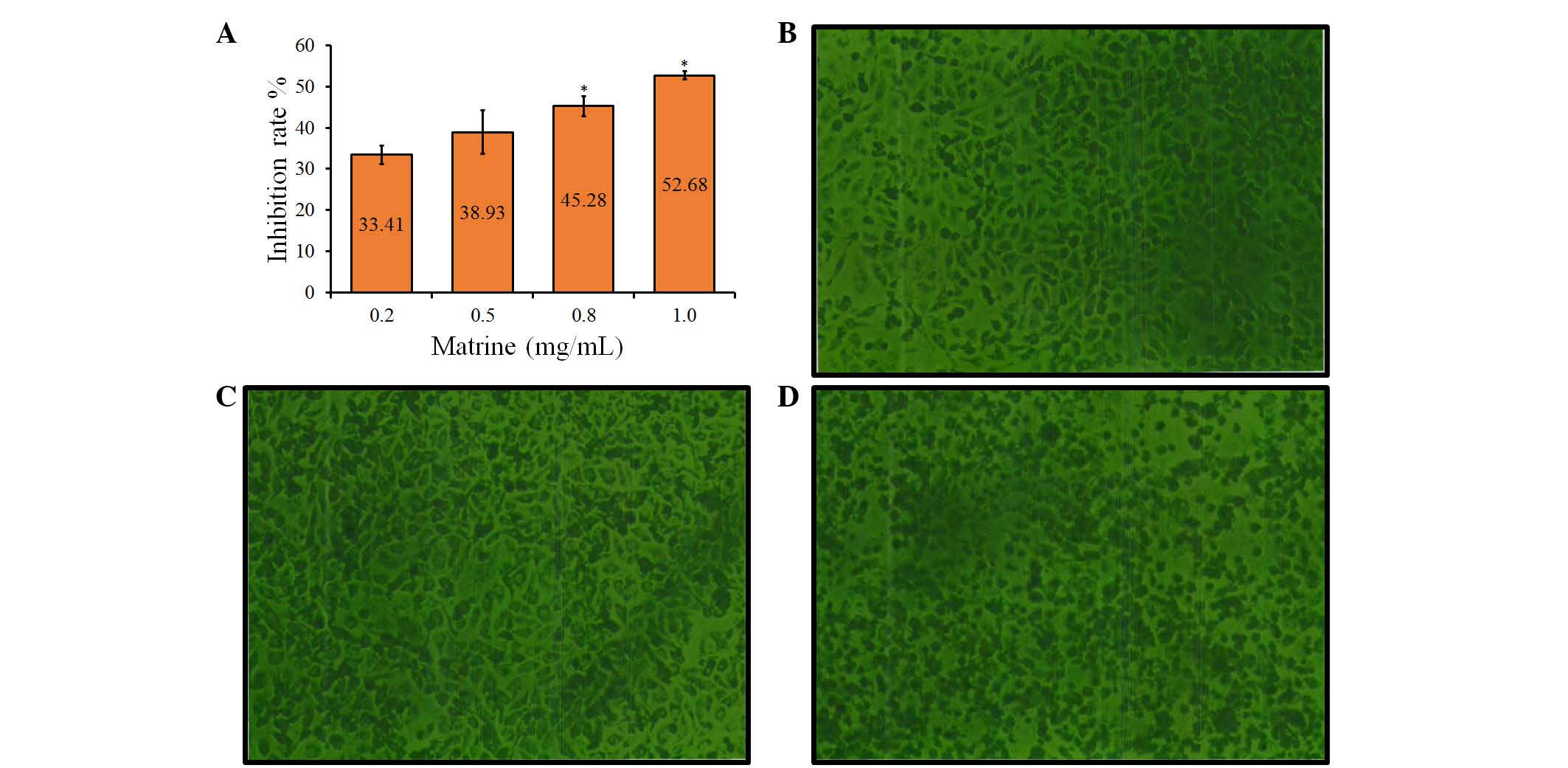
Matrine induces growth inhibition of
A549 cells
To examine the growth-modulatory effects of matrine
on A549 cells, a wide concentration range of matrine (0.2, 0.5, 0.8
and 1.0 mg/ml) was used. Following treatment with matrine for 48 h,
significant (P<0.05) inhibition of cell growth was observed at
high concentrations of 0.8 and 1.0 mg/ml, compared with the control
groups, with average rates of growth inhibition of 45.28±4.18 and
52.68±3.32%, respectively (Fig.
