Introduction
Hepatitis C virus (HCV) infection is one of the
primary causes of chronic liver disease worldwide (1). It is estimated that ~3% of the
world's population is chronically infected with HCV (2), although the frequency of infection
varies between populations and geographic regions (3). In Western Europe, the prevalence of
HCV ranges between 0.4 and 3%. In Eastern Europe and the Middle
East, the prevalence is higher, although the exact prevalence
remains to be fully elucidated (4). The highest worldwide prevalence is in
Egypt, which has a national prevalence of 9% and a prevalence of up
to 50% in rural areas (5).
HCV is transmitted parenterally. Prior to the 1990s,
the principal routes of HCV transmission were blood transfusion,
intravenous drug use and unsafe injection procedures (6). In industrialized countries, these
modes of transmission account for ~70% of HCV infections (6). Since 1992, screening of blood donors
has essentially eradicated the transmission of HCV by transfusion.
Currently, new HCV infections are caused by intravenous or nasal
drug use and, to a lesser degree, unsafe medical or surgical
procedures (7). Sexual
transmission of HCV among men who have intercourse with men was
also a major route of transmission, and data indicates that
promiscuous male homosexual activity is associated with HCV
infection (8).
China has a population of 1,300,000,000; the
frequency of HCV infection has been reported to be 3.2% nationally
and 3.1% in rural areas (9,10).
However, the prevalence of HCV infection differs between regions
(11). The transmission of HCV via
contaminated blood was previously a serious issue in China. Between
1994 and 1996, a plasma campaign in Henan province resulted in the
infection of 500,000 blood donors with HCV and the infection of
300,000 donors with human immunodeficiency virus (12–15).
Similar experiences were also reported in other provinces of China
(14).
At present in China (since 1992), the principal
routes of transmission for newly diagnosed HCV infections are blood
transfusions, surgical procedures and intravenous drug use
(16,17).
Guangdong is a province on the South China Sea coast
of China. A previous study from a medical center in Guangdong
province sampled 393 patients with chronic hepatitis C and showed
that the predominant HCV subtypes were subtypes 1b, 6a, 2a, 3a and
3b, which accounted for 65.9, 17.1, 7.4, 3.6 and 3.3% of cases,
respectively (18).
Zijin County is located in the north of Guangdong
province and has a population of ~800,000. The present study
focussed on a previous outbreak in which dozens of cases of HCV
infection were newly diagnosed in the same local street. It did not
appear that the principle routes of HCV transmission, including
blood transfusion or intravenous drug use, were responsible for
this outbreak. To determine the transmission routes of this
specific outbreak, the present study conducted an emergency survey
of the region.
Materials and methods
Study design
In late February 2012, an emergency survey was
conducted investigate the HCV epidemic of Xiangshui Road in Zijin
County, Heyuan. A questionnaire regarding lifetime risk factors for
HCV infection was administered to all local residents. Blood
samples were obtained from all respondents (n=736) and the serum
was separated from the samples by centrifugation (2,200 × g,
10 min, 4°C). Healthcare workers from a recently closed local
medical clinic were interviewed regarding procedures at the clinic.
Informed consent was obtained from all participants. The present
study was performed in accordance with the 1964 Declaration of
Helsinki and later amendments. The protocol was approved by the
ethics committee of the Third Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China).
Analysis of serum samples
Determination of HCV infection
An HCV enzyme immunoassay (HCV EIA 3.0; Abbott GmbH,
Wiesbaden, Germany) was used to detect anti-HCV antibodies in the
serum samples. Individuals positive for anti-HCV antibodies were
further assessed for the presence of HCV RNA using the COBAS
AMPLICOR HCV Monitor 2.0 assay (Roche Diagnostics, Branchburg, NJ,
USA). If the result was negative, a second serum sample was
acquired from the respondents for analysis 1 month following the
initial sample analysis.