1A). However, low concentrations of matrine (0.2 and 0.5 mg/ml)
had no significant effect on cell growth, compared with the control
groups. These results indicated that the growth inhibitory effect
of matrine on A549 cells was concentration-dependent (Fig. 1A).
Cellular morphological changes
Following treatment with matrine for 48 h, the
morphology of the cells was observed using a fluorescence-imaging
micro-spectrophotometer. Compared with the control group (Fig. 1B), treatment with matrine at a low
concentration (0.2 mg/ml had no significant effect on cellular
morphology (Fig. 1C), whereas
marked morphological changes, including cell shrinkage, disruption
and destruction were observed in the cells treated with a high
concentration of matrine (1.0 mg/ml), compared with the control
group (Fig. 1D). Therefore, a high
concentration of matrine led to a decrease in cell colony size,
which was also indicated by the decrease in cell diopter (Fig. 1C).
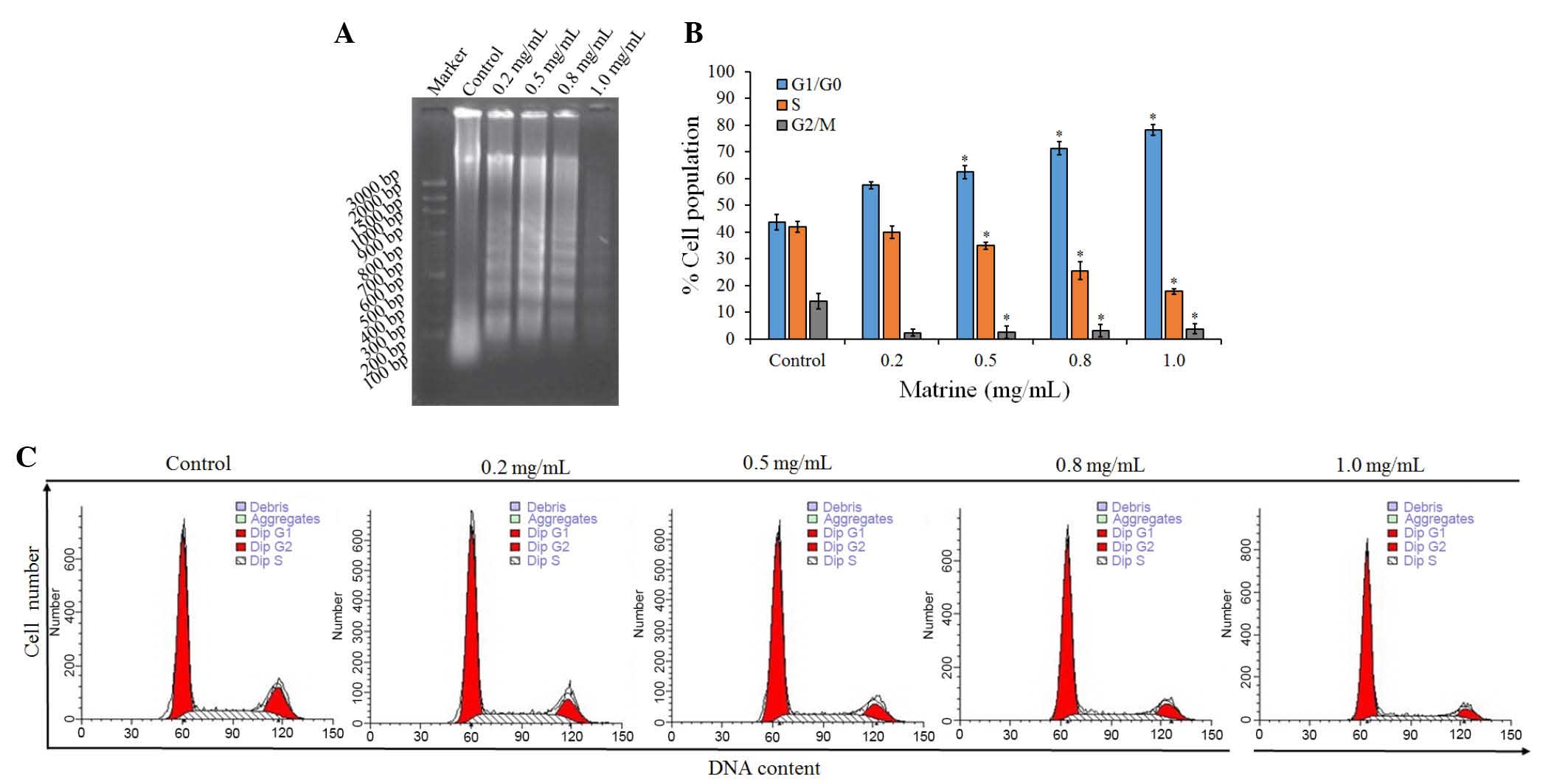
Cell apoptosis is induced by
matrine
To examine the mechanism responsible for the
matrine-mediated inhibition of cell proliferation, cell-cycle
distribution was evaluated using FCM. Compared with the control
groups, treatment with matrine led to a significant (P<0.05)
increase in the ratio of cells in the G1/G0 phase, and significant
(P<0.05) decreases in the ratios of cells in the S and G2/M
phases, the majority of cells remained at the G1 phase following
treatment with matrine at low and high concentrations (0.2 and 1.0