Sequencing of HCV RNA
The HCV RNA was extracted from serum samples
identified as positive for HCV RNA. HCV RNA sequencing was
performed as described previously (19). In brief, the HCV RNA was extracted
from the serum samples using the RNAiso™ Plus extraction kit
(Takara Biotechnologoy Co., Ltd., Dalian, China). The HCV RNA was
then reverse transcribed into cDNA using the ReverTra Ace-α-reverse
transcription kit (Toyobo, Shanghai, China), according to the
manufacturer's protocol. The core and nonstructural protein 5B
(NS5B) regions of the HCV were amplified using a nested polymerase
chain reaction. The core outer primers were as follows: Forward,
5′-ACTGCCTGATAGGGTGCTTGC-3′ and reverse,
5′-ATGTACCCCATGAGGTCGGC-3′; the inner primers were:
Forward,5′-AGGTCTCGTAGACCGTGCA-3′ and reverse,
5′-CATGTGAGGGTATCGATGAC-3′. The NS5B outer degenerate primers were:
Forward, 5′-CCACATCMRCTCCGTGTGTGG-3′ and reverse,
5′-GGRGCDGARTACCTRGTCAT-3′; the inner degenerate primers were:
Forward, 5′-ACMCCAATWSMCACBACCATCATG-3′ and reverse,
5′-TACCTGGTCATAGCCTCCGTGA-3′. PCR was conducted using the Takara
Taq™ PCR kit (Takara Biotechnologoy Co., Ltd.). The outer
PCR system (30 µl) consisted of: 3 µl 10X PCR buffer, 2 µl 2.5 mM
dNTP, 17.6 µl dH2O, 1.5 µl of each primer (10 pmol/µl),
0.4 µl Taq enzyme (2.5 U/µl) and 4 µl template cDNA. Inner
PCR system (30 µl) consisted of: 3 µl 10X PCR buffer, 2 µl 2.5 mM
dNTP, 19.6 µl dH2O, 1.5 µl of each primer (10 pmol/µl),
0.4 µl Taq enzyme (2.5 U/µl) and 2 µl template cDNA. PCR
conditions were as follows: 94°C for 5 min; followed by 30 cycles
at 94°C for 30 sec, 55°C for 1 min and 72°C for 40 sec; and a final
step at 72°C for 10 min. DNA was sequenced in both directions using
an ABI Prism 3,730 genetic analyzer (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The experiment was
performed twice, at an interval of 2 months, to confirm the
results.
Phylogenetic analysis of core and NS5B
alignments
The sequences of the HCV strains were aligned using
the ClustalW 1.8 software package (20), with a reference panel of sequences
representative of each subtype (21) retrieved from the HCV database
(http://hcv.lanl.gov/content/index).
Prior to phylogenetic analysis, the jModeltest program (22) was used to determine the optimal
substitution model based on the Akaike information criterion
(23). The results indicated that
K2+G was the optimal model for the Core-E1 and NS5B datasets. Under
this model, maximum-likelihood trees were estimated using the
subtree pruning and regrafting and nearest neighbor interchange
algorithms in PhyML (24).
Bootstrap support values (1,000 repetitions) of >70% were
accepted as defining clusters.
Statistical analysis
Differences between groups were examined using a
χ2 test. To identify the factors significantly
associated with HCV infection, univariate logistic regression
analysis was performed, and the variables with P<0.15 in
univariable analysis were selected for multiple binary logistic
regression analyses with backwards elimination forced in the final
model. Statistical analysis was performed using SPSS version 19.0
(IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
HCV infection and risk factors
A total of 736 residents from Xiangshui Road, Zijin,
Guangdong, were included in the survey. The included residents had
an average age of 40.3±19.6 years old (range, 4–90 years old) and
51.4% were men (378/736 respondents). Of the 736 residents included
in the survey, 50.8% were positive for the anti-HCV antibody. The
characteristics of the HCV-positive and HCV-negative survey
respondents are shown in Table
I.
 | Table I.Univariate and multivariate logistic
regression analyses of risk factors associated with HCV
infection. |
Table I.
Univariate and multivariate logistic
regression analyses of risk factors associated with HCV
infection.
| Characteristics and
risk factors | HCV positive
(n=374) | HCV negative
(n=362) | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Male | 182 (48.7%) | 193 (53.3%) | 0.83 (0.62–1.11) | 0.207 |
|
|
| Age (≥40 years
old) | 205 (54.8%) | 184 (50.8%) | 1.17 (0.88–1.57) | 0.279 | 1.37 (0.94–1.99) | 0.100 |
| HBsAg | 28 (7.5%) | 29 (8.0%) | 0.93 (0.54–1.60) | 0.790 |
|
|
| Blood donor | 19 (5.1%) | 23 (6.4%) | 0.79 (0.42–1.48) | 0.457 |
|
|
| Blood
transfusion | 22 (5.9%) | 12 (3.3%) | 1.82
(0.89–3.7) | 0.097 | 9.14
(4.04–20.67) | <0.001 |
| Blood product | 45 (12.0%) | 27 (7.5%) | 1.70
(1.03–2.80) | 0.037 | 2.99
(1.54–5.78) | 0.001 |
| Drug use
(injection) | 14 (3.7%) | 7 (1.9%) | 1.97
(0.79–4.94) | 0.140 | 14.98
(5.52–40.64) | <0.001 |
| Surgery
history | 28 (7.5%) | 34 (9.4%) | 0.78
(0.46–1.32) | 0.352 |
|
|
| Intravenous
injectiona | 303 (81.0%) | 93 (25.7%) | 12.34
(8.70–17.51) | <0.001 | 20.63
(13.64–31.21) | <0.001 |
| Visited a
dentist | 36 (9.6%) | 32 (8.8%) | 1.10
(0.67–1.81) | 0.713 |
|
|
| Family
infectionb | 98 (26.2%) | 55 (15.2%) | 1.98
(1.37–2.86) | <0.001 |
|
|
| Sexual
historyc | 14 (3.7%) | 11 (3.0%) | 1.24
(0.56–2.77) | 0.598 |
|
|
Using univariate logistic regression analysis, the
exposure of an individual to a number of risk factors, including
blood product transfusion, intravenous injection at a local clinic
and ≥2 infected family members, were significantly associated with
the risk of HCV infection. Gender, age, HBV surface antigen
(HBsAg), blood donor history, blood transfusion history,
intravenous drug use, surgery history, dental visit history and
number of sexual partners were not significantly associated with
the risk of HCV infection. Of the infected patients, 86.6%
(324/374) had multiple risk factors, and 86.7% (85/98) of the
individuals with ≥2 family members infected with HCV had a history
of intravenous injection at a local clinic. To further examine the
association between risk factors and HCV infection, multivariate
logistic regression analysis was performed (Table II). Following adjustment for blood
transfusions, blood product transfusion, intravenous drug use and
intravenous injection, the number of family members infected with
HCV was not a significant risk factor for HCV infection.
 | Table II.Univariate and multivariate logistic
regression analyses of risk factors associated with a positive HCV
result. |
Table II.
Univariate and multivariate logistic
regression analyses of risk factors associated with a positive HCV
result.
| Characteristics and
risk factors | HCV RNA (+) (n=264)
n (%) | HCV RNA (−) (n=110)
n (%) | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Male | 126 (47.7) | 56 (50.9) | 0.88
(0.56–1.37) | 0.575 |
|
|
| Age (≥40 years
old) | 156 (59.1) | 44 (40.0) | 3.42
(2.07–5.65) | <0.001 | 2.24
(1.38–3.64) | 0.001 |
| HBsAg | 20 (7.6) | 8 (7.3) | 1.05
(0.45–2.45) | 0.919 |
|
|
| Blood
transfusion | 15 (5.7) | 7 (6.4) | 0.89
(0.35–2.24) | 0.798 | 0.89
(0.30–2.61) | 0.832 |
| Blood product | 29 (11.0) | 16 (14.5) | 0.73
(0.38–1.40) | 0.335 | 0.68
(0.35–1.34) | 0.265 |
| Drug use
(injection) | 9 (3.4) | 8 (7.3) | 0.45
(0.17–1.20) | 0.102 | 0.64
(0.173–2.37) | 0.504 |
| Intravenous
injectiona | 225 (85.2) | 78 (70.9) | 2.37
(1.39–4.04) | 0.001 | 1.64
(0.80–3.37) | 0.177 |
A total of 53.8% (396/736) of the survey respondents
indicated a history of visiting a local clinic where they had
received intravenous injection. The clinic had closed 3 months
prior to the initiation of the survey. When the healthcare workers
from the closed clinic were interviewed, they stated that they had
reused glass syringes for intravenous injections and that equipment
was cleaned through scalding. Following each injection, the needle
and syringe were washed by filling it with clean water several
times and shaking vigorously for 30 sec. This cleaning process was
repeated twice. The syringe was later detached from the needle and
boiled for 15 min with 2% sodium carbonate for 15 min. However,
this procedure was not always observed by the survey respondents,
particularly when there were numerous patients attending the
clinic.
Positivity for HCV RNA
The 374 respondents who were positive for anti-HCV
antibodies were examined for the presence of HCV RNA using reverse
transcription-polymerase chain reaction analysis. The results
showed that 264 (70.5%) were positive for HCV RNA.
In the HCV RNA-positive survey respondents, only age
was significantly associated with the risk of being HCV RNA
positive (odds ratio, 2.22; 95% confidence interval, 1.41–3.50;
P<0.001). In the multivariate logistic regression analysis,
having ≥2 sexual partners, or exposure to intravenous injection,
intravenous drug use, blood product transfusion or blood
transfusion were not found to be significantly associated with the
risk of a positive HCV RNA result.
HCV subtype and phylogenetic
grouping
RNA was extracted from the serum of the survey
respondents identified as positive for the presence of HCV RNA (264
samples). Core and NS5B sequences were amplified successfully in
237 and 222 cases, respectively. Core and NS5B sequences were
obtained in 213 respondents, core sequences were obtained in 24
respondents and NS5B sequences were obtained in nine respondents.
Together, the amplification of either or both sequence regions was
successful in 246 cases.
A total of four HCV subtypes were identified in the
HCV RNA-positive samples: Subtype 1b in nine (3.7%) cases, subtype
2a in 84 (34.1%) cases, subtype 3b in two (0.8%) cases and subtype
6a in 151 (61.4%) cases. The genotypes determined by the core
sequences were consistent with those determined by NS5B
sequences.