mg/ml; Table I). The proportion of
diploid cells increased marginally, whereas that of aneuploid cells
decreased. Cell debris increased, compared with the control
group.
 | Table I.Changes in cell cycle induced by
matrine. |
Table I.
Changes in cell cycle induced by
matrine.
| Group | Cell debris (%) | Apoptosis (%) | Diploid (%) | Aneuploid (%) |
|---|
| Control | 1.76±0.1891 | 0.0309±0.0165 | 93.9335±3.977 | 6.0665±4.3721 |
| Matrine |
|
|
|
|
| 0.2
(mg/ml) | 18.8967±3.9119 | 0.0400±0.0693 | 98.7367±1.4318 | 1.2401±1.4673 |
| 1.0
(mg/ml) | 47.0597±4.9622 | 0.07097±0.0689 | 98.9799±0.1605 | 1.0201±0.1394 |
The effect of matrine on the induction of apoptosis
in A549 cells was also examined using a DNA fragmentation assay.
Agarose gel electrophoresis at 48 h showed that matrine treatment
resulted in the formation of DNA fragments in the A549 cells
(Fig. 2A). Quantitative evaluation
was also performed using a TUNEL assay to detect DNA-strand breaks.
Compared with the control cells at 48 h, treatment with 1.0 mg/ml
matrine induced apoptosis of ~30% of the A549 cells (Fig. 2B). The results showed that treating
the cells with matrine caused a significant inhibition of
cell-cycle progression in the A549 cells (Fig. 2C), resulting in an increase of the
percentage of cells in the G1 phase, compared with the control.
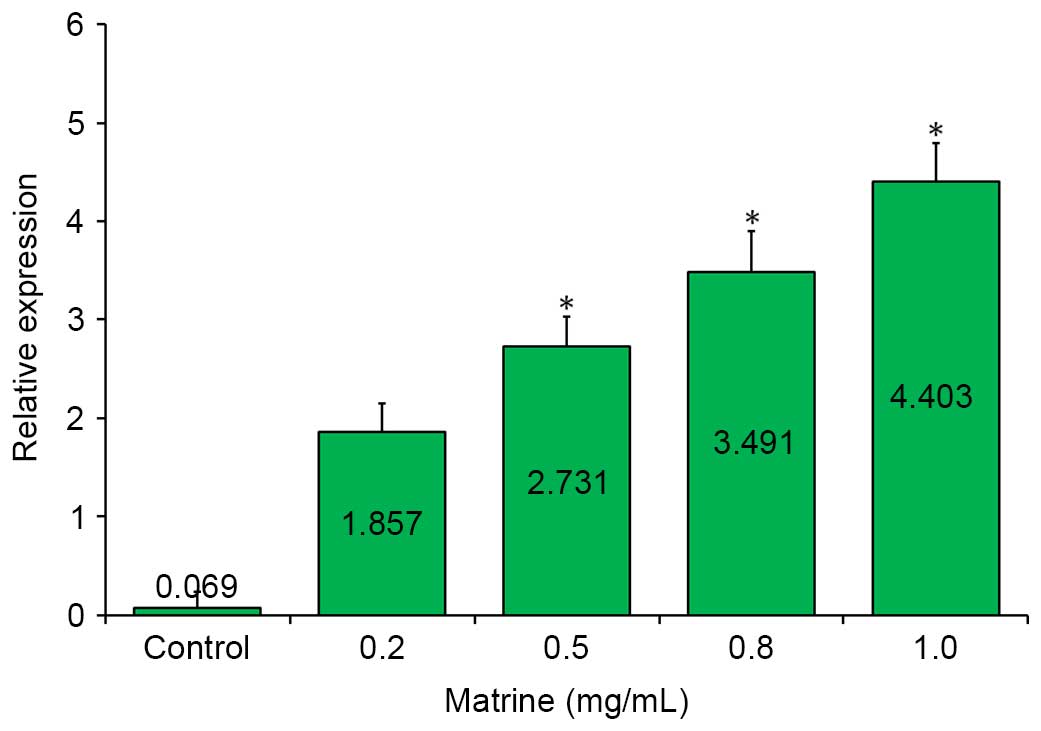
miR-126 is upregulated in
matrine-treated cells
To examine the correlation between matrine treatment
and the expression of miR-126, the relative expression of