HCV subtypes 2a and 6a were further analyzed through
the generation of NS5B trees, since these subtypes accounted for
95.5% of genotypes confirmed. The NS5B sequences of subtype 2a were
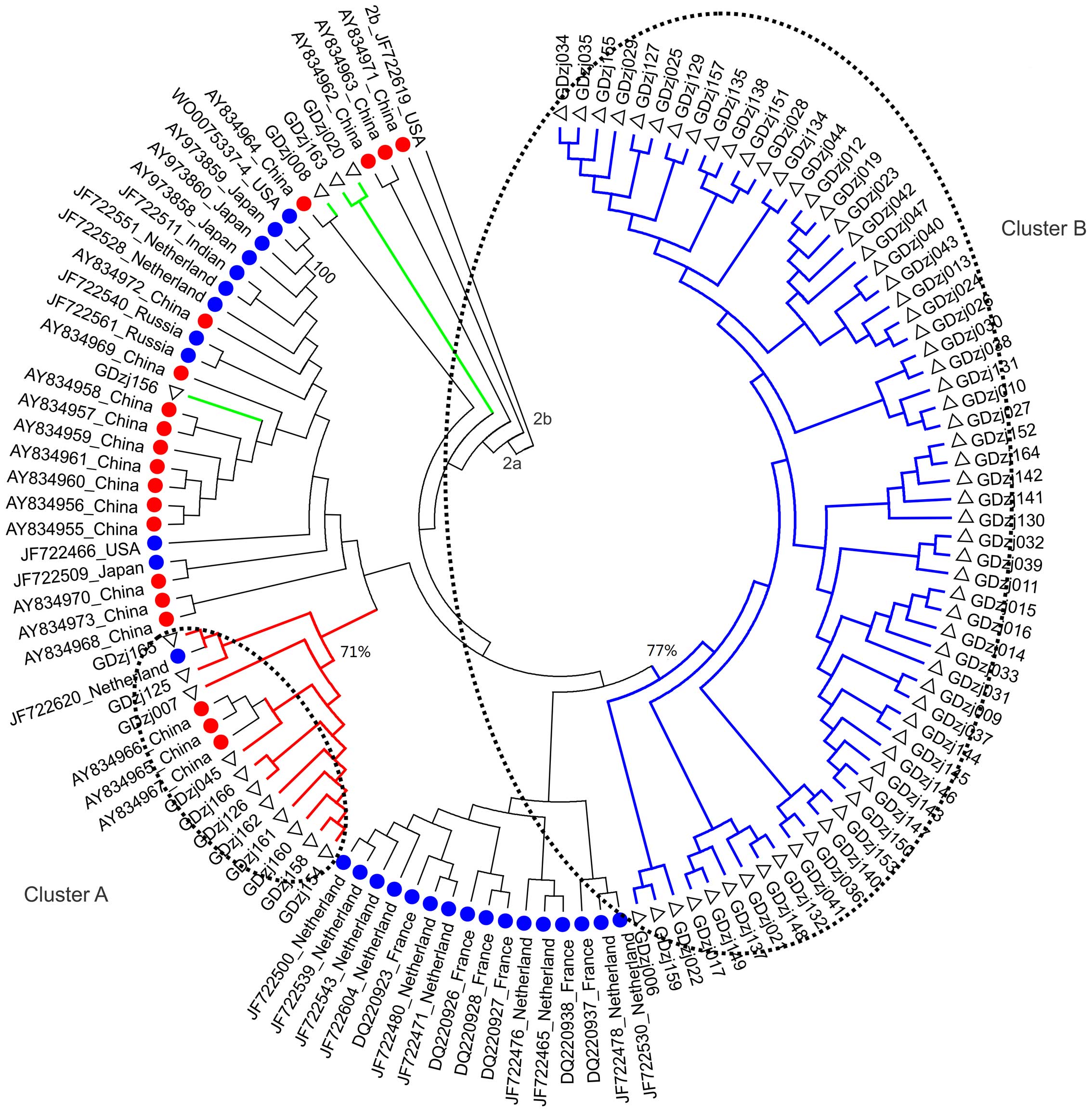
grouped into clusters A and B, which contained the sequences of 11
and 63 samples, respectively (Fig.
1). The bootstrap scores of clusters A and B were 71 and 77%,
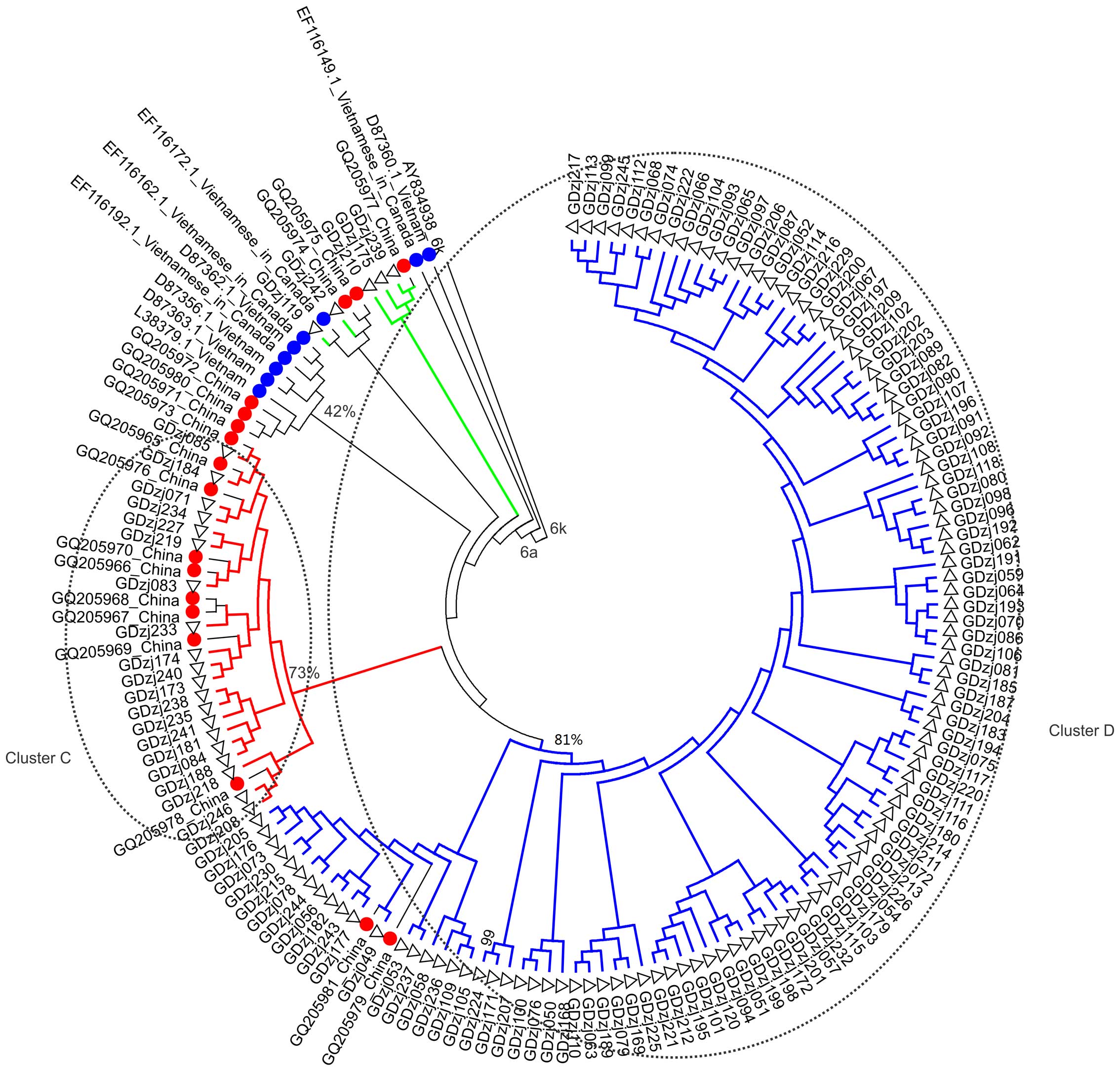
respectively. The NS5B sequences of subtype 6a were grouped into
cluster C and D, which contained the sequences of 20 and 112
samples, respectively (Fig. 2).
The bootstrap scores of cluster C and D were 73 and 81%,
respectively. The patients exposed to intravenous injection were
more likely to distribute in clusters A, B, C and D, compared with
those exposed to other risk factors (179/186, vs. 23/36;
P<0.001; Table III).
 | Table III.Hepatitis C virus subtype
distribution by risk factor. |
Table III.
Hepatitis C virus subtype
distribution by risk factor.
|
| Subtype 2a | Subtype 6a |
|
|---|
|
|
|
|
|
|---|
| Risk factor | Cluster A | Cluster B | Cluster C | Cluster D | Remaining
group |
|---|
| Other risk
factorsa | 1 | 4 | 2 | 6 | 1 |
| Blood
transfusion | 0 | 2 | 1 | 1 | 7 |
| Blood product
transfusion | 0 | 1 | 1 | 1 | 3 |
| Drug use
(injection) | 0 | 0 | 2 | 1 | 2 |
| Intravenous
injectionb | 9 | 55 | 13 | 102 | 7 |
| Total | 10 | 62 | 19 | 111 | 20 |
Discussion
An outbreak of HCV infection was identified on a
single road, Xiangshui Road, Zijin, Guangdong. Of the 736
respondents of the survey distributed to local residents, 50.8%
were HCV-seropositive. Analysis of the risk factors for HCV
infection among the survey respondents found that blood
transfusion, intravenous drug use and intravenous injection at a
local clinic were associated with HCV infection. Phylogenetic
analysis of HCV NS5B sequences identified four clusters, suggesting
a common mode of transmission, with the majority of patients
reporting injection at a local clinic. The present study
hypothesized that transmission may have occurred through unsafe
injection practices.