miR-126 was detected among the samples by RT-qPCR analysis.
As shown in Fig. 3, a markedly
upregulated expression of miR-126 was observed in the
matrine-treated A549 cells, compared with the control in the
RT-qPCR assay. This upregulation was further enhanced by increasing
the concentration of matrine, and reached its peak (~4.4-fold),
when the matrine concentration was increased to 1.0 mg/ml.
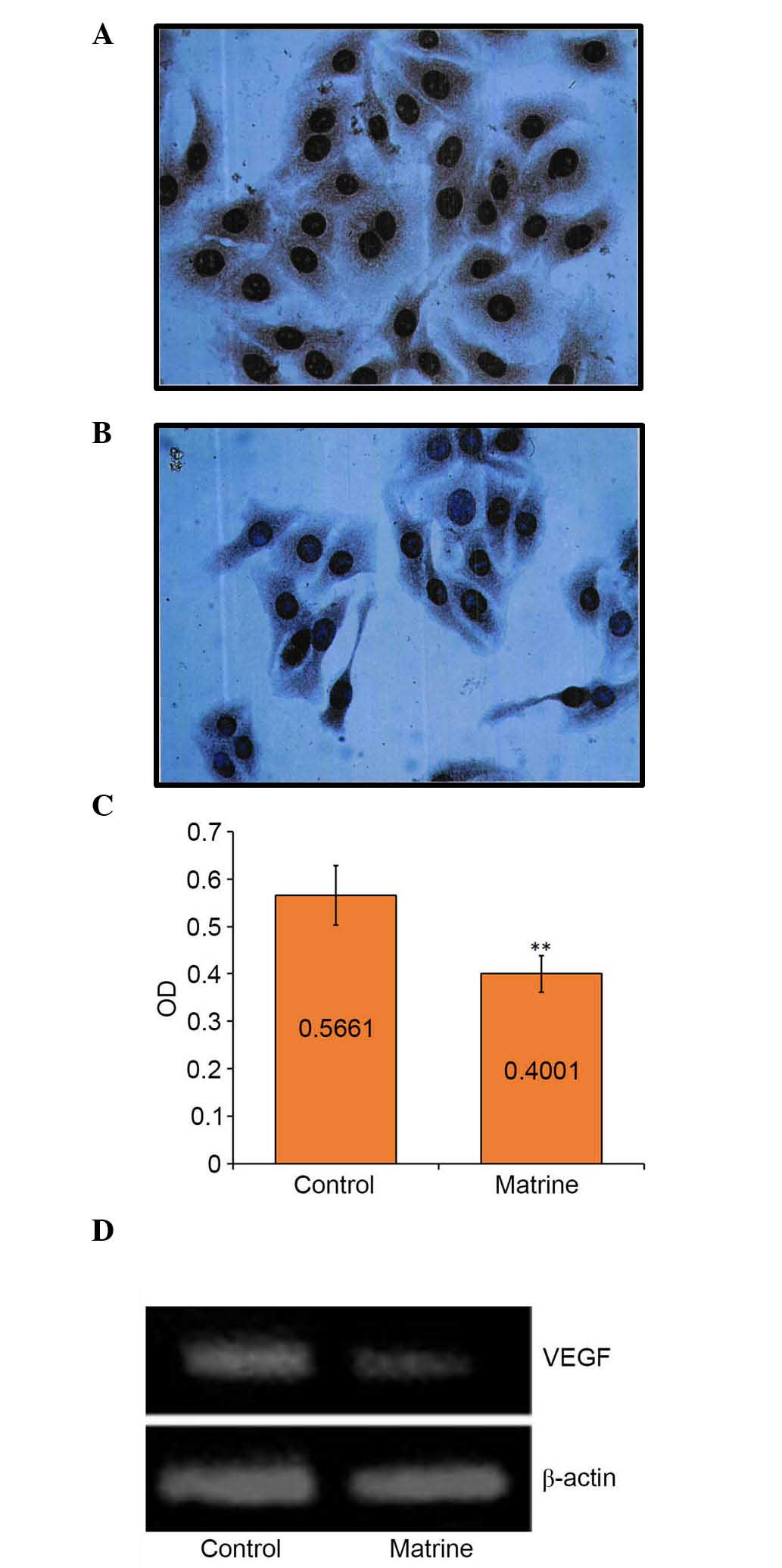
Downregulation of the miR-126 target,
VEGF, is induced by matrine treatment
IHC staining showed that almost all the cell nuclei
and brown cytoplasmic particles were sepia-colored following
treatment with matrine at a low concentration (0.2 mg/ml) for 48 h
(Fig. 4A and B). The mean optical
density (OD) value of each group indicated the expression level of
the VEGF gene. The mean OD of the control group was
0.5661±0.0298;, whereas that of the matrine-treated group was
0.4001±0.0766. The mean OD of the matrine-treated group was
significantly (P<0.01) higher, compared with that of the control
group (Fig. 4C), suggesting that
the low concentration of matrine was sufficient to inhibit the gene
expression of VEGF. These results were also confirmed using
western blot analysis (Fig. 4D).
The band of the control cells was brighter (~1.5-fold), compared
with that of the matrine-treated cells (Fig. 4D).
Discussion
Although traditional chemotherapy remains the
primary method of cancer therapy, cancer cells often develop drug