In the present study, blood donation, surgery,
dental visits, and having ≥2 sexual partners were not significantly
associated with HCV infection. In China, paid blood donation has
been banned since 1998, and HCV infection rates in blood donors
have decreased from 12.87 to 1.71% (25,26).
HCV screening prior to surgery has been routine practice since
1992, thus transmission of HCV via surgery or dental care is well
controlled. It is known that sexual transmission of HCV accounts
for <5% of HCV infections (27), as HCV is rarely present in semen or
vaginal fluid. The risk of HCV infection for an individual who is
in an unprotected sexual relationship with an HCV-infected
individual for 20 years is 2.5% (28). Although it has been reported that
the prevalence of HCV infection in the intravenous drug use
population was 61.4% in China (9),
and the infection rate was substantially higher in southwestern
China, only a marginal proportion of the survey respondents had a
history of intravenous drug use (21/736). These results suggested
that blood donation, surgery, dental visits and having ≥2 sexual
partners were not the primary routes of HCV transmission in the
individuals in the present study.
The spontaneous clearance of HCV occurs in 15–30% of
cases of acute HCV (29) and is
associated with interleukin 28B gene variation (30,31).
The frequency of the rs12979860 C allele, which contributes to
viral clearance in the Chinese Han population ranges between 74 and
98% (32). Thus, it may be that
70.5% of the anti-HCV antibody-positive respondents who were HCV
RNA-positive in the present study was a result of spontaneous
clearance.
HCV has been classified into seven genotypes and 82
subtypes (http://talk.ictvonline.org/ictv_wikis/w/sg_flavi/35.table-1-confirmed-hcv-genotypessubtypes-november-2014.aspx).
Genotypes differ from each other by 31–33% at the nucleotide level,
whereas subtypes differ by 20–25% (33). Despite the genetic diversity of
HCV, all genotypes share collinearity in the large open reading
frame, and the genetic associations of HCV variants consistent
throughout the genome (33).
Through genotyping of the HCV core and NS5B sequences of the
infected patients, the present study showed that the genotypes
determined by core sequences were consistent with those determined
by NS5B sequences.
HCV genotypes have varied geographic distribution
patterns. HCV subtypes 1a, 1b, 2a, 2b and 3a are distributed
globally, whereas all other subtypes are restricted predominantly
to certain geographic regions. Genotype 6 and its subtypes are
found predominantly in Southeast Asia (34). Studies in Southern China have
reported that HCV-6a accounts for 49.7% of cases detected in blood
donors and 17.1% in patients with chronic HCV infection, and its
overall proportion is increasing (35,36).
In Guangdong, the predominant HCV subtypes in patients with chrnoic
HCV are subtypes 1b, 6a, 2a, 3a and 3b (18). However, the genotypic distribution
in the present study, which predominantly consisted of genotype
subtypes 2a and 6a, differed from previous reports in South China
(18,35). This genotypic distribution may have
resulted from a specific transmission pattern.
It has been shown that different genotypes are
transmitted by different routes. For example, blood transfusion and
surgery are more common risk factors for HCV infection by genotypes
1 and 2. By contrast, blood transfusion and surgery are less common
risk factors for infection by genotypes 3 or 6 and
lifestyle-associated risk factors, including intravenous drug use,
tattoos and piercings, are more common risk factors (32). The subtypes found in the present
study were clustered into four homologous groups, and the majority
of patients who reported a history of intravenous injection at a
local clinic were allocated into these four homologous clusters.
The consistency between the epidemiological history and the results
of the phylogenetic analysis may indicate a causal association.
As the clinic had closed, it was not possible to
confirm intravenous procedures by the healthcare workers. From
interviews with former employees of the clinic, it was revealed
that intravenous injections were performed using reused glass
syringes. As HCV is able to survive outside the body for at least 4
days and the virus is able to survive for weeks in blood collected
inside a needle or syringe (6),
the reuse of glass syringes at this clinic increased the risk of
HCV transmission. The reuse of glass syringes has been reported in
other provinces of China. In a survey examining esophageal cancer
in Anyang (Henan, China) between 2006 and 2008, it was shown that
intravenous injection with reusable glass syringes and needles was
the primary risk factor for HCV infection (37). Similarly, in Maqiao (Henan, China),
86 HCV infections were found to be caused by intravenous injections
with reusable glass syringes at a local clinic (38). Therefore, the outbreak of HCV
infections in the present study may have been caused by intravenous
injection in the local clinic where glass syringes were reused.
The present study showed the epidemiological
characteristic of the outbreak of HCV in Zijin County. The presence
of the HCV genotype 6a in the patients reflected the problem of the
increasing spread of HCV genotype 6a in South China. Although the
available evidence is not of sufficient quality to confirm
intravenous injection in a local clinic as the causal factor, the
present study confirmed the serious health problem associated with
reusing contaminated syringes. The present study was limited by the
inability to perform the investigation prior to closure of the
local clinic to demonstrate the cause of the outbreak. All surveys
were performed retrospectively in respondents and the recall bias
was unavoidable.