resistance, which significantly reduces the efficiency of
chemotherapeutic treatment. Furthermore, due to the poor prognosis
associated with certain types of lung cancer and limited treatment
options besides surgery, patients may seek alternative treatments,
including TCMs, alone or in combination with standard care
(28). In previous years, the
potential of natural products from the medicinal plants used in TCM
has been recognized by the scientific community in Western
countries. Several natural products and derivatives thereof belong
to the standard repertoire of cancer chemotherapeutic agents
(29). Novel TCM-derived
anticancer drugs include arsenic trioxide, camptothecin,
cantharidin, homoharringtonine, podophyllotoxin, vinblastine and
vincristine (27). There is
evidence that these anticancer TCMs function as inducers of
apoptosis to inhibit cell growth through genes involved in
regulating cell proliferation, angiogenesis or apoptosis (30,31),
and immune-enhancers to improve immunological function or
resistance against tumors and viruses (32).
The results of the present study suggested that
matrine was important in suppressing tumor cell growth in the A549
human NSCLC cell line. Matrine decreased the survival of A549 cells
in a dose-dependent manner. The present study found that a low
concentration (0.2 mg/ml) of matrine was sufficient to inhibit A549
cell growth; this cell growth arrest was concentration-dependent,
with an elevated inhibitory effect observed when the concentration
of matrine increased (Fig. 1A).
However, no significant changes in cell morphology were observed at
a low concentration (0.2 mg/ml) until the concentration increased
to 1.0 mg/ml (Fig. 2). In addition
to cell growth arrest, A549 cell apoptosis was induced following
matrine treatment for 48 h, as indicated by changes in the cell
cycle, compared with the untreated control, detected using FCM
(Fig. 2). Significant (P<0.05)
decreases in the proportion of cells in the S and G2/M phases,
followed by an increase in proportion of cells in the G1/G0 phase
were observed following treatment with matrine at low and high
concentrations (0.2 and 1.0 mg/ml), which was in agreement with the
MTT assay (Table I and Fig. 1A). These results were similar to
the previously reported inhibitory effect of matrine on murine H22
cell proliferation (12). In
contrast to the concentration-dependent effect of matrine on the
murine H22 cells, in which inhibition occurred following treatment
with 0.5 mg/ml matrine for 48 h, treatment with a low concentration
of matrine (0.2 mg/ml) for 48 h was sufficient to induce apoptosis
of the A549 NSCLC cells in the present study. These results
indicated that a low concentration of matrine inhibited A549 cell
proliferation by retarding cell growth to prolong the cell
cycle.
The proportion of cell apoptosis in the present
study was low (Table I),
indicating that the inhibitory effect of matrine on the A549 cells
was predominantly caused by cell cycle retardation, comprising cell
growth extension and decelerated proliferation, rather than cell
apoptosis. The increase of cells in the G1/G0 phase, and decrease
of cells in the S and G2/M phases suggested that the inhibitory
effect of matrine on A549 proliferation was predominantly
attributed to cell cycle arrest at the G1 phase. However, further
investigations are required to confirm these results and to
investigate the underlying mechanism.
miR-126 is often downregulated in
NSCLC-derived cell lines (24).
Using RT-qPCR analysis, the expression of miR-126 was
determined in the present study. Compared with the untreated A549
control cells, a dose-dependent upregulation of miR-126 was
observed in the matrine-treated groups (Fig. 3) suggesting a correlation between
matrine and miR-126 in the A549 cells. This was supported by
the downregulation of VEGF, a target of miR-126
(24) observed using western blot
analysis (Fig. 4D). A low
concentration of matrine (0.2 mg/ml) inhibited the expression of
VEGF, which was ~1.5-fold lower, compared with that in the
control group, and was inversely correlated with the expression of
miR-126, by ~1.857-fold (Fig.
3). These results indicated that the antitumor TCM matrine
induced cell cycle arrest and apoptosis, and recovery of the
expression of miR-126 in the A549 NSCLC cell line.
References
|
1
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: Non-small cell lung cancer. J Natl Compr Canc
Netw. 10:1236–1271. 2012.PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biesalski HK, de Mesquita B Bueno, Chesson
A, Chytil F, Grimble R, Hermus RJ, Köhrle J, Lotan R, Norpoth K,
Pastorino U and Thurnham D: European consensus statement on lung
cancer: Risk factors and prevention. Lung cancer panel. CA Cancer J
Clin. 48:167–176; discussion 164–166. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Secretan B, Straif K, Baan R, Grosse Y, El
Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part E: Tobacco,
areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol.
10:1033–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martínez-Sánchez JM, Fernández E, Fu M,
Gallus S, Martínez C, Sureda X, La Vecchia C and Clancy L: Smoking
behaviour, involuntary smoking, attitudes towards smoke-free
legislations, and tobacco control activities in the European Union.
PLoS One. 5:e138812010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fossella FV, DeVore R, Kerr RN, Crawford
J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al:
TAX Non-Small-Cell Lung Cancer Study Group: Randomized phase III
trial of docetaxel versus vinorelbine or ifosfamide in patients
with advanced non-small-cell lung cancer previously treated with
platinum-containing chemotherapy regimens. JCO. 18:2354–2362.
2000.
|
|
7
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avila MA, Berasain C, Sangro B and Prieto
J: New therapies for hepatocellular carcinoma. Oncogene.