Acknowledgements
This study was supported by funding from the
National Science and Technology Major Project (grant no.
2012ZX10002003) and the Sun Yat-Sen University Clinical Research
5010 Program (grant no. 2010011).
References
|
1
|
Lavanchy D: The global burden of hepatitis
C. Liver Int. 29:(Suppl 1). S74–S81. 2009. View Article : Google Scholar
|
|
2
|
Shepard CW, Finelli L and Alter MJ: Global
epidemiology of hepatitis C virus infection. Lancet Infect Dis.
5:558–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sievert W, Altraif I, Razavi HA, Abdo A,
Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, et
al: A systematic review of hepatitis C virus epidemiology in Asia,
Australia and Egypt. Liver Int. 31:(Suppl 2). S61–S80. 2011.
View Article : Google Scholar
|
|
4
|
Esteban JI, Sauleda S and Quer J: The
changing epidemiology of hepatitis C virus infection in Europe. J
Hepatol. 48:148–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamal SM and Nasser IA: Hepatitis C
genotype 4: What we know and what we don't yet know. Hepatology.
47:1371–1383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
European Association for Study of Liver, .
EASL Clinical Practice Guidelines: Management of hepatitis C virus
infection. J Hepatol. 60:392–420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alter MJ: HCV routes of transmission: What
goes around comes around. Semin Liver Dis. 31:340–346. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van de Laar TJ, Matthews GV, Prins M and
Danta M: Acute hepatitis C in HIV-infected men who have sex with
men: An emerging sexually transmitted infection. AIDS.
24:1799–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo-Liang Xia, Chong-Bai Liu, Hui-Lin Cao,
Sheng-Li Bi, Mei-Yun Zhan, Chong-Ao Su, Jun-Hua Nan and Xiao-Qui
Qi: Prevalence of hepatitis B and C virus infections in the general
Chinese population. Results from a nationwide cross-sectional
seroepidemiologic study of hepatitis A, B, C, D and E virus
infections in China, 1992. International Hepatology Communications.
5:62–73. 1996. View Article : Google Scholar
|
|
10
|
Shimbo S, Zhang ZW, Gao WP, Hu ZH, Qu JB,
Watanabe T, Nakatsuka H, Matsuda-Inokuchi N, Higashikawa K and
Ikeda M: Prevalence of hepatitis B and C infection markers among
adult women in urban and rural areas in Shaanxi Province, China.
Southeast Asian J Trop Med Public Health. 29:263–268.
1998.PubMed/NCBI
|
|
11
|
Tang S: Seroepidemiological study on
hepatitis C virus infection among blood donors from various regions
in China. Zhonghua Liu Xing Bing Xue Za Zhi. 14:271–274. 1993.(In
Chinese). PubMed/NCBI
|
|
12
|
Fu Y, Xia W, Wang Y, Tian L, Pybus OG, Lu
L and Nelson K: The seroprevalence of hepatitis C virus (HCV) among
559,890 first-time volunteer blood donors in China reflects
regional heterogeneity in HCV prevalence and changes in blood donor
recruitment models. Transfusion. 50:1505–1511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams V, Erwin K and Le PV: Public health
works: Blood Donation in Urban China. Soc Sci Med. 68:410–418.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan H, Wang JX, Ren FR, Zhang YZ, Zhao
HY, Gao GJ, Ji Y and Ness PM: Blood banking in China. Lancet.
360:1770–1775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi XL, Ren QH, Zhu ZY, Qu DM, Ji Y, Peng
DH and Ni SQ: Hepatitis C virus infection in blood donors in the
People's Republic of China. Transfusion. 39:9131999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia X, Luo J, Bai J and Yu R: Epidemiology
of hepatitis C virus infection among injection drug users in China:
Systematic review and meta-analysis. Public Health. 122:990–1003.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong ZX, Zhou HJ, Wang JH, Xiang XG,
Zhuang Y, Guo SM, Gui HL, Zhao GD, Tang WL, Wang H and Xie Q:
Distribution of hepatitis C virus genotypes in Chinese patients
with chronic hepatitis C: Correlation with patients'
characteristics and clinical parameters. J Dig Dis. 13:564–570.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu L, Tong W, Yuan M, Lu T, Li C and Lu L:
An increased diversity of HCV isolates were characterized among 393
patients with liver disease in China representing six genotypes, 12
subtypes, and two novel genotype 6 variants. J Clin Virol.
57:311–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai Q, Zhao Z, Liu Y, Shao X and Gao Z:
Comparison of three different HCV genotyping methods: Core, NS5B
sequence analysis and line probe assay. Int J Mol Med. 31:347–352.