25:3866–3884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taron M, Rosell R, Felip E, Mendez P,
Souglakos J, Ronco MS, Queralt C, Majo J, Sanchez JM, Sanchez JJ
and Maestre J: BRCA1 mRNA expression levels as an indicator of
chemoresistance in lung cancer. Hum Mol Genet. 13:2443–2449. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan Z, Jikai J, Xiaoshan L, et al:
Differentiation and apoptosis in K562 erythroleukemia cells induced
by matrine. Natural Med. 52:295–299. 1998.
|
|
14
|
Li CQ, Zhu YT, Zhang FX, Fu LC, Li XH,
Cheng Y and Li XY: Anti-HBV effect of liposome-encapsulated matrine
in vitro and in vivo. World J Gastroenterol. 11:426–428. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Zhu M, Shi R and Yang M: Radix
Sophorae flavescentis for chronic hepatitis B: A systematic review
of randomized trials. Am J Chin Med. 31:337–354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JP, Zhang M, Zhou JP, Liu FT, Zhou
B, Xie WF and Guo C: Antifibrotic effects of matrine on in vitro
and in vivo models of liver fibrosis in rats. Acta Pharmacol Sin.
22:183–186. 2001.PubMed/NCBI
|
|
17
|
Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu
XS, Xu XR, Liu BZ and He YJ: Effects of matrine on proliferation
and differentiation in K-562 cells. Leuk Res. 25:793–800. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang YQ, Huang GS, Wang Z, Guo Y and
Zhang HY: Effects of matrine on the relative molecules expression
of proliferation and apoptosis in K562 cells. Zhongguo Yi Xue Ke
Xue Yuan Xue Bao. 23:333–336. 2001.(In Chinese). PubMed/NCBI
|
|
19
|
Wang Y, Peng C, Zhang G, Liu Y, Li H and
Shan J: Study on invasion and metastasis related factors in
differentiation of SMMC-7721 cells induced by matrine. Zhong Yao
Cai. 26:566–569. 2003.(In Chinese). PubMed/NCBI
|
|
20
|
Han Y, Zhang S, Wu J, Yu K, Zhang Y, Yin L
and Bi L: Matrine induces apoptosis of human multiple myeloma cells
via activation of the mitochondrial pathway. Leuk Lymphoma.
51:1337–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo C, Zhu Y, Jiang T, Lu X, Zhang W, Jing
Q, Li J, Pang L, Chen K, Qiu F, et al: Matrine induced gastric
cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules
of Bcl-2 family. Toxicology. 229:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Wang T, Wen X, Wei Y, Peng X, Li
H and Wei L: Effect of matrine on HeLa cell adhesion and migration.
Eur J Pharmacol. 563:69–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou G, Li N, Ma Y-H, Chen X-B, Wang X-F
and Luo S-X: Expression of Survivin and P-glycoprotein in non-small
cell lung cancer and their relationship with the effectiveness of
Matrine and pacilitaxel. Chin J Cancer Prev Treat. 20:350–353.
2013.
|
|
24
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Janmaat ML, Kruyt FA, Rodriguez JA and
Giaccone G: Response to epidermal growth factor receptor inhibitors
in non-small cell lung cancer cells: Limited antiproliferative
effects and absence of apoptosis associated with persistent
activity of extracellular signal-regulated kinase or Akt kinase
pathways. Clin Cancer Res. 9:2316–2326. 2003.PubMed/NCBI
|
|
26
|
Danielsen T, Hvidsten M, Stokke T, Solberg
K and Rofstad EK: Hypoxia induces p53 accumulation in the S-phase
and accumulation of hypophosphorylated retinoblastoma protein in
all cell cycle phases of human melanoma cells. Br J Cancer.
78:1547–1558. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeuchi K, Choi YL, Togashi Y, Soda M,
Hatano S, Inamura K, Takada S, Ueno T, Yamashita Y, Satoh Y, et al:
KIF5B-ALK, a novel fusion oncokinase identified by an
immunohistochemistry-based diagnostic system for ALK-positive lung
cancer. Clin Cancer Res. 15:3143–3149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu P, Dugoua JJ, Eyawo O and Mills EJ:
Traditional Chinese medicines in the treatment of hepatocellular
cancers: A systematic review and meta-analysis. J Exp Clin Cancer
Res. 28:1122009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
31
|
Efferth T, Sauerbrey A, Olbrich A, Gebhart
E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, et
al: Molecular modes of action of artesunate in tumor cell lines.
Mol Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ooi LS, Wang H, Luk CW and Ooi VE:
Anticancer and antiviral activities of Youngia japonica (L.) DC
(Asteraceae, Compositae). J Ethnopharmacol. 94:117–122. 2004.
View Article : Google Scholar : PubMed/NCBI
|