2013.PubMed/NCBI
|
|
20
|
Chenna R, Sugawara H, Koike T, Lopez R,
Gibson TJ, Higgins DG and Thompson JD: Multiple sequence alignment
with Clustal series of programs. Nucleic Acids Res. 31:3497–3500.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith DB, Bukh J, Kuiken C, Muerhoff AS,
Rice CM, Stapleton JT and Simmonds P: Expanded classification of
hepatitis C virus into 7 genotypes and 67 subtypes: Updated
criteria and genotype assignment web resource. Hepatology.
59:318–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Posada D: jModelTest: Phylogenetic model
averaging. Mol Biol Evol. 25:1253–1256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aho K, Derryberry D and Peterson T: Model
selection for ecologists: The worldviews of AIC and BIC. Eclogy.
95:631–636. 2014. View Article : Google Scholar
|
|
24
|
Guindon S and Gascuel O: A Simple, fast,
and accurate algorithm to estimate large phylogenies by maximum
likelihood. Syst Biol. 52:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao X, Cui Q, Shi X, Su J, Peng Z, Chen X,
Lei N, Ding K, Wang L, Yu R and Wang N: Prevalence and trend of
hepatitis C virus infection among blood donors in Chinese mainland:
A systematic review and meta-analysis. BMC Infect Dis. 11:882011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui Y and Jia J: Update on epidemiology of
hepatitis B and C in China. J Gastroenterol Hepatol. 28:(Suppl).
S7–S10. 2013. View Article : Google Scholar
|
|
27
|
Alter MJ, Kruszon-Moran D, Nainan OV,
McQuillan GM, Gao F, Moyer LA, Kaslow RA and Margolis HS: The
prevalence of hepatitis C virus infection in the United States 1998
through 1994. N Eng J Med. 341:556–562. 1999. View Article : Google Scholar
|
|
28
|
Arrese M, Riquelme A and Soza A: Insulin
resistance, hepatic steatosis and hepatitis C: A complex
relationship with relevant clinical implications. Ann Hepatol.
9:(Suppl). S112–S118. 2010.
|
|
29
|
Lee MH, Yang HI, Yuan Y, L'Italien G and
Chen CJ: Epidemiology and natural history of hepatitis C virus
infection. World J Gastroenterol. 20:9270–9280. 2014.PubMed/NCBI
|
|
30
|
Knapp S, Warshow U, Ho KM, Hegazy D,
Little AM, Fowell A, Alexander G, Thursz M, Cramp M and Khakoo SI:
A polymorphism in IL28B distinguishes exposed, uninfected
individuals from spontaneous resolvers of HCV infection.
Gastroenterology. 141:320–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Ma H, Chen S, Wang J, Liu G, Xu M,
Ke L and He M: Interleukin-28B genetic variations and spontaneous
clearance of hepatitis C antibody-positive blood donors in China.
Transfusion. 53:2498–2504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao HY, Sun DG, Jiang D, Yang RF, Guo F,
Wang JH, Liu F, Zhang HY, Zhang HH, Du SC, et al: IL28B genetic
variants and gender are associated with spontaneous clearance of
hepatitis C virus infection. J Viral Hepat. 19:173–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simmonds P, Bukh J, Combet C, Deléage G,
Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens
G, et al: Consensus proposals for a unified system of nomenclature
of hepatitis C virus genotypes. Hepatology. 42:962–973. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson B, Myers G, Howard C, Brettin T,
Bukh J, Gaschen B, Gojobori T, Maertens G, Mizokami M, Nainan O, et
al: Classification, nomenclature, and database development for
hepatitis C virus (HCV) and related viruses: Proposals for
standardization. International committee on virus taxonomy. Arch
Virol. 143:2493–2503. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu Y, Qin W, Cao H, Xu R, Tan Y, Lu T,
Wang H, Tong W, Rong X, Li G, et al: HCV 6a prevalence in Guangdong
province had the origin from Vietnam and recent dissemination to
other regions of China: Phylogeographic analyses. PLoS One.
7:e280062012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fu Y, Wang Y, Xia W, Pybus OG, Qin W, Lu L
and Nelson K: New trends of HCV infection in China revealed by
genetic analysis of viral sequences determined from first-time
volunteer blood donors. J Viral Hepat. 18:42–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu F, Chen K, He Z, Ning T, Pan Y, Cai H
and Ke Y: Hepatitis C seroprevalence and associated risk factors,
Anyang, China. Emerg Infect Dis. 15:1819–1822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo YH, Fan JX, Wang Z, Sun DY, Wang HF,
Li ML, Liu J, Cui WG, Liu GH and Guo WS: Sero-prevalence and
associated risk factors on hepatitis C in Maqiao township, Henan
province of China. Zhonghua Liu Xing Bing Xue Za Zhi. 33:722–725.
2012.(In Chinese). PubMed/NCBI
